Development and Characterization of Double-Antibody Sandwich ELISA for Detection of Zika Virus Infection
Abstract
1. Introduction
2. Materials and Methods
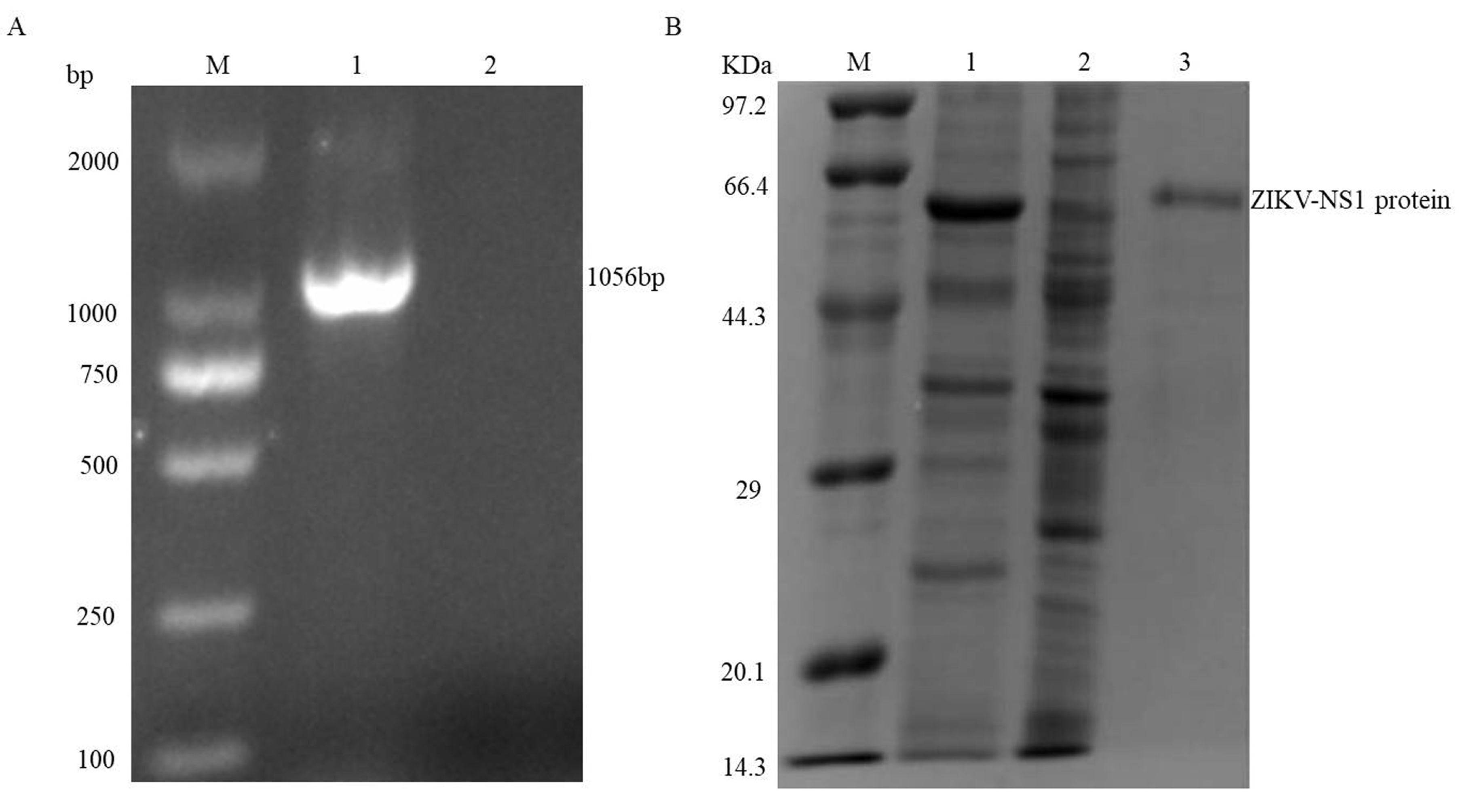
2.1. Expression and Purification of the NS1 Protein
2.2. Ethics Statement
2.3. Production of Monoclonal and Polyclonal Antibodies Against the ZIKV-NS1 Protein
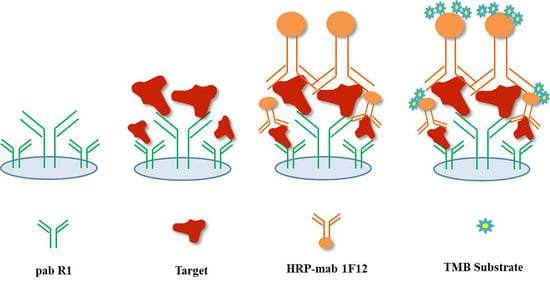
2.4. Detection of Recombinant ZIKV-NS1 Protein by the DAS-ELISA
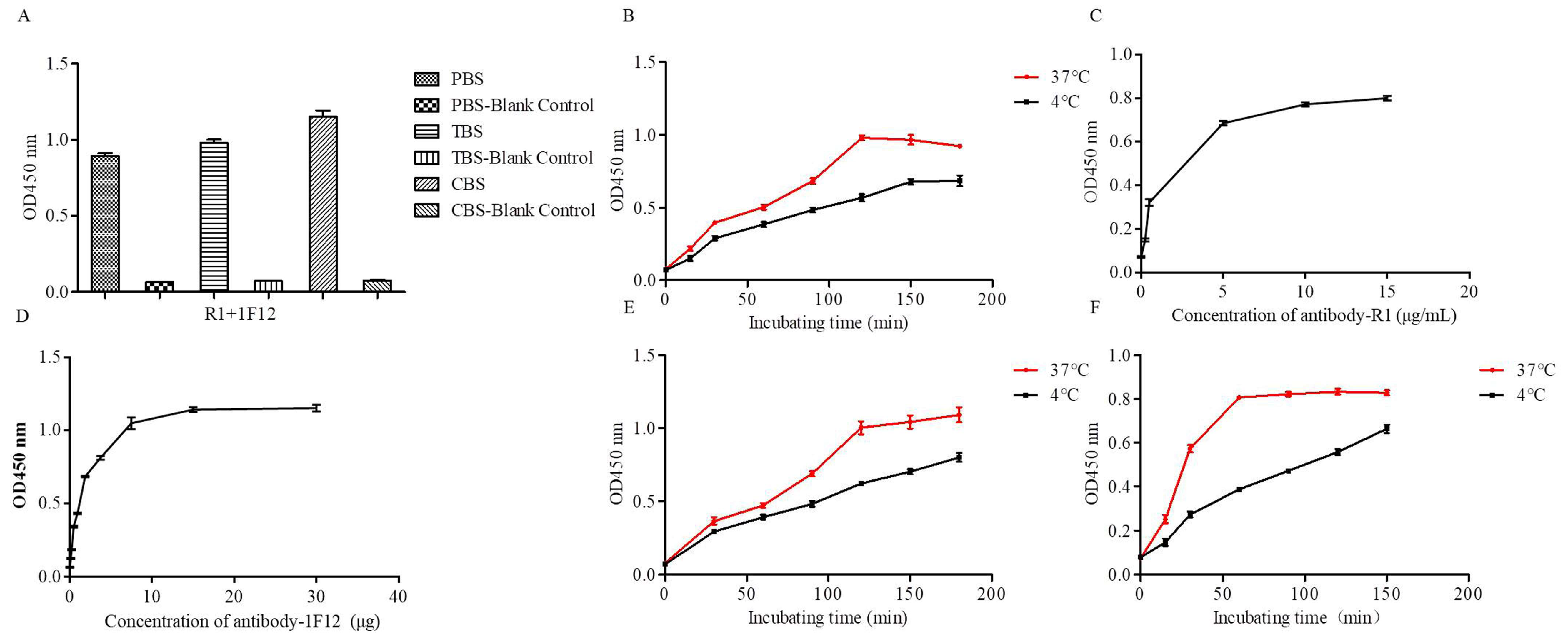
2.5. Titer and Binding Affinity of mAb 1F12 and pAb R1 Based on the ELISA
2.6. Western Blot Assay
2.7. Indirect Immunofluorescent Assay
2.8. Establishment of the DAS-ELISA Based on pAb R1 and mAb 1F12 Probe
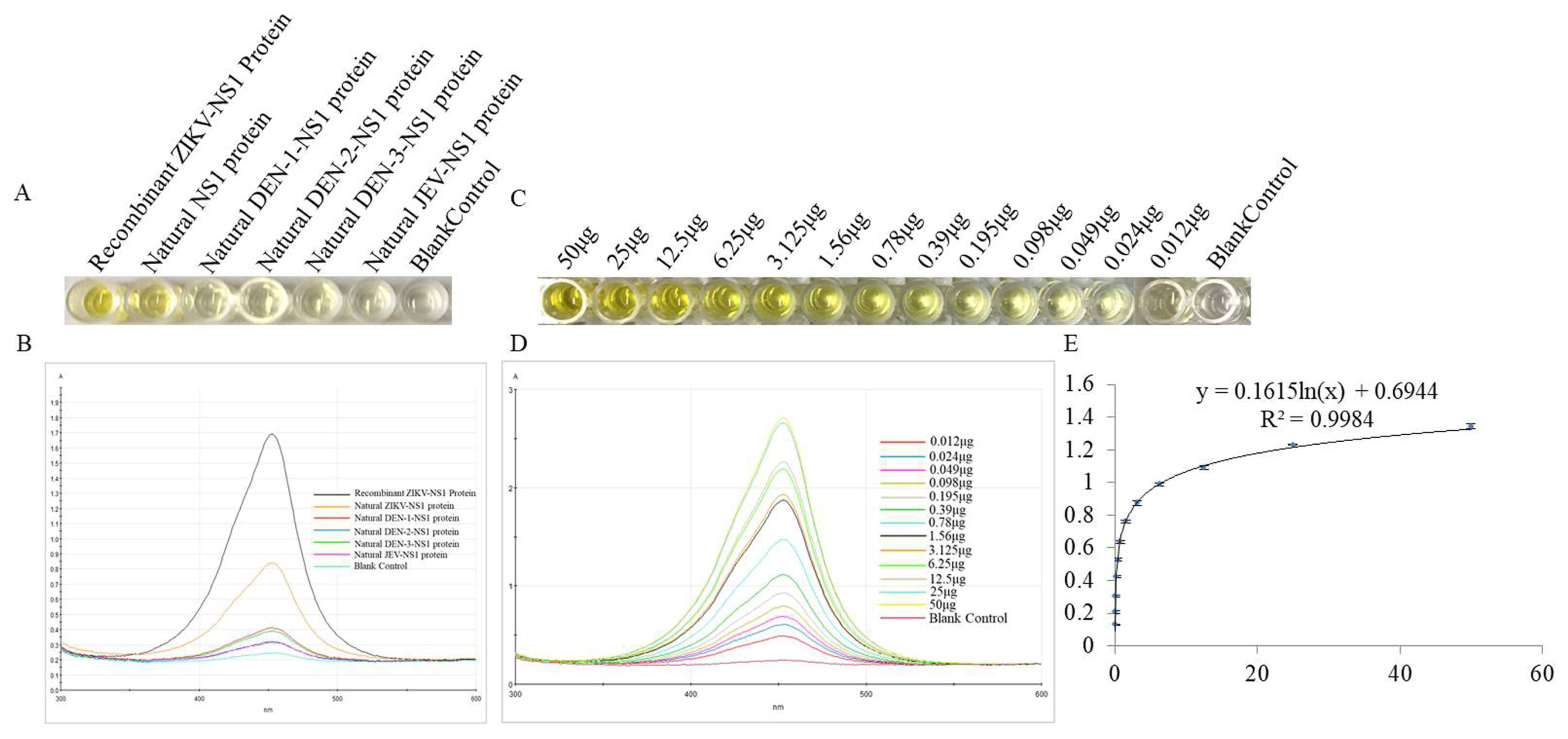
2.9. Specificity and Sensitivity of the DAS-ELISA
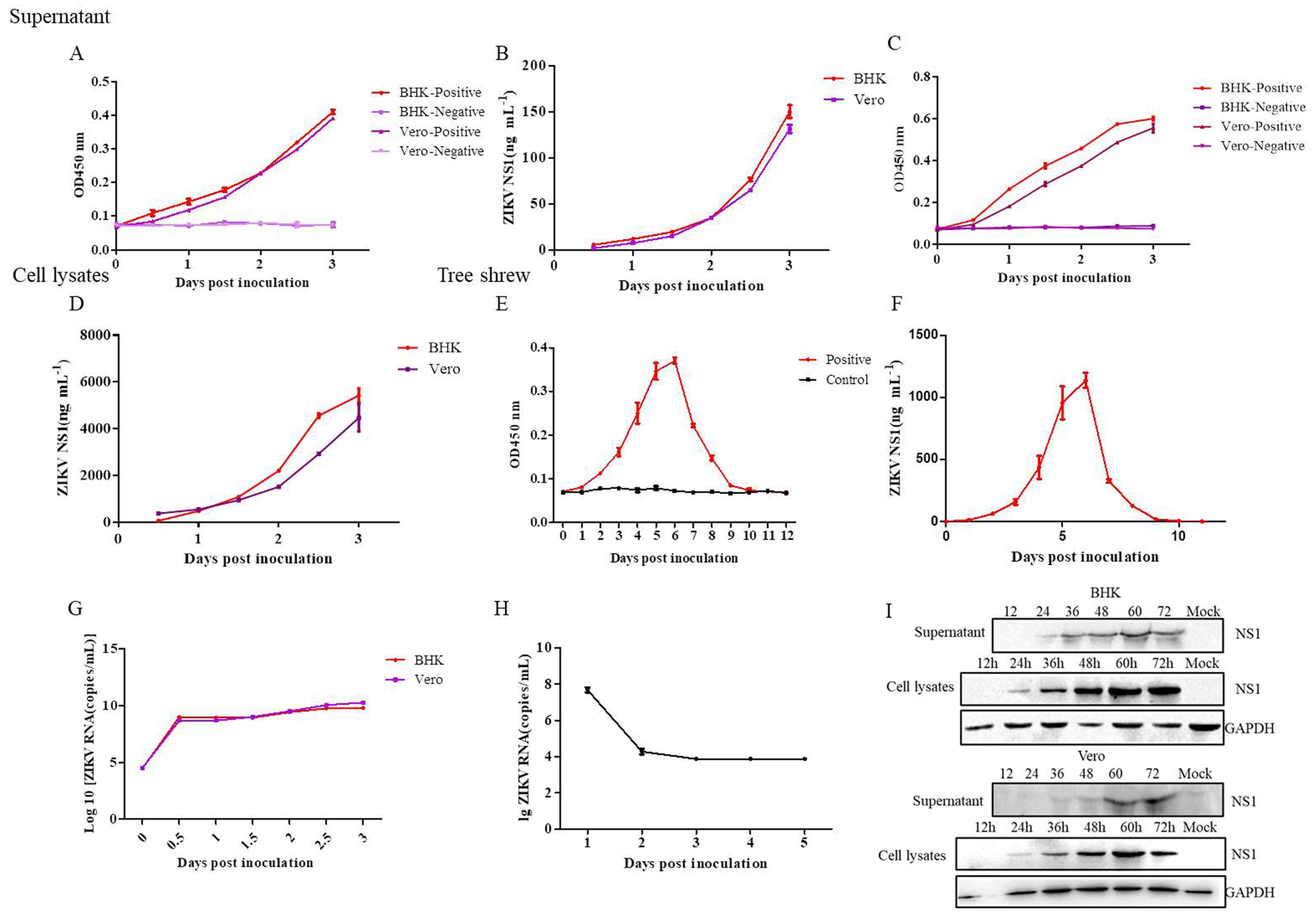
2.10. Application of the DAS-ELISA for the Detection of ZIKV Infection in Cells and Tree Shrews
3. Results
3.1. Expression and Purification of the ZIKV-NS1 Protein
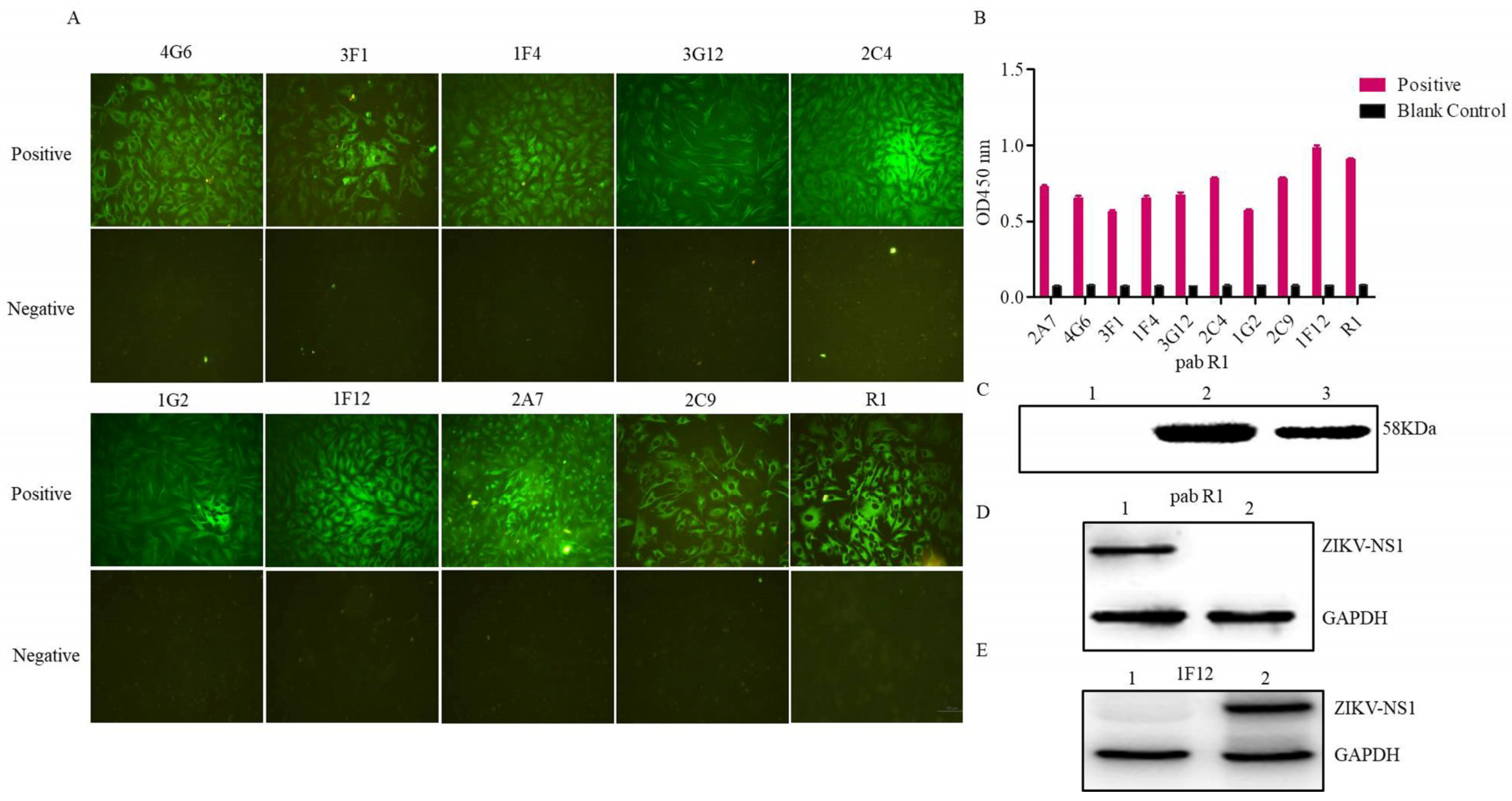
3.2. Evaluation of the Rabbit Antiserum
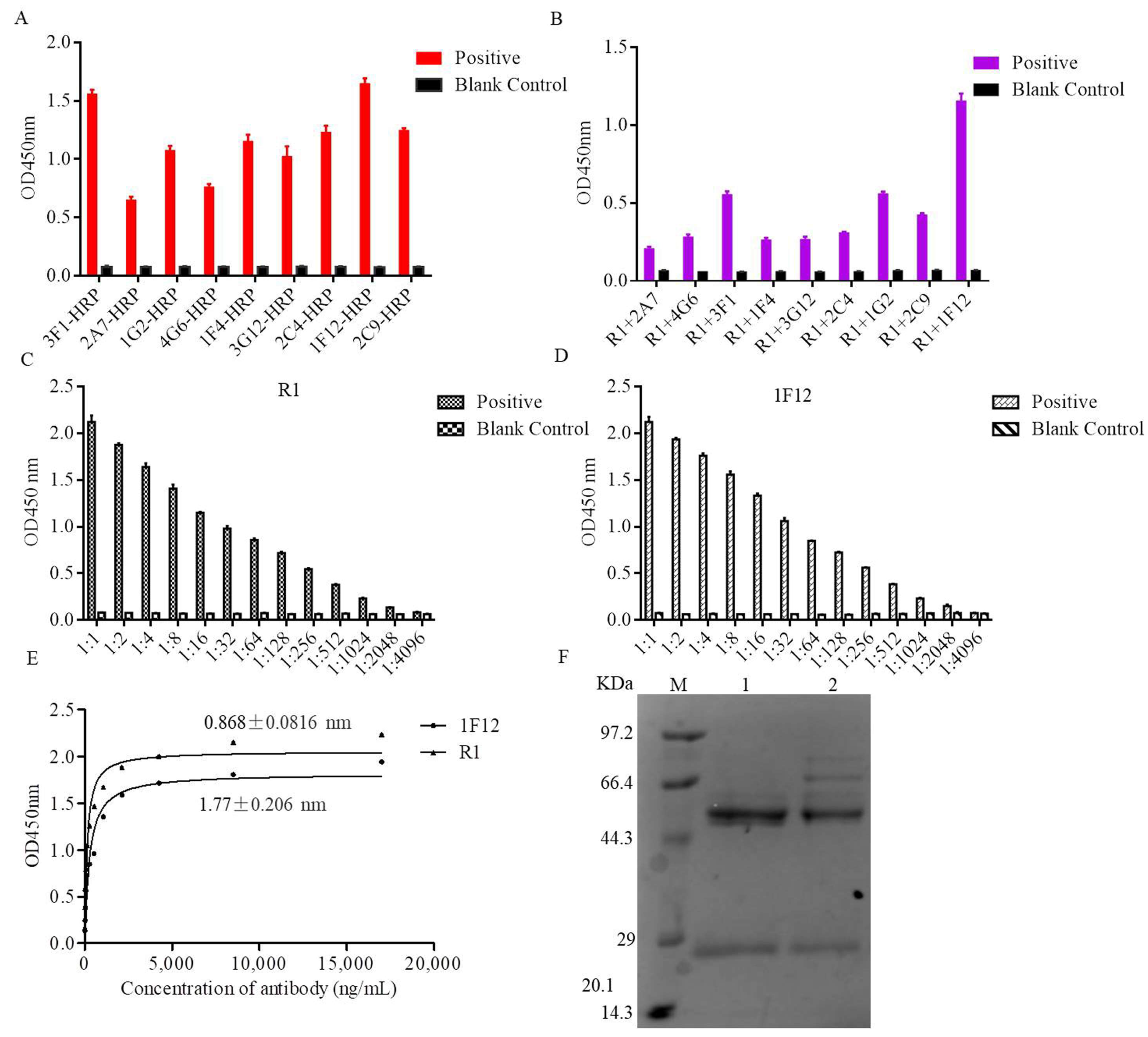
3.3. Characterization of the Ascites Against NS1 Protein
3.4. Establishment of the DAS-ELISA
3.5. Optimization Operations
