Abstract
To guide the cultivation of superior Chinese fir plantlets, we designed an L16(4)4 orthogonal experiment to determine how leaf age and exogenous hormones influence key growth processes in leaf cuttings. Hormone concentration and treatment duration significantly affected leaf cuttings in all three age categories; 6-benzylaminopurine (6-BA), 1-naphthaleneacetic acid (NAA), and treatment time exerted the strongest effects on callus initiation rates. Additionally, NAA had the largest effect on the rooting rate across all cuttings, and all three hormones significantly influenced the bud germination rate. Based on our experimental results, expected optimal treatments for callus initiation were 10 mg∙L−1 indole-3-butyric acid (IBA) for 10 min, 30 mg∙L−1 NAA for 15 min, and 10 mg∙L−1 NAA plus 30 mg∙L−1 IBA for 10 min. For the rooting rate, the expected optimal treatment was 50 mg∙L−1 NAA and 40 mg∙L−1 IBA for 5–20 min. Finally, for bud germination, optimal treatments were 20 min of immersion in water, 30 mg∙L−1 6-BA plus 50 mg∙L−1 NAA for 15 min, and 30 mg∙L−1 6-BA for 5 min. Plantlet formation only occurred in the <one-year-old leaves, and at very low rates (maximum 5.8%); this outcome is likely attributable to the mother plant’s relatively old age (five years). Plantlet formation from cuttings is dependent on ensuring the rooting rate after callus initiation. Therefore, to promote rooting rates and bud germination, we recommend leaving more xylem at the base of leaf cuttings.
1. Introduction
Chinese fir (Cunninghamia lanceolata (Lamb.) Hook.) is a coniferous species endemic to southern China that has been cultivated for approximately 1000 years. As the most abundant Chinese timber species, its current planting area is greater than 1.10 × 107 ha, accounting for 24% of the nation’s total forests [1]. With its desirable features (fast growth, high timber quality, and pest/disease resistance), the Chinese fir is widely used in construction, furniture, shipbuilding, pulp production, medicine, and landscape or residential horticulture [2]. With heightened economic development and a concurrent surge in timber demand, annual shortages in high-quality Chinese-fir plantlets have become an increasing concern. Currently, Chinese fir plantations are mainly produced from seedlings, and numerous seed orchards are established in China. However, seed breeding easily causes genetic recombination in offspring, raising difficulties in preserving desirable parental traits [3]. Furthermore, seed orchards tend to have an unstable yield because seeds are vulnerable to environmental variation, and older seeds have high abortive rates coupled with low germination rates [4,5]. Therefore, cultivating numerous high-quality plantlets is essential to the stability and productivity of Chinese fir plantations [6].
Asexual propagation involves using plant parts (roots, leaves, stems, and bulbs) to generate new plants, commonly through techniques including tissue culture, cutting, and layer propagation [5,7]. The success of tissue culture propagation strictly depends on efficient, reliable, and stable regeneration protocols that decrease contamination and improve survival [8]. Due to the implementation difficulties of such protocols, plant breeders frequently turn to cutting propagation in cereal crops, medicinal herbs, and ornamental plants [9,10,11]. For forestation programs, cutting plantlets are a better option than sexual (seed-grown) and tissue-culture propagation due to several reasons. First, compared to seed propagation, cultivars from cuttings maintain excellent parental traits that are passed on to the next generation. It has been reported that cultivars have significant advantages over seed-grown plantlets in terms of tree height, diameter at breast height (DBH), and single tree volume [12,13]. Furthermore, tissue culture propagation requires strict protocol to optimize the media components and training of professional workers to master the tissue culture technology. Indeed, calli formation from Chinese fir organs using the tissue culture method is difficult, and cannot be subcultured for more than two generations [14]. Cutting materials are abundant and easy to obtain. Successful cutting propagation is associated with cutting collection period [12], auxin concentration [15], treatment method [16], and organ positions [17]. The numerous and complex factors influencing cutting propagation success suggest that more research is necessary to refine the process. Indeed, only a few studies have examined the cutting propagation of trees due to low rooting and plantlet formation rates [18]. Intriguingly, one study found that rooting traits were greatly improved under a new propagation technique that pretreated stem cuttings with quick dips in 2000 mg∙L−1 indole-3-butyric acid (IBA) [9].
