Coordination and Determinants of Leaf Community Economics Spectrum for Canopy Trees and Shrubs in a Temperate Forest in Northeastern China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Species Selection
2.2. Leaf Traits Collections and Measurements
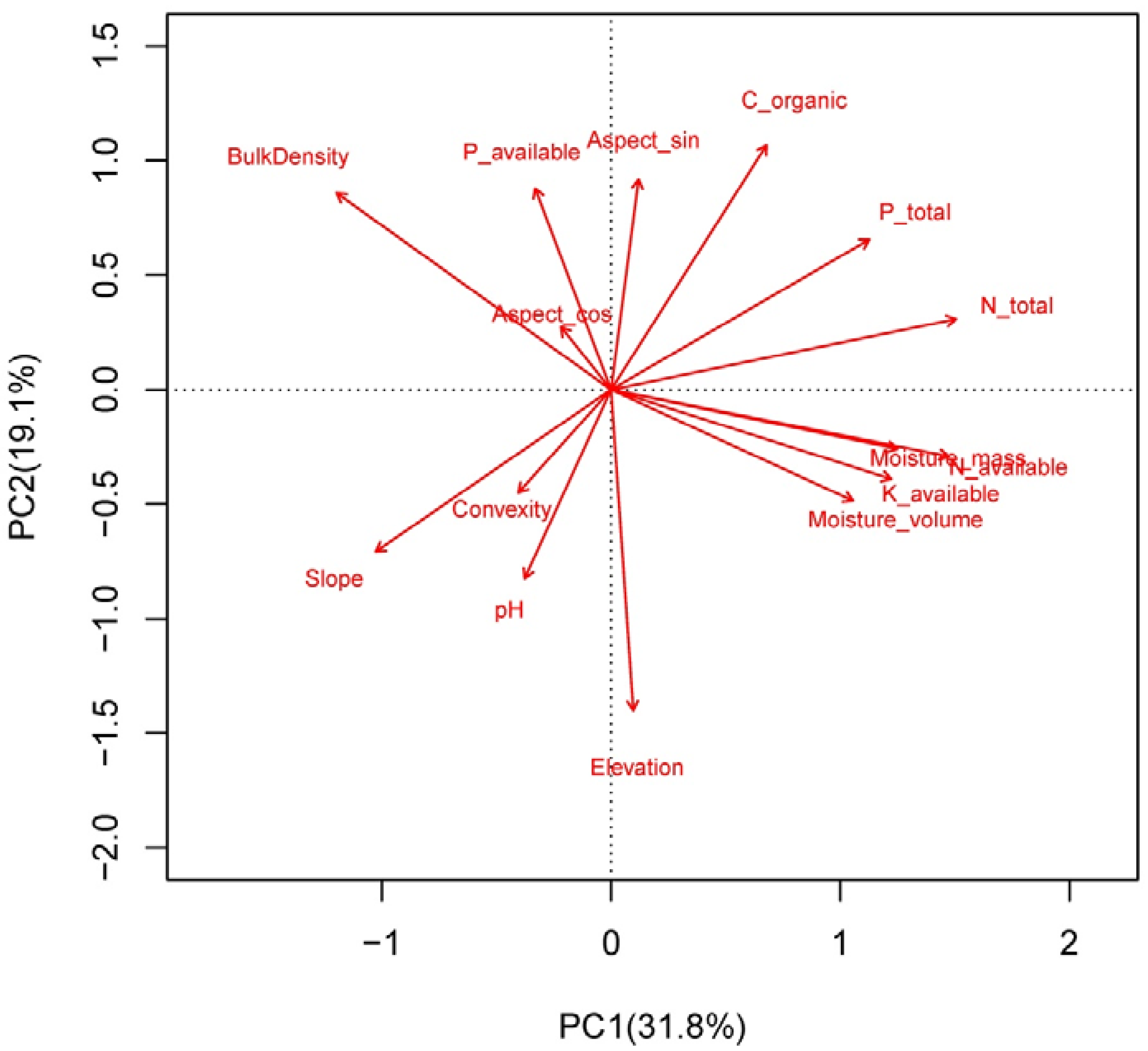
2.3. Topography and Soil Properties Collections
2.4. Statistical Analysis
3. Results
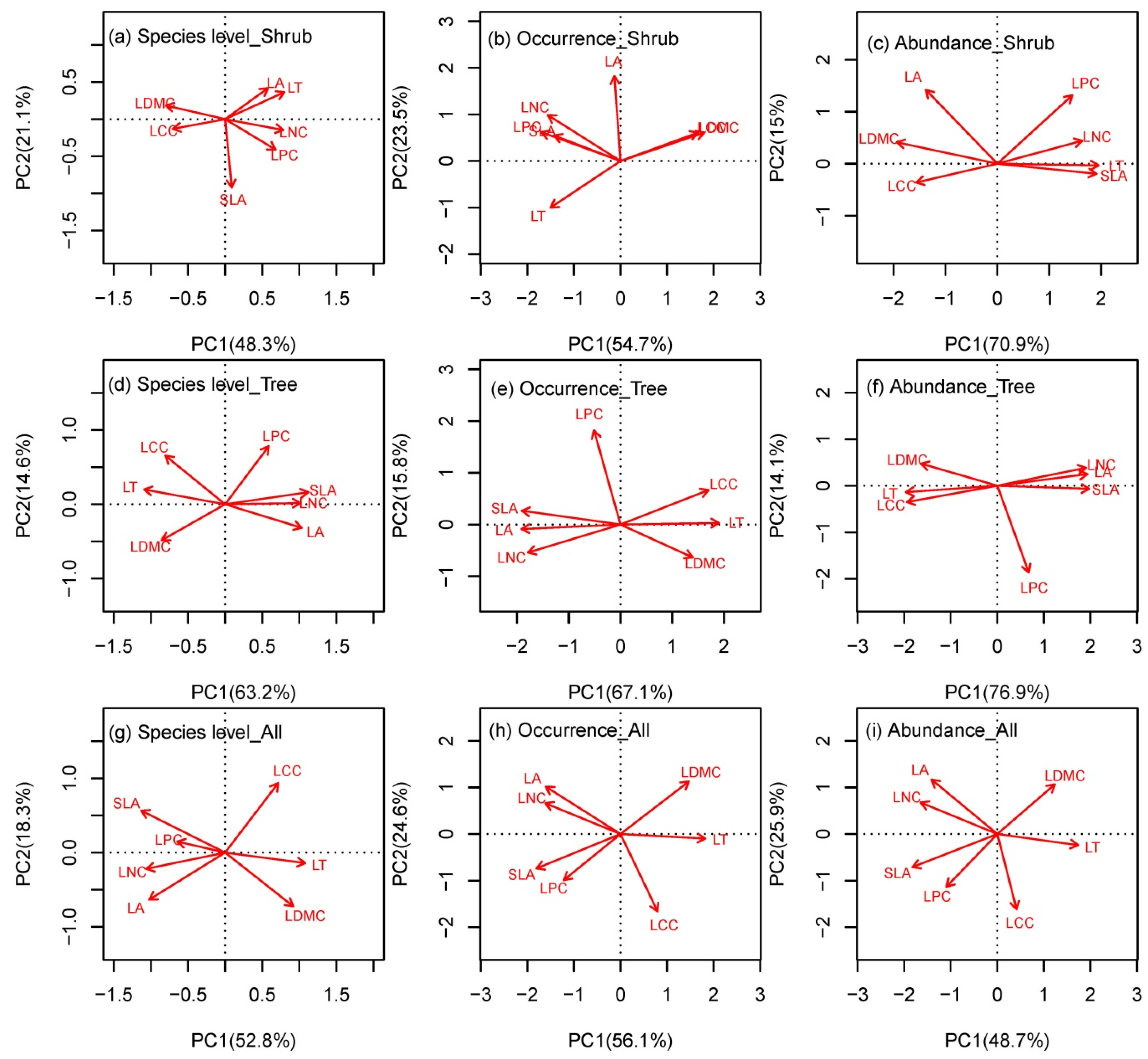
3.1. Coordination among Leaf Traits at the Species and Community Levels
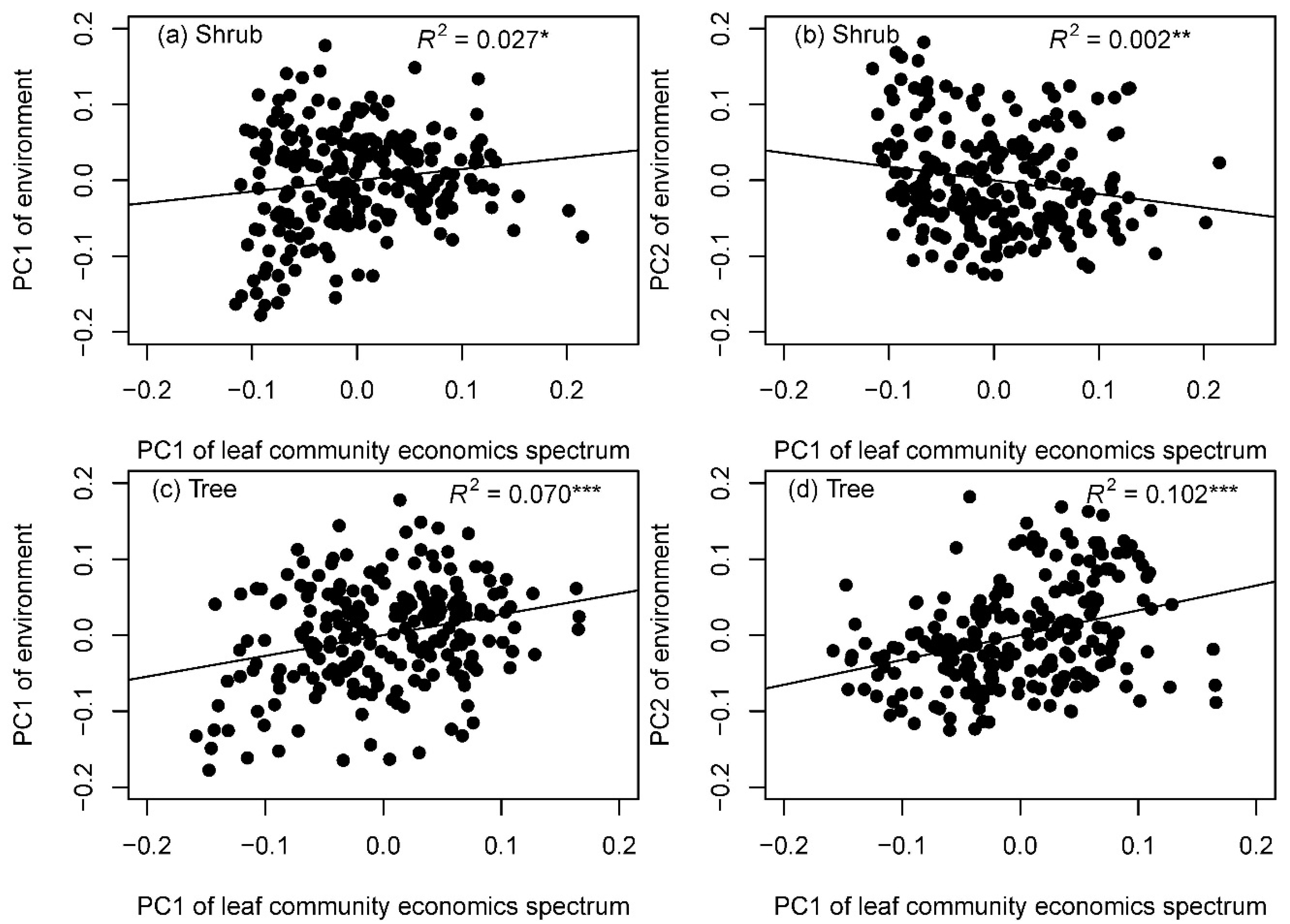
3.2. Effects of Environment on LES Traits Distributions at the Community Level
4. Discussion
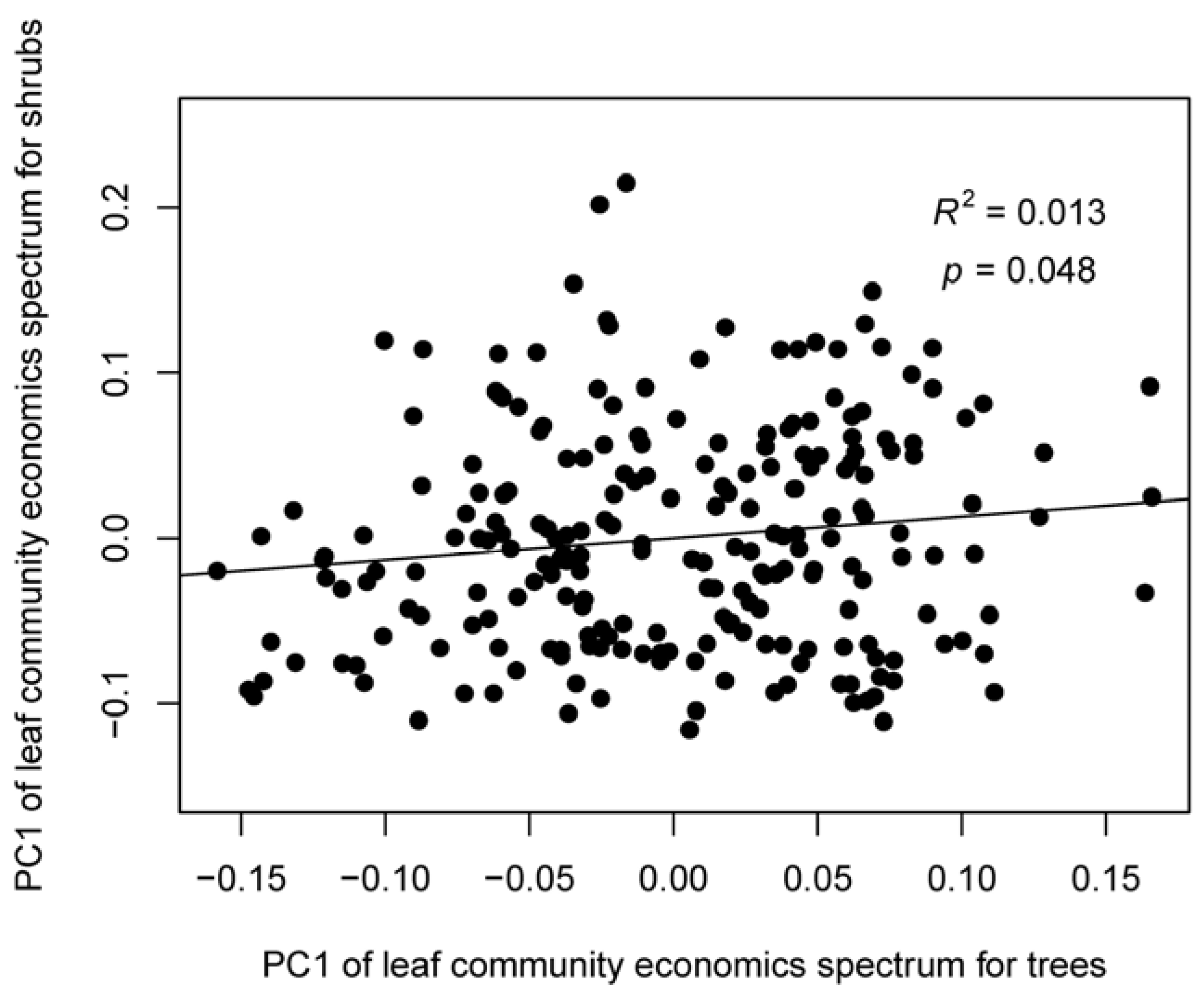
4.1. The Leaf Community Economics Spectrum
4.2. Determinants of Leaf Community Economics Spectrum: Hints for Community Assembly
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Violle, C.; Navas, M.-L.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the concept of trait be functional! Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- Wright, S.J.; Kitajima, K.; Kraft, N.J.; Reich, P.B.; Wright, I.J.; Bunker, D.E.; Condit, R.; Dalling, J.W.; Davies, S.J.; Díaz, S. Functional traits and the growth-mortality trade-off in tropical trees. Ecology 2010, 91, 3664–3674. [Google Scholar] [CrossRef] [PubMed]
- Swenson, N.G.; Stegen, J.C.; Davies, S.J.; Erickson, D.L.; Forero-Montaña, J.; Hurlbert, A.H.; Kress, W.J.; Thompson, J.; Uriarte, M.; Wright, S.J. Temporal turnover in the composition of tropical tree communities: Functional determinism and phylogenetic stochasticity. Ecology 2012, 93, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Kraft, N.J.; Godoy, O.; Levine, J.M. Plant functional traits and the multidimensional nature of species coexistence. Proc. Natl. Acad. Sci. USA 2015, 112, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Laughlin, D.C.; Wilson, S. The intrinsic dimensionality of plant traits and its relevance to community assembly. J. Ecol. 2014, 102, 186–193. