Abstract
Tropical forests play an important role in regulating the global climate and the carbon cycle. With the changing temperature and moisture along the elevation gradient, the Luquillo Experimental Forest in Northeastern Puerto Rico provides a natural approach to understand tropical forest ecosystems under climate change. In this study, we conducted a soil translocation experiment along an elevation gradient with decreasing temperature but increasing moisture to study the impacts of climate change on soil organic carbon (SOC) and soil respiration. As the results showed, both soil carbon and the respiration rate were impacted by microclimate changes. The soils translocated from low elevation to high elevation showed an increased respiration rate with decreased SOC content at the end of the experiment, which indicated that the increased soil moisture and altered soil microbes might affect respiration rates. The soils translocated from high elevation to low elevation also showed an increased respiration rate with reduced SOC at the end of the experiment, indicating that increased temperature at low elevation enhanced decomposition rates. Temperature and initial soil source quality impacted soil respiration significantly. With the predicted warming climate in the Caribbean, these tropical soils at high elevations are at risk of releasing sequestered carbon into the atmosphere.
1. Introduction
Soil respiration, defined as CO2 emission from the soil surface through the activities of soil microbes, plant roots, and other organisms, is one of the major pathways to release carbon fixed by vegetation into the atmosphere [1,2]. Because the quantity of carbon stored in soils is double that stored in either the atmosphere or the terrestrial vegetation, soil respiration is a critical component in the global carbon cycle [1,3]. Tropical biosphere stores 46% of the world’s living terrestrial carbon and 11% of the world’s soil carbon [4] and is sensitive to changes in climate [5,6,7], therefore, it plays an important role in global C dynamics [8]. At steady state, the carbon emission from soil, as the second largest carbon flux between the atmosphere and the terrestrial biomes, can be balanced by CO2 net uptake by plants (net primary production, NPP) [1,9]. However, any small changes in soil respiration and carbon caused by climate change could have huge impacts on the global carbon cycle and future global climate [2,9]. With the ongoing and predicted warming/drought in the tropics [10,11], tropical ecosystems might change from a C sink to a C source.
Soil respiration involves the interactions among plant roots, rhizosphere, soil microbes, soil fauna, and physicochemical conditions [12]. Previous experiments have indicated that changes in environmental conditions would affect the soil respiration rate and might have a significant impact on the global carbon cycle [9,13,14,15]. Soil temperature, moisture, and the carbon substrate available for microorganisms (which is related to vegetation type) are major factors influencing the soil respiration rate at a given site [13,16,17,18].
Temperature is thought to be the primary driver of soil respiration [3,19,20,21]. Soil respiration generally increases exponentially with temperature [14,15,22] to maintain the increased metabolism of plant roots, soil microbes, and soil fauna [23,24,25]. When temperature reaches a certain maximum point, most of the enzymatic activity involved in respiration will be inhibited due to enzyme malfunction, and soil respiration stops increasing [26,27]. The optimal temperature for cryophiles is below 20 °C whereas that for mesophiles and thermophiles is 20 °C–40 °C and above 40 °C, respectively [28].
Soil moisture is generally considered to positively correlate with soil respiration [14,26]. Soil respiration, especially root respiration, is relatively low in dry conditions, and increases to a maximum in intermediate moisture conditions [14,17]. Although some microorganisms develop strategies to survive and grow under low soil moisture conditions [27,29], water deficiency prevails to limit activities of soil fauna and microbes. On the other hand, when soil is saturated, oxygen can be limited. Anaerobic conditions suppress aerobic microbial activities, resulting in limited soil respiration [15,26,27]. Soil moisture also affects the availability of soil nutrients [27,30]. Some of the nutrients available to plants and soil microorganisms need to be dissolved in water (e.g., N) [22,31,32]. The influence of soil moisture on soil respiration varies greatly among ecosystems. Changes in soil moisture can have a significant influence in semiarid ecosystems [16], but may not significantly alter soil respiration for humid ecosystems, except during warm and dry seasons [14]. Furthermore, the effects of moisture combined with temperature were suggested experimentally to be more reliable predictors [9,16,33].
The availability of soil carbon substrates is also an important factor controlling decomposition and heterotrophic respiration [34,35,36]. Heterotrophic respiration (i.e., CO2 emissions produced through soil organic matter (SOM) decomposition), is primarily driven by the activities of soil microorganisms and soil fauna, and their richness and abundance primarily control the decomposition rate of SOM [23,24,25]. The spatial distribution of soil microbes is, to a great extent, affected by the availability of carbon substrates [2,32,35]. Moreover, soil microbes themselves have particular C:N balance needs [35,36]. Therefore, soil carbon substrates can affect soil respiration by controlling the distribution and activities of soil fauna and microbes.
Soil respiration varies with vegetation types [15,22,37]. Global mean soil respiration rates vary widely among major vegetation biomes, and the lowest rates occur in tundra and northern bogs, while the highest rates occur in tropical moist forests [22]. Although the distribution pattern of soil respiration may be partially affected by temperature and moisture, substrate quality and species composition of fauna and microbes may differ substantially among vegetation types, and could partly explain the difference in soil respiration [22,37,38].