Semi-lignified twigs have been successfully used to propagate Chinese-fir cutting [19]. Ortet age and cutting length are often the most important factors affecting rooting and early ramet growth [20]. Plantlet formation rates of one-year-old stem cuttings decreased as the parental age increased (87.3%, 84.6%, and 45% for 5, 10, and 15 years old), and rose with a greater cutting length (1.6%–53.0% for 1–6 cm). Plantlet rates also decreased from 99.0% to 74.5% as stem cutting density per unit area increased from 67 to 200 m2 [21]. In contrast to shoots and roots, leaf cuttings are not as easily propagated, mainly because they lack dormant meristems. Indeed, most tree and grass species cannot root from leaf cuttings [22]. However, leaves have been used for the cutting propagation of Chinese fir since the 1970s. The Fuzhou Forestry Ministry was the first to attempt using Chinese fir leaves as cutting materials for forestation (Forestry Ministry, Fushun district, 1975) [23]. They demonstrated that when leaf cuttings were immersed in 50 ppm IBA for 24 h before being planted, callus and rooting rates reached 80% and 45%, respectively. Additionally, spring appears to be the optimum period for Chinese-fir leaf cuttings, with bud germination rates varying from 60%–70%, although the rooting rate is low at 20% [23]. The sampling position of leaves on the ortet directly affects the plantlet formation rate in a phenomenon called topophysis, whereby plant parts retain specific characteristics for a period of time [24]. Plantlets form at a high rate of 65.1% when the leaf cuttings with axillary buds are from plagiotropic branching of less than one-year old. However, plantlet rates were low if leaf cuttings came from two-year-old branches (44.5%). Beyond these studies, we have little data on using Chinese-fir leaves for cutting propagation because of general low rooting and plantlet formation success. However, in our previous study, needles from three superior Chinese-fir clones formed calli 15 days post-cutting at a high callus initiation rate (maximum 90%). They also rooted 50 days post-cutting, but at lower rates (averaging 17%) [25]. Therefore, using Chinese fir needles may allow successful propagation and ease seedling shortage on plantations.
In this study, we hypothesized that leaf age and hormone-combination treatments would significantly affect callus initiation, rooting, bud germination, and plantlet formation. We used an orthogonal L16(4)4 design to determine the conditions (age and hormone combination) for optimizing the rates of the four propagation processes.
2. Materials and Methods
2.1. Greenhouse Management and Leaf-Cutting Collection
The experiment was conducted at the greenhouse of Fujian Agriculture and Forestry University (FAFU), Fuzhou, south China (26°4′52.5″ N, 119°14′48.6″ E; 38 m a.s.l.). The site is characterized by a typical subtropical monsoon climate with an average annual temperature of 20–25 °C and average precipitation of 900~2100 mm. The highest mean temperature was 33–37 °C in July or August, with an extreme mean temperature of 42.3 °C in summer and of −2.5 °C in winter. The greenhouse seedbed matrix comprised homogeneous river sand that had been disinfected with 0.3% potassium permanganate one week before testing. A shade net covered the greenhouse to maintain light transmittance at 30%–50%. The sand bed was kept moist (relative humidity ~85%) using an overhead boom automatic irrigation system (15 s per 30 min in the day) (Figure 1). After the sand bed was treated with compound fertilizer and irrigated with tap water, grooves were dug with a wooden stick for planting cuttings.

Figure 1.
Leaf cutting propagation of Chinese fir in the greenhouse.
All leaves were from one five-year-old mother plant on a hill in FAFU. All leaves were picked from the first plagiotropic branch and were divided into three groups (<one-year-old, one-year-old, and two-year-old) based on existing categorization [26]. Chinese fir leaves form a new node annually (with some variation due to ambient temperature). Thus, the apex needle in the first node was <one-year old, while subsequent needles farther along the branch were one and two years old, respectively. Chinese-fir leaves were picked on a sunny morning (21 August 2011). Leaves with xylem at the base (5 mm long) were removed using single-sided blades. All samples were then immersed in exogenous hormone for different times according to the experimental design, and were then planted in the pre-dug grooves at 10 cm × 10 cm spacing. Two-thirds of the leaves were outside the seedbed.
2.2. Orthogonal Experimental Design
To determine appropriate hormone concentrations for treatment, we performed a preliminary leaf-cutting experiment based on a Chinese fir study in Guizhou (Leaf Cutting Study Groups 1979) [27]. The experiment was an L16(4)4 orthogonal design. Sixteen treatments of three hormones (6-BA (6-benzylaminopurine), NAA (1-naphthaleneacetic acid), and IBA) at four concentrations (0, 50, 100, and 150 mg∙L−1) and immersion times (10, 20, 60, and 120 min) were tested. Each treatment had 30 replicates, and a total of 1440 leaf cuttings were used (16 treatments × 30 replicates × 3 leaf ages). The results of this preliminary study showed that the callus initiation rate was highest at 50 mg·L−1 each of IBA and NAA, but decreased greatly at 100 and 150 mg∙L−1. Additionally, the callus initiation rate decreased with increasing treatment time. Therefore, in the main experiment (Table 1), 6-BA, NAA, and IBA concentrations were set to 0, 10, 30, and 50 mg·L−1, respectively, at immersion times of 5, 10, 15, and 20 min. The following indicators were measured 12 weeks after cutting propagation:

Table 1.