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.; Diemer, M. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Diaz, S.; Kattge, J.; Cornelissen, J.H.; Wright, I.J.; Lavorel, S.; Dray, S.; Reu, B.; Kleyer, M.; Wirth, C.; Prentice, I.C.; et al. The global spectrum of plant form and function. Nature 2016, 529, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Poorter, L.; Wright, S.J.; Paz, H.; Ackerly, D.D.; Condit, R.; Ibarra-Manríquez, G.; Harms, K.E.; Licona, J.C.; Martínez-Ramos, M.; Mazer, S.J.; et al. Are functional traits good predictors of demographic rates? Evidence from five neotropical forests. Ecology 2008, 89, 1908–1920. [Google Scholar] [CrossRef] [PubMed]
- Onoda, Y.; Wright, I.J.; Evans, J.R.; Hikosaka, K.; Kitajima, K.; Niinemets, U.; Poorter, H.; Tosens, T.; Westoby, M. Physiological and structural tradeoffs underlying the leaf economics spectrum. New Phytol. 2017, 214, 1447–1463. [Google Scholar] [CrossRef] [PubMed]
- Vasseur, F.; Violle, C.; Enquist, B.J.; Granier, C.; Vile, D. A common genetic basis to the origin of the leaf economics spectrum and metabolic scaling allometry. Ecol. Lett. 2012, 15, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Blonder, B.; Violle, C.; Enquist, B.J.; Cornelissen, H. Assessing the causes and scales of the leaf economics spectrum using venation networks inpopulus tremuloides. J. Ecol. 2013, 101, 981–989. [Google Scholar] [CrossRef]
