Late Spring Frost in Mediterranean Beech Forests: Extended Crown Dieback and Short-Term Effects on Moth Communities
Abstract
1. Introduction
2. Materials and Methods
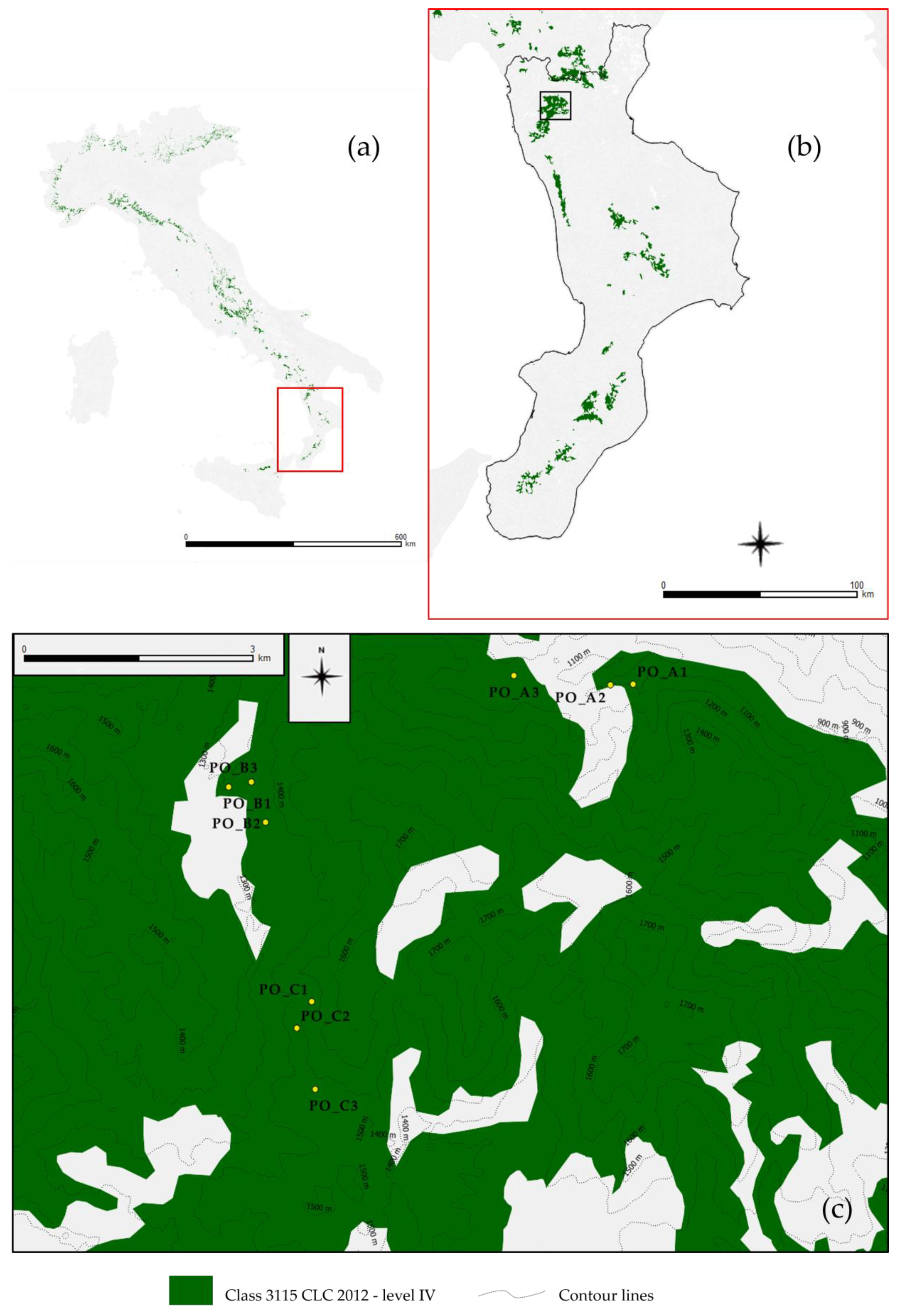
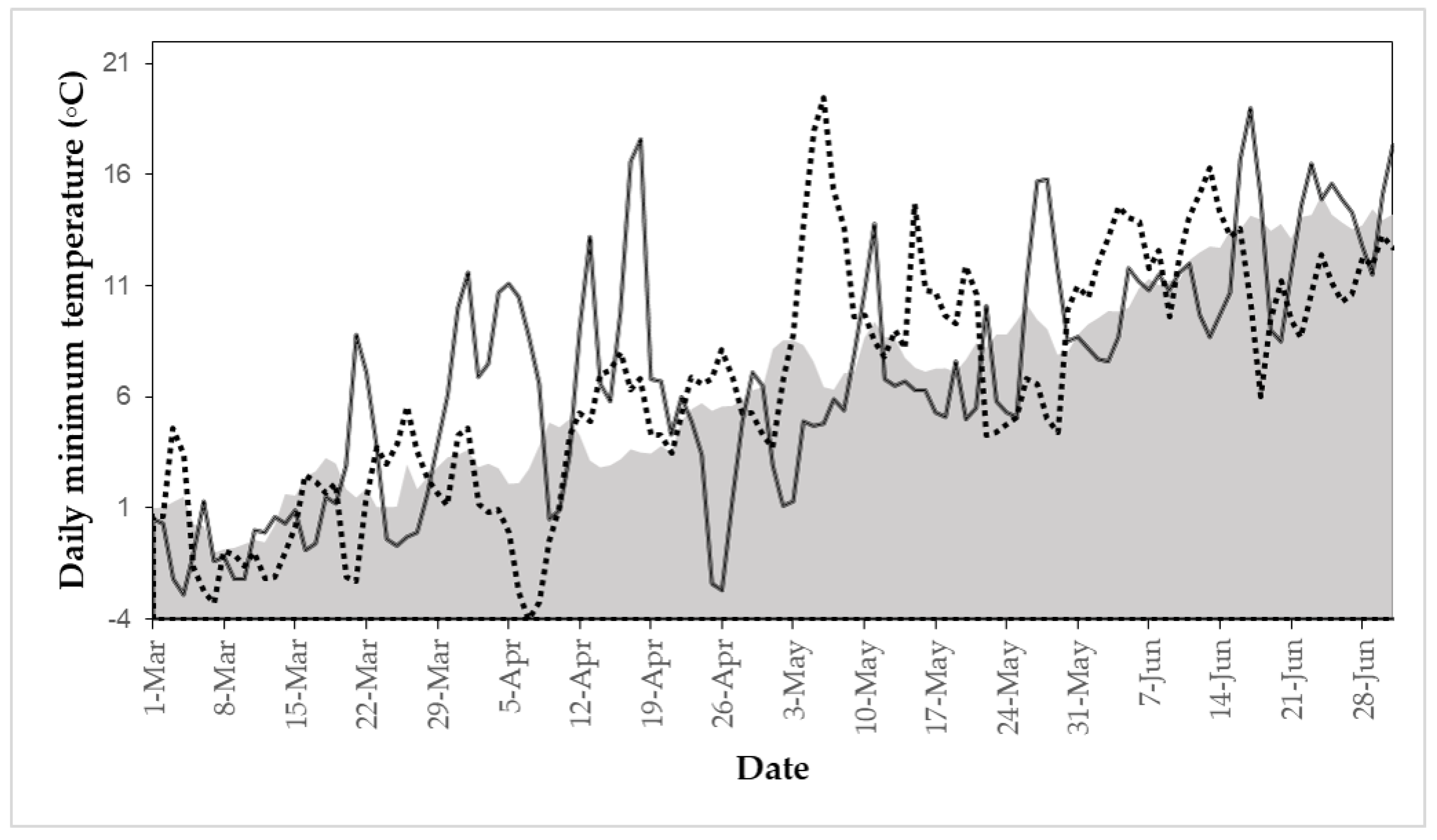
2.1. Study Area and the 2016 Spring EWE
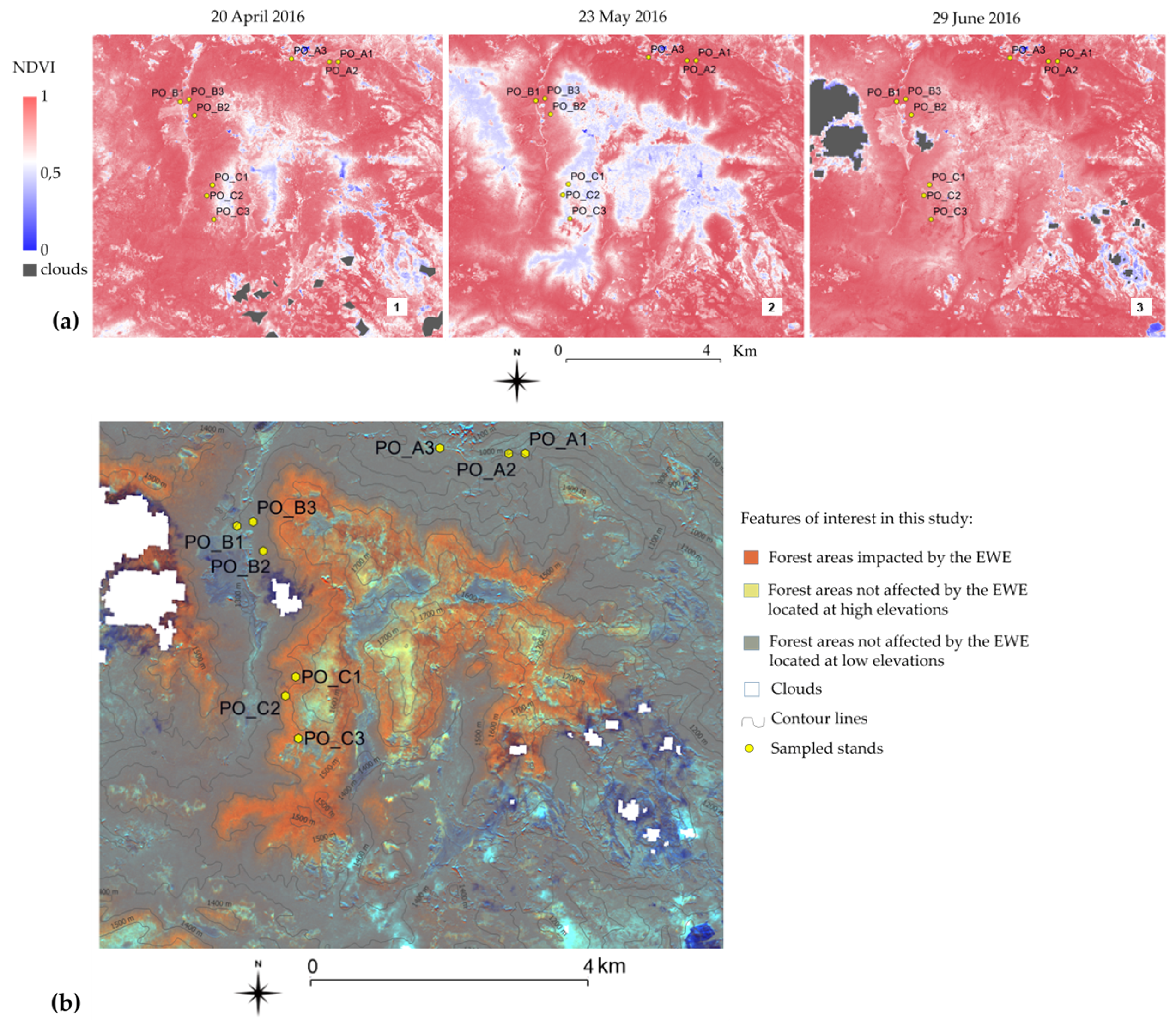
2.2. Satellite Images and Supplementary Data
2.3. Methods Devised to Identify Forest Areas Impacted by the EWE
2.4. Moth Sampling and Dataset Organization
- -
- Dataset 1 contained samples from the three stands of PO_C site collected from May to November 2015 and 2016; in total, we used 42 samples to compare species richness and abundance before and after the EWE at high elevation.