Almost all the environmental conditions influencing soil respiration (e.g., forest type, solar radiation, temperature and moisture, and soil fauna such as earthworms) have distinct elevation patterns in the Luquillo Experimental Forest (LEF). In general, temperature, plant species richness and abundance, and NPP decrease with elevation, whereas SOM, soil organic carbon (SOC), precipitation, and soil moisture increase [39,40,41,42]. The number of earthworm species also significantly increased along the elevation from low to top [43]. The elevation gradient provides a natural in situ simulation of climate change [14,17,44]. Therefore, studying the variation in soil carbon along an elevation gradient in LEF is an ideal approach to investigate the impacts of climate change on tropical carbon processes. This paper describes a soil translocation experiment conducted along the elevation gradient in the LEF. We hypothesized that (1) soil respiration will increase but SOC will decrease with enhanced soil temperature and moisture; and (2) soils originated from high elevation with high SOC content will respire more than those from low elevation under similar climatic conditions. Warming in the tropics might boost soil respiration, especially in soils at high elevations with large SOC content, therefore this study may contribute to better understanding of C dynamics in tropical regions in the context of global change.
2. Materials and Methods
2.1. Study Area
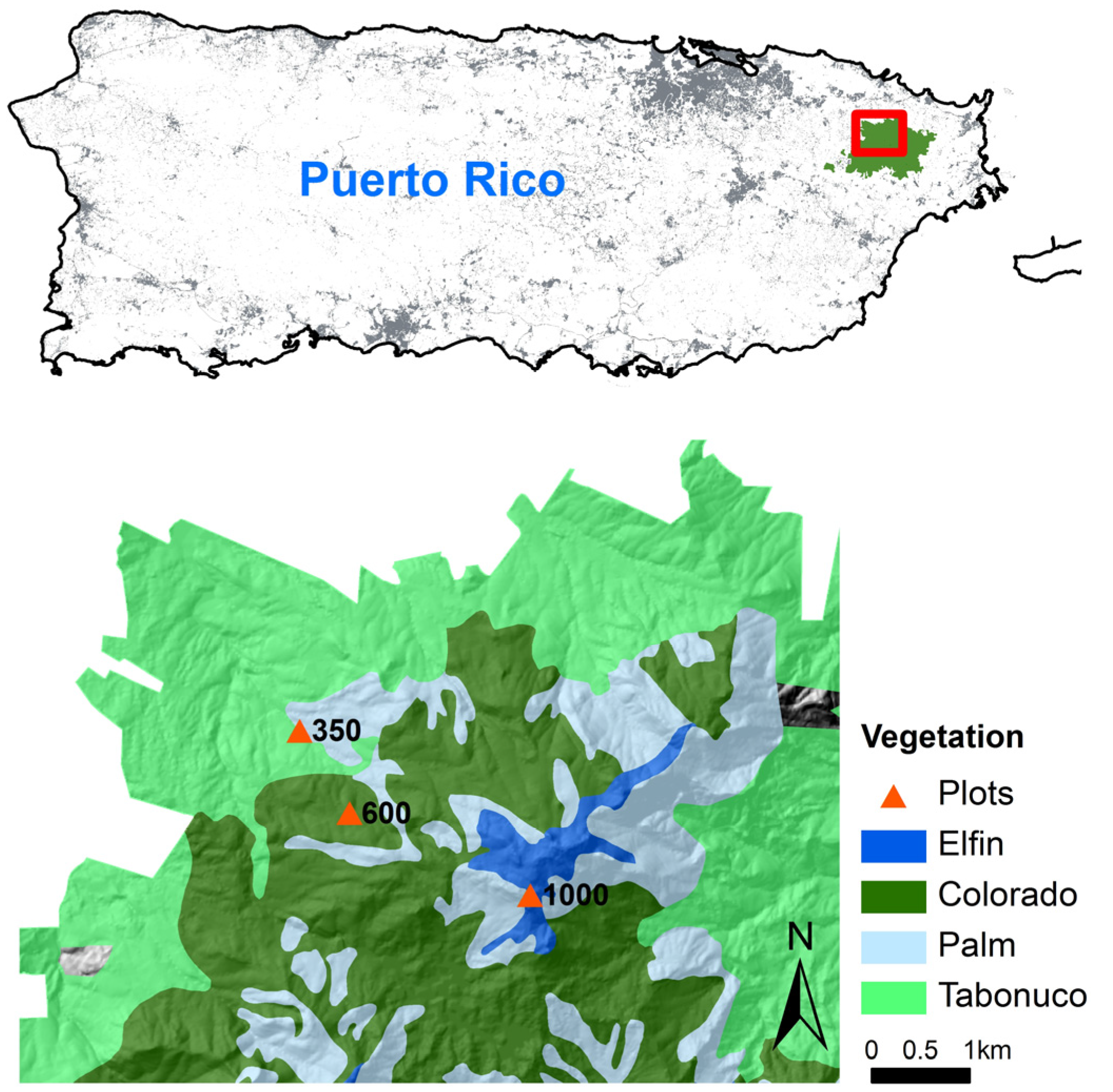
All three experiment sites are located along an elevation gradient in LEF (18°20′ N, 65°49′ W), northeastern Puerto Rico (Figure 1), a tropical wet montane forest. Annual rainfall ranges from an average of 3537 mm at low elevation to 4849 mm at high elevation, and monthly temperatures in January and September change from 23.5 °C and 27 °C at low elevation to 17 °C and 20 °C at high elevation, respectively [45]. Soils are mainly derived from volcaniclastic sediments and are classified as “clay” based on their particle size distribution, except for one high-elevation area where the soils are derived from quartz diorite with lower clay content and are classified as “clay loam” [42]. The distribution of vegetation in LEF exhibits a distinct elevation pattern. Tabonuco forest, Palm forest, Palo Colorado, and Elfin woodland distribute along the elevation gradient from the foothill to the top [41,46]. The three sites are facing north to northwest with the slopes of 5.0, 26.0, and 9.7° at 350, 600, and 1000 m, respectively. We chose a relatively flat place at each site to implement the soil translocations. The soils are acidic with the pH of 4.2, 4.6, and 4.4 at the three sites. Soil clay contents are 53%, 56%, and 36%, and soil bulk densities are 0.8, 0.6, and 0.9 g·cm−3 at 350, 600, and 1000 m, respectively. The soil Ca contents are 0.6, 0.2, and 0.2 mg·g−1, and soil P contents are 0.3, 0.2, and 0.4 mg·g−1 at the three sites, respectively. The lowest site (18°19′24′′ N, 65°49′3′′ W) is within the Tabonuco forest, a subtropical wet forest dominated by Dacryodes excelsa and Prestoea montana. The middle site (18°18′56′′ N, 65°48′46′′ W) is within the Palo Colorado and adjacent to the Tabonuco forest. Palo Colorado is a lower montane wet forest dominated by Cyrilla racemiflora, Prestoea montana, Henriettea squamulosa, and Magnolia splendens. The highest site (18°18′29′′ N, 65°47′43′′ W) is within the Elfin woodland characterized by stunted dwarf trees and dominated by Cyathea bryophila, Eugenia borinquensis, Ocotea spathulata, and Tabebuia rigida [47].