- Blonder, B.; Vasseur, F.; Violle, C.; Shipley, B.; Enquist, B.J.; Vile, D. Testing models for the leaf economics spectrum with leaf and whole-plant traits in arabidopsis thaliana. AoB Plants 2015, 7, plv049. [Google Scholar] [CrossRef] [PubMed]
- Siefert, A.; Violle, C.; Chalmandrier, L.; Albert, C.H.; Taudiere, A.; Fajardo, A.; Aarssen, L.W.; Baraloto, C.; Carlucci, M.B.; Cianciaruso, M.V.; et al. A global meta-analysis of the relative extent of intraspecific trait variation in plant communities. Ecol. Lett. 2015, 18, 1406–1419. [Google Scholar] [CrossRef] [PubMed]
- Messier, J.; McGill, B.J.; Enquist, B.J.; Lechowicz, M.J. Trait variation and integration across scales: Is the leaf economic spectrum present at local scales? Ecography 2017, 40, 685–697. [Google Scholar] [CrossRef]
- Messier, J.; Lechowicz, M.J.; McGill, B.J.; Violle, C.; Enquist, B.J.; Cornelissen, H. Interspecific integration of trait dimensions at local scales: The plant phenotype as an integrated network. J. Ecol. 2017, in press. [Google Scholar] [CrossRef]
- Kraft, N.J.; Valencia, R.; Ackerly, D.D. Functional traits and niche-based tree community assembly in an amazonian forest. Science 2008, 322, 580–582. [Google Scholar] [CrossRef] [PubMed]
- Cornwell, W.K.; Ackerly, D.D. Community assembly and shifts in plant trait distributions across an environmental gradient in coastal California. Ecol. Monogr. 2009, 79, 109–126. [Google Scholar] [CrossRef]
- Pérez-Ramos, I.M.; Roumet, C.; Cruz, P.; Blanchard, A.; Autran, P.; Garnier, E.; Aerts, R. Evidence for a ‘plant community economics spectrum’ driven by nutrient and water limitations in a mediterranean rangeland of southern France. J. Ecol. 2012, 100, 1315–1327. [Google Scholar] [CrossRef]