- -
- Dataset 2 contained samples from all of the stands of PO_A, PO_B, and PO_C sites collected from September to November 2015 and 2016; in total, we used 54 samples to search for changes in community structures and in abundance patterns of individual species.
- -
- Dataset 3 contained wing measurements of all the specimens belonging to the two dominant species collected during all sampling sessions.
2.5. Methods to Evaluate the EWE Impact on Moth Communities
3. Results
3.1. The Impact of EWE on Beech Forests
3.2. The Impact of EWE on Moth Communities
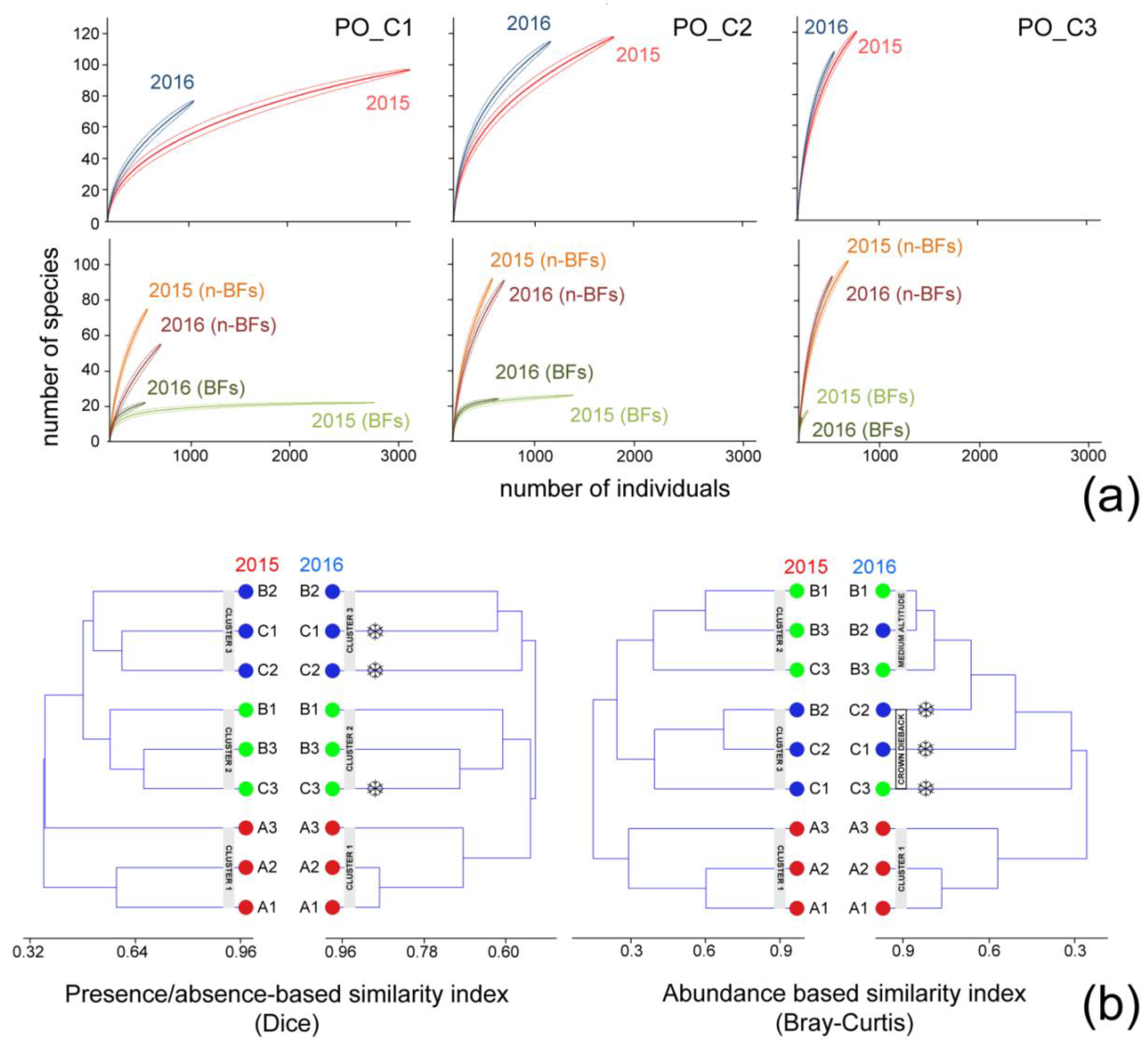
3.2.1. Diversity and Abundance before and after the EWE
3.2.2. Consistency of Autumn Moth Communities between Impacted and Non-Impacted Sites
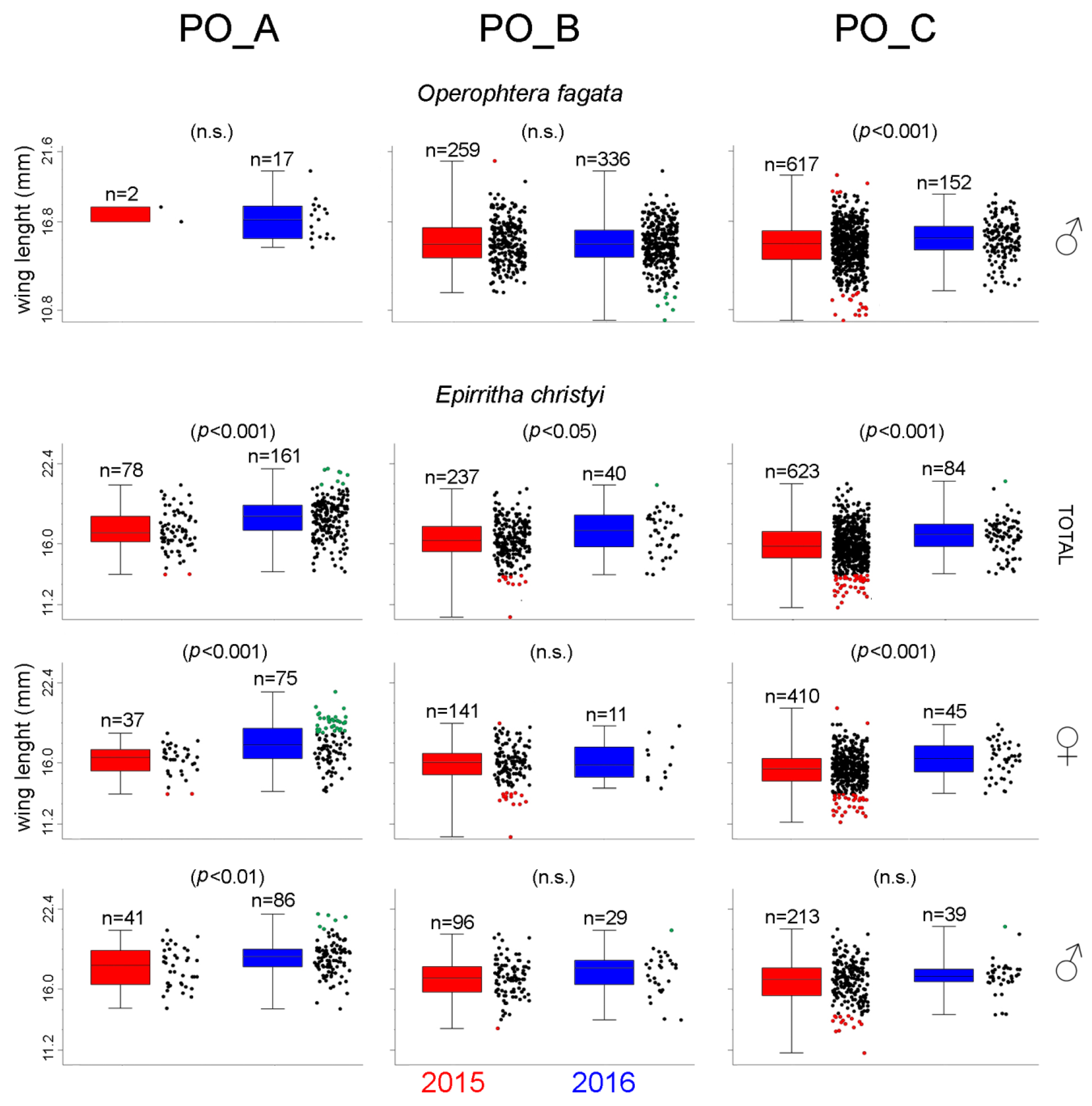
3.2.3. Changes in the Wing Measurements of Epirrita christyi and Operophtera fagata
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Field, C.B.; Barros, V.R. Climate Change 2014: Impacts, Adaptation, and Vulnerability: Working Group II Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: New York, NY, USA, 2014. [Google Scholar]
- Hänninen, H. Does climatic warming increase the risk of frost damage in northern trees? Plant Cell Environ. 1991, 14, 449–454. [Google Scholar] [CrossRef]
- Kramer, K.; Friend, A.; Leinonen, I. Modelling comparison to evaluate the importance of phenology and spring frost damage for the effects of climate change on growth of mixed temperate-zone deciduous forests. Clim. Res. 1996, 5, 31–41. [Google Scholar] [CrossRef]
- Jentsch, A.; Kreyling, J.; Beierkuhnlein, C. A new generation of climate-change experiments: Events, not trends. Front. Ecol. Environ. 2007, 5, 365–374. [Google Scholar] [CrossRef]
- Rigby, J.R.; Porporato, A. Spring frost risk in a changing climate. Geophys. Res. Lett. 2008, 35. [Google Scholar] [CrossRef]
- Hansen, J.; Ruedy, R.; Sato, M.; Lo, K. Global surface temperature change. Rev. Geophys. 2010, 48. [Google Scholar] [CrossRef]
- Rosenzweig, C.; Iglesias, A.; Yang, X.B.; Epstein, P.R.; Chivian, E. Climate change and extreme weather events; implications for food production, plant diseases, and pests. Glob. Change Hum. Health 2001, 2, 90–104. [Google Scholar] [CrossRef]
- Jentsch, A.; Beierkuhnlein, C. Research frontiers in climate change: Effects of extreme meteorological events on ecosystems. C. R. Geosci. 2008, 340, 621–628. [Google Scholar] [CrossRef]
- Sala, O.E.; Chapin, F.S.; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A. Global biodiversity scenarios for the year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef] [PubMed]
- Piessens, K.; Adriaens, D.; Jacquemyn, H.; Honnay, O. Synergistic effects of an extreme weather event and habitat fragmentation on a specialised insect herbivore. Oecologia 2009, 159, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Jiguet, F.; Brotons, L.; Devictor, V. Community responses to extreme climatic conditions. Curr. Zool. 2011, 57, 406–413. [Google Scholar] [CrossRef]
- Melillo, J.M.; Callaghan, T.V.; Woodward, F.I.; Salati, E.; Sinha, S.K. Effects on ecosystems. Clim. Chang. IPCC Sci. Assess. 1990, 283–310. [Google Scholar]
- Parmesan, C.; Root, T.L.; Willig, M.R. Impacts of extreme weather and climate on terrestrial biota. Bull. Am. Meteorol. Soc. 2000, 81, 443–450. [Google Scholar] [CrossRef]
- Parmesan, C.; Ryrholm, N.; Stefanescu, C.; Hill, J.K. Poleward shifts in geographical ranges of butterfly species associated with regional warming. Nature 1999, 399, 579. [Google Scholar] [CrossRef]