3.6. The Specificity and Sensitivity of ZIKV Detection by DAS-ELISA
3.7. Application of the DAS-ELISA for ZIKV Detection in the Supernatants, Cell Lysates of Cells, and the Sera of Tree Shrews
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Dick, G.W.A.; Kitchen, S.F.; Haddow, A.J. Zika Virus (I). Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 509–520. [Google Scholar] [CrossRef]
- Rasmussen, S.A.; Jamieson, D.J.; Honein, M.A.; Petersen, L.R. Zika Virus and Birth Defects—Reviewing the Evidence for Causality. N. Engl. J. Med. 2016, 374, 1981–1987. [Google Scholar] [CrossRef] [PubMed]
- Coyne, C.B.; Lazear, H.M. Zika virus- reigniting the TORCH. Nat. Rev. Microbiol. 2016, 14, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Brasil, P.; Pereira, J.P., Jr.; Moreira, M.E.; Nogueira, R.M.R.; Damasceno, L.; Wakimoto, M.; Rabello, R.S.; Valderramos, S.G.; Halai, U.A.; Salles, T.S. Zika Virus Infection in Pregnant Women in Rio de Janeiro. N. Engl. J. Med. 2016, 375, 2321–2334. [Google Scholar] [CrossRef] [PubMed]
- Caolormeau, V.M.; Blake, A.; Mons, S.; Lastère, S.; Roche, C.; Vanhomwegen, J.; Dub, T.; Baudouin, L.; Teissier, A.; Larre, P. Guillain-Barre Syndrome outbreak associated with Zika virus infection in French Polynesia: A case-control study. Lancet 2016, 387, 1531–1539. [Google Scholar] [CrossRef]