- De la Riva, E.G.; Tosto, A.; Pérez-Ramos, I.M.; Navarro-Fernández, C.M.; Olmo, M.; Anten, N.P.R.; Marañón, T.; Villar, R.; de Bello, F. A plant economics spectrum in mediterranean forests along environmental gradients: Is there coordination among leaf, stem and root traits? J. Veg. Sci. 2016, 27, 187–199. [Google Scholar] [CrossRef]
- Domínguez, M.T.; Aponte, C.; Pérez-Ramos, I.M.; García, L.V.; Villar, R.; Marañón, T. Relationships between leaf morphological traits, nutrient concentrations and isotopic signatures for mediterranean woody plant species and communities. Plant Soil 2012, 357, 407–424. [Google Scholar] [CrossRef]
- Keddy, P.A. Assembly and response rules: Two goals for predictive community ecology. J. Veg. Sci. 1992, 3, 157–164. [Google Scholar] [CrossRef]
- Zhao, Y.T.; Ali, A.; Yan, E.R. The plant economics spectrum is structured by leaf habits and growth forms across subtropical species. Tree Physiol. 2017, 37, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Anderson-Teixeira, K.J.; Davies, S.J.; Bennett, A.C.; Gonzalez-Akre, E.B.; Muller-Landau, H.C.; Wright, S.J.; Abu Salim, K.; Almeyda Zambrano, A.M.; Alonso, A.; Baltzer, J.L.; et al. Ctfs-forestgeo: A worldwide network monitoring forests in an era of global change. Glob. Chang. Biol. 2015, 21, 528–549. [Google Scholar] [CrossRef] [PubMed]
- Piao, T.; Comita, L.S.; Jin, G.; Kim, J.H. Density dependence across multiple life stages in a temperate old-growth forest of northeast China. Oecologia 2013, 172, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.K.; Pan, X.; Liu, G.F.; Li, W.B.; Dai, W.H.; Tang, S.L.; Zhang, Y.L.; Xiao, T.; Chen, L.Y.; Xiong, W.; et al. Novel evidence for within-species leaf economics spectrum at multiple spatial scales. Front. Plant Sci. 2015, 6, 901. [Google Scholar] [CrossRef] [PubMed]