- Parmesan, C. Ecological and Evolutionary Responses to Recent Climate Change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef]
- Hellmann, J.J.; Pelini, S.L.; Prior, K.M.; Dzurisin, J.D.K. The response of two butterfly species to climatic variation at the edge of their range and the implications for poleward range shifts. Oecologia 2008, 157, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Diamond, S.E.; Frame, A.M.; Martin, R.A.; Buckley, L.B. Species’ traits predict phenological responses to climate change in butterflies. Ecology 2011, 92, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Scalercio, S.; Bonacci, T.; Mazzei, A.; Pizzolotto, R.; Brandmayr, P. Better up, worse down: Bidirectional consequences of three decades of climate change on a relict population of Erebia cassioides. J. Insect Conserv. 2014, 18, 643–650. [Google Scholar] [CrossRef]
- Jentsch, A.; Beierkuhnlein, C. Global climate change and local disturbance regimes as interacting drivers for shifting altitudinal vegetation patterns. Erdkunde 2003, 57, 216–231. [Google Scholar] [CrossRef]
- Walck, J.L.; Hidayati, S.N.; Dixon, K.W.; Thompson, K.; Poschlod, P. Climate change and plant regeneration from seed. Glob. Chang. Biol. 2011, 17, 2145–2161. [Google Scholar] [CrossRef]
- Kramer, K.; Leinonen, I.; Loustau, D. The importance of phenology for the evaluation of impact of climate change on growth of boreal, temperate and Mediterranean forests ecosystems: An overview. Int. J. Biometeorol. 2000, 44, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolström, M.; et al. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- De Feudis, M.; Cardelli, V.; Massaccesi, L.; Lagomarsino, A.; Fornasier, F.; Westphalen, D.; Cocco, S.; Corti, G.; Agnelli, A. Influence of Altitude on Biochemical Properties of European Beech (Fagus sylvatica L.) Forest Soils. Forests 2017, 8, 213. [Google Scholar] [CrossRef]
- Inouye, D.W. Effects of climate change on phenology, frost damage, and floral abundance of montane wildflowers. Ecology 2008, 89, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Bascietto, M.; Bajocco, S.; Mazzenga, F.; Matteucci, G. Assessing spring frost effects on beech forests in Central Apennines from remotely-sensed data. Agric. For. Meteorol. 2018, 248, 240–250. [Google Scholar] [CrossRef]
- Dittmar, C.; Fricke, W.; Elling, W. Impact of late frost events on radial growth of common beech (Fagus sylvatica L.) in Southern Germany. Eur. J. For. Res. 2006, 125, 249–259. [Google Scholar] [CrossRef]
- Giesecke, T.; Hickler, T.; Kunkel, T.; Sykes, M. T.; Bradshaw, R.H.W. Towards an understanding of the Holocene distribution of Fagus sylvatica L. J. Biogeogr. 2007, 34, 118–131. [Google Scholar] [CrossRef]
- Augspurger, C.K. Spring 2007 warmth and frost: Phenology, damage and refoliation in a temperate deciduous forest: Warmth and frost. Funct. Ecol. 2009, 23, 1031–1039. [Google Scholar] [CrossRef]
- Augspurger, C.K. Reconstructing patterns of temperature, phenology, and frost damage over 124 years: Spring damage risk is increasing. Ecology 2013, 94, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Kreyling, J.; Thiel, D.; Nagy, L.; Jentsch, A.; Huber, G.; Konnert, M.; Beierkuhnlein, C. Late frost sensitivity of juvenile Fagus sylvatica L. differs between southern Germany and Bulgaria and depends on preceding air temperature. Eur. J. For. Res. 2012, 131, 717–725. [Google Scholar] [CrossRef]
