Identification of Potential Metabolic Markers for the Selection of a High-Yield Clone of Quercus acutissima in Clonal Seed Orchard
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Sample Preparation
2.2. Metabolic Profiling and Hormone Analysis
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wu, T.; Wang, G.G.; Wu, Q.; Cheng, X.; Yu, M.; Wang, W.; Yu, X. Patterns of leaf nitrogen and phosphorus stoichiometry among Quercus acutissima provenances across China. Ecol. Complex. 2014, 17, 32–39. [Google Scholar] [CrossRef]
- Kang, K.S.; Kim, C.S.; El-Kassaby, Y.A. Clonal variation in acorn production and its effect on the effective population size in a Quercus acutissima seed orchard. Silvae Genet. 2010, 59, 170–175. [Google Scholar] [CrossRef][Green Version]
- Hwang, J.T.; Choi, H.K.; Kim, S.H.; Chung, S.; Hur, H.J.; Park, J.H.; Chung, M.Y. Hypolipidemic activity of Quercus acutissima fruit ethanol extract is mediated by inhibition of acetylation. J. Med. Food 2017, 20, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Lee, T. Coloured Flora of Korea; Hayangmunsa: Seoul, Korea, 2003. [Google Scholar]
- Darley-Hill, S.; Johnson, W.C. Acorn dispersal by blue jay (Cyanocitta cristata). Oecologia 1981, 50, 231–232. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.E.; Koo, C.D.; Kim, J.S.; Park, J.I.; Shin, W.S.; Shin, C.S. Ecological characteristics of below-ground ectomycorrhizal colony of Sarcodon aspratus in oak tree stands. J. Korean For. Soc. 2002, 91, 457–464. [Google Scholar]
- Yim, K.B.; Min, Y.T.; Kim, Y.M.; Han, S.D.; Kwon, H.M. Oak Trees; Institute of Forest Genetics: Suwon, Korea, 1995; pp. 35–52. [Google Scholar]
