How to Bloom the Green Desert: Eucalyptus Plantations and Native Forests in Uruguay beyond Black and White Perspectives
Abstract
1. Introduction
2. Materials and Methods
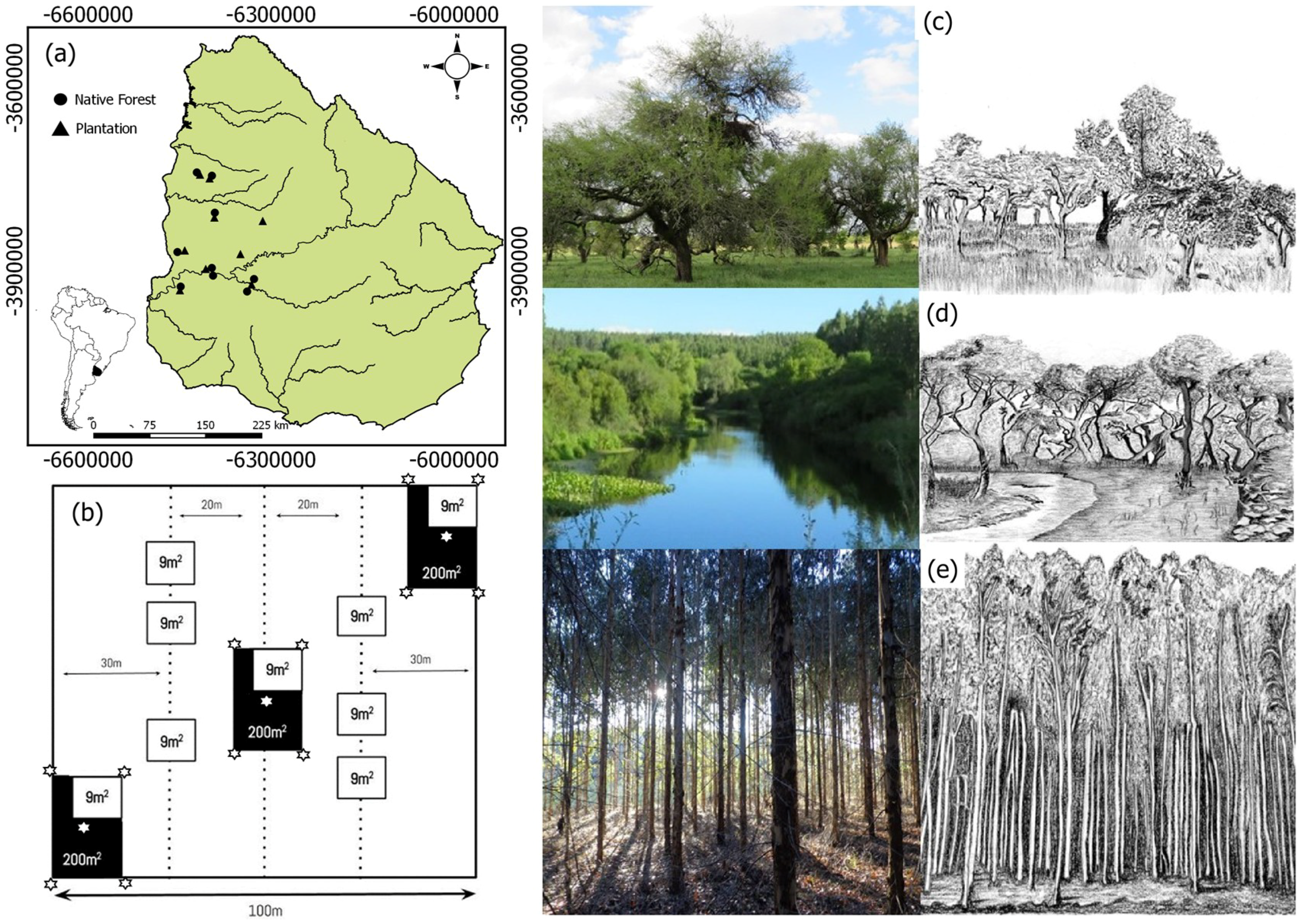
2.1. Study Area
2.2. Field Inventory Design
2.3. Data Analysis
3. Results
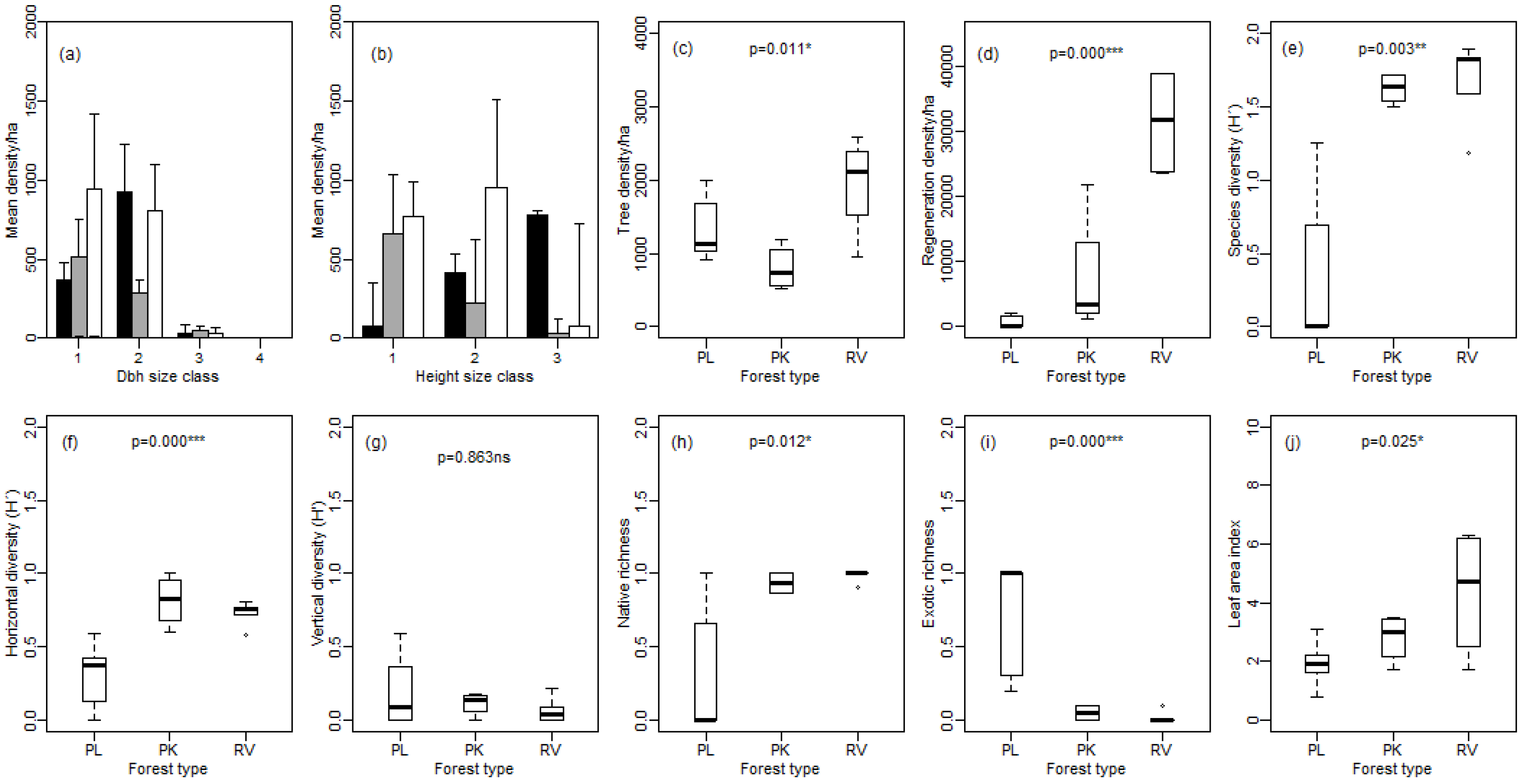
3.1. Forest Structure and Regeneration
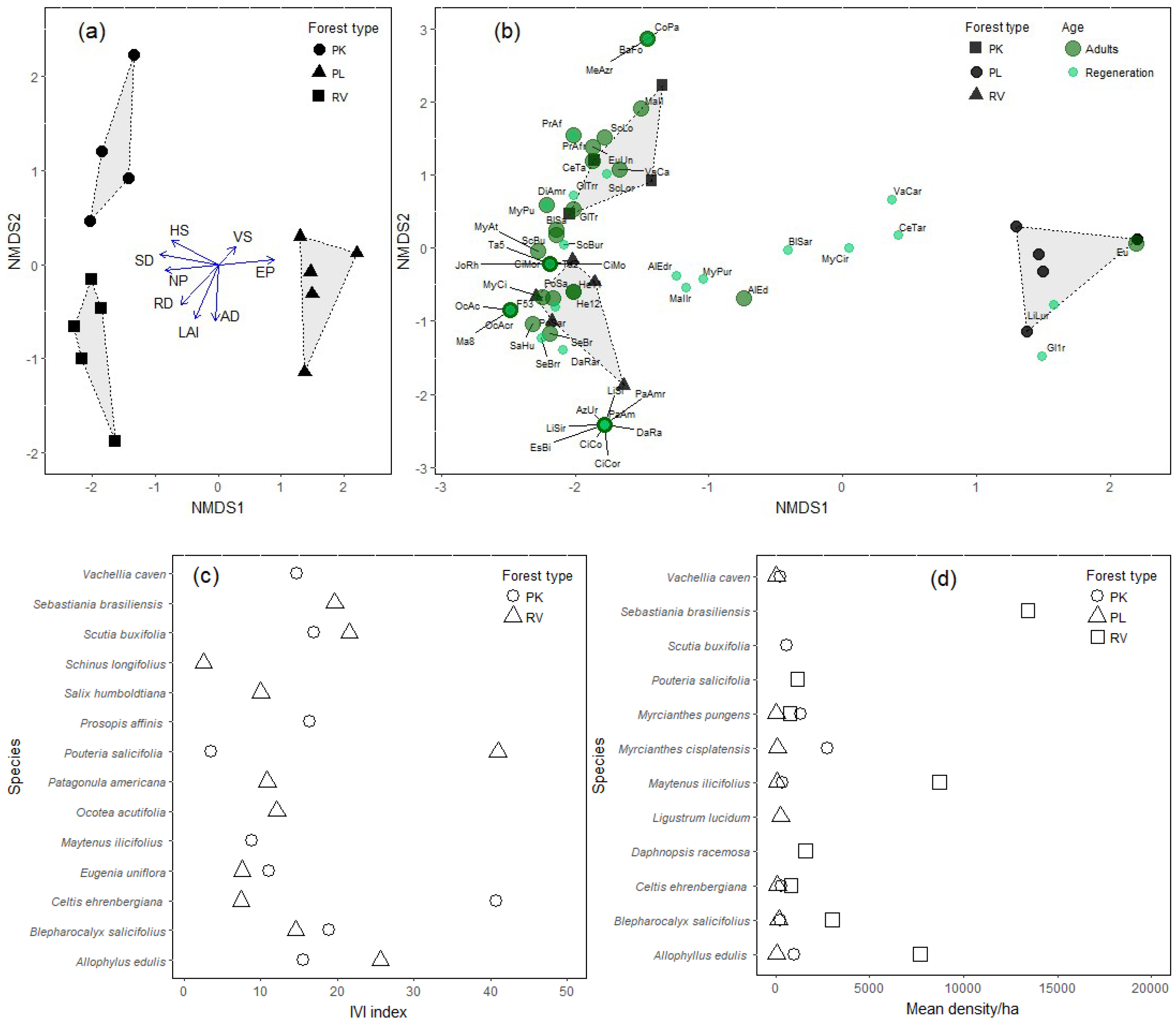
3.2. Diversity and Composition
3.3. Importance Value and Potential Use of Native Species
4. Discussion
4.1. Forest Structure and Regeneration
4.2. Forest Diversity and Composition
4.3. Native Species Importance Value Index and Potential Use
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
Supplementary References for Table 1 Species Composition and Potential Use of Tree Species in Different Uruguayan Forest Types
- A1
- de Aguiar, M.D.; da Silva, A.C.; Higuchi, P.; Negrini, M.; Fert Neto, J. Potencial de uso de espécies arbóreas de uma floresta secundária em Lages, Santa Catarina. Rev. Ciênc. Agroveterinárias 2012, 11, 238–247.
- A2
- Araujo, I.C.L.; Dziedzic, M.; Maranho, L.T. Management of the Environmental Restoration of Degraded Areas. Braz. Arch. Biol. Technol. 2014, 57, 284–294, doi:10.1590/S1516-89132014000200018.