- García-Plazaola, J.I.; Becerril, J.M. Effects of drought on photoprotective mechanisms in European beech (Fagus sylvatica L.) seedlings from different provenances. Trees 2000, 14, 485–490. [Google Scholar] [CrossRef]
- Subramanian, N.; Bergh, J.; Johansson, U.; Nilsson, U.; Sallnäs, O. Adaptation of Forest Management Regimes in Southern Sweden to Increased Risks Associated with Climate Change. Forests 2016, 7, 8. [Google Scholar] [CrossRef]
- De Marco, A.; Fioretto, A.; Giordano, M.; Innangi, M.; Menta, C.; Papa, S.; Virzo De Santo, A. C Stocks in Forest Floor and Mineral Soil of Two Mediterranean Beech Forests. Forests 2016, 7, 181. [Google Scholar] [CrossRef]
- Walentowski, H.; Müller-Kroehling, S.; Bergmeier, E.; Bernhardt-Römermann, M.; Gossner, M.M.; Reif, A.; Schulze, E.-D.; Bußler, H.; Strätz, C.; Adelmann, W. Faunal diversity of Fagus sylvatica forests: A regional and European perspective based on three indicator groups. Ann. For. Res. 2014, 57, 215–231. [Google Scholar]
- Scalercio, S.; Bonacci, T.; Turco, R.; Bernardini, V. Relationships between Psychidae communities (Lepidoptera: Tineoidea) and the ecological characteristics of old-growth forests in a beech dominated landscape. Eur. J. Entomol. 2016, 113, 113–121. [Google Scholar] [CrossRef]
- Greco, S.; Coluzzi, R.; Imbrenda, V.; Infusino, M.; Simoniello, T.; Scalercio, S. Relationships between landscape and moth diversity in Mediterranean mountain forest ecosystems at multiple spatial scales. Forest@ 2017, 14, 75–83. [Google Scholar] [CrossRef]
- Summerville, K.S.; Ritter, L.M.; Crist, T.O. Forest moth taxa as indicators of lepidopteran richness and habitat disturbance: A preliminary assessment. Biol. Conserv. 2004, 116, 9–18. [Google Scholar] [CrossRef]
- Boggs, C.L.; Murphy, D.D. Community composition in mountain ecosystems: Climatic determinants of montane butterfly distributions. Glob. Ecol. Biogeogr. Lett. 1997, 6, 39–48. [Google Scholar] [CrossRef]
- Cooke, B.J.; Roland, J. The Effect of Winter Temperature on Forest Tent Caterpillar (Lepidoptera: Lasiocampidae) Egg Survival and Population Dynamics in Northern Climates. Environ. Entomol. 2003, 32, 299–311. [Google Scholar] [CrossRef]
- Matter, S.F.; Doyle, A.; Illerbrun, K.; Wheeler, J.; Roland, J. An assessment of direct and indirect effects of climate change for populations of the Rocky Mountain Apollo butterfly (Parnassius smintheus Doubleday). Insect Sci. 2011, 18, 385–392. [Google Scholar] [CrossRef]
- Boggs, C.L.; Inouye, D.W. A single climate driver has direct and indirect effects on insect population dynamics. Ecol. Lett. 2012, 15, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Roland, J.; Matter, S.F. Variability in winter climate and winter extremes reduces population growth of an alpine butterfly. Ecology 2013, 94, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, P.R.; Breedlove, D.E.; Brussard, P.F.; Sharp, M.A. Weather and the “regulation” of subalpine populations. Ecology 1972, 53, 243–247. [Google Scholar] [CrossRef]
- Singer, M.C.; Thomas, C.D. Evolutionary Responses of a Butterfly Metapopulation to Human- and Climate-Caused Environmental Variation. Am. Nat. 1996, 148, S9–S39. [Google Scholar] [CrossRef]
- Thomas, C.D.; Singer, M.C.; Boughton, D.A. Catastrophic extinction of population sources in a butterfly metapopulation. Am. Nat. 1996, 148, 957–975. [Google Scholar] [CrossRef]
- Easterling, D.R.; Evans, J.L.; Groisman, P.Y.; Karl, T.R.; Kunkel, K.E.; Ambenje, P. Observed variability and trends in extreme climate events: A brief review. Bull. Am. Meteorol. Soc. 2000, 81, 417–425. [Google Scholar] [CrossRef]