- Ackerly, D.; Knight, C.; Weiss, S.; Barton, K.; Starmer, K. Leaf size, specific leaf area and microhabitat distribution of chaparral woody plants: Contrasting patterns in species level and community level analyses. Oecologia 2002, 130, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Baraloto, C.; Timothy Paine, C.E.; Poorter, L.; Beauchene, J.; Bonal, D.; Domenach, A.M.; Herault, B.; Patino, S.; Roggy, J.C.; Chave, J. Decoupled leaf and stem economics in rain forest trees. Ecol. Lett. 2010, 13, 1338–1347. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; McCormack, M.L.; Ma, C.; Kong, D.; Zhang, Q.; Chen, X.; Zeng, H.; Niinemets, U.; Guo, D. Leaf economics and hydraulic traits are decoupled in five species-rich tropical-subtropical forests. Ecol. Lett. 2015, 18, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- Bao, S.D. Soil Agricultural Chemical Analysis; China Agricultural Press: Beijing, China, 2000. [Google Scholar]
- Legendre, P.; Mi, X.; Ren, H.; Ma, K.; Yu, M.; Sun, I.-F.; He, F. Partitioning beta diversity in a subtropical broad-leaved forest of China. Ecology 2009, 90, 663–674. [Google Scholar] [CrossRef] [PubMed]
- De Cáceres, M.; Legendre, P.; Valencia, R.; Cao, M.; Chang, L.-W.; Chuyong, G.; Condit, R.; Hao, Z.; Hsieh, C.-F.; Hubbell, S.; et al. The variation of tree beta diversity across a global network of forest plots. Glob. Ecol. Biogeogr. 2012, 21, 1191–1202. [Google Scholar] [CrossRef]
- Jones, M.M.; Tuomisto, H.; Borcard, D.; Legendre, P.; Clark, D.B.; Olivas, P.C. Explaining variation in tropical plant community composition: Influence of environmental and spatial data quality. Oecologia 2008, 155, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Gao, W.; Cai, H.; Jin, G. Spatial variation of soil respiration is linked to the forest structure and soil parameters in an old-growth mixed broadleaved-Korean pine forest in northeastern China. Plant Soil 2015, 400, 263–274. [Google Scholar] [CrossRef]