The L16(4)4 orthogonal experimental design including different growth hormone combinations and treatment durations. 6-BA: 6-benzylaminopurine; NAA: 1-naphthaleneacetic acid; IBA: indole-3-butyric acid.
- (1)
- Callus initiation rate = leaf cuttings with callus/total number of leaves,
- (2)
- Rooting rate = leaf cuttings with roots/total number of leaves,
- (3)
- Bud germination rate = leaf cuttings with buds/total number of leaves,
- (4)
- Plantlet formation rate = leaf cuttings with buds and roots/total number of leaves.
2.3. Histology
The root structure of leaf cuttings was observed with the paraffin method.
First, leaf cuttings were fixed with FAA solution (70% alcohol: 90 mL, glacial acetic acid: 5 mL, formalin: 5 mL) for 24 h, and then rinsed three times (1 h per rinse) with 70% ethanol. They were then dehydrated in an increasing ethanol series, as follows: 85% ethanol (1 h) → 95% ethanol (1 h) → 100% ethanol (30 min) → 100% ethanol (30 min). Next, samples were cleared with a 1:1 ethanol: xylene solution for 1 h, followed by two separate xylene baths (30 min each). Fixed tissues were dipped in a 1:1 xylene: paraffin mixture for 1 h, then twice in paraffin (30 min each), heated to 62 °C, placed in a box containing paraffin, and cooled in water for 5–10 min.
Second, the tissue-embedded paraffin block was placed on a glass plate and fixed on the sample stage with a heated dissection knife. A rotary slicer was used to cut 10 μm sections that were patched on slides using a glycerol coating, and these were then placed on a 50 °C table, ensuring that the slices were completely flat.
Third, sliced samples were dewaxed with two xylene washes (5 min each), before being rehydrated in a decreasing ethanol series (5 min per step): 1:1 ethanol: xylene solution → 100% ethanol → 100% ethanol → 85% ethanol → 70% ethanol → 50% ethanol → 30% ethanol → distilled water. Next, samples were dyed with 1% red water solution for 1 h, followed by an increasing alcohol series and a xylene seal: 5 min in 35% alcohol, 5 min in 50% alcohol, 3 min in 70% alcohol, 3 min in 80% alcohol, 1 min in 100% alcohol, 3 min again in 100% alcohol, 5 min in 1:1 pure alcohol: xylene solution, and 5 min in xylene.
Finally, images of the tissue slices were taken with a Ti-S200 inverted fluorescence microscope (Nikon, Tokyo, Japan), supplied by the Fujian Chinese Fir Engineering Technology Research Center.
2.4. Statistical Analysis
Two-way ANOVA was used to test for significant effects of hormone treatment, leaf age, and their interactions on callus initiation, rooting, bud germination, and plantlet formation. Mean comparisons were performed using the least significant differences (LSD) test. To calculate the optimal combination and factor effects for different values, we used range analysis with the formula /4, RX = max{KiX} − min{KiX }, where X (factor) = A, B, C, or D and i (level) = 1, 2, 3, or 4. Here, K is the average of values per factor to determine the optimal level, and R is the gap between the maximum and minimum values per factor to determine their effects. All analyses were conducted in SPSS 17.0 for Windows (SPSS Ins., Chicago, IL, USA). Significance was set at p < 0.05.
3. Results
3.1. Callus Initiation Rate and Rooting Rate
Callus initiation and rooting rates varied significantly between different leaf ages and treatments (p < 0.001, ANOVA; Table 2). The interaction between leaf age and treatment was significant (p < 0.001) (Figure 2a,b). Range analysis indicated that the major factors affecting the callus initiation rate were 6-BA, NAA, and immersion time for <one-year-old, one-year-old, and two-year-old cuttings, respectively (Table 2). Average callus initiation rates increased with increasing leaf age (67.9%, 76.9%, and 77.2%) (Figure 3a). Optimal hormone combinations for the callus initiation rate from young to older cuttings were treatments 11, 16, and 14, respectively, with treatment 16 (A4B4C1D3) being the maximum (93.3% for one-year-olds) (Table 3).

Table 2.
Summary of two-way ANOVA for the main effects of leaf age and treatment, and their interaction on callus initiation, rooting, bud germination, and plantlet formation of Chinese fir leaf cuttings.

Figure 2.
Growth of Chinese fir cuttings. (a) Callus initiation; (b) root formation; (c) bud germination; and (d) plantlet formation.
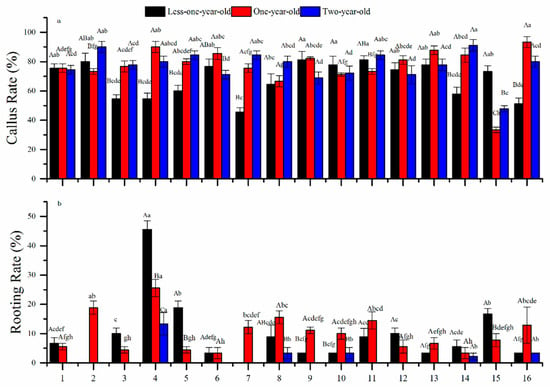
Figure 3.