- A3
- Bennadji, Z.; Fagúndez, C.; Puppo, M.; Nuñez, P.; Alfonso, M.; Rodríguez, F. Identificación y caracterización de especies arbóreas nativas y exóticas para la implementación de proyectos en el marco del mecanismo de desarrollo limpio (MDL) en el Uruguay: Algunos resultados preliminares. Rev. INIA 2007, 30–33.
- A4
- Bertucci, A.; Haretche, F.; Olivaro, C.; Vázquez, A. Prospección química del bosque de galería del río Uruguay. Braz. J. Pharmacogn. 2008, 18, 21–25, doi:10.1590/S0102-695X2010005000044.
- A5
- Bueno, N.R.; Castilho, R.O.; da Costa, R.B.; Pott, A.; Pott, V.J.; Scheidt, G.N.; Batista, M.d.S. Medicinal plants used by the Kaiowá and Guarani indigenous populations in the Caarapó Reserve, Mato Grosso do Sul, Brazil. Acta Bot. Bras. 2005, 19, 39–44, doi:10.1590/S0102-33062005000100005.
- A6
- Castillo, D.; Bennadji, Z.; Alfonso, M. Revista INIA. 2014, pp. 62–66.
- A7
- Coelho, G.; Benvenuti-Ferreira, G.; Schirmer, J.; Lucchese, O. Survival, growth and seed mass in a mixed tree species planting for Atlantic Forest restoration. AIMS Environ. Sci. 2016, 3, 382–394, doi:10.3934/environsci.2016.3.382.
- A8
- Delfino, L.; Nicoli, N.; Muñoz, F.; Gago, J.; Rodríguez, R.; Gracía, A. Manual del Curso de Flora Indigena; Museo y Jardin Botanico Atilio Lombardo: Montevideo, Uruguay, 2014.