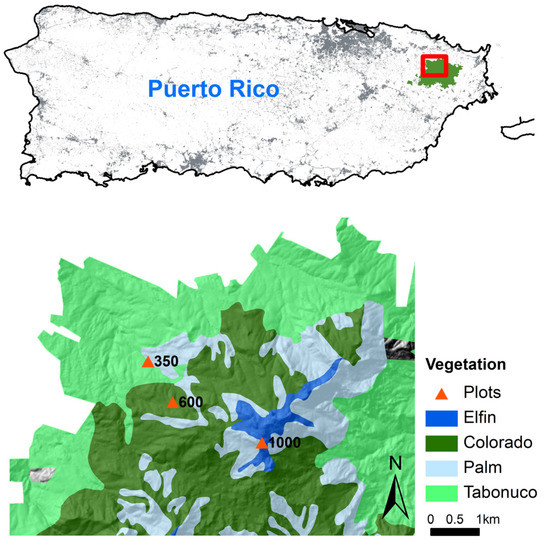
Figure 1.
The soil translocation experiment sites were located along the elevation gradient (350, 600, and 1000 m) within different dominant vegetation types in the Luquillo Experimental Forest, northeastern Puerto Rico.
2.2. Soil Translocation Experiment
Nine soil cores were excavated from each site, six of them were translocated to the other two sites (three for each), and three soil cores remained at the source site but at different places. Before collecting the samples, all the aboveground litter was removed. The organic-rich soils (0–5 cm) were collected separately, and later put back on the top of soil cores after reinstallation in the translocated sites. The soil cores were taken using polyvinyl (PVC) tubes 10 cm in diameter and 15 cm in length. When excavating the soil cores, the tubes were inserted vertically into the subsoil to take an intact soil monolith with a depth of 15 cm. When soil cores were reinstalled, the soils were kept intact in the tubes and separated from the surrounding soils. The bottom of the coring tube was covered with iron mesh (63-μm) to further prevent large roots from growing and to balance the effects of temperature and moisture in the soil within and outside the tubes.
In each of the three soil translocation sites, one soil temperature and one moisture probe were installed at both surface and 15 cm in-depth in an undisturbed place and connected to a data logger (HOBO® Micro station, ONSET Computer Corporation, Bourne, MA, USA). Soil temperature and moisture were recorded every 30 min from July 2011 to March 2012. Due to a battery failure, data were missing for the site at 600 m in November 2011 and February–March 2012, and the site at 1000 m in November 2011 and March 2012. Temperature measurements at the 1000-m site in December 2011–March 2012 were recalibrated using a mobile temperature probe because of a disturbance to the installed probe in December 2011. The automatically measured soil temperature and moisture were only used to display the naturally occurred gradients in temperature and moisture along the elevation gradient.
Soil respiration rates (Rs) were measured monthly with a portable InfraRed Gas Analyzer (EMG4, PP Systems, Amesbury, MA, USA), with a cylindrical cuvette (CPY-2) inserted in soil cores. The soil respiration fluxes were recorded for 2–3 min after the CO2 concentration in the closed chamber increased steadily. The real-time temperature and moisture for both soil and air were measured with external sensors or a thermometer. All the measurements were repeated three times.
At the end of the soil translocation experiment in May 2012, the SOC and SOM contents of all the soil cores translocated were measured at the International Institute of Tropical Forestry (IITF) of USDA Forest Service Chemistry Laboratory. The soil samples were oven dried to constant mass at 50 °C, and then ground and passed through a 20 mesh sieve (size of 0.84 mm) to screen coarse root detritus and other organic materials. SOC was determined using the LECO TruSpec CN Analyzer (LECO®, LECO Corporation, Saint Joseph, MI, USA). There are no carbonates found in these soils. Total carbon (TC) is equal to total organic carbon. Soil organic matter (SOM) was estimated using loss on ignition (LOI). LOI was determined using the LECO TGA-701 Analyzer at 490 °C for two hours (or until constant weight is reached at less than 2.5% variability) in an oxygen saturated environment. Soil total nitrogen content was determined using the LECO CN Analyzer.
2.3. Statistical Analyses
To test our hypotheses of impacts of soil source quality, translocation, and associated climate shift on soil respiration, we applied paired-t, ANOVA, and linear mixed-effects models on the measured soil respiration rates. Soil respiration rates were examined for normality (Shapiro–Wilk normality test), and log transformation was performed if needed. Multiple paired-t was used to test the impacts of soil source quality, such as SOC and SOM contents, on Rs. For example, in order to compare the Rs of the soils originating from 350 m with those from 1000 m, we took the paired Rs of R350,350 ~ R1000,350, R350,600 ~ R1000,600 and R350,1000 ~ R1000,1000, for which the first subscript indicates the soil source and the second subscript is for the translocated site. By pairing the Rs of soils originating from two different sites but translocated at the same sites, we virtually excluded the impacts of microclimate. ANOVA was applied to test the impacts of translocation site on soil respiration. We also used t-tests to compare the respirations of the soils with the same source but translocated to different elevations. The linear mixed-effects model was finally used as a synthetic analysis to test the impacts of translocated sites and microclimate on Rs, with soil source set as a random effect. Statistical analyses were run in R software [48].