- Smart, S.M.; Ellison, A.M.; Bunce, R.G.H.; Marrs, R.H.; Kirby, K.J.; Kimberley, A.; Scott, A.W.; Foster, D.R. Quantifying the impact of an extreme climate event on species diversity in fragmented temperate forests: The effect of the October 1987 storm on British broadleaved woodlands. J. Ecol. 2014, 102, 1273–1287. [Google Scholar] [CrossRef]
- McDermott Long, O.; Warren, R.; Price, J.; Brereton, T.M.; Botham, M.S.; Franco, A.M.A. Sensitivity of UK butterflies to local climatic extremes: Which life stages are most at risk? J. Anim. Ecol. 2017, 86, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Palmer, G.; Platts, P.J.; Brereton, T.; Chapman, J.W.; Dytham, C.; Fox, R.; Pearce-Higgins, J.W.; Roy, D.B.; Hill, J.K.; Thomas, C.D. Climate change, climatic variation and extreme biological responses. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160144. [Google Scholar] [CrossRef] [PubMed]
- Infusino, M.; Greco, S.; Turco, R.; Bernardini, V.; Scalercio, S. Managed mountain forests as diversity reservoirs in Mediterranean landscapes: New data on endemic species and faunistic novelties of moths. Bull. Insectology 2016, 69, 249–258. [Google Scholar]
- Piovesan, G.; Biondi, F.; Bernabei, M.; Di Filippo, A.; Schirone, B. Spatial and altitudinal bioclimatic zones of the Italian peninsula identified from a beech (Fagus sylvatica L.) tree-ring network. Acta Oecologica 2005, 27, 197–210. [Google Scholar] [CrossRef]
- Lanfredi, M.; Coppola, R.; D’Emilio, M.; Imbrenda, V.; Macchiato, M.; Simoniello, T. A geostatistics-assisted approach to the deterministic approximation of climate data. Environ. Model. Softw. 2015, 66, 69–77. [Google Scholar] [CrossRef]
- Hanes, J.M. Spring leaf phenology and the diurnal temperature range in a temperate maple forest. Int. J. Biometeorol. 2014, 58, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Wato, Y.A.; Heitkönig, I.M.A.; van Wieren, S.E.; Wahungu, G.; Prins, H.H.T.; van Langevelde, F. Prolonged drought results in starvation of African elephant (Loxodonta africana). Biol. Conserv. 2016, 203, 89–96. [Google Scholar] [CrossRef]
- Rouse, J.W., Jr.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring the Vernal Advancement and Retrogradation (Green Wave Effect) of Natural Vegetation; Progress Report RSC 1978-1, NTIS No. E73-10693; Remote Sensing Center and A and M University: College Station, TX, USA, 1974. [Google Scholar]
- Simoniello, T.; Lanfredi, M.; Liberti, M.; Coppola, R.; Macchiato, M. Estimation of vegetation cover resilience from satellite time series. Hydrol. Earth Syst. Sci. Discuss. 2008, 5, 511–546. [Google Scholar] [CrossRef]
- Simoniello, T.; Lanfredi, M.; Coppola, R.; Imbrenda, V.; Macchiato, M. Correlation of Vegetation and Air Temperature Seasonal Profiles-Spatial Arrangement and Temporal Variability, Phenology and Climate Change. Available online: https://www.intechopen.com/books/phenology-and-climate-change/correlation-of-vegetation-and-temperature-seasonal-profiles-spatial-arrangement-and-temporal-variabi (accessed on 21 March 2012).
- Lanfredi, M.; Coppola, R.; Simoniello, T.; Coluzzi, R.; D’Emilio, M.; Imbrenda, V.; Macchiato, M. Early Identification of Land Degradation Hotspots in Complex Bio-Geographic Regions. Remote Sens. 2015, 7, 8154–8179. [Google Scholar] [CrossRef]