- A9
- Diniz, M.E.d.R.; Buschini, M.L.T. Pollen analysis and interaction networks of floral visitor bees of Eugenia uniflora L. (Myrtaceae), in Atlantic Forest areas in southern Brazil. Arthropod-Plant Interact. 2015, 9, 623–632, doi:10.1007/s11829-015-9400-1.
- A10
- Garcez, F.R.; Garcez, W.S.; Yoshida, N.C.; Figueiredo, P.O. A diversidade dos constituintes químicos da flora de Mato Grosso do Sul e sua Relevância como Fonte de substâncias bioativas. Rev. Virtual Quimica 2016, 8, 97–129, doi:10.5935/1984-6835.20160008.
- A11
- Gomes, J.P.; Dacoregio, H.M.; da Silva, K.M.; da Rosa, L.H.; Bortoluzzi, R.L.d.C. Myrtaceae na bacia do rio Caveiras: Características ecológicas e usos não madeireiros. Floresta E Ambiente 2017, 24, 1–10, doi:10.1590/2179-8087.011115.
- A12
- Grings, M.; Brack, P. Árvores na vegetação nativa de Nova Petrópolis, Rio Grande do Sul1. Iheringia Sér. Botânica 2009, 64, 5–22.
- A13
- Montanha, J.A.; Schenkel, E.P.; Cardoso-Taketa, A.T.; Dresch, A.P.; Langeloh, A.; Dallegrave, E. Chemical and anti-ulcer evaluation of Jodina rhombifolia (Hook. & Arn.) Reissek extracts. Braz. J. Pharmacogn. 2009, 19, 29–32, doi:10.1590/S0102-695X2009000100007.