Effects of growth hormones at differing concentrations on the (a) callus initiation rate and (b) rooting rate of Chinese fir leaf cuttings. Different lowercase letters above the columns indicate significant differences (p < 0.05) between different treatments. Different capital letters indicate significant differences (p < 0.05) between different leaf ages. The numbers 1 to 16 on the horizontal axis refer to the 16 treatments in Table 1.

Table 3.
Range analysis of results from the L16(4)4 orthogonal experiment examining the callus initiation rate, rooting rate, bud germination rate, and plantlet formation rate. * represented the optimal level in each variable.
The most important determinant factor for rooting rates was NAA (Table 2). Average rooting rates were low and decreased with age (<one-year-old: 10.6%, one-year-old: 10.1%, and two-year old: 5.1%; Figure 3b). Treatment 4 (A1B4C4D4) resulted in the maximum rooting rate for all three ages (45.6%, 25.6%, and 13.3%; Table 2).
3.2. Bud Germination Rate and Plantlet Formation Rate
Bud germination rates and plantlet formation rates significantly varied between different ages and hormone treatments (p < 0.001, ANOVA; Table 2). The interaction between leaf age and treatment was also significant (p < 0.001; Figure 2c). Range analysis showed that 6-BA had the strongest effect on the bud germination rate for <one-year-olds and one-year-olds, while NAA was most effective on two-year-olds (Table 2). Average bud germination rates were 46.1%, 15.1%, and 25.8%, respectively, for the three age groups. Maximum germination rates for the three age groups were 71.1%, 45.6%, and 44.4% in treatments 13, 16, and 1, respectively (Figure 4a). However, the optimal hormone combination for bud germination rates was A3B1C1D4, A3B2C1D3, and A3B1C1D1 for <one-year-olds, one-year-olds, and two-year-olds, respectively (Table 2).
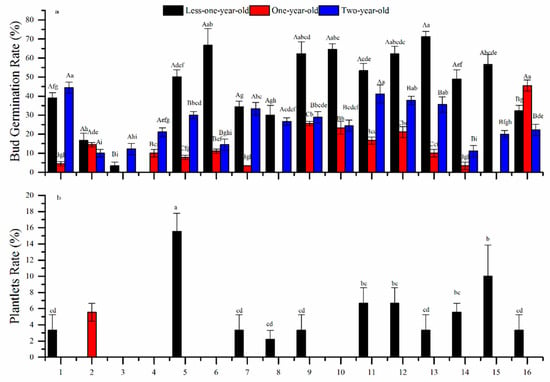
Figure 4.
Effects of growth hormones at differing concentrations on the (a) bud germination rate and (b) plantlet formation rate of Chinese-fir leaf cuttings. Different lowercase letters above the columns indicate significant differences (p < 0.05) between different treatments. Different capital letters indicate significant differences (p < 0.05) between different leaf ages. The numbers 1 to 16 on the horizontal axis refer to the 16 treatments in Table 1.
Only <one-year-olds exhibited plantlet formation, and the rate was very low (average 5.8%) (Figure 4d). The most effective to least effective hormone-and-immersion-time combinations were C > B > A > D (Table 2). Plantlet formation rate was highest in treatment 5 (A2B1C2D3) at 15.5%, but the optimal combinations for the plantlet formation rate were A3B1C2D1 and A3B1C2D3 (Table 2).
3.3. Root Anatomical Characteristics of Chinese-Fir Leaf Cuttings
In a previous study, we found that leaf tissue did not contain primitive latent roots [24,25]. Darker cell clusters (calli) appeared at the base of leaf cuttings and slowly expanded 40 d post-cutting (Figure 2a). Calli cells originated from cortical parenchyma cells, which formed root primordial cells with a pyramid-like contour (Figure 5). At around 50 days post-cutting, these subcuticular primordial cells then volumetrically expanded and elongated, breaking the callus surface and forming adventitious roots. Therefore, calli appearance is the basis of adventitious root formation, which in turn promoted primordial root cell differentiation and growth. This process is clearly of great importance in the successful rooting of Chinese-fir leaf cuttings.
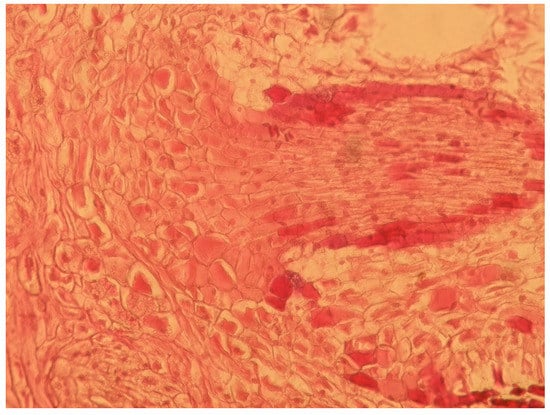
Figure 5.
