Invasive Species May Disrupt Protected Area Networks: Insights from the Pine Wood Nematode Spread in Portugal
Abstract
:1. Introduction
2. Materials and Methods
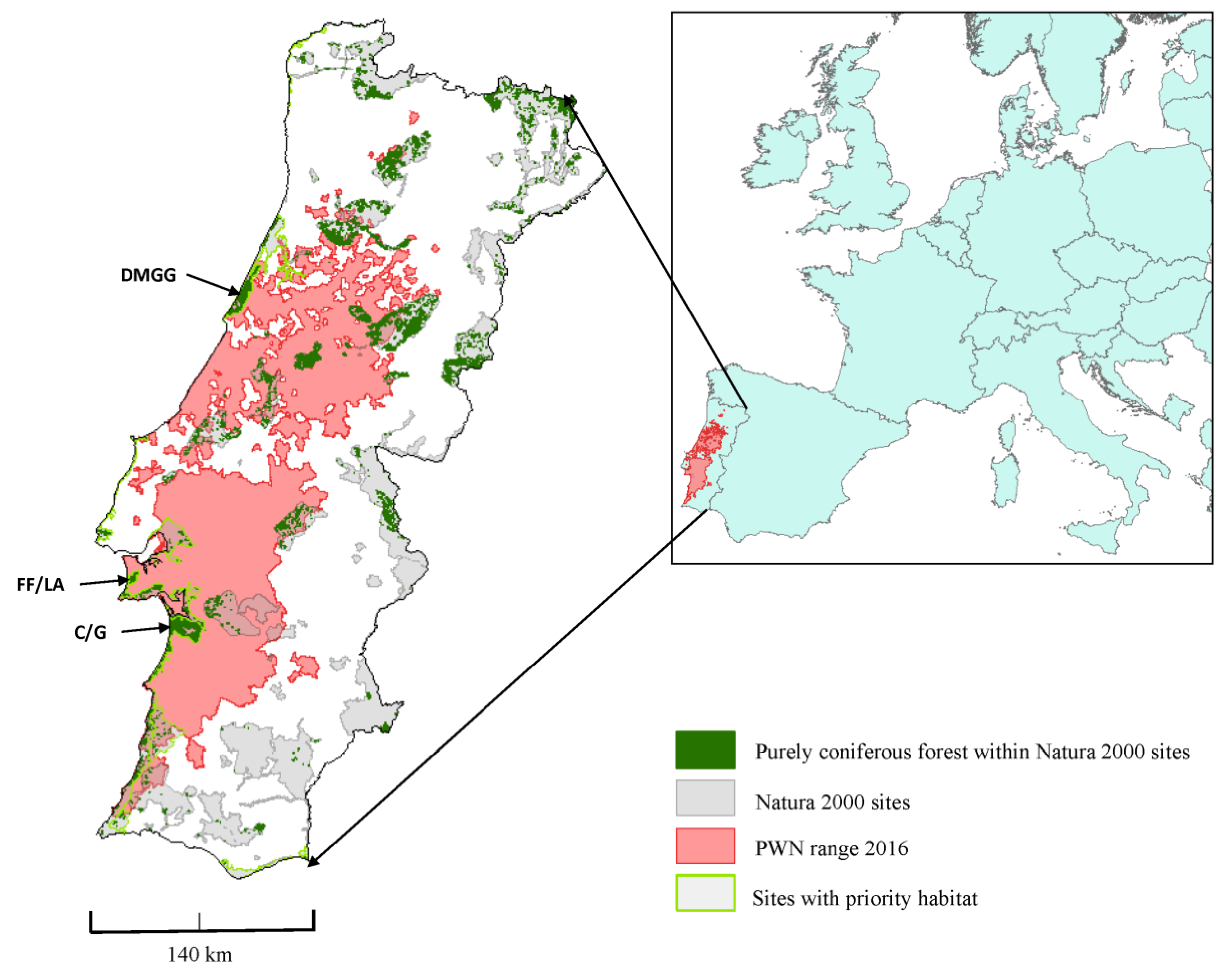
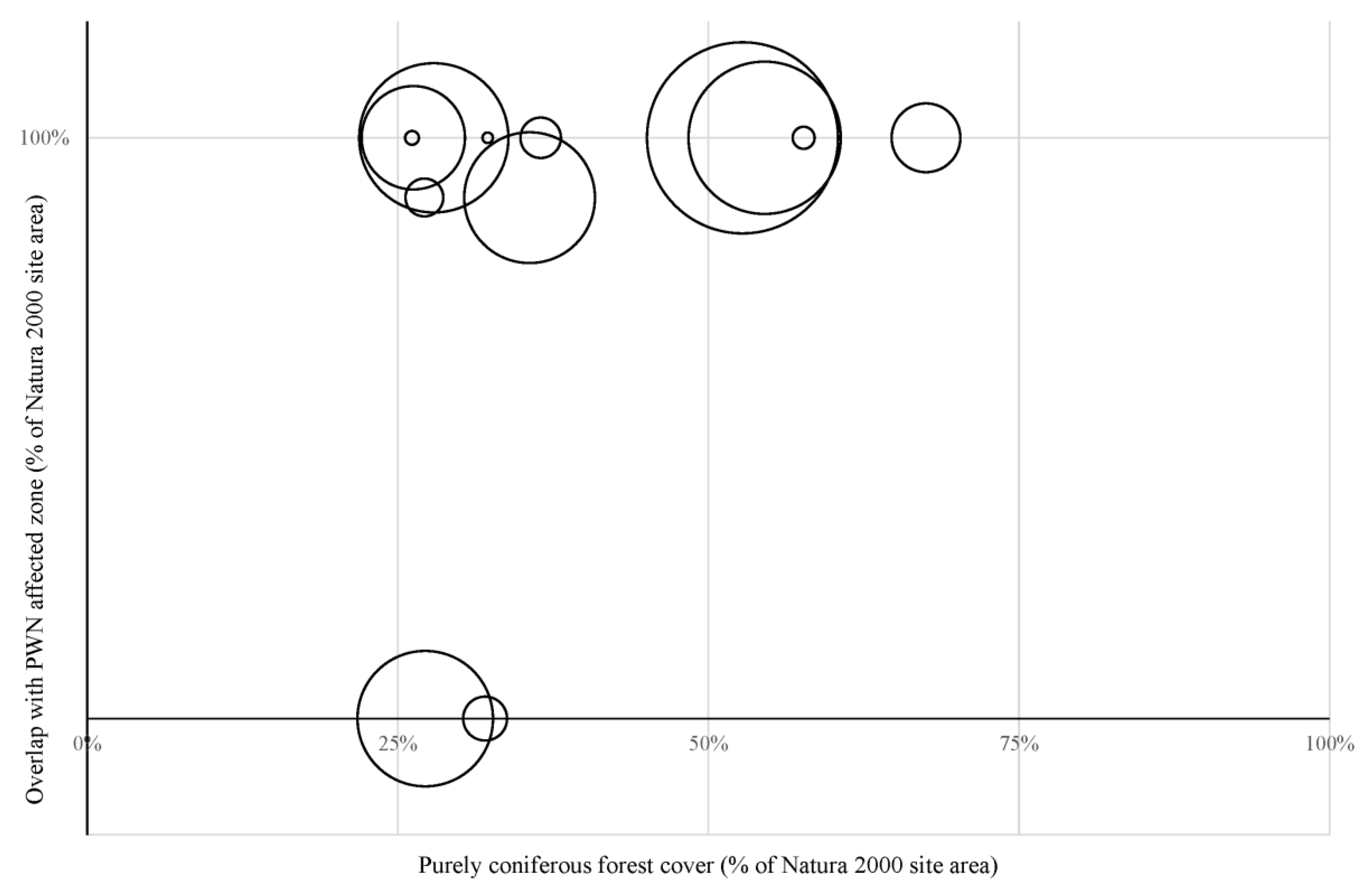
2.1. Natura 2000 Sites and Their Rate of Infection by the PWN
2.2. Mapping Corridors between Natura 2000 Forest Sites
2.2.1. Focal Locations within Natura 2000 Forest Sites
2.2.2. Landscape Resistance to Movement
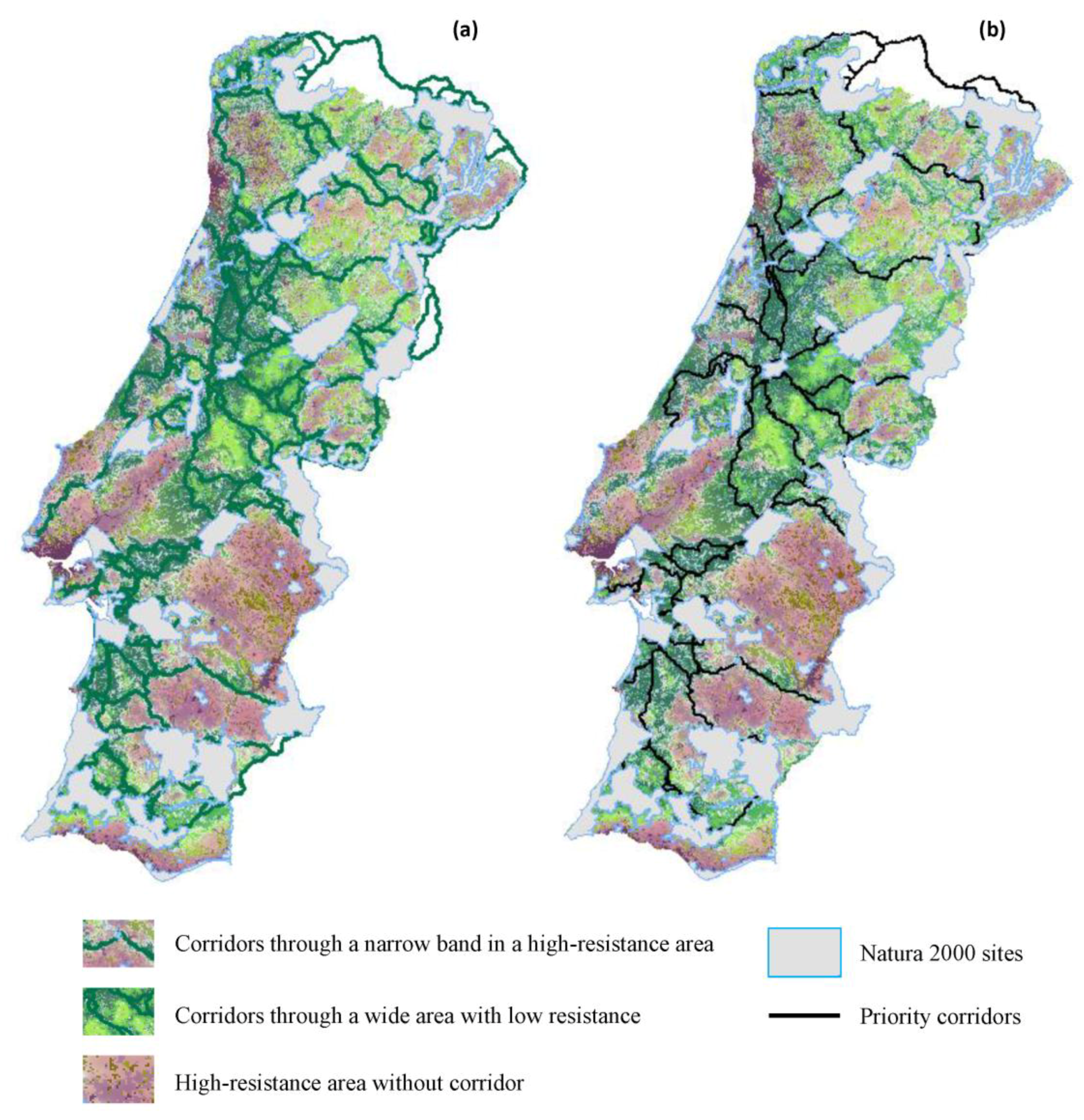
2.2.3. Corridors between PAs
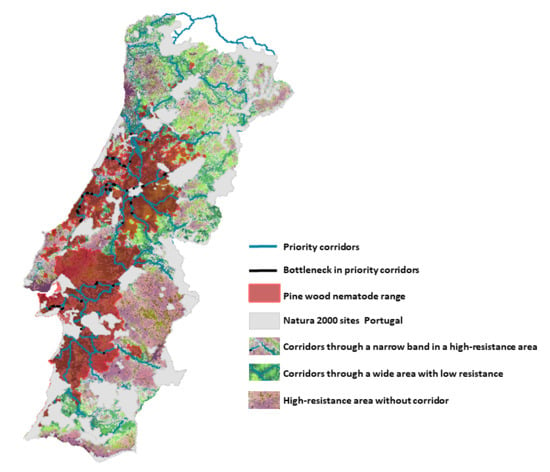
2.2.4. Priority Corridors for Conservation
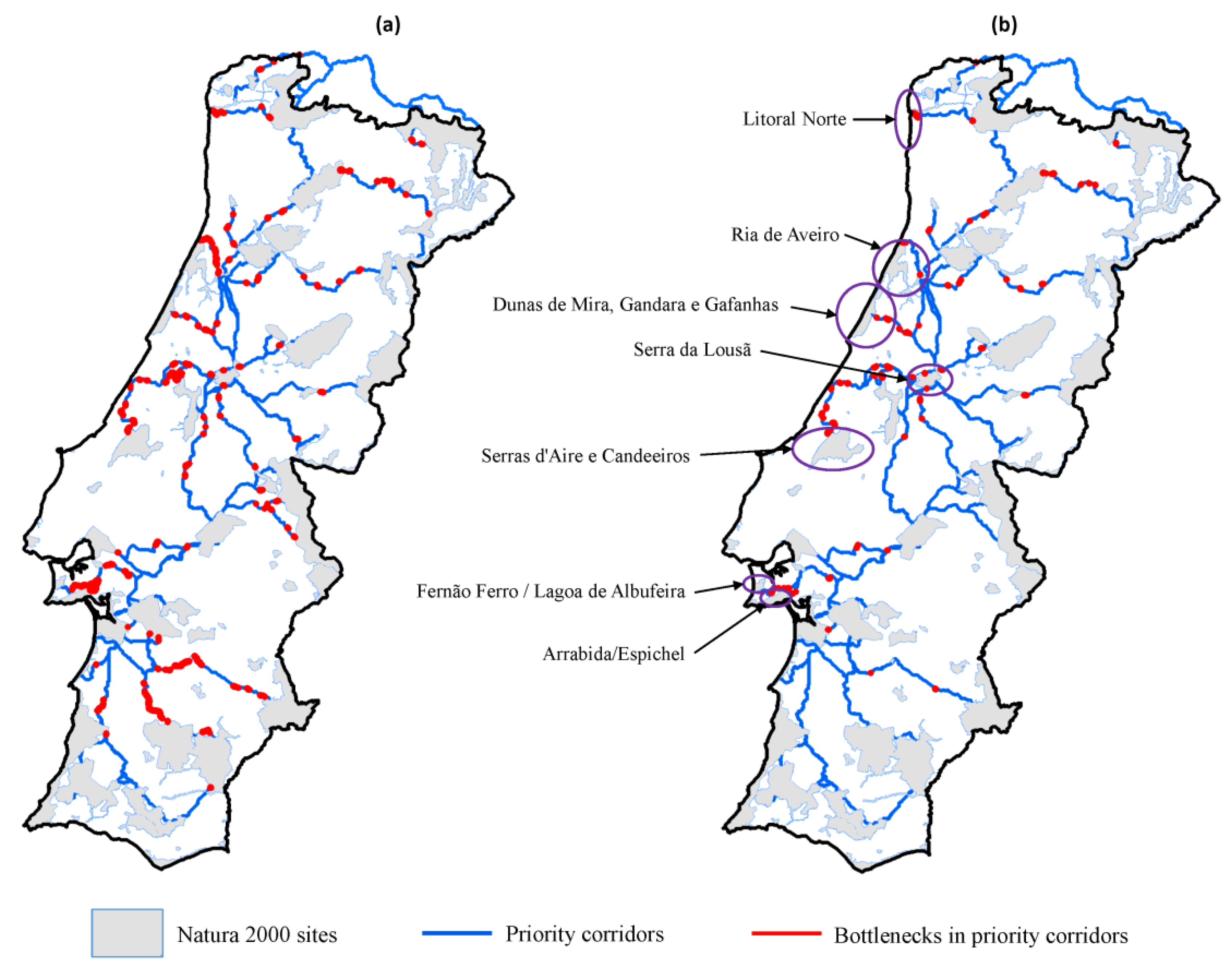
2.2.5. Bottlenecks (Weak Points) along Priority Corridors
3. Results
3.1. Impacts of PWN on the Natura 2000 Sites
3.2. Corridors between Natura 2000 Forest Sites
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Pimentel, D.; Zuniga, R.; Morrison, D. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol. Econ. 2005, 52, 273–288. [Google Scholar] [CrossRef]
- Soliman, T.; Mourits, M.C.; Van Der Werf, W.; Hengeveld, G.M.; Robinet, C.; Lansink, A.G.O. Framework for modelling economic impacts of invasive species, applied to pine wood nematode in Europe. PLoS ONE 2012, 7, e45505. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, D.; Lach, L.; Zuniga, R.; Morrison, D. Environmental and economic costs of nonindigenous species in the United States. BioScience 2000, 50, 53–65. [Google Scholar] [CrossRef]
- Colautti, R.I.; Bailey, S.A.; Van Overdijk, C.D.; Amundsen, K.; MacIsaac, H.J. Characterised and projected costs of nonindigenous species in Canada. Biol. Invasions 2006, 8, 45–59. [Google Scholar] [CrossRef]
- Pimentel, D. (Ed.) Biological Invasions: Economic and Environmental Costs of Alien Plant, Animal, and Microbe Species; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Gao, R.; Shi, J.; Huang, R.; Wang, Z.; Luo, Y. Effects of pine wilt disease invasion on soil properties and Masson pine forest communities in the Three Gorges reservoir region, China. Ecol. Evol. 2015, 5, 1702–1716. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Luo, Y.; Zeng, F.; Chen, W.; Yan, X.; Jiang, P. Impact of invasion of pine wood nematode on the niche of main populations in Masson pine community. J. Beijing Forest Univ. 2004, 27, 76–82. [Google Scholar]
- Shi, J.; Luo, Y.Q.; Wu, H.W.; Yan, X.S.; Jiang, P.; Chen, W.P. Impact of invasion of pine wood nematode on the growth of dominant shrub Pleioblastus amarus in Pinus massoniana communities. For. Stud. China 2009, 11, 61–63. [Google Scholar]