- A14
- Root-Bernstein, M.; Jaksic, F. The chilean espinal: Restoration for a sustainable silvopastoral system. Restor. Ecol. 2013, 21, 409–414, doi:10.1111/rec.12019.
- A15
- Roussy, L.; Keil1, G.; Refort, M.; Iaconis, A.; Abedini, W. Propiedades tecnológicas de la madera de Citharexylum montevidense (Spreng.) Mol. “Espina de bañado”. Quebracho 2013, 21, 58–66.
- A16
- Scipioni, M.C.; Galvão, F.; Longhi, S.J. Composição florística e estratégias de dispersão e regeneração de grupos florísticos em florestas estacionais deciduais no rio grande do sul. Floresta 2013, 43, 241–254, doi:10.5380/rf.v43i2.27098.
- A17
- da Silva, E.R.; Diedrich, D.; Bolzan, R.C.; Giacomelli, S.R. Toxicological and pharmacological evaluation of discaria Americana Gillies & hook (Rhamnaceae) in mice. Braz. J. Pharm. Sci. 2012, 48, 273–280, doi:10.1590/S1984-82502012000200011.
- A18
- De Sousa, M.J.; Alves, O. Espécies úteis da família Euphorbiaceae no Brasil Especies de interés de familia Euphorbiaceae en Brasil Species from the Euphorbiaceae family used for medicinal purposes in Brazil; Embrapa Amazonia Oriental: Belém-PA, Brazil 2014; Volume 19.
- A19
- Tempel, E.; Romano, C.M.; Barbieri, R.L.; Heiden, G.; Zitzke, F.S.; Brisolara Correa, L. Características ornamentais de plantas do Bioma Pampa. Rev. Bras. Hortic. Ornam. 2009, 15, 46–62, doi:10.14295/rbho.v15i1.435.
- A20
- Zuchiwschi, E.; Fantini, A.C.; Alves, A.C.; Peroni, N. Limitações ao uso de espécies florestais nativas pode contribuir com a erosão do conhecimento ecológico tradicional e local de agricultores familiares. Acta Bot. Bras. 2010, 24, 270–282, doi:10.1590/S0102-33062010000100029.
References
- Payn, T.; Carnus, J.M.; Freer-Smith, P.; Kimberley, M.; Kollert, W.; Liu, S.; Orazio, C.; Rodriguez, L.; Silva, L.N.; Wingfield, M.J. Changes in planted forests and future global implications. For. Ecol. Manag. 2015, 352, 57–67. [Google Scholar] [CrossRef]
- Rappaport, D.; Montagnini, F. Tree species growth under a rubber (Hevea brasiliensis) plantation: Native restoration via enrichment planting in southern Bahia, Brazil. New For. 2014, 45, 715–732. [Google Scholar] [CrossRef]
- Yang, X.; Bauhus, J.; Both, S.; Fang, T.; Härdtle, W.; Kröber, W.; Ma, K.; Nadrowski, K.; Pei, K.; Scherer-Lorenzen, M.; et al. Establishment success in a forest biodiversity and ecosystem functioning experiment in subtropical China (BEF-China). Eur. J. For. Res. 2013, 132, 593–606. [Google Scholar] [CrossRef]
- Miah, D.; Uddin, M.F.; Bhuiyan, M.K.; Koike, M.; Shin, M.Y. Carbon sequestration by the indigenous tree species in the reforestation program in Bangladesh-aphanamixis polystachya Wall. and Parker. For. Sci. Technol. 2009, 5, 62–65. [Google Scholar] [CrossRef][Green Version]
- Arias, D.; Calvo-Alvarado, J.; de B. Richter, D.; Dohrenbusch, A. Productivity, aboveground biomass, nutrient uptake and carbon content in fast-growing tree plantations of native and introduced species in the Southern Region of Costa Rica. Biomass Bioenergy 2011, 35, 1779–1788. [Google Scholar] [CrossRef]
- Evans, J.; Turnbull, J. Plantation Forestry in the Tropics. The Role, Silviculture, and Use of Planted Forests for Industrial, Social, Environmental, and Agroforestry Purposes, 3rd ed.; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- FAO. Estado actual de la información sobre árboles fuera del bosque; FAO: Montevideo, Uruguay, 2007. [Google Scholar]
- Payret, C.C.; Pineiro, G.; Achkar, M.; Gutierrez, O.; Panario, D. The irruption of new agro-industrial technologies in Uruguay and their environmental impacts on soil, water supply and biodiversity: A review. Int. J. Environ. Health 2009, 3, 175–197. [Google Scholar] [CrossRef]
- Redo, D.J.; Aide, T.M.; Clark, M.L.; Andrade-Núñez, M.J. Impacts of internal and external policies on land change in Uruguay, 2001–2009. Environ. Conserv. 2012, 39, 122–131. [Google Scholar] [CrossRef]
- Piñeiro, D.E. Land grabbing: Concentration and “foreignisation” of land in Uruguay. Can. J. Dev. Stud. 2012, 33, 471–489. [Google Scholar] [CrossRef]
- FAO. Paquete de informe sobre los bosques 2015; FAO: Montevideo, Uruguay, 2014. [Google Scholar]
- MGAP. Anuario Estadístico Agropecuario 2016; MGAP: Montevideo, Uruguay, 2016. [Google Scholar]