- Heymann, D.L.; Hodgson, A.; Sall, A.A.; Freedman, D.O.; Staples, J.E.; Althabe, F.; Baruah, K.; Mahmud, G.; Kandun, N.; Vasconcelos, P.F.C. Zika virus and microcephaly: Why is this situation a PHEIC. Lancet 2016, 387, 719–721. [Google Scholar] [CrossRef]
- Fauci, A.S.; Morens, D.M. Zika Virus in the Americas—Yet Another Arbovirus Threat. N. Engl. J. Med. 2016, 374, 601–604. [Google Scholar] [CrossRef] [PubMed]
- Maurer-Stroh, S.; Mak, T.M.; Ng, Y.K.; Phuah, S.P.; Huber, R.G.; Marzinek, J.K.; Holdbrook, D.A.; Lee, R.T.; Cui, L.; Lin, R.T. South-east Asian Zika virus strain linked to cluster of cases in Singapore, August 2016. Eurosurveillance 2016, 21, 3407. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.A.; Mier-Y-Teran-Romero, L.; Reefhuis, J.; Gilboa, S.M.; Hills, S.L. Zika and the Risk of Microcephaly. N. Engl. J. Med. 2016, 375, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, W.; Wong, G.; Bi, Y.; Yan, J.; Yi, S.; Chen, E.; Hao, Y.; Lou, X.; Mao, H. Highly diversified Zika viruses imported to China, 2016. Protein Cell 2016, 7, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Haddow, A.D.; Schuh, A.J.; Yasuda, C.Y.; Kasper, M.R.; Heang, V.; Huy, R.; Guzman, H.; Tesh, R.B.; Weaver, S.C. Genetic characterization of Zika virus strains: Geographic expansion of the Asian lineage. PLoS Negl. Trop. Dis. 2012, 6, e1477. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.K. Zika virus: Epidemiology, current phobia and preparedness for upcoming mass gatherings, with examples from World Olympics and Pilgrimage. Pak. J. Med. Sci. 2016, 32, 1038–1043. [Google Scholar] [CrossRef] [PubMed]
- Bhavani, K.G.; Krishna, K.B.M.; Srinivasu, N.; Ramachandran, D.; Raman, N.V.V.S.S.; Babu, B.H. Determination of genotoxic impurity in atazanavir sulphate drug substance by LC-MS. J. Pharm. Biomed. Anal. 2017, 132, 156–158. [Google Scholar]
- Nicolini, A.M.; Mccracken, K.E.; Yoon, J.Y. Future developments in biosensors for field-ready Zika virus diagnostics. J. Biol. Eng. 2017, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Musso, D.; Gubler, D.J. Zika Virus. Clin. Microbiol. Rev. 2016, 29, 487–524. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.T. Update: Interim Guidance for Prevention of Sexual Transmission of Zika Virus—United States, July 2016. Morb. Mortal. Wkly. Rep. 2016, 65, 323–325. [Google Scholar] [CrossRef] [PubMed]