- Osipova, E.; Shadie, P.; Zwahlen, C.; Osti, M.; Shi, Y.; Kormos, C.; Bertzky, B.; Murai, M.; Van Merm, R.; Badman, T. IUCN World Heritage Outlook 2: A Conservation Assessment of all Natural World Heritage Sites; IUCN: Gland, Switzerland, 2017; p. 92. [Google Scholar]
- Laurance, W.F.; Useche, D.C.; Rendeiro, J.; Kalka, M.; Bradshaw, C.J.A.; Arroyo-Rodriguez, V. Averting biodiversity collapse in tropical forest protected areas. Nature 2012, 489, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.D.; Gillingham, P.K.; Bradbury, R.B.; Roy, D.B.; Hodgson, J.A. Protected areas facilitate species’ range expansions. Proc. Natl. Acad. Sci. USA 2012, 109, 14063–14068. [Google Scholar] [CrossRef] [PubMed]
- Convention on Biological Diversity (CBD). Decision UNEP/CBD/COP/DEC/X/2 Adopted by the Conference of the Parties to the Convention on Biological Diversity at Its Tenth Meeting. 2010. Available online: https://www.cbd.int/decision/cop/?id=12268 (accessed on 25 April 2018).
- Saura, S.; Bertzky, B.; Bastin, L.; Battistella, L.; Mandrici, A.; Dubois, G. Protected area connectivity: Shortfalls in global targets and country-level priorities. Biol. Conserv. 2018, 219, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Sousa, E.; Rodrigues, J.; Bonifácio, L.; Naves, P.; Rodrigues, A. Management and control of the Pine Wood Nematode, Bursaphelenchus xylophilus, in Portugal. In Nematodes: Morphology, Functions and Management Strategies; Boeri, F., Chung, J., Eds.; Nova Science Publishers: New York, NY, USA, 2011; ISBN 978-1-61470-784-4. [Google Scholar]
- Kuroda, K.; Yamada, T.; MIineo, K.; Tamura, H. Effects of cavitation on the development of pine wilt disease caused by Bursaphelenchus xylophilus. Jpn. J. Phytopathol. 1988, 54, 606–615. [Google Scholar] [CrossRef]
- Hu, G.; Xu, X.; Wang, Y.; Lu, G.; Feeley, K.J.; Yu, M. Regeneration of different plant functional types in a Masson pine forest following pine wilt disease. PLoS ONE 2012, 7, e36432. [Google Scholar] [CrossRef] [PubMed]
- Evans, H.F.; McNamara, D.G.; Braasch, H.; Chadoeuf, J.; Magnusson, C. Pest risk analysis (PRA) for the territories of the European Union (as PRA area) on Bursaphelenchus xylophilus and its vectors in the genus Monochamus. EPPO Bull. 1996, 26, 199–249. [Google Scholar] [CrossRef]