- Cubbage, F.; Balmelli, G.; Bussoni, A.; Noellemeyer, E.; Pachas, A.N.; Fassola, H.; Colcombet, L.; Rossner, B.; Frey, G.; Dube, F.; et al. Comparing silvopastoral systems and prospects in eight regions of the world. Agrofor. Syst. 2012, 86, 303–314. [Google Scholar] [CrossRef]
- Brussa, C.A.; Grela, I.A. Flora arbórea del Uruguay. Con énfasis en las especies de Rivera y Tacuarembó; Empresa Gráfica Mosca: Montevideo, Uruguay, 2007. [Google Scholar]
- Haretche, F.; Mai, P.; Brazeiro, A. Woody flora of Uruguay: inventory and implication within the Pampean region. Acta Bot. Bras. 2012, 26, 537–552. [Google Scholar] [CrossRef]
- Bennadji, Z.; Puppo, B.M.; Alfonso, M.; Núñez, F.R.P.; Rodríguez, F. Potencial de uso del pecan como especie forestal multipropósito en Uruguay. Revista INIA; INIA: Montevideo, Uruguay, 2012; pp. 38–42. [Google Scholar]
- Castillo, D.; Bennadji, Z.; Alfonso, M. Potencial socioeconómico de especies forestales nativas del Uruguay: avances en bioprospección de algarrobos y palo de jabón. Revista INIA; INIA: Tacuarembó, Uruguay, 2014; pp. 62–66. [Google Scholar]
- Bremer, L.L.; Farley, K.A. Does plantation forestry restore biodiversity or create green deserts? A synthesis of the effects of land-use transitions on plant species richness. Biodivers. Conserv. 2010, 19, 3893–3915. [Google Scholar] [CrossRef]
- Gautreau, P. Forestación, territorio y ambiente: 25 años de silvicultura transnacional en Uruguay, Brasil y Argentina; Primera edición; Ediciones Trilce: Montevideo, Uruguay, 2014; ISBN 978-9974-32-627-9. [Google Scholar]
- Boulmane, M.; Oubrahim, H.; Halim, M.; Bakker, M.R.; Augusto, L. The potential of Eucalyptus plantations to restore degraded soils in semi-arid Morocco (NW Africa). Ann. For. Sci. 2017, 74. [Google Scholar] [CrossRef]
- Six, L.J.; Bakker, J.D.; Bilby, R.E. Vegetation dynamics in a novel ecosystem: Agroforestry effects on grassland vegetation in Uruguay. Ecosphere 2014, 5, 1–15. [Google Scholar] [CrossRef]
- Hall, J.S.; Love, B.E.; Garen, E.J.; Slusser, J.L.; Saltonstall, K.; Mathias, S.; van Breugel, M.; Ibarra, D.; Bork, E.W.; Spaner, D.; et al. Tree plantations on farms: Evaluating growth and potential for success. For. Ecol. Manag. 2011, 261, 1675–1683. [Google Scholar] [CrossRef]
- Fayolle, A.; Ouédraogo, D.Y.; Ligot, G.; Daïnou, K.; Bourland, N.; Tekam, P.; Doucet, J.L. Differential performance between two timber species in forest logging gaps and in plantations in Central Africa. Forests 2015, 6, 380–394. [Google Scholar] [CrossRef]
- O’Hara, K.L. What is close-to-nature silviculture in a changing world? Forestry 2016, 89, 1–6. [Google Scholar] [CrossRef]
- Gautreau, P.; Bartesaghi, L.; Commagnac, L.; de Souza Lindenmaier, D.; Haretche, F.; Liagre, R.; Pérez, N.; Rios, M. El macizo forestal del Queguay. Informe sobre la constitución de una base de datos para un análisis de la vegetación leñosa; Universidad de Lille-DINAMA–MVOTMA: Montevideo, Uruguay, 2008. [Google Scholar]
- Guido, A.A.; Mársico, L.L. Composición florística y estructura del componente leñoso del bosque asociado al Río Queguay Grande (Paysandú, Uruguay). Recur. Rurais 2011, 7, 59–65. [Google Scholar]
- Asner, G.P.; Scurlock, J.M.O.; A. Hicke, J. Global synthesis of leaf area index observations: implications for ecological and remote sensing studies. Glob. Ecol. Biogeogr. 2003. [Google Scholar] [CrossRef]
- Krebs, C.J. Ecological Methodology, 2nd ed.; Addison Wesley Longman: Menlo Park, CA, USA, 1999. [Google Scholar]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication. Math. Theory Commun. 1949, 27, 117. [Google Scholar] [CrossRef]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Minchin, P.R. An evaluation of the relative robustness of techniques for ecological ordination. Vegetatio 1987. [Google Scholar] [CrossRef]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001. [Google Scholar] [CrossRef]
- Kent, M.; Coker, P. Vegetation Description and Data Analysis: A Practical Approach; John Wiley and Sons: Chichester, UK, 1994; ISBN 978-0471490937. [Google Scholar]
- Oksanen, J.; Guillaume Blanchet, F.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymus, P.; et al. Community Ecology Package, Package ‘vegan’. 2017. Available online: https://CRAN.R-project.org/package=vegan (accessed on 8 October 2018).