- Garnier, E.; Cortez, J.; Billès, G.; Navas, M.-L.; Roumet, C.; Debussche, M.; Laurent, G.; Blanchard, A.; Aubry, D.; Bellmann, A.; et al. Plant functional markers capture ecosystem properties during secondary succession. Ecology 2004, 85, 2630–2637. [Google Scholar] [CrossRef]
- De la Riva, E.G.; Pérez-Ramos, I.M.; Tosto, A.; Navarro-Fernández, C.M.; Olmo, M.; Marañón, T.; Villar, R. Disentangling the relative importance of species occurrence, abundance and intraspecific variability in community assembly: A trait-based approach at the whole-plant level in mediterranean forests. Oikos 2016, 125, 354–363. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016; Available online: https://www.R-project.org/ (accessed on 14 April 2016).
- Peppe, D.J.; Royer, D.L.; Cariglino, B.; Oliver, S.Y.; Newman, S.; Leight, E.; Enikolopov, G.; Fernandez-Burgos, M.; Herrera, F.; Adams, J.M.; et al. Sensitivity of leaf size and shape to climate: Global patterns and paleoclimatic applications. New Phytol. 2011, 190, 724–739. [Google Scholar] [CrossRef] [PubMed]
- Jung, V.; Violle, C.; Mondy, C.; Hoffmann, L.; Muller, S. Intraspecific variability and trait-based community assembly. J. Ecol. 2010, 98, 1134–1140. [Google Scholar] [CrossRef]
- Cosme, L.H.; Schietti, J.; Costa, F.R.; Oliveira, R.S. The importance of hydraulic architecture to the distribution patterns of trees in a central amazonian forest. New Phytol. 2017, in press. [Google Scholar] [CrossRef] [PubMed]
- Kawai, K.; Okada, N.; Watling, J. How are leaf mechanical properties and water-use traits coordinated by vein traits? A case study in Fagaceae. Funct. Ecol. 2016, 30, 527–536. [Google Scholar] [CrossRef]