- Infusino, M.; Brehm, G.; Di Marco, C.; Scalercio, S. Assessing the efficiency of UV LEDs as light sources for sampling the diversity of macro-moths (Lepidoptera). Eur. J. Entomol. 2017, 114, 25–33. [Google Scholar] [CrossRef]
- Berio, E. Lepidoptera Noctuidae. I. Generalities. Hadeninae, Cucullinae; Officine Grafiche Calderini: Bologna, Italy, 1985; Volume XXII, ISBN 88-7019-235-0. [Google Scholar]
- Berio, E. Lepidoptera Noctuidae II: Sezione Quadrifide; Officine Grafiche Calderini: Bologna, Italy, 1991; Volume XXVII, ISBN 88-7019-469-8. [Google Scholar]
- Marini, M.; Trentini, M. I Macrolepidetteri dell’appennino lucchese; Arti Grafiche Tamari: Bologna, Italy, 1986. [Google Scholar]
- Bertaccini, E.; Fiumi, G.; Provera, P. Bombici e sfingi d’Italia: (Lepidoptera Heterocera); Natura-Giuliano Russo Editore: Bologna, Italy, 1995; Volume I. [Google Scholar]
- Bertaccini, E.; Fiumi, G.; Provera, P. Bombici e sfingi d’Italia: (Lepidoptera Heterocera); Natura-Giuliano Russo Editore: Bologna, Italy, 1997; Volume II. [Google Scholar]
- Rákosy, L. Die Noctuiden Rumäniens: Lepidoptera Noctuidae; Oberösterreichisches Landesmuseum: Linz, Austria, 1996. [Google Scholar]
- Flamigni, C.; Fiumi, G.; Parenzan, P. Lepidotteri eteroceri d’Italia: Geometridae Ennominae; Natura Edizioni Scientifiche: Bologna, Italy, 2007. [Google Scholar]
- Hellmann, F.; Parenzan, P. I macrolepidotteri del Piemonte; Museo Regionale di Scienze Naturali: Torino, Italy, 2010. [Google Scholar]
- Hausmann, A.; Viidalepp, J. Larentiinae I (Perizomini and Eupitheciini). Geom. Moths Eur. 2012, 3, 1–743. [Google Scholar]
- Scalercio, S.; Infusino, M.; Hausmann, A. Nothocasis rosariae sp. n., a new sylvicolous, montane species from southern Europe (Lepidoptera: Geometridae, Larentiinae). Zootaxa 2016, 4161, 177. [Google Scholar] [CrossRef] [PubMed]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST-palaeontological statistics, ver. 1.89. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Altwegg, R.; Visser, V.; Bailey, L.D.; Erni, B. Learning from single extreme events. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160141. [Google Scholar] [CrossRef] [PubMed]
- Imbrenda, V.; D’emilio, M.; Lanfredi, M.; Macchiato, M.; Ragosta, M.; Simoniello, T. Indicators for the estimation of vulnerability to land degradation derived from soil compaction and vegetation cover. Eur. J. Soil Sci. 2014, 65, 907–923. [Google Scholar] [CrossRef]
- Puletti, N.; Chianucci, F.; Castaldi, C. Use of Sentinel-2 for forest classification in Mediterranean environments. Ann. Silvic. Res. 2017, 42, 1. [Google Scholar]
- Khani, A.; Moharramipour, S.; Barzegar, M. Cold tolerance and trehalose accumulation in overwintering larvae of the codling moth, Cydia pomonella (Lepidoptera: Tortricidae). Eur. J. Entomol. 2007, 104, 385. [Google Scholar] [CrossRef]
- Parenzan, P. Contributi alla conoscenza della Lepidotterofauna dell’Italia meridionale: XVII. Geometridae. Entomologica, Bari 1994, 28, 99–246. [Google Scholar]
- Scalercio, S.; Infusino, M.; Tuscano, J. I macrolepidotteri notturni della faggeta di Monte Curcio, Sila Grande (Calabria, Italia meridionale) (Lepidoptera). Quad. Della Stazione Ecol. Civ. Mus. Storia Nat. Ferrara 2008, 18, 5–19. [Google Scholar]
- Shirai, Y. Longevity, flight ability and reproductive performance of the diamondback moth, Plutella xylostella (L.)(Lepidoptera: Yponomeutidae), related to adult body size. Res. Popul. Ecol. 1995, 37, 269–277. [Google Scholar] [CrossRef]