- Han, Y.C.; Chung, H.G.; Min, Y.T.; Kang, K.S. Plus Tree Handbook; Forest Genetics Research Institute: Suwon, Korea, 1996; pp. 180–195. [Google Scholar]
- Sork, V.L.; Bramble, E. Prediction of acorn crops in three species of North American oaks: Quercus alba, Q. rubra and Q. velutina. Ann. Sci. For. 1993, 50, 128s–136s. [Google Scholar] [CrossRef]
- Gilland, K.E.; Keiffer, C.H.; McCarthy, B.C. Seed production of mature forest-grown American chestnut (Castanea dentata (Marsh.) Borkh). J. Torrey Bot. Soc. 2012, 139, 283–289. [Google Scholar] [CrossRef]
- Pourhashemi, M.; Panahi, P.; Zandebasiri, M. Application of visual surveys to estimate acorn production of Brant's oak (Quercus brantii Lindl.) in northern Zagros Forests of Iran. Caspi. J. Environ. Sci. 2013, 11, 85–95. [Google Scholar]
- Kim, H.T.; Kang, J.W.; Lee, W.Y.; Han, S.U.; Park, E.J. Estimation of acorn production capacity using growth characteristics of Quercus acutissima in a clonal seed orchard. For. Sci. Technol. 2016, 12, 51–54. [Google Scholar]
- Fernandez, O.; Urrutia, M.; Bernillon, S.; Giauffret, C.; Tardieu, F.; Le Gouis, J.; Langlade, N.; Charcosset, A.; Moing, A.; Gibon, Y. Fortune telling: Metabolic markers of plant performance. Metabolomics 2016, 12, 158. [Google Scholar] [CrossRef] [PubMed]
- Weckwerth, W. Integration of metabolomics and proteomics in molecular plant physiology-coping with the complexity by data-dimensionality reduction. Physol. Plant. 2008, 132, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Hamzehzarghani, H.; Kushalappa, A.C.; Dion, Y.; Rioux, S.; Comeau, A.; Yaylayan, V.; Marshall, W.D.; Mather, D. Metabolic profiling and factor analysis to discriminate quantitative resistance in wheat cultivars against fusarium head blight. Physiol. Mol. Plant Pathol. 2005, 66, 119–133. [Google Scholar] [CrossRef]