- Mamiya, Y. History of pine wilt disease in Japan. J. Nematol. 1988, 20, 219–226. [Google Scholar] [PubMed]
- Mota, M.M.; Braasch, H.; Bravo, M.A.; Penas, A.C.; Burgermeister, W.; Metge, K.; Sousa, E. First report of Bursaphelenchus xylophilus in Portugal and in Europe. Nematology 1999, 1, 727–734. [Google Scholar] [CrossRef]
- Sousa, E.; Bravo, M.A.; Pires, J.; Naves, P.; Penas, A.C.; Bonifacio, L.; Mota, M.M. Bursaphelenchus xylophilus (Nematoda; aphelenchoididae) associated with Monochamus galloprovincialis (Coleoptera; Cerambycidae) in Portugal. Nematology 2001, 3, 89–91. [Google Scholar] [CrossRef]
- Rodrigues, J.M. National eradication programme for the pinewood nematode. In Pine Wilt Disease: A Worldwide Threat to Forest Ecosystems; Springer: Dordrecht, The Netherlands, 2008; pp. 5–14. [Google Scholar]
- Holmes, T.P.; Aukema, J.E.; Von Holle, B.; Liebhold, A.; Sills, E. Economic impacts of invasive species in forests. Ann. N. Y. Acad. Sci. 2009, 1162, 18–38. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Mid-term Review of the EU Biodiversity Strategy. In Natura 2000 Nature and Biodiversity Newsletter. 39, January; 2016. Available online: http://ec.europa.eu/environment/nature/info/pubs/docs/nat2000newsl/nat39_en.pdf (accessed on 25 April 2018).