- HilleRisLambers, J.; Adler, P.B.; Harpole, W.S.; Levine, J.M.; Mayfield, M.M. Rethinking community assembly through the lens of coexistence theory. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 227–248. [Google Scholar] [CrossRef]
- Chase, J.M.; Leibold, M.A. Ecological Niches: Linking Classical and Contemporary Approaches; University of Chicago Press: Chicago, IL, USA, 2003. [Google Scholar]
- Hubbell, S.P. The Unified Neutral Theory of Biodiversity and Biogeography; Princeton University Press: Princeton, NJ, USA, 2001. [Google Scholar]
| SLA | LA | LT | LDMC | LCC | LNC | LPC | |
|---|---|---|---|---|---|---|---|
| SLA | −0.26 ns | −0.29 ns | −0.24 ns | 0.01 ns | 0.21 ns | 0.36 ns | |
| LA | −0.67 (0.10 ns) | 0.46 ns | −0.20 ns | −0.31 ns | 0.55 ns | 0.32 ns | |
| LT | 0.84 (0.17) | −0.68 (−0.22) | −0.79 | −0.61 | 0.43 ns | 0.38 ns | |
| LDMC | −0.97 (−0.63) | 0.77 (0.24) | −0.89 (−0.80) | 0.47 ns | −0.56 | −0.53 ns | |
| LCC | −0.54 (−0.39) | 0.48 (0.16) | −0.86 (−0.78) | 0.58 (0.68) | −0.51 ns | −0.33 ns | |
| LNC | 0.83 (0.57) | −0.31 (0.48) | 0.69 (0.36) | −0.80 (−0.50) | −0.42 (−0.54) | 0.56 | |
| LPC | 0.62 (0.65) | −0.06 ns (0.29) | 0.67 (0.43) | −0.55 (−0.66) | −0.68 (−0.55) | 0.63 (0.70) |
| SLA | LA | LT | LDMC | LCC | LNC | LPC | |
|---|---|---|---|---|---|---|---|
| SLA | 0.74 | −0.90 | −0.80 | −0.48 | 0.77 | 0.42 | |
| LA | 0.90 (0.84) | −0.80 | −0.43 | −0.68 | 0.71 | 0.33 ns | |
| LT | −0.97 (−0.93) | −0.92 (−0.88) | 0.54 | 0.63 | −0.73 | −0.32 ns | |
| LDMC | −0.80 (−0.74) | −0.71 (−0.56) | 0.69 (0.52) | 0.30 ns | −0.51 | −0.40 | |
| LCC | −0.91 (−0.67) | −0.94 (−0.81) | 0.92 (0.71) | 0.75 (0.50) | −0.50 | −0.13 ns | |
| LNC | 0.90 (0.77) | 0.93 (0.85) | −0.95 (−0.85) | −0.61 (−0.38) | −0.92 (−0.79) | 0.41 | |