- Witta, S.; Galiciab, L.; Liseca, J.; Cairnsc, J.; Tiessend, A.; Arause, J.L.; Rojasb, N.P.; Ferniea, A.R. Metabolic and phenotypic responses of greenhouse-grown maize hybrids to experimentally controlled drought stress. Mol. Plant. 2012, 5, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.R.; Gheneim, R.; Kozak, R.A.; Ellis, D.D.; Mansfield, S.D. The potential of metabolite profiling as a selection tool for genotype discrimination in Populus. J. Exp. Bot. 2005, 56, 2807–2819. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.R.; Ukrainetz, N.K.; Kang, K.Y.; Mansfield, S.D. Metabolite profiling of Douglas-fir (Pseudotsuga menziesii) field trials reveals strong environmental and weak genetic variation. New Phytol. 2007, 174, 762–773. [Google Scholar] [CrossRef] [PubMed]
- Ossipov, V.; Ossipova, S.; Bykov, V.; Oksanen, E.; Koricheva, J.; Haukioja, E. Application of metabolomics to genotype and phenotype discrimination of birch trees grown in a long-term open-field experiment. Metabolomics 2008, 4, 39–51. [Google Scholar] [CrossRef]
- Farrow, S.C.; Emery, R.N. Concurrent profiling of indole-3-acetic acid, abscisic acid, and cytokinins and structurally related purines by high-performance-liquid-chromatography tandem electrospray mass spectrometry. Plant Methods 2012, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Kusaba, S.; Masashi, F.; Chikako, H.; Isomaro, Y.; Tomoaki, S.; Yuriko, K.M. Decreased GA1 Content Caused by the Overexpression ofOSH1 Is Accompanied by Suppression of GA 20-Oxidase Gene Expression. Plant Physiol. 1998, 117, 1179–1184. [Google Scholar] [CrossRef] [PubMed]