3. Results
3.1. Naturally Occurred Gradient in Soil Temperature and Moisture and Changes in Soil C and N Contents before and after the Translocation Experiment along the Elevation Gradient
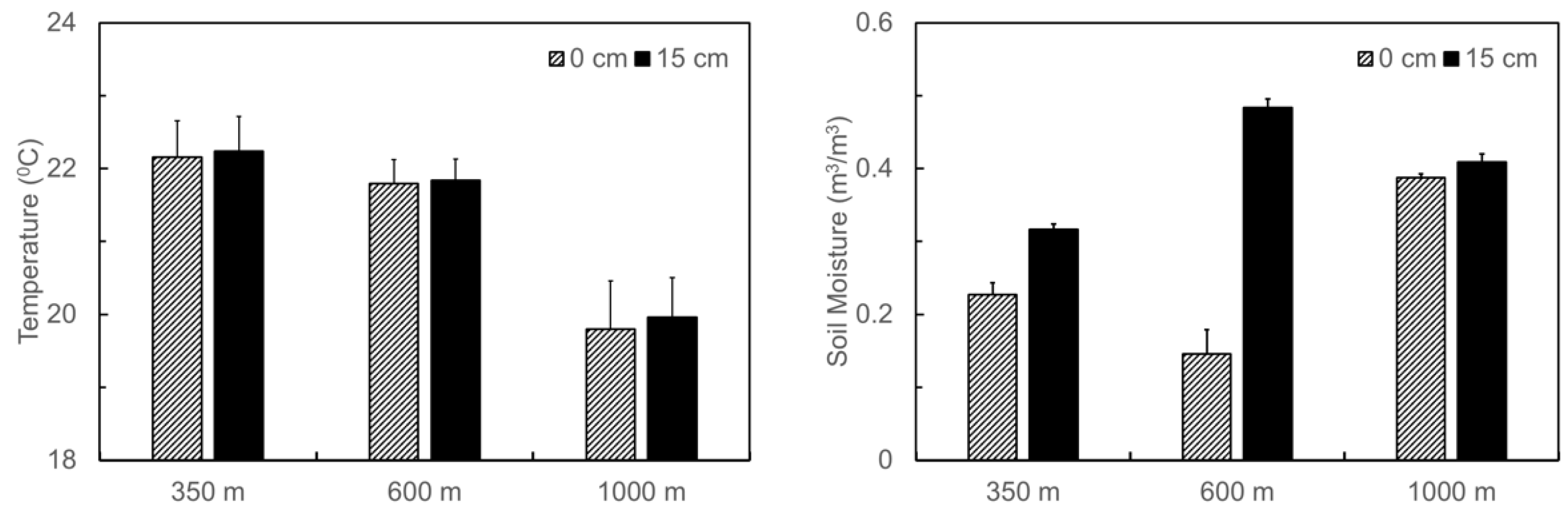
Soil temperature and moisture at the three translocation sites, on average, followed the naturally occurring gradients in climate along the elevation gradient, i.e., decreased temperature but increased moisture from the foothill to the top (Figure 2). The averaged soil temperature within the depth of 0–15 cm during the period July 2011–March 2012 decreased from 22.2 °C at 350 m, to 21.8 °C at 600 m, and to 19.9 °C at 1000 m. On the other hand, the averaged soil moisture increased from 0.27 m3·m−3 at 350 m, to 0.31 m3·m−3 at 600 m, and to 0.4 m3·m−3 at 1000 m. The difference in soil moisture between the surface and the depth of 15 cm was larger at the site of 600 m than at the other two sites.
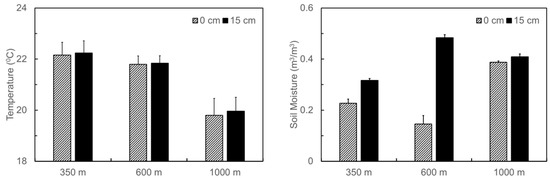
Figure 2.
Averaged soil temperature and moisture at 0 and 15 cm in depth throughout the experiment period at the three soil translocation sites (with elevations at 350 m, 600 m, and 1000 m, respectively) in the Luquillo Experimental Forest, Puerto Rico. Bars stand for standard errors.