- Sekar, S. A meta-analysis of the traits affecting dispersal ability in butterflies: Can wingspan be used as a proxy? Factors affecting dispersal ability in butterflies. J. Anim. Ecol. 2012, 81, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Wickman, P.O.; Karlsson, B. Abdomen Size, Body Size and the Reproductive Effort of Insects. Oikos 1989, 56, 209. [Google Scholar] [CrossRef]
- Tammaru, T.; Kaitaniemi, P.; Ruohomäki, K.; Ruohomaki, K. Realized Fecundity in Epirrita autumnata (Lepidoptera: Geometridae): Relation to Body Size and Consequences to Population Dynamics. Oikos 1996, 77, 407. [Google Scholar] [CrossRef]
- Bell, J.R.; Bohan, D.A.; Shaw, E.M.; Weyman, G.S. Ballooning dispersal using silk: World fauna, phylogenies, genetics and models. Bull. Entomol. Res. 2005, 95, 69–114. [Google Scholar] [CrossRef] [PubMed]
- Awaya, Y.; Tanaka, K.; Kodani, E.; Nishizono, T. Responses of a beech (Fagus crenata Blume) stand to late spring frost damage in Morioka, Japan. For. Ecol. Manag. 2009, 257, 2359–2369. [Google Scholar] [CrossRef]
- Higgins, K. Metapopulation extinction risk: Dispersal’s duplicity. Theor. Popul. Biol. 2009, 76, 146–155. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, J.F.; Hellmann, J.J.; Boggs, C.L.; Ehrlich, P.R. Climate change hastens population extinctions. Proc. Natl. Acad. Sci. 2002, 99, 6070–6074. [Google Scholar] [CrossRef] [PubMed]
- Oliver, T.H.; Marshall, H.H.; Morecroft, M.D.; Brereton, T.; Prudhomme, C.; Huntingford, C. Interacting effects of climate change and habitat fragmentation on drought-sensitive butterflies. Nat. Clim. Chang. 2015, 5, 941–945. [Google Scholar] [CrossRef]
- Malinowska, A.H.; van Strien, A.J.; Verboom, J.; WallisdeVries, M.F.; Opdam, P. No Evidence of the Effect of Extreme Weather Events on Annual Occurrence of Four Groups of Ectothermic Species. PLoS ONE 2014, 9, e110219. [Google Scholar] [CrossRef] [PubMed]

| TOT | BFs | n-BFs | Operophtera fagata | Epirrita christyi | ||
|---|---|---|---|---|---|---|
| PO_A(1+2+3) | 2015/2016 | 574/607 | 227/225 | 347/383 | 9/10 | 75/152 |
| ∆% | 6 | −1 | 10 | 11 | 103 | |
| PO_B(1+2+3) | 2015/2016 | 828/480 | 759/377 | 69/103 | 400/338 | 257/23 |
| ∆% | −42 | −50 | 49 | −16 | −91 | |
| PO_C(1+2+3) | 2015/2016 | 3188/500 | 3066/268 | 122/232 | 2260/161 | 654/70 |
| ∆% | −84 | −91 | 90 | −93 | −89 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greco, S.; Infusino, M.; De Donato, C.; Coluzzi, R.; Imbrenda, V.; Lanfredi, M.; Simoniello, T.; Scalercio, S. Late Spring Frost in Mediterranean Beech Forests: Extended Crown Dieback and Short-Term Effects on Moth Communities. Forests 2018, 9, 388. https://doi.org/10.3390/f9070388
Greco S, Infusino M, De Donato C, Coluzzi R, Imbrenda V, Lanfredi M, Simoniello T, Scalercio S. Late Spring Frost in Mediterranean Beech Forests: Extended Crown Dieback and Short-Term Effects on Moth Communities. Forests. 2018; 9(7):388. https://doi.org/10.3390/f9070388
Chicago/Turabian StyleGreco, Silvia, Marco Infusino, Carlo De Donato, Rosa Coluzzi, Vito Imbrenda, Maria Lanfredi, Tiziana Simoniello, and Stefano Scalercio. 2018. "Late Spring Frost in Mediterranean Beech Forests: Extended Crown Dieback and Short-Term Effects on Moth Communities" Forests 9, no. 7: 388. https://doi.org/10.3390/f9070388
APA StyleGreco, S., Infusino, M., De Donato, C., Coluzzi, R., Imbrenda, V., Lanfredi, M., Simoniello, T., & Scalercio, S. (2018). Late Spring Frost in Mediterranean Beech Forests: Extended Crown Dieback and Short-Term Effects on Moth Communities. Forests, 9(7), 388. https://doi.org/10.3390/f9070388