- Pearce, D.W.; Rood, S.B.; Wu, R. Phytohormones and shoot growth in a three-generation hybrid poplar family. Tree Physiol. 2004, 24, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Pharis, R.P.; Yeh, F.C.; Bruce, P.D. Superior growth potential in trees: What is its basis, and can it be tested for at an early age? Can. J. For. Res. 1991, 21, 368–374. [Google Scholar] [CrossRef]
- Todorova, D.; Genkov, T.; Vaseva-Gemisheva, I.; Alexieva, V.; Karanov, E.; Smith, A.; Hall, M. Effect of temperature stress on the endogenous cytokinin content in Arabidopsis thaliana (L.) Heynh plants. Acta Physiol. Plant. 2005, 27, 13–18. [Google Scholar] [CrossRef]
- Scott, I.M.; Horgan, R.; McGaw, B.A. Zeatin-9-glucoside, a major endogenous cytokinin of Vinca rosea L. crown gall tissue. Planta 1980, 149, 472–475. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.C.; Mok, M.C.; Mok, D.W.S. A gene encoding the cytokinin enzyme zeatinO-xylosyltransferase of Phaseolus vulgaris. Plant Physiol. 1999, 120, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Horgan, R. A new cytokinin metabolite. Biochem. Biophys. Res. Commun. 1975, 65, 358–363. [Google Scholar] [CrossRef]
- Parker, C.W.; Letham, D.S.; Gollnow, B.I.; Summons, R.E.; Duke, C.C.; MacLeod, J.K. Regulators of cell division in plant tissues XXV. Metabolism of zeatin by lupin seedlings. Planta 1978, 142, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Duke, C.C.; Letham, D.S.; Parker, C.W.; MacLeod, J.K.; Summons, R.E. The complex of 0-glucosylzeatin derivatives formed in Populus species. Phytochemistry 1979, 18, 819–824. [Google Scholar] [CrossRef]