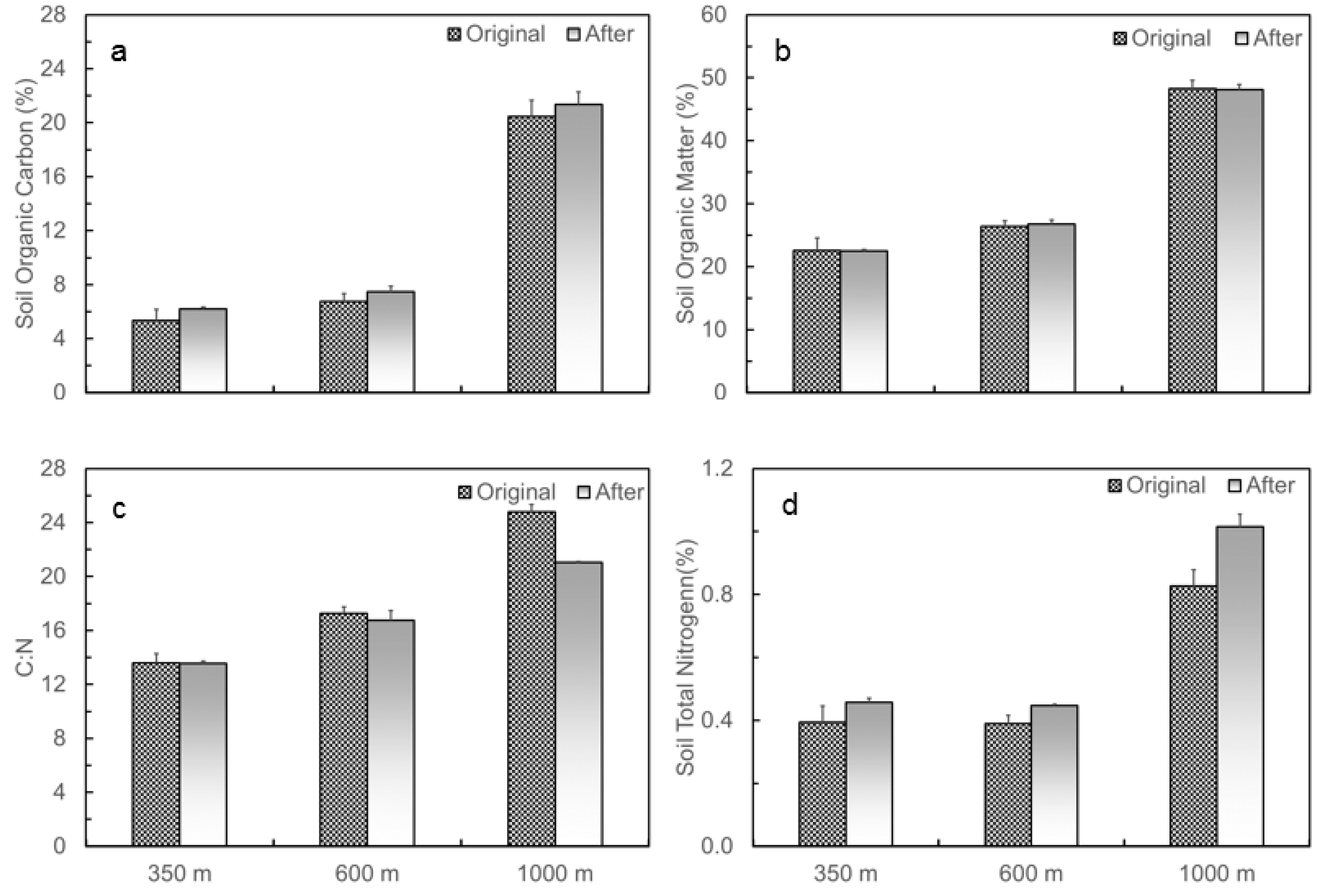
Initial patterns of SOC, SOM, and C:N all showed increased trends along the elevation gradient from low to top (Figure 3a–c). SOC increased from 5.4% at 350 m to 6.8% at 600 m, and to 20.5% at 1000 m, and SOM increased from 22.5% to 26.4% and to 48.2%, respectively. C:N increased from 13.6, to 17.2, and to 24.8 at the sites of 350, 600, and 1000 m, respectively. Soil total nitrogen was higher at 1000 m than at 350 or 600 m (Figure 3d). When measured 11 months after translocation, SOC increased to 6.2%, 7.5%, and 21.3% for the soils originating from 350, 600, and 1000 m, respectively. SOC originating from 350 m changed to 6.4% and 5.9% when translocated to 350 and 1000 m, respectively. For soils originating from 1000 m, SOC changed to 20.6% and 23.2% when translocated to 350 and 1000 m, respectively. Soil total nitrogen also increased after the soil translocation experiment. For the soils with the same source but translocated to different elevations, changes in soil total nitrogen follow the pattern of changes in SOC.
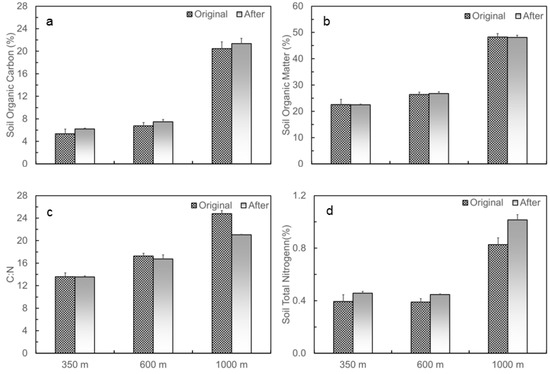
Figure 3.
Changes in (a) soil organic carbon, (b) soil organic matter, (c) C to N ratio, and (d) soil total nitrogen of the soils at 0–15 cm at the three soil translocation sites (with elevations of 350, 600, and 1000 m), measured before and after the soil translocation experiment in the Luquillo Experimental Forest, Puerto Rico.
3.2. Impacts of Soil Source Quality on Soil Respiration
We took a natural logarithm transformation of the soil respiration rate before performing the multiple paired-t tests because the original Rs did not pass the normality test, i.e., Shapiro–Wilk test in R. Paired-t tests on the log-transformed Rs were only applied to the comparisons between the soil source at 1000 m and that at 350 m or 600 m, according to the result of the normality test. Soils originating from 1000 m have a significantly larger respiration rate than those from 600 m (estimated difference = 0.31, t = 2.3, df = 21, p = 0.03) and from 350 m (estimated difference = 0.23, t = 1.7, df = 24, p = 0.1).