- Ripley, B.; Venables, B.; Bates, D.M.; Hornik, K.; Gebhardt, A.; Firth, D. Support Functions and Datasets for Venables and Ripley’s MASS, Package ‘MASS’. 2017. Available online: http://www.et.bs.ehu.es/cran/web/packages/MASS/index.html (accessed on 8 October 2018).
- Nebel, J.P.; Porcile, J.F. La contaminación del bosque nativo por especies arbóreas y arbustivas exóticas; MGAP: Montevideo, Uruguay, 2006. [Google Scholar]
- Brockerhoff, E.G.; Jactel, H.; Parrotta, J.A.; Quine, C.P.; Sayer, J. Plantation forests and biodiversity: Oxymoron or opportunity? Biodivers. Conserv. 2008, 17, 925–951. [Google Scholar] [CrossRef]
- Calviño-Cancela, M.; Rubido-Bará, M.; van Etten, E.J.B. Do eucalypt plantations provide habitat for native forest biodiversity? For. Ecol. Manag. 2012, 270, 153–162. [Google Scholar] [CrossRef]
- Zhang, C.; Li, X.; Chen, Y.; Zhao, J.; Wan, S.; Lin, Y.; Fu, S. Effects of Eucalyptus litter and roots on the establishment of native tree species in Eucalyptus plantations in South China. For. Ecol. Manag. 2016, 375, 76–83. [Google Scholar] [CrossRef]
- Sun, Z.; Huang, Y.; Yang, L.; Schaefer, V.; Chen, Y. Plantation age, understory vegetation, and species-specific traits of target seedlings alter the competition and facilitation role of Eucalyptus in South China. Restor. Ecol. 2017. [Google Scholar] [CrossRef]
- Shiferaw, A.; Pavlis, J. Native Woody Plants Diversity and Density under Eucalyptus camaldulensis Plantation, in Gibie Valley, South Western Ethiopia. Open J. For. 2012, 2, 232–239. [Google Scholar] [CrossRef]
- Hartley, M.J. Rationale and methods for conserving biodiversity in plantation forests. For. Ecol. Manag. 2002, 155, 81–95. [Google Scholar] [CrossRef]
- De, L.; Abruzzi, M.; Grings, M.; Richter, F.S.; Backes, A.R. Composição, estrutura e fatores edáficos condicionantes da distribuição das espécies do componente arbóreo em floresta ribeirinha do rio Ibirapuitã, Bioma Pampa. Iheringia Sér. Botânica 2016, 70, 245–263. [Google Scholar]
- Budke, J.C.; Giehl, E.L.H.; Athayde, E.A.; Eisinger, S.M.; Záchia, R.A. Florística e fitossociologia do componente arbóreo de uma floresta ribeirinha, arroio Passo das Tropas, Santa Maria, RS, Brasil. Acta Bot. Bras. 2004, 18, 581–589. [Google Scholar] [CrossRef]
- Chu, C.; Mortimer, P.E.; Wang, H.; Wang, Y.; Liu, X.; Yu, S. Allelopathic effects of Eucalyptus on native and introduced tree species. For. Ecol. Manag. 2014, 323, 79–84. [Google Scholar] [CrossRef]
- Rodrigues, A.S.L.; Brooks, T.M. Shortcuts for Biodiversity Conservation Planning: The Effectiveness of Surrogates. Annu. Rev. Ecol. Evol. Syst. 2007, 38, 713–737. [Google Scholar] [CrossRef]
- de Pinho Júnior, G.V.; Nascimento, A.R.T.; Valverde, B.T.; Clemente, L.H. Brazilian savanna re-establishment in a monoculture forest: diversity and environmental relations of native regenerating understory in Pinus caribaea Morelet. stands. J. For. Res. 2015, 26, 571–579. [Google Scholar] [CrossRef]
- Donaldson, L.; Wilson, R.J.; Maclean, I.M.D. Old concepts, new challenges: adapting landscape-scale conservation to the twenty-first century. Biodivers. Conserv. 2017, 26, 527–552. [Google Scholar] [CrossRef]
- Gossner, M.M.; Schall, P.; Ammer, C.; Ammer, U.; Engel, K.; Schubert, H.; Simon, U.; Utschick, H.; Weisser, W.W. Forest management intensity measures as alternative to stand properties for quantifying effects on biodiversity. Ecosphere 2014, 5. [Google Scholar] [CrossRef]
- González, S.E. Estudio de la composición florística y estructura de los bosques ribereños del río Uruguay al norte y al sur de la represa de Salto Grande, en los departamentos de Artigas, Salto y Paysandú (Uruguay). Master’s Thesis, Universidad de la República (UdelaR), Montevideo, Uruguay, 2013. [Google Scholar]
- Costa, N.R.; Delgado, G.S. Análisis de planes de manejo en bosques naturales de Uruguay y estudio de caso en una comunidad serrana, departamento de Lavalleja. Master’s Thesis, Universidad de la República (UdelaR), Montevideo, Uruguay, 2001. [Google Scholar]