- Suthar, M.S.; Diamond, M.S.; Gale, M., Jr. West Nile virus infection and immunity. Nat. Rev. Microbiol. 2013, 11, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Klema, V.; Padmanabhan, R.; Choi, K. Flaviviral Replication Complex: Coordination between RNA Synthesis and 5′-RNA Capping. Viruses 2015, 7, 4640–4656. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.C.; Akey, D.L.; Konwerski, J.R.; Tarrasch, J.T.; Skiniotis, G.; Kuhn, R.J.; Smith, J.L. Extended surface for membrane association in Zika virus NS1 structure. Nat. Struct. Mol. Biol. 2016, 23, 865–867. [Google Scholar] [CrossRef] [PubMed]
- Young, P.R.; Hilditch, P.A.; Bletchly, C.; Halloran, W. An Antigen Capture Enzyme-Linked Immunosorbent Assay Reveals High Levels of the Dengue Virus Protein NS1 in the Sera of Infected Patients. J. Clin. Microbiol. 2000, 38, 1053–1057. [Google Scholar] [PubMed]
- Cecchetto, J.; Fernandes, F.C.B.; Lopes, R.; Bueno, P.R. The capacitive sensing of NS1 Flavivirus biomarker. Biosens. Bioelectron. 2017, 87, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Parkash, O.; Shueb, R.H. Diagnosis of Dengue Infection Using Conventional and Biosensor Based Techniques. Viruses 2015, 7, 5410–5427. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.J.; Furuya, A.; Zou, J.; Xie, X.; Ii, A.P.D.; Kramer, L.D.; Shi, P.Y. A Multiplex Microsphere Immunoassay for Zika Virus Diagnosis. Ebiomedicine 2017, 16, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Stettler, K.; Beltramello, M.; Espinosa, D.A.; Graham, V.; Cassotta, A.; Bianchi, S.; Vanzetta, F.; Minola, A.; Jaconi, S.; Mele, F. Specificity, cross-reactivity and function of antibodies elicited by Zika virus infection. Science 2016, 353, 823–826. [Google Scholar] [CrossRef] [PubMed]
- Musso, D.; Roche, C.; Nhan, T.X.; Robin, E.; Teissier, A.; Caolormeau, V.M. Detection of Zika virus in saliva. J. Clin. Virol. 2015, 68, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Bingham, A.M. Comparison of Test Results for Zika Virus RNA in Urine, Serum, and Saliva Specimens from Persons with Travel-Associated Zika Virus Disease—Florida, 2016. Morb. Mortal. Wkly. Rep. 2016, 65, 475–478. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Sun, T.; Xia, X.; Wei, Q.; Song, Y.; Han, Q.; Chen, Q.; Hu, J.; Zhang, J. Optimized Expression, Purification of Herpes B Virus gD Protein in Escherichia coli, and Production of Its Monoclonal Antibodies. Jundishapur J. Microbiol. 