3.3. Impacts of Soil Translocated Site and Associated Microclimate on Soil Respiration
An ANOVA test on the log-transformed Rs among the three translocated sites indicated that the treatment of the translocation site had significant effects on soil respiration (F(2,71) = 6.07, p = 0.004). Soils translocated to the site at 350 m had a higher respiration rate than those translocated to 600 or 1000 m.
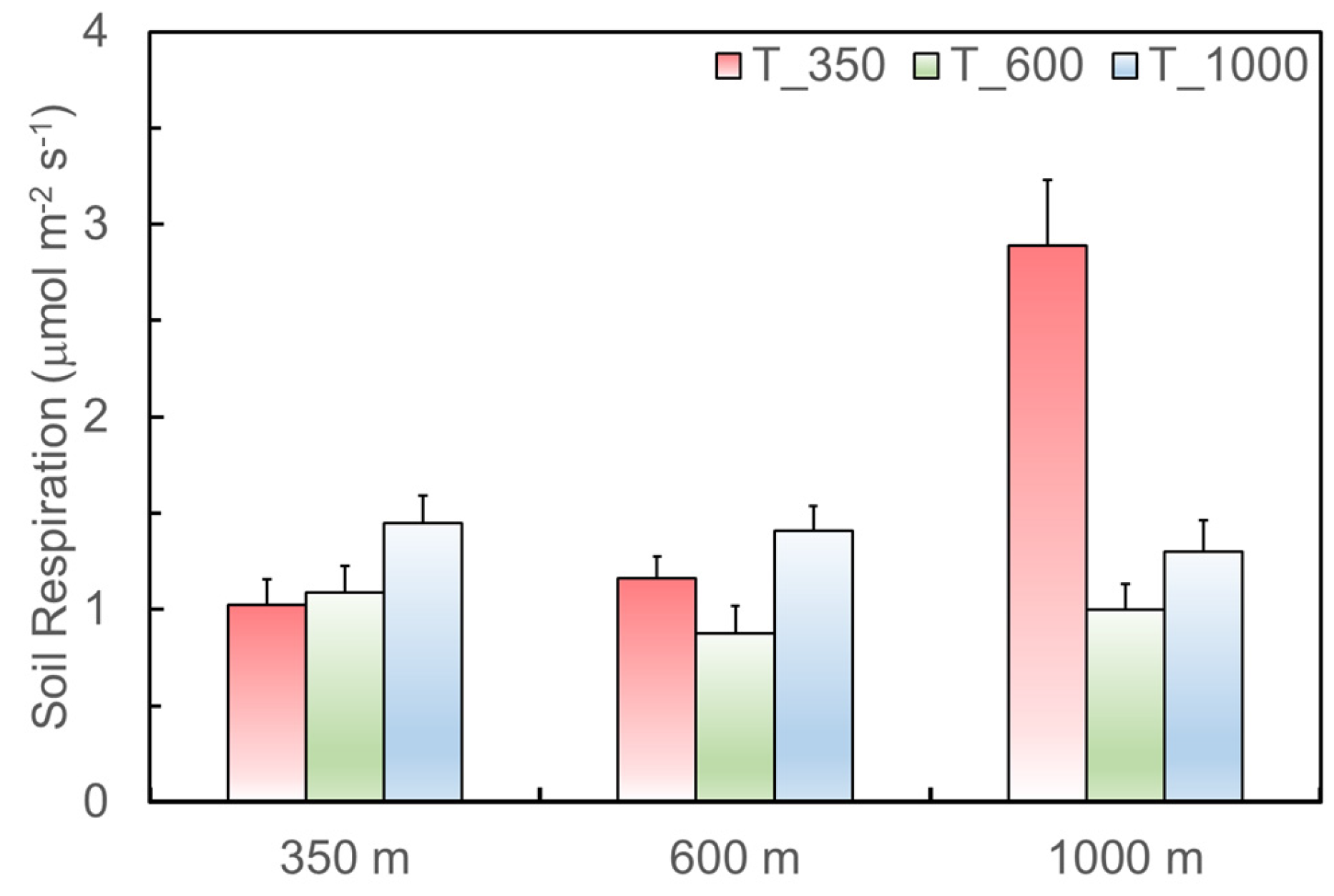
As for the soils with the same source but translocated to different elevations (Figure 4), soils originating from 350 m have significantly higher respiration rates when translocated to 1000 m, as indicated by the comparison of the sample mean of 1.447 versus 1.024 μmol·m−2·s−1 (t = 2.17, df = 16, p = 0.05). Soils originating from 1000 m also have significantly higher respiration rates when translocated to 350 m, 2.890 versus 1.298 μmol·m−2·s−1 in mean (t = 4.2, df = 10, p = 0.002).
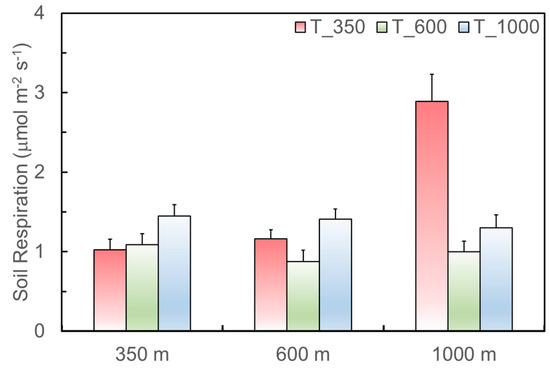
Figure 4.
Soil respiration of the soils originating from 350, 600, and 1000 m (x axis), but translocated to 350 (T_350), 600 (T_600), and 1000 m (T_1000) in the Luquillo Experimental Forest, Puerto Rico, from August 2011 to May 2012.