| LPC | 0.34 (0.28) | 0.22 (0.24) | −0.28 (−0.24) | −0.30 (−0.26) | −0.16 (0.03ns) | 0.18 (0.07 ns) |
| Abiotic Factors | Shrub | Tree | |
|---|---|---|---|
| Topography | Elevation | 0.09 | −0.30 * |
| Slope | −0.05 | −0.34 * | |
| Convexity | −0.14 * | −0.26 * | |
| Sin (Aspect) | −0.31 * | 0.11 | |
| Cos (Aspect) | −0.22 * | −0.02 | |
| Soil | Bulk density | −0.14 * | 0.02 |
| Available N | 0.25 * | 0.20 * | |
| Available P | −0.09 | 0.09 | |
| Available K | 0.20 * | 0.31 * | |
| Organic C | 0.00 | 0.37 * | |
| Total N | 0.09 | 0.29 * | |
| Total P | 0.08 | 0.45 * | |
| pH | 0.21 * | 0.04 | |
| Volumetric moisture | 0.10 | −0.02 | |
| Mass moisture | 0.10 | −0.03 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, F.; Xun, Y.; Cai, H.; Jin, G. Coordination and Determinants of Leaf Community Economics Spectrum for Canopy Trees and Shrubs in a Temperate Forest in Northeastern China. Forests 2017, 8, 202. https://doi.org/10.3390/f8060202
Jiang F, Xun Y, Cai H, Jin G. Coordination and Determinants of Leaf Community Economics Spectrum for Canopy Trees and Shrubs in a Temperate Forest in Northeastern China. Forests. 2017; 8(6):202. https://doi.org/10.3390/f8060202
Chicago/Turabian StyleJiang, Feng, Yanhan Xun, Huiying Cai, and Guangze Jin. 2017. "Coordination and Determinants of Leaf Community Economics Spectrum for Canopy Trees and Shrubs in a Temperate Forest in Northeastern China" Forests 8, no. 6: 202. https://doi.org/10.3390/f8060202
APA StyleJiang, F., Xun, Y., Cai, H., & Jin, G. (2017). Coordination and Determinants of Leaf Community Economics Spectrum for Canopy Trees and Shrubs in a Temperate Forest in Northeastern China. Forests, 8(6), 202. https://doi.org/10.3390/f8060202