Root formation from calli, observed using light microscopy. Here, the callus begins to differentiate into sharp and long primordial tissues.
4. Discussion
The results of this study confirm our hypothesis that leaf age and exogenous hormones combinations significantly (p < 0.001) affect the rates of callus formation, rooting, bud germination, and plantlet formation during leaf-cutting propagation of Chinese fir. We found low rooting rates (5.1%–10.6%) that decreased with increasing leaf age. However, callus initiation rates and bud germination rates were relatively higher (67.9%–77.2% and 15.1%–46.1%, respectively). Not all the plants can be propagated from a leaf or a section of a leaf, and leaf cuttings are commonly used for propagating some herbaceous and succulent plants [28]. Previous experiments showed that leaf cuttings with axillary buds were able to form new plants, like citrus, Eucalyptus, Populus plants, etc. [29,30]. Our findings concur with the existing understanding of a low rooting rate being the primary challenge facing Chinese-fir propagation through leaf cuttings. Treatments with exogenous hormones (e.g., 6-BA, NAA, and IBA) are a common method employed to address this problem [31,32], and here we identified 50 mg·L−1 NAA + 50 mg·L−1 IBA + 20 min immersion time as the optimal hormone treatment for boosting rooting rates. In comparison, 1 h in an IBA solution effectively stimulated rooting in cuttings from four olive cultivars [33]. Additionally, the best overall rooting for Gardenia augusta ‘Radicans’ cuttings was obtained using a foliar spray or basal quick-dip of 123.0 μM IBA + 67.1 μM NAA [16]. Among Chinese fir, optimal hormone treatments varied greatly depending on leaf-cutting age [34], and this was borne out by our observation that the highest callus formation rate (82.9%) occurred with <one-year-old treated by 50 mg·kg−1 NAA and 50 mg·kg−1 IBA.
In our study, only leaf cuttings of <one-year-olds developed into new plantlets, and at low rates (maximum 5.8%). These results are somewhat consistent with previous reports that the plantlet formation rate was higher from one-year-old twigs (65%) than from two-year-old twigs (45%), although both rates are considerably higher than ours [24]. Because leaf age affects the locations from which cuttings are made, the outcomes are similar to the effects of different cutting positions in leafless branch cuttings. Specifically, branch cuttings from the distal end rooted better than the middle and proximal end, probably due to higher endogenous auxin levels [35]. Moreover, the rooting number and rates were higher in one-year-old stem cuttings than in two-year-old cuttings of Picea likiangensis (Franch.) Pritz. [36]. For Azadirachta indica A. Juss and Pongamia pinnata L., distal-end cuttings had 100% rooting success, probably because endogenous auxin was higher in that region [35,37]. Similarly, leaves with a petiole and stems with an attached axillary bud develop into plants at a high frequency [38]. The longer stem contains more parenchymal and cambial cells in the xylem that has high potential for root development, while the axillary bud can easily form shoots [38,39]. For instance, in pineapple (Ananas comosus L. [Merr.]) var.), medium-sized crown-leaf-bud cuttings greatly improved rapid multiplication [40]. Additionally, the petiole or hypocotyl tissue supplies carbohydrates and other elements that benefit bud germination and rooting. Indeed, the rooting ability of stem cuttings is closely related to carbohydrate content (particularly soluble sugars) during adventitious root formation [41].
Parent plant age is another important factor for plantlet formation. In our study, the mother Chinese fir was five years old, likely contributing to the observed low rooting rates of leaf cuttings (average 5.1%–10.6%). Previous research showed that leaf cuttings from one-year-old seedlings had a higher regeneration ability and nearly 50% plantlet formation rate, while cuttings from five-year-old seedlings experienced difficulties rooting unless hormone treatment was used [27]. Likewise, leaf cuttings from 15-year-old adults seldom formed roots and buds, even with hormonal induction [27]. When using base-tiller cuttings from one-year-old ortets of Chinese fir, the rooting rate was 90%, but dropped to 50% when cuttings were from 15- or 20-year-olds [42]. When using stem cuttings with leaves retained, a three-year-old Ilex paraguariensis resulted in higher rooting rates than a 20-year-old [43]. Therefore, successful propagation is more likely for younger plant material, whereas mature tissues have less ability to root or form plantlets [44,45]. The inhibitory effects of elder ortets on rooting may be linked to a reduction in endogenous hormones or an increasing lack of sensitivity to exogenous hormone treatment. Mature cuttings also have a lower phenolic content, thus limiting auxin function and in turn, rooting ability [46].