The linear mixed effects model on the log-transformed Rs with translocation sites as a fixed effect and soil source as a random effect confirmed that the translocation site has a significant effect on soil respiration (upper part in Table 1). When temperature, moisture, and their interaction were added to the fixed model, the effects of both translocated site and temperature were significant. However, the effects of moisture and its interaction with temperature were not significant (lower part in Table 1). The AIC and BIC (Akaike or Bayesian Information Criterion) of the later model are smaller when temperature and moisture were added as additional explanatory variables (Table 1).

Table 1.
The results of linear mixed effects models on the log-transformed soil respiration rate (μmol·m−2·s−1) with translocation sites (upper part) or translocation sites, soil temperature, moisture, and interaction of temperature and moisture (lower part) as fixed effects, and soil source as random effects. Temp. and M. stand for temperature and moisture, respectively. AIC and BIC stand for Akaike and Bayesian Information Criterion, respectively.
4. Discussion
Tropical forests play important roles in the interaction between terrestrial ecosystems and the global climate system, such as C dynamics. Warming and drought have the potential to change tropical forests from a C sink to a C source. Soil translocation experiments along altitudinal gradients provide an approach to assess the impacts of climate change on soil C dynamics. Our soil translocation experiment in tropical forests in northeastern Puerto Rico showed that both soil C and the soil respiration rates were altered by changes in temperature, moisture, and initial soil organic C.
Soil organic carbon, organic matter, total nitrogen, and C:N all increased with elevation (Figure 3), which conformed with other altitudinal studies involving naturally occurring gradients of decreasing temperature but increasing moisture [49]. Compared to the content before translocation, SOC increased slightly at the end of the experiment, which might be caused by the degradation of dead roots and litter in the soil cores. With the same source location, the changes in SOC after the translocation experiment followed the patterns of Rs. The greater the Rs at the translocated site, the greater the decrease in soil carbon.
Increasing SOC, SOM, and C:N along the altitudinal gradient (Figure 3) also implied improved substrate quantity/quality for soil respiration. By virtually excluding the effects of microclimate using the paired-t test, our study highlights that soil source has significant impacts on soil respiration. The soils originating from high elevation with high SOC, SOM, and C:N had a much larger respiration rate than those originating from low elevation with low SOC, SOM, and C:N. The results support our hypothesis on the important role of soil substrate in decomposition.
In addition to soil substrates, difference in microenvironment at the translocated sites along the elevation gradient could also differentiate the soil respiration. Our analysis on Rs via linear mixed effects models revealed that translocation significantly impacted soil respiration (Table 1). As one important factor influencing carbon processes, temperature is considered to have positive effects on SOM decomposition and soil respiration rates [33,50,51]. Our experiment supported the hypothesis of positive temperature effect on soil respiration. Soils translocated to the lowest site, thus with the highest temperature, had a higher mean respiration rate than those translocated to the middle and the highest elevations. This is particularly prominent for the soils originating from high elevation, thus having high SOC, SOM, and C:N (right columns in Figure 4 with soils from 1000 m). The linear mixed-effects model result confirmed a significantly positive effect of temperature on Rs (Table 1). Our estimated Q10 values also revealed high Rs sensitivity to the temperature of these soils, i.e., 3.1, 6.9, and 8.7 at 350, 600, and 1000 m, respectively.
However, environmental controls on soil carbon processes are complex. Temperature alone could not explain the pattern of soil respiration rates in LEF as signaled by the increases in Rs of the soils from 350 m but translocated to 1000 m with lowered temperature (left columns in Figure 4). Although not significant, the effect of soil moisture on Rs is indicated as being positive (Table 1). Soil moisture increases with elevation and reaches the highest at 1000 m (Figure 2), which might stimulate the decomposition rate during the dry season as mentioned above. Existing studies also suggested the effects of soil fauna and microbial biomass on Rs, and showed that soil fauna and microbes varied with microclimate conditions, soil properties (e.g., clay), and vegetation [34,52,53,54]. The soil fauna and microbes from high elevation might prefer the SOC and SOM with lower C:N originating from low elevation [26] and thus accelerate the decomposition. Particularly, soil microbial mass positively relates to SOM [34,52]. Since SOM increases with elevation in LEF (Figure 3), soil microbial mass might also increase along the elevation from low to top in LEF. Therefore, the increased Rs of the soils translocated from low elevation to high elevation might be related to the fact that the positive impacts of soil moisture, specific microbes, and microbial biomass at high elevation outweighed the limitation of decreased temperature.
Our conclusions of complex environmental controls on soil respiration along an elevation gradient are consistent with others. Kane et al. (2003) measured soil respiration rates along a gradient in the Olympic National Park, Washington, USA, where soil temperature at a high elevation site was 4.5 °C lower than that at low elevation. However, there was no significant relation detected between soil respiration and temperature [14]. Similarly, in a study on climate dependence of heterotrophic soil respiration along a 3000-m elevation gradient in a tropical forest in Peru, Zimmermann et al. (2009) also concluded that the soil respiration rate did not vary significantly along the elevation gradient with decreasing temperature, although SOC stocks increased linearly with increased elevation [17].
The ecosystem carbon balance primarily depends on the differences between the responses of productivity and those of respiration to climate change, especially warming [51,55,56]. Existing studies found resource quality could significantly affect the temperature sensitivity of SOM decomposition [26,57]. The labile SOM is sensitive to climate change and generally decomposes rapidly, which can largely contribute to increases in soil CO2 fluxes [58,59]. Our estimated Q10 values, a widely-used parameter to describe the temperature sensitivity of soil C, increased with elevation. The Q10 value at high elevation, with low temperature, high moisture, SOC, SOM, and C:N, is more than double that at low elevation. Therefore, understanding the environment controls on the temperature sensitivity of soil respiration is critical to predict the responses of carbon processes to climate change [50,58,60,61,62].