- ICNF. 6° Inventário Florestal Nacional. In Áreas dos Usos do Solo e das Espécies Florestais de Portugal Continental. Resultados Preliminaries; Instituto da Conservação da Natureza e das Florestas: Lisboa, Portugal, 2013; p. 34. [Google Scholar]
- De la Fuente, B.; Mateo-Sánchez, M.C.; Rodríguez, G.; Gastón, A.; Pérez de Ayala, R.; Colomina-Pérez, D.; Melero, M.; Saura, S. Natura 2000 sites, public forests and riparian corridors: The connectivity backbone of forest green infrastructure. Land Use Policy 2018, 75, 429–441. [Google Scholar] [CrossRef]
- Gastón, A.; Blázquez-Cabrera, S.; Garrote, G.; Mateo-Sánchez, M.C.; Beier, P.; Simón, M.A.; Saura, S. Response to agriculture by a woodland species depends on cover type and behavioural state: Insights from resident and dispersing Iberian lynx. J. Appl. Ecol. 2016, 53, 814–824. [Google Scholar] [CrossRef]
- Ziółkowska, E.; Ostapowicz, K.; Radeloff, V.C.; Kuemmerle, T.; Sergiel, A.; Zwijacz-Kozica, T.; Selva, N. Assessing differences in connectivity based on habitat versus movement models for brown bears in the Carpathians. Landsc. Ecol. 2016, 31, 1863–1882. [Google Scholar] [CrossRef]
- Adriaensen, F.; Chardon, J.P.; De Blust, G.; Swinnen, E.; Villalba, S.; Gulinck, H.; Matthysen, E. The application of ‘least-cost’ modelling as a functional landscape model. Landsc. Urban Plan 2003, 64, 233–247. [Google Scholar] [CrossRef]
- Gurrutxaga, M.; Rubio, L.; Saura, S. Key connectors in protected forest area networks and the impact of highways: A transnational case study from the Cantabrian Range to the Western Alps (SW Europe). Landsc. Urban Plan 2011, 101, 310–320. [Google Scholar] [CrossRef]
- Gurrutxaga, M.; Saura, S. Prioritizing highway defragmentation locations for restoring landscape connectivity. Environ. Conserv. 2014, 41, 157–164. [Google Scholar] [CrossRef]
- Ministerio de Agricultura, Alimentación y Medio Ambiente. Identificación de Áreas a Desfragmentar para Reducir los Impactos de las Infraestructuras Lineales de Transporte en la Biodiversidad; Documentos para la reducción de la fragmentación de hábitats causada por infraestructuras de transporte; número 6; Madrid, Spain. 2013, p. 260. Available online: http://www.magrama.gob.es/es/biodiversidad/servicios/banco-datos-naturaleza/informacion-disponible/fragmentacion-habitats-desfragmentacion.aspx (accessed on 25 April 2018).