2016, 9, e32183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, C.; Dai, L.; Zhang, L.; Xu, K.; Song, Y.; Xia, X.; Han, Q.; Chen, Q.; Zhang, J. Efficient Capture and Detection of Zika Virion by Polyclonal Antibody Against Prokaryotic Recombinant Envelope Protein. Jundishapur J. Microbiol. 2018, 11, e68858. [Google Scholar] [CrossRef]
- Musso, D.; Nilles, E.J.; Cao-Lormeau, V.M. Rapid spread of emerging Zika virus in the Pacific area. Clin. Microbiol. Infect. 2014, 20, O595–O596. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Zeng, H. Aptamer-Based ELISA Assay for Highly Specific and Sensitive Detection of Zika NS1 Protein. Anal. Chem. 2017, 89, 12743–12748. [Google Scholar] [CrossRef] [PubMed]
- Langerak, T.; Yang, H.; Baptista, M.; Doornekamp, L.; Kerkman, T.; Codrington, J.; Roosblad, J.; Vreden, S.G.; De Bruin, E.; Mogling, R.; et al. Zika Virus Infection and Guillain-Barre Syndrome in Three Patients from Suriname. Front. Neurol. 2016, 7, 233. [Google Scholar] [CrossRef] [PubMed]
- Gourinat, A.C.; O’Connor, O.; Calvez, E.; Goarant, C.; Dupontrouzeyrol, M. Detection of Zika Virus in Urine. Emerg. Infect. Dis. 2015, 21, 84–86. [Google Scholar] [CrossRef] [PubMed]
| Name | Sequence | Length (bp) |
|---|---|---|