Our examination of root anatomical characteristics revealed that adventitious roots mainly originated from cortical parenchyma cells rather than phloem cambium and pith. These findings are consistent with some previous studies on Chinese-fir leaf cuttings [27], but not with others [19,46]. In contrast with leaf cuttings, the roots of stem cuttings originate from both cortical parenchyma cells and vascular cambium cells. These distinct origins are related to the structure of different cutting samples [46]. Stem cuttings preserve more cortical and xylem tissues, whereas leaf cuttings retain only a small amount of xylem. Hence, rooting tends to be easier for stem cuttings than for leaf cuttings. Therefore, we suggest that rooting would be improved if Chinese-fir leaf cuttings retained more xylem at the base and had longer stems.
5. Conclusions
In this study, we employed an orthogonal experimental design to study how leaf age and hormone treatments affect the growth of Chinese fir leaf cuttings. We demonstrated that hormone concentrations and treatment duration significantly influenced leaf-cutting growth regardless of age, but the exact effect differed. Range analysis showed that the most important factors affecting callus initiation rates were 6-BA, NAA, and treatment time for <one, one-, and two-year-old leaf cuttings, respectively. Regardless of cutting age, NAA significantly improved the rooting rates. Both 6-BA and NAA had the greatest impact on bud germination rates. We also observed relatively high callus initiation rates overall (67.9%–77.2%), but low rooting rates that decreased further with age (5.1%~10.6%). Plantlet formation rates were very low (maximum 5.8%), likely because the mother plant was five years old. Histological analysis revealed that root primordium differentiated from cortical parenchyma cells and breaks calli to form adventitious roots. Future studies should especially focus on promoting the development of calli into adventitious roots, a process that is crucial to the successful propagation of Chinese fir leaf cuttings.
Author Contributions
S.L. (Shubin Li) and S.L. (Sizu Lin) conceived and designed the study. P.H. carried out the experiment. L.Z. and G.D. analyzed and prepared the manuscript. S.D.A.-D. and Z.M. provided suggestions on figure preparation.
Funding
The research was funded by the Major Special Project of Industry-University-Research, Cooperation of Fujian Province (Grant No. 2012N0010), the China Postdoctoral Science Foundation (Grant No. 2016T90593), the open project of Fujian Provincial Colleges and University Engineering Research Center of Plantation Sustainable Management (PSM-2017002) and the National Natural Science Foundation of Fujian Province (2018J05059).
Acknowledgments
We thank P. Tang, M. Sun, and L.L. Zheng for their assistance with the experiments. We also thank Liufeng Chi for help with figure preparation.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- State Forestry Administration of the People’s Republic of China. Forest resources in China: The 8th National Forest Inventory. Available online: http://211.167.243.162:8085/8/book/jiankuang/index.html (accessed on 1 February 2014).
- Mei, G.Y.; Sun, Y.J.; Lin, F.; Wang, Y.F. Study on evaluation index system of Cunninghamia lanceolata scenic and recreational forests based on analytic network process. J. Cent. South Univ. For. Technol. 2013, 33, 110–114. (In Chinese) [Google Scholar]
- Wu, P.F.; He, Y.L.; Ma, X.Q.; Huang, M.S. Factors affecting rooting and germinating ability of cuttings of Chinese fir clone. J. Fujian Agric. For. Univ. (Nat. Sci. Ed.) 2008, 37, 51–56. (In Chinese) [Google Scholar]
- Yu, H.; Wang, W.; Wang, Y.; Hou, B. High frequency wheat regeneration from leaf tissue explants of regenerated plantlets. Adv. Biosci. Biotechnol. 2012, 3, 46–50. [Google Scholar] [CrossRef]
- Romero, J.L. A review of propagation programs for Gmelina arborea. New For. 2014, 28, 245–254. [Google Scholar] [CrossRef]