- Grela, I.; Brussa, C. Relevamiento florístico y análisis comparativo de comunidades arbóreas de Sierra de Ríos (Cerro Largo—Uruguay ). Agrocienc. Urug. 2003, 7, 11–26. [Google Scholar]
- Piaggio, M.; Delfino, L. Florística y fitosociología de un bosque fluvial en Minas de Corrales, Rivera, Uruguay. Iheringia 2009, 64, 45–51. [Google Scholar]
- Traversa-Tejero, I.P.; Alejano-Monge, M.R. Caracterización, distribución y manejo de los bosques nativos en el norte de Uruguay. Rev. Mex. Biodivers. 2013, 84, 249–262. [Google Scholar] [CrossRef]
- Ghersa, C.M.; De la Fuente, E.; Suarez, S.; Leon, R.J.C. Woody species invasion in the rolling pampa grasslands, Argentina. Agric. Ecosyst. Environ. 2002, 88, 271–278. [Google Scholar] [CrossRef]
- Chaneton, E.J.; Mazía, N.; Batista, W.B.; Rolhauser, A.G.; Ghersa, C.M. Woody plant invasions in Pampa Grasslands: A biogeographical and community assembly perspective. In Ecotones between Forest and Grassland; Springer: Berlin, Germany, 2013; pp. 115–144. ISBN 978-1-4614-3797-0. [Google Scholar]
- Plaza Behr, M.C.; Pérez, C.A.; Goya, J.F.; Azcona, M.; Arturi, M.F. Plantación de Celtis ehrenbergiana como técnica de recuperación de bosques invadidos por Ligustrum lucidum en los talares del NE de Buenos Aires. Ecol. Austral 2016, 26, 171–177. [Google Scholar]
- Sosa, B.; Caballero, N.; Carvajales, A.; Fernández, G.; Mello, A.L.; Achkar, M. Control de Gleditsia triacanthos en el parque nacional esteros de farrapos e islas del río Uruguay. Ecol. Austral 2015, 25, 250–254. [Google Scholar]
- Prata, E.; Pinto, S.; Assis, M. Fitossociologia e distribuição de espécies arbóreas em uma floresta ribeirinha secundária no mnicípio de Rio Claro, SP, Brasil. Rev. Bras. Botânica 2011, 34, 159–168. [Google Scholar] [CrossRef]
- Watzlawick, L.F.; Longhi, S.J.; Schneider, P.R.; Finger, C.A.G. Aspectos da vegetação arbórea em fragmento de estepe estacional savanícola, barra do quaraí-RS, Brasil. Cienc. Flor. 2014, 24, 23–36. [Google Scholar] [CrossRef]
- FAO. Forest Health Project—Uruguay. Available online: http://www.fao.org/forestry/49410/en/ury/ (accessed on 27 July 2018).
- García-Fernández, C.; Ruiz-Pérez, M.; Wunder, S. Is multiple-use forest management widely implementable in the tropics? For. Ecol. Manag. 2008, 256, 1468–1476. [Google Scholar] [CrossRef]
- Pokorny, B.; Pacheco, P. Money from and for forests: A critical reflection on the feasibility of market approaches for the conservation of Amazonian forests. J. Rural Stud. 2014, 36, 441–452. [Google Scholar] [CrossRef]
- Araujo, I.C.L.; Dziedzic, M.; Maranho, L.T. Management of the environmental restoration of degraded areas. Braz. Arch. Biol. Technol. 2014, 57, 284–294. [Google Scholar] [CrossRef]
- Root-Bernstein, M.; Jaksic, F. The chilean espinal: Restoration for a sustainable silvopastoral system. Restor. Ecol. 2013, 21, 409–414. [Google Scholar] [CrossRef]
- Gomes, J.P.; Dacoregio, H.M.; da Silva, K.M.; da Rosa, L.H.; da Costa Bortoluzzi, R.L. Myrtaceae na bacia do rio Caveiras: Características ecológicas e usos não madeireiros. Floresta E Ambiente 2017, 24, 1–10. [Google Scholar] [CrossRef]
- dos Reis Diniz, M.E.; Buschini, M.L.T. Pollen analysis and interaction networks of floral visitor bees of Eugenia uniflora L. (Myrtaceae), in Atlantic Forest areas in southern Brazil. Arthropod-Plant Interact. 2015, 9, 623–632. [Google Scholar] [CrossRef]
- McFadden, T.N.; Dirzo, R. Opening the silvicultural toolbox: A new framework for conserving biodiversity in Chilean timber plantations. For. Ecol. Manag. 2018, 425, 75–84. [Google Scholar] [CrossRef]
- Felton, A.; Nilsson, U.; Sonesson, J.; Felton, A.M.; Roberge, J.M.; Ranius, T.; Ahlström, M.; Bergh, J.; Björkman, C.; Boberg, J.; et al. Replacing monocultures with mixed-species stands: Ecosystem service implications of two production forest alternatives in Sweden. Ambio 2016, 45, 124–139. [Google Scholar] [CrossRef] [PubMed]
- Mertz, O.; Mertens, C.F. Land Sparing and Land Sharing Policies in Developing Countries – Drivers and Linkages to Scientific Debates. World Dev. 2017, 98, 523–535. [Google Scholar] [CrossRef]