- Gurrutxaga, M.; Lozano, P.J.; del Barrio, G. GIS-based approach for incorporating the connectivity of ecological networks into regional planning. J. Nat. Conserv. 2010, 18, 318–326. [Google Scholar] [CrossRef]
- Ruiz-González, A.; Gurrutxaga, M.; Cushman, S.A.; Madeira, M.J.; Randi, E.; Gómez-Moliner, B.J. Landscape genetics for the empirical assessment of resistance surfaces: The European Pine Marten (Martes martes) as a target-species of a regional ecological network. PLoS ONE 2014, 9, e110552. [Google Scholar] [CrossRef] [PubMed]
- Beier, P.; Majka, D.; Newell, S.; Garding, E. Best management practices for wildlife corridors. North. Ariz. Univ. 2008, 1, 3. [Google Scholar]
- Verbeylen, G.; De Bruyn, L.; Adriaensen, F.; Matthysen, E. Does matrix resistance influence Red squirrel (Sciurus vulgaris L. 1758) distribution in an urban landscape? Landsc. Ecol. 2003, 18, 791–805. [Google Scholar] [CrossRef]
- Chetkiewicz, C.L.B.; Boyce, M.S. Use of resource selection functions to identify conservation corridors. J. Appl. Ecol. 2009, 46, 1036–1047. [Google Scholar] [CrossRef]
- McRae, B.H.; Kavanagh, D.M. Linkage Mapper Connectivity Analysis Software; The Nature Conservancy: Seattle, WA, USA, 2011; Available online: http://www.circuitscape.org/linkagemapper (accessed on 25 April 2018).
- Saura, S.; Pascual-Hortal, L. A new habitat availability index to integrate connectivity in landscape conservation planning: Comparison with existing indices and application to a case study. Landsc. Urban Plan 2007, 83, 91–103. [Google Scholar] [CrossRef]
- Saura, S.; Rubio, L. A common currency for the different ways in which patches and links can contribute to habitat availability and connectivity in the landscape. Ecography 2010, 33, 523–537. [Google Scholar] [CrossRef]
- Saura, S.; De la Fuente, B. Connectivity as the amount of reachable habitat: Conservation priorities and the roles of habitat patches in landscape networks. In Learning Landscape Ecology, 2nd ed.; Gergel, S.E., Turner, M.G., Eds.; Springer: New York, NY, USA, 2017; Available online: http://dx.doi.org/10.1007/978-1-4939-6374-4 (accessed on 25 April 2018).
- Saura, S.; Bastin, L.; Battistella, L.; Mandrici, A.; Dubois, G. Protected areas in the world’s ecoregions: How well connected are they? Ecol. Indic. 2017, 76, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Saura, S.; Torné, J. Conefor Sensinode 2.2: A software package for quantifying the importance of habitat patches for landscape connectivity. Environ. Modell. Softw. 2009, 24, 135–139. [Google Scholar] [CrossRef]
- De la Fuente, B.; Saura, S.; Beck, P.S.A. Predicting the spread of an invasive tree pest: The pine wood nematode in Southern Europe. J. Appl. Ecol. 2018. [Google Scholar] [CrossRef]
- Bergdahl, D.R. Impact of pinewood nematode in North America: Present and future. J. Nematol. 1988, 20, 260. [Google Scholar] [PubMed]
- Webster, J.; Mota, M. Pine Wilt Disease: Global Issues, Trade and Economic Impact; Springer: Heidelberg, Germany, 2008; pp. 315–316. ISBN 978-1-4020-8454-6. [Google Scholar]
- Evans, H.F. Plant health risk and monitoring evaluation (PHRAME) final report. In PHRAME Final Report and Conclusions; 2007. Available online: https://www.forestry.gov.uk/fr/infd-7xrfx9 (accessed on 25 April 2018).