- Su, Z.N.; Chen, S.W.; Yang, J.H.; Tan, L.; Luo, Y.Z.; Yang, M. Effects of rooting agents on root morphology of cuttings of Chinese fir clone. Genom. Appl. Biol. 2013, 32, 787–794. (In Chinese) [Google Scholar]
- Palanisamy, K.; Ansari, S.A.; Kumar, P.; Gupta, B.N. Adventitious rooting in shoot cuttings of Azadirachta indica and Pongamia pinnata. New For. 1998, 16, 81–88. [Google Scholar] [CrossRef]
- Khan, U.W.; Ahmed, R.; Shahzadi, I.; Shah, M.M. Some important factors influencing tissue culture response in wheat. Sarhad J. Agric. 2015, 31, 199–209. [Google Scholar] [CrossRef]
- Guo, X.F.; Fu, X.L.; Zang, D.K.; Ma, Y. Effect of auxin treatments, cuttings’ collection date and initial characteristics on Paeonia, ‘Yang Fei Chu Yu’ cutting propagation. Sci. Hort. 2009, 119, 177–181. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, P.; Ansari, S.A. A simple method for large-scale propagation of Dendrocalamus asper. Sci. Hort. 2004, 100, 251–255. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, J.Y.; Liu, Z.L.; Li, L.; Pan, R.C.; Jin, L.H. Exogenous auxin effects on growth and phenotype of normal and hairy roots of Pueraria lobata (Willd.) Ohwi. Plant Growth Regul. 2002, 38, 37–43. [Google Scholar] [CrossRef]
- Hu, B.Z.; Li, R.L.; Feng, J.G. Comparison of plantations established by seedlings and clones of Chinese fir. J. Nanjing For. Univ. 1999, 23, 74–75. (In Chinese) [Google Scholar]
- You, S.L.; Dai, S.G.; Ma, G.L.; Yan, X.Z.; Wang, G.J. Genetic variation and excellent clonal selection of Chinese fir clones. J. Sichuan For. Sci. Technol. 2001, 22, 60–64. (In Chinese) [Google Scholar]
- Zhu, M.L.; Wang, Q.; Wei, Z.M. A comparative study of Chinese fir regeneration system. J. Mol. Cell Biol. 2007, 40, 239–244. (In Chinese) [Google Scholar]
- Zeng, D.X.; Yin, W.L.; Wang, Y.H.; Zhao, X.Q.; Wang, H.F. Propagation with etiolated softwood cuttings of five dwarf cultivars of Chinese tree peony. Acta Hortic. Sin. 2005, 32, 725–728. (In Chinese) [Google Scholar]
- Blythe, E.K.; Sibley, J.L.; Ruter, J.M.; Tilt, K.M. Cutting propagation of foliage crops using a foliar application of auxin. Sci. Hort. 2004, 103, 31–37. [Google Scholar] [CrossRef]
- Wang, J.H.; Zhang, J.G.; Zhang, S.Z.; Xu, Y.; Yang, H.Q.; Zhang, J.X.; Sun, G.P. Research of hormone, age and position effect of hardwood cutting in Picea crassifolia Kom. J. Northwest Sci.-Tech. Univ. Agric. For. (Nat. Sci. Ed.) 2006, 34, 65–71. (In Chinese) [Google Scholar]
- Pêgo, R.G.; Grossi, J.A.S.; Honorato, P.R.; Alves, C.M.L. Leaf cutting propagation of Seemania sylvatica (Kunth) hants. Acta Hortic. 2013, 1000, 251–256. [Google Scholar] [CrossRef]
- He, Z.X.; Jiang, S.; Ye, Z.H.; Shi, J.S. The rooting mechanism of cutting propagation of Chinese fir clones. J. Zhejiang For. Coll. 1994, 11, 38–44. (In Chinese) [Google Scholar]
- Tan, J.Z.; Li, F.Q.; Yuan, Z.Q.; Yang, C.H.; Kong, Y.Q. Experiment of Chinese fir short cuttings on seedlings. Guangdong For. Sci. Technol. 1994, S1, 30–33. (In Chinese) [Google Scholar]
- He, G.P.; Chen, Y.T.; Zhang, X.F.; Feng, J.Y.; Du, J.J.; Li, G. Density study of Chinese fir cuttings. For. Sci. Technol. 1997, 7, 9–11. (In Chinese) [Google Scholar]
- Gorelick, R. Why Vegetative propagation of leaf Cuttings is possible in succulent and semi-succulent plants. Haseltonia 2015, 20, 51–57. [Google Scholar] [CrossRef]
- Fuzhou Forestry Institute. Reports of leaf cuttings of Chinese fir. Jiangxi For. Sci. Technol. 1975, 4, 10–13. (In Chinese) [Google Scholar]
- Chen, F.M. The experimental conclusion of Chinese fir cuttings using leaf bud in different positions. J. Zhejiang For. Sci. Technol. 1979, 6, 9–11. (In Chinese) [Google Scholar]
- Li, S.B.; Huang, P.; Ding, G.C.; Zhou, L.L.; Tang, P.; Sun, M.; Zheng, Y.Y.; Lin, S.Z. Optimization of hormone combinations for root growth and bud germination in Chinese fir (Cunninghamia lanceolata) clone leaf cuttings. Sci. Rep. 2017, 7, 5046. [Google Scholar] [CrossRef] [PubMed]
- Kikuzawa, K.; Lechowicz, M.J. Quantifying Leaf Longevity. In Ecology of Leaf Longevity; Springer: Tokyo, Japan, 2011; pp. 23–39. [Google Scholar]