| ZIKV-ASF | GGTCAGCGTCCTCTCTAATAAACG | 24 |
| ZIKV-ASR | GCACCCTAGTGTCCACTTTTTCC | 23 |
| ZIKV-Probe | FAM-AGCCATGACCGACACCACACCGT-BQ1 | 23 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Du, X.; Chen, C.; Chen, Z.; Zhang, L.; Han, Q.; Xia, X.; Song, Y.; Zhang, J. Development and Characterization of Double-Antibody Sandwich ELISA for Detection of Zika Virus Infection. Viruses 2018, 10, 634. https://doi.org/10.3390/v10110634
Zhang L, Du X, Chen C, Chen Z, Zhang L, Han Q, Xia X, Song Y, Zhang J. Development and Characterization of Double-Antibody Sandwich ELISA for Detection of Zika Virus Infection. Viruses. 2018; 10(11):634. https://doi.org/10.3390/v10110634
Chicago/Turabian StyleZhang, Liding, Xuewei Du, Congjie Chen, Zhixin Chen, Li Zhang, Qinqin Han, Xueshan Xia, Yuzhu Song, and Jinyang Zhang. 2018. "Development and Characterization of Double-Antibody Sandwich ELISA for Detection of Zika Virus Infection" Viruses 10, no. 11: 634. https://doi.org/10.3390/v10110634
APA StyleZhang, L., Du, X., Chen, C., Chen, Z., Zhang, L., Han, Q., Xia, X., Song, Y., & Zhang, J. (2018). Development and Characterization of Double-Antibody Sandwich ELISA for Detection of Zika Virus Infection. Viruses, 10(11), 634. https://doi.org/10.3390/v10110634