- Toubiana, D.; Fait, A. Metabolomics-assisted crop breeding towards improvement in seed quality and yield. In Seed Development: Omics Technologies Toward Improvement of Seed Quality and Crop Yield; Springer: Dordrecht, The Netherlands, 2012; pp. 453–475. [Google Scholar]
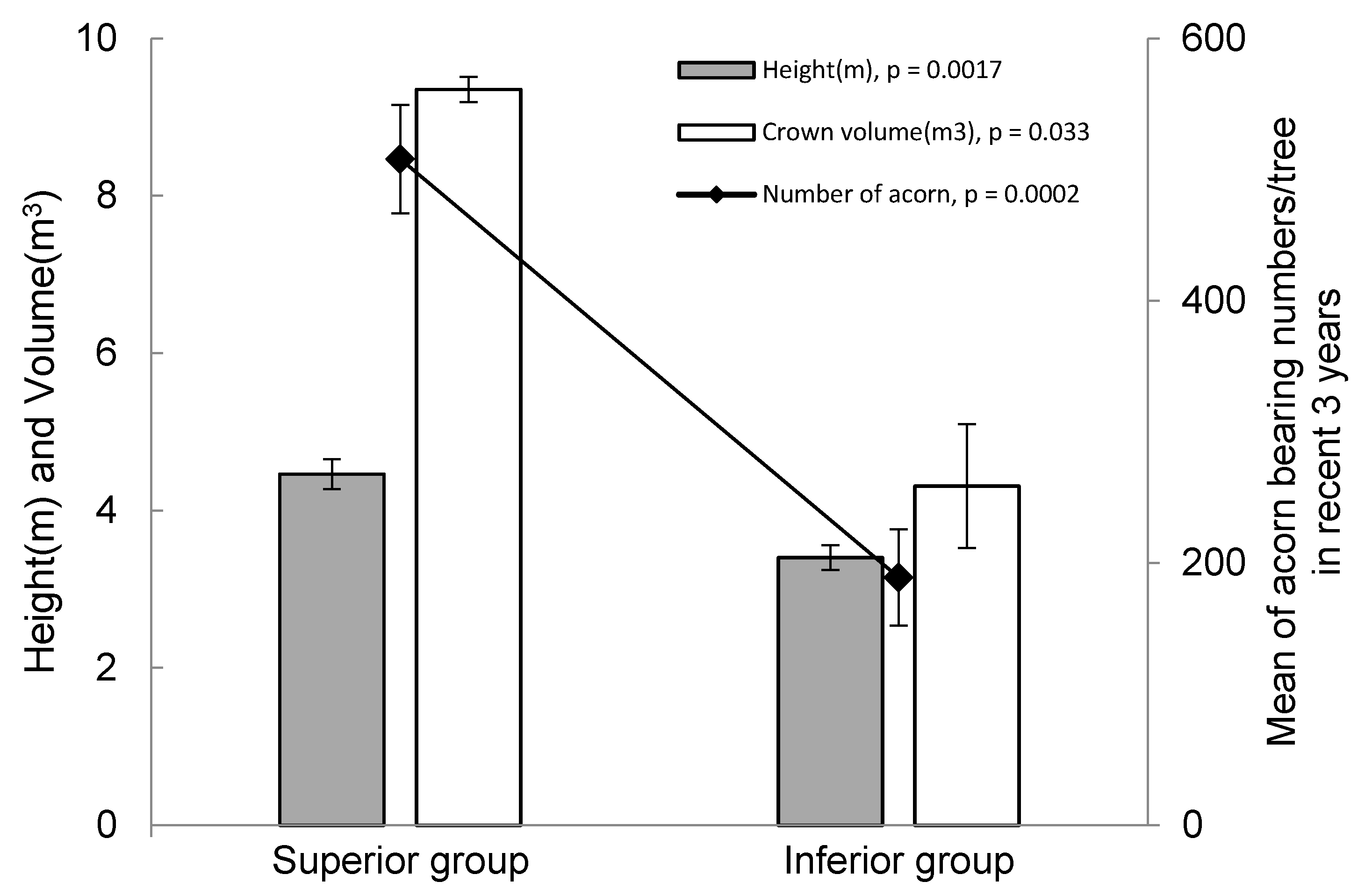
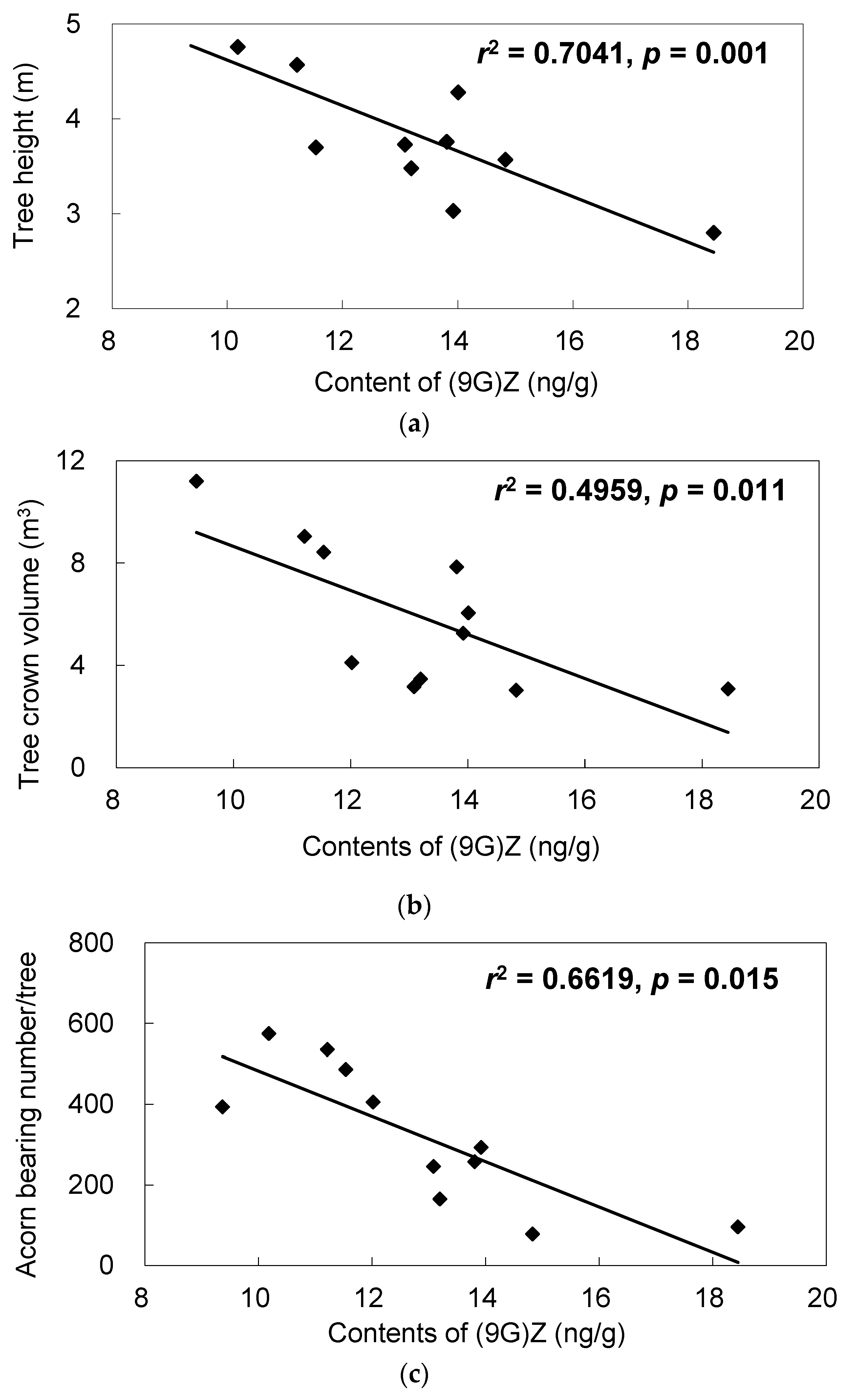
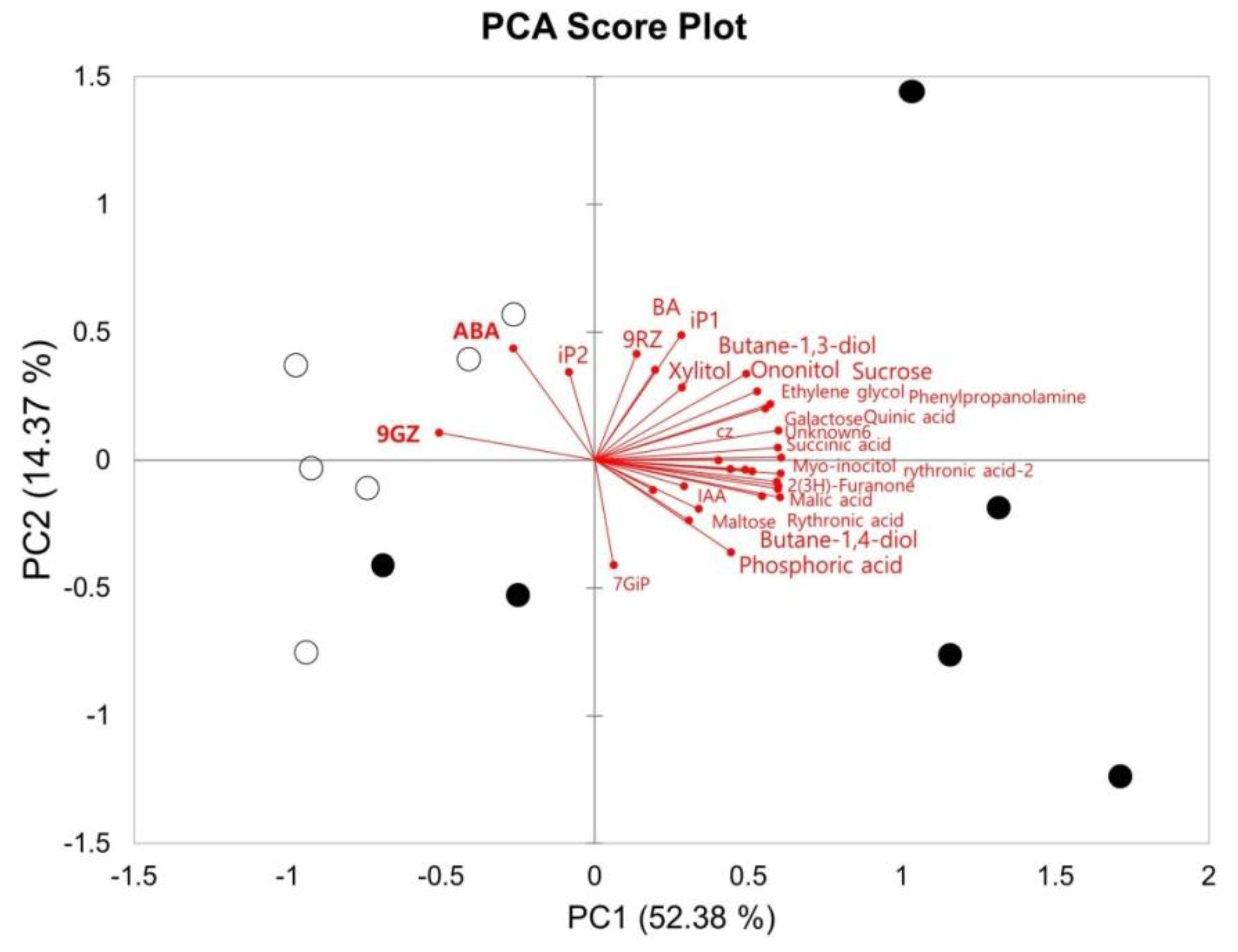
| Metabolites | Relative Contents (Mean) | Fold Changes (Superior/Inferior) | t-Test p-Value | |
|---|---|---|---|---|
| Superior Group | Inferior Group | |||
| <leaves> | ||||
| Ethylene glycol | 74 | 34 | 2.18 | 0.045 |
| Butane-1,3-diol | 509 | 194 | 2.62 | 0.049 |
| 2(3H)-Furanone | 62 | 23 | 2.70 | 0.042 |
| Malic acid | 3514 | 1520 | 2.31 | 0.048 |
| Butane-1,4-diol | 264 | 100 | 2.64 | 0.019 |
| Rythronic acid-2 | 53 | 25 | 2.12 | 0.046 |
| Ononitol | 10,663 | 4997 | 2.13 | 0.048 |
| Phenylpropanolamine | 32,444 | 11,389 | 2.85 | 0.021 |
| Quinic acid | 22,284 | 9341 | 2.39 | 0.026 |
| D-Ribose | 1727 | 968 | 1.78 | 0.002 |
| Galactose | 205 | 75 | 2.73 | 0.044 |
| Myo-inositol | 172 | 90 | 1.91 | 0.037 |
| Muco-inositol | 282 | 97 | 2.91 | 0.017 |
| Sucrose | 39,070 | 14,068 | 2.78 | 0.025 |
| Maltose | 456 | 153 | 2.98 | 0.035 |
| <stems> | ||||
| Phosphoric acid | 146 | 86 | 1.70 | 0.043 |
| Succinic acid | 10 | 5 | 2.02 | 0.000 |
| Rythronic acid | 42 | 30 | 1.40 | 0.021 |
| Xylitol | 15 | 10 | 1.51 | 0.033 |
| Unknown 6 | 9 | 5 | 1.79 | 0.018 |
| Metabolites | Acorn Bearing Numbers/Tree | Crown Volume |
|---|---|---|
| Correlation Coefficient | Correlation Coefficient | |
| Phosphoric acid | 0.4301 * | 0.2380 |
| Succinic acid | 0.5317 * | 0.2252 |
| Malic acid | 0.5430 ** | 0.2821 |
| Butane-1,3-diol | 0.5951 ** | 0.3848 |
| Xylitol | 0.4308 * | 0.3070 |
| Isocitric acid | 0.4738 * | 0.6522 ** |