| Species/Author/Code | Mean AD/ha | Mean RD/ha | Potential Use |
|---|---|---|---|
| Schinus longifolius (Lindl.) Speg. (ScLo) | 250 PK 3 RV | 185 PK 20 RV | med 9 or 9 |
| Patagonula americana L. (PaAm) | 90 RV | 99 RV | re 7 w 8,20 |
| Maytenus ilicifolia Mart. ex Reissek (MaIl) | 9 PK | 370 PK 1175 RV 493 PL | med 8 |
| Escallonia bífida Link & Otto (EsBi) | 17 RV | or 8 | |
| Sebastiania brasiliensis Spreng. (SeBr) | 447 RV | 317 RV | re 1,2 med 1,18 or 18 |
| Citronella gongonha (Mart.) R.A. Howard (CiCo) | 3 RV | 40 RV | zo 8,16 |
| Ocotea acutifolia (Nees) Mez (OcAc) | 197 RV | 119 RV | w 8 med 10 |
| Bauhinia forficate Link (BaFo) | 8 PK | med 16 or 8 | |
| Gleditsia triacanthos L. (GlTr) | 8 PK 13 RV | 185 PK 40 RV | ex |
| Prosopis affinis Spreng. (PrAf) | 4 PK | 154 PK | bee 8,6 re 14 zo 6 w 8,3 |
| Vachellia caven (Molina) Seigler & Ebinger (VaCa) | 25 PK | 247 PK 246 PL | bee 6 re 14 zo 6 |
| Melia azedarach L. (MeAzr) | 31 PK | ex | |
| Blepharocalyx salicifolius (Kunth) O. Berg (BlSa) | 129 PK 150 RV | 247 PK 479 RV 1358 PL | zo 8,12,16 re 1,11 med 1,11 |
| Eugenia uniflora L. (EuUn) | 38 PK 37 RV | zo 8–12,16,20 re 7 bee 9,11 or 8 med 4,5,20 | |
| Myrcianthes cisplatensis (Cambess.) O. Berg (MyCi) | 8 PK 350 RV | 2746 PK 188 RV 740 PL | zo 11 bee 11 med 4 |
| Myrcianthes pungens (O. Berg) D. Legrand (MyPu) | 4 PK 70 RV | 1296 PK 260 RV 246 PL | zo 8,11,12,16,20 re 1,7 bee 11 med 5 |
| Myrrhinium atropurpureum Schott (MyAt) | 8 PK 17 RV | zo 12,16 re 11 w 8 or 8 med 11 | |
| Ligustrum lucidum W.T. Aiton (LiSi) | 4 PK 87 RV | 12395 RV 2222 PL | ex |
| Colletia paradoxa (Spreng.) Escal. (CoPa) | 4 PK | bee 8 or 19 | |
| Scutia buxifolia Reissek (ScBu) | 70 PK 157 RV | 556 PK 96 RV | zo 16 re 1 w 8 med 1 |
| Discaria Americana Gillies & Hook. (DiAmr) | 185 PK | med 17 | |
| Azara uruguayensis (AzUr) | 27 RV | or 8 | |
| Salix humboldtiana Willd. (SaHu) | 17 RV | w 8 | |
| Jodina rhombifolia (Hook. & Arn.) Reissek (JoRh) | 3 RV | med 13 | |
| Allophylus edulis (A. St.-Hil., A. Juss. & Cambess.) Hieron. ex Niederl. (AlEd) | 38 PK 103 RV | 987 PK 7679 RV 370 PL | zo 12,16, 20 re 2, 7 med 20 |
| Pouteria salicifolia (Spreng.) Radlk. (PoSa) | 8 PK 353 RV | 31 PK 247 RV | med 4 |
| Daphnopsis racemosa Griseb. (DaRa) | 27 RV | 353 RV | fb 8, or 19 |
| Celtis ehrenbergiana (Klotzsch) Liebm. (CeTa) | 133 PK 33 RV | 278 PK 290 RV 740 PL | zo 8 |
| Citharexylum montevidense (Spreng.) Moldenke (CiMo) | 10 RK | 10 RV | zo 16 w 15 or 8 |
| NMDS | ANOVA | |||
|---|---|---|---|---|
| Parameters | r2 | p | F | p |
| Tree density (AD) | 0.04 | 0.701ns | 6.2 | 0.0106 * |
| Regeneration density (RD) | 0.50 | 0.002 ** | 22.9 | 0.000 *** |
| Species diversity (SD) | 0.94 | 0.001 *** | 8.2 | 0.003 ** |
| Horizontal structure (HS) | 0.54 | 0.004 ** | 16.1 | 0.000 *** |
| Vertical structure (VS) | 0.17 | 0.237ns | 0.1 | 0.863ns |
| Native proportion (NP) | 0.86 | 0.001 *** | 6.0 | 0.0119 * |
| Exotic proportion (EP) | 0.90 | 0.001 *** | 23.4 | 0.000 *** |
| Leaf Area Index (LAI) | 0.39 | 0.020* | 4.7 | 0.025 * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pozo, P.; Säumel, I. How to Bloom the Green Desert: Eucalyptus Plantations and Native Forests in Uruguay beyond Black and White Perspectives. Forests 2018, 9, 614. https://doi.org/10.3390/f9100614
Pozo P, Säumel I. How to Bloom the Green Desert: Eucalyptus Plantations and Native Forests in Uruguay beyond Black and White Perspectives. Forests. 2018; 9(10):614. https://doi.org/10.3390/f9100614
Chicago/Turabian StylePozo, Paola, and Ina Säumel. 2018. "How to Bloom the Green Desert: Eucalyptus Plantations and Native Forests in Uruguay beyond Black and White Perspectives" Forests 9, no. 10: 614. https://doi.org/10.3390/f9100614
APA StylePozo, P., & Säumel, I. (2018). How to Bloom the Green Desert: Eucalyptus Plantations and Native Forests in Uruguay beyond Black and White Perspectives. Forests, 9(10), 614. https://doi.org/10.3390/f9100614