- Soliman, T.; Hengeveld, G.M.; Robinet, C.; Mourits, M.; van der Werf, W.; Oude Lansink, A. A Risk Assessment Model on Pine Wood Nematode in the EU. In EAAE 2011 Congress—Change and Uncertaint—Challenges for Agriculture, Food and Natural Resources; ETH Zurich: Zurich, Switzerland, 2011. [Google Scholar]
- Hunter, M.L. Maintaining Biodiversity in Forest Ecosystems; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Lindenmayer, D.B.; Laurance, W.F.; Franklin, J.F.; Likens, G.E.; Banks, S.C.; Blanchard, W.; Manning, A.D. New policies for old trees: Averting a global crisis in a keystone ecological structure. Conserv. Lett. 2014, 7, 61–69. [Google Scholar] [CrossRef]
- Stephenson, N.L.; Das, A.J.; Condit, R.; Russo, S.E.; Baker, P.J.; Beckman, N.G.; Alvarez, E. Rate of tree carbon accumulation increases continuously with tree size. Nature 2014, 507, 90. [Google Scholar] [CrossRef] [PubMed]
- OECD. Environmental Performance Reviews: Portugal 2001; OECD Publishing: Paris, France, 2001. [Google Scholar]
- Heller, N.E.; Zavaleta, E.S. Biodiversity management in the face of climate change: A review of 22 years of recommendations. Biol. Conserv. 2009, 142, 14–32. [Google Scholar] [CrossRef]
- Robinet, C.; Roques, A.; Pan, H.; Fang, G.; Ye, J.; Zhang, Y.; Sun, J. Role of human-mediated dispersal in the spread of the pinewood nematode in China. PLoS ONE 2009, 4, e4646. [Google Scholar] [CrossRef] [PubMed]
- Hudgins, E.J.; Liebhold, A.M.; Leung, B. Predicting the spread of all invasive forest pests in the United States. Ecol. Lett. 2017, 20, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Vicente, C.; Espada, M.; Vieira, P.; Mota, M. Pine wilt disease: A threat to European forestry. Eur. J. Plant Pathol. 2012, 133, 89–99. [Google Scholar] [CrossRef]
- Muzika, R.M.; Liebhold, A.M. A critique of silvicultural approaches to managing defoliating insects in North America. Agric. For. Entomol. 2000, 2, 97–105. [Google Scholar] [CrossRef]
- Jactel, H.; Menassieu, P.; Vetillard, F.; Gaulier, A.; Samalens, J.C.; Brockerhoff, E.G. Tree species diversity reduces the invasibility of maritime pine stands by the bast scale, Matsucoccus feytaudi. Can. J. For. Res. 2006, 36, 314–323. [Google Scholar] [CrossRef]
- Liebhold, A.M. Forest pest management in a changing world. Int. J. Pest Manag. 2012, 58, 289–295. [Google Scholar] [CrossRef]
- Gaspar, D.; Trindade, C.; Usié, A.; Meireles, B.; Barbosa, P.; Fortes, A.M.; Ramos, A.M. Expression profiling in Pinus pinaster in response to infection with the pine wood nematode Bursaphelenchus xylophilus. Forests 2017, 8, 279. [Google Scholar] [CrossRef]
- Menéndez-Gutiérrez, M.; Alonso, M.; Toval, G.; Díaz, R. Testing of selected Pinus pinaster half-sib families for tolerance to pinewood nematode (Bursaphelenchus xylophilus). Forestry 2017, 91, 38–48. [Google Scholar] [CrossRef]
- Ellison, A.M.; Chen, J.; Díaz, D.; Kammerer-Burnham, C.; Lau, M. Changes in ant community structure and composition associated with hemlock decline in New England. In Proceedings of the 3rd Symposium on Hemlock Woolly Adelgid in the Eastern United States, Asheville, NC, USA, 1–3 February 2005; USDA Forest Service: Morgantown, WV, USA, 2005. [Google Scholar]
- Boyd, I.L.; Freer-Smith, P.H.; Gilligan, C.A.; Godfray, H.C.J. The consequence of tree pests and diseases for ecosystem services. Science 2013, 342, 1235773. [Google Scholar] [CrossRef] [PubMed]
- Nunes da Silva, M.; Solla, A.; Sampedro, L.; Zas, R.; Vasconcelos, M.W. Susceptibility to the pinewood nematode (PWN) of four pine species involved in potential range expansion across Europe. Tree Physiol. 2015, 35, 987–999. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, C.S.; Gonçalves, E.V.; Firmino, P.N.; Calvão, T.; Fonseca, L.; Abrantes, I.; Máguas, C. Differences in constitutive and inducible defences in pine species determining susceptibility to pinewood nematode. Plant Pathol. 2017, 66, 131–139. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De la Fuente, B.; Beck, P.S.A. Invasive Species May Disrupt Protected Area Networks: Insights from the Pine Wood Nematode Spread in Portugal. Forests 2018, 9, 282. https://doi.org/10.3390/f9050282
De la Fuente B, Beck PSA. Invasive Species May Disrupt Protected Area Networks: Insights from the Pine Wood Nematode Spread in Portugal. Forests. 2018; 9(5):282. https://doi.org/10.3390/f9050282
Chicago/Turabian StyleDe la Fuente, Begoña, and Pieter S. A. Beck. 2018. "Invasive Species May Disrupt Protected Area Networks: Insights from the Pine Wood Nematode Spread in Portugal" Forests 9, no. 5: 282. https://doi.org/10.3390/f9050282
APA StyleDe la Fuente, B., & Beck, P. S. A. (2018). Invasive Species May Disrupt Protected Area Networks: Insights from the Pine Wood Nematode Spread in Portugal. Forests, 9(5), 282. https://doi.org/10.3390/f9050282