- Leaf Cutting Study Groups of Chinese fir in Guizhou Agriculture College. Research on the leaf cutting nursery of Chinese fir. For. Sci. Technol. 1979, 2, 4–6. (In Chinese) [Google Scholar]
- Ou, Y.Y.; Lu, J.F.; Silva, J.A.T.D.; Ma, G.H. Vegetative propagation of Metabriggsia ovalifolia W. T. Wang using leaf cuttings and petiole segments. J. Pomol. Horticult. Sci. 2015, 90, 724–727. [Google Scholar]
- Singh, K.K. Propagation of citrus species through cutting: A review. J. Med. Plants Stud. 2018, 6, 167–172. [Google Scholar]
- Almeida, M.R.D.; Aumond, M.; Costa, C.T.D.; Schwambach, J.; Ruedell, C.M.; Correa, L.R.; Fett-Neto, A.G. Environmental control of adventitious rooting in Eucalyptus, and Populus, cuttings. Trees 2017, 31 (Suppl. 7), 1–14. [Google Scholar] [CrossRef]
- Wang, J.H.; Zhang, J.G.; Zhang, S.Z.; Xu, Y.; Li, R.J.; Qi, X.L.; Hou, X.Z. Effects of several factors on rooting of cutting propagation of Picea balfouriana. J. Nanjing For. Univ. 2007, 31, 51–54. (In Chinese) [Google Scholar]
- Li, X.; Wu, M.; Jian, H.Y.; Gui, M.; Long, J.; Xiong, L. The effects of cutting season, plant growth regulators and their concentration on the rooting of Leucadendron cv. Inca Gold cuttings. Chin. Agric. Sci. Bull. 2009, 25, 143–147. (In Chinese) [Google Scholar]
- Inocente, V.H.H.; Nienow, A.A.; Tre, L. Time of treatment with IBA in Olive cultivars rooting. Rev. Bras. Frutic. 2018, 40. [Google Scholar] [CrossRef]
- Peng, Z.Q. Research of Cunninghamia lanceolata Leaf cutting techonology. Anhui Agric. Sci. Bull. 2017, 23, 42–44. (In Chinese) [Google Scholar]
- Palanisamy, K.; Kumar, P. Effect of position, size of cuttings and environmental factors on adventitious rooting in neem (Azadirachta indica A. Juss). For. Ecol. Manag. 1997, 98, 277–280. [Google Scholar] [CrossRef]
- Wang, J.H.; Zhang, J.G.; Zhang, S.Z.; Xu, Y.; Li, R.F.; Qi, X.L.; Hou, X.Z. Study on the technology of hard-branch cutting propagation and rooting properties in Picea likiangensis (Franch.) Pritz. J. Northwest Sci.-Tech. Univ. Agric. For. (Nat. Sci. Ed.) 2006, 34, 97–101. (In Chinese) [Google Scholar]
- Heloir, M.C.; Kevers, C.; Hausman, J.F.; Gaspar, T. Changes in the concentrations of auxins and polyamines during rooting of in-vitro-propagated walnut shoots. Tree Physiol. 1996, 16, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Miedema, P. Vegetative propagation of Beta vulgaris by leaf cuttings with axillary buds. Euphytica 1982, 31, 771–772. [Google Scholar] [CrossRef]
- You, W.Z.; Dong, J.; Lu, A.J.; Yun, L.L. Effects of different cuttings length on rooting by tender branch cutting of Larch. J. Anhui Agric. Sci. 2007, 35, 4714–4715. (In Chinese) [Google Scholar]
- Tassew, A.A. Evaluation of leaf bud cuttings from different sized Crowns for Rapid Propagation of Pineapple (Ananas Comosus L. [Merr.]). J. Biol. Agric. Healthc. 2014, 4, 27. [Google Scholar]
- Sergio, T.; Alberto, P.; Stefano, P.; Daniela, F. Influence of light and shoot development stage on leaf photosynthesis and carbohydrate status during the adventitious root formation in cuttings of Corylus avellana L. Front. Plant Sci. 2015, 6, 1–14. [Google Scholar]
- Qiu, J.Q. Study on the rooting growth of Chinese fir cuttings under different treatments. J. Cent. South For. Univ. 1998, 18, 46–51. (In Chinese) [Google Scholar]
- Tarrago, J.; Sansberro, P.; Filip, R.; Lópezb, P.; Gonzáleza, A.; Lunaa, C.; Mroginskia, L. Effect of leaf retention and flavonoids on rooting of Ilex paraguariensis cuttings. Sci. Hortic. 2005, 103, 479–488. [Google Scholar] [CrossRef]
- Kleinschmit, J.; Schmidt, J. Experiences with Picea abies cutting propagation in germany and problem s connected with large scale application. Silvae Genet. 1997, 26, 526. [Google Scholar]
- Saranga, J.; Cameron, R. Adventitious root formation in Anacardium occidentale L. in response to phytohormones and removal of roots. Sci. Hortic. 2007, 111, 164–172. [Google Scholar] [CrossRef]
- Ma, Y.; Li, M.H.; Zhang, X.H.; Yan, M.X. The anatomic studies of adventitious root formation of Chinese fir twig cuttings. J. Huazhong Agric. Univ. 1998, 17, 81–83. (In Chinese) [Google Scholar]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).