| Glucitol | 0.4591 * | 0.4317 * |
| Maltose | 0.4110 * | 0.3657 |
| Rythronic acid | 0.2458 | 0.4498 * |
| Characteristic of Acorn Production | Hormone Contents (ng/g Dry Weight ) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (9G)Z | (9R)Z | (7G)iP | iP | (9R) DZ | BA | cis-Z | IAA | ABA | IAA/ ABA | |
| Superior group | 11.4 ± 1.6 | 108.8 ± 34.7 | 57.2 ± 25.5 | 150.4 ± 102.0 | 19.5 ± 6.1 | 6.8 ± 2.6 | 1.9 ± 0.6 | 280.5 ± 166.0 | 1,802.9 ± 666.3 | 0.19 ± 0.15 |
| Inferior group | 14.5 ± 2.0 | 107.2 ± 30.4 | 47.1 ± 22.5 | 94.3 ± 40.7 | 18.4 ± 5.6 | 6.2 ± 4.0 | 1.5 ± 0.6 | 299.2 ± 72.1 | 2,197.6 ± 576.2 | 0.15 ± 0.05 |
| Sig. | ** | - | - | - | - | - | - | - | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, J.W.; Lee, H.; Lim, H.; Lee, W.Y. Identification of Potential Metabolic Markers for the Selection of a High-Yield Clone of Quercus acutissima in Clonal Seed Orchard. Forests 2018, 9, 116. https://doi.org/10.3390/f9030116
Kang JW, Lee H, Lim H, Lee WY. Identification of Potential Metabolic Markers for the Selection of a High-Yield Clone of Quercus acutissima in Clonal Seed Orchard. Forests. 2018; 9(3):116. https://doi.org/10.3390/f9030116
Chicago/Turabian StyleKang, Jun Won, Hyunseok Lee, Hyemin Lim, and Wi Young Lee. 2018. "Identification of Potential Metabolic Markers for the Selection of a High-Yield Clone of Quercus acutissima in Clonal Seed Orchard" Forests 9, no. 3: 116. https://doi.org/10.3390/f9030116
APA StyleKang, J. W., Lee, H., Lim, H., & Lee, W. Y. (2018). Identification of Potential Metabolic Markers for the Selection of a High-Yield Clone of Quercus acutissima in Clonal Seed Orchard. Forests, 9(3), 116. https://doi.org/10.3390/f9030116