With the ongoing and predicted warming climate in the Caribbean region [11], tropical soils are at severe risk of releasing large amounts of CO2 into the atmosphere. Our soil translocation experiment improved the understanding of the impacts of environmental conditions on soil carbon processes by highlighting the significant effects of soil source and temperature, the nonsignificant positive effect of moisture, and overall complex environmental controls along the altitudinal gradient. Further long-term and multi-factor soil translocation experiments at the ecosystem level, incorporating, additionally, soil microbes, fauna, and litter inputs, should be established to study the impacts of climate change on tropical soil carbon balance, as well as their feedback to the climate.
5. Conclusions
Tropical forests play important roles in the interactions between terrestrial ecosystems and the global climate system, and have the potential to change from a C sink to a C source under climate change. Soil translocation experiments along the altitudinal gradient provide an approach to assess the impacts of climate change on soil C dynamics. Our soil translocation experiment at tropical forests in northeastern Puerto Rico showed that both soil C and soil respiration rates were altered by variations in temperature, moisture, and initial soil organic C. When soils were translocated to a lower elevation, the increased temperature enhanced soil respiration and therefore less soil organic C was left at the end of the experiment. Such an effect is more significant for those soils with an initial high content of soil organic C, i.e., soils originating from high elevations. On the other hand, soil respiration could also be enhanced when soils were translocated to higher elevation due to altered moisture conditions and soil microbes. Further comprehensive studies involving soil microbial composition and biomass and the quality of C substrates would improve the mechanistic understanding of soil respiration in response to climate change, and advance the earth system modeling.
Acknowledgments
This research was supported by the grant DEB-1546686 from the National Science Foundation to the Institute for Tropical Ecosystem Studies (ITES), Department of Environmental Science, University of Puerto Rico, and to the International Institute of Tropical Forestry (IITF), USDA Forest Service, as part of the Luquillo Long-Term Ecological Research Program. The USDA Forest Service and University of Puerto Rico gave additional field and lab support. Technicians at the El Verde Field Station helped with experiment set-up. Technicians (María M. Rivera, Maysaá Ittayem, and Mary Jane Sánchez) at IITF USDA Forest Service provided assistance on field work and laboratory analyses.
Author Contributions
M.Y., G.G., and X.Z. conceived, designed the experiments, and reviewed various versions of the manuscript; D.C. performed the experiments and field work; D.C., M.Y., and Q.G. analyzed the data; G.G. contributed lab analyses, equipment/materials, and field work; D.C., M.Y., and Q.G. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Townsend, A.R.; Vitousek, P.M.; Trumbore, S.E. Soil organic-matter dynamics along gradients in temperature and land-use on the island of Hawaii. Ecology 1995, 76, 721–733. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Andrews, J.A. Soil respiration and the global carbon cycle. Biogeochemistry 2000, 48, 7–20. [Google Scholar] [CrossRef]
- Silver, W.L. The potential effects of elevated CO2 and climate change on tropical forest soils and biogeochemical cycling. Clim. Chang. 1998, 39, 337–361. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A large and persistent carbon sink in the world's forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Meir, P.; Wood, T.E.; Galbraith, D.R.; Brando, P.M.; da Costa, A.C.L.; Rowland, L.; Ferreira, L.V. Threshold responses to soil moisture deficit by trees and soil in tropical rain forests: Insights from field experiments. Bioscience 2015, 65, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Rowland, L.; da Costa, A.C.L.; Galbraith, D.R.; Oliveira, R.S.; Binks, O.J.; Oliveira, A.A.R.; Pullen, A.M.; Doughty, C.E.; Metcalfe, D.B.; Vasconcelos, S.S.; et al. Death from drought in tropical forests is triggered by hydraulics not carbon starvation. Nature 2015, 528, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Gatti, L.V.; Gloor, M.; Miller, J.B.; Doughty, C.E.; Malhi, Y.; Domingues, L.G.; Basso, L.S.; Martinewski, A.; Correia, C.S.C.; Borges, V.F.; et al. Drought sensitivity of amazonian carbon balance revealed by atmospheric measurements. Nature 2014, 506, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Anderegg, W.R.L.; Ballantyne, A.P.; Smith, W.K.; Majkut, J.; Rabin, S.; Beaulieu, C.; Birdsey, R.; Dunne, J.P.; Houghton, R.A.; Myneni, R.B.; et al. Tropical nighttime warming as a dominant driver of variability in the terrestrial carbon sink. Proc. Natl. Acad. Sci. USA 2015, 112, 15591–15596. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Norby, R.J.; Ledford, J.; Weltzin, J.F. Responses of soil respiration to elevated CO2, air warming, and changing soil water availability in a model old-field grassland. Glob. Chang. Biol. 2007, 13, 2411–2424. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; p. 1535. [Google Scholar]
- Yu, M.; Gao, Q.; Gao, C.; Wang, C. Extent of night warming and spatially heterogeneous cloudiness differentiate temporal trend of greenness in mountainous tropics in the new century. Sci. Rep. 2017, 7, 41256. [Google Scholar] [CrossRef] [PubMed]
- Baggs, E.M. Partitioning the components of soil respiration: A research challenge. Plant Soil 2006, 284, 1–5. [Google Scholar] [CrossRef]
- Cramer, W.; Bondeau, A.; Woodward, F.I.; Prentice, I.C.; Betts, R.A.; Brovkin, V.; Cox, P.M.; Fisher, V.; Foley, J.A.; Friend, A.D.; et al. Global response of terrestrial ecosystem structure and function to CO2 and climate change: Results from six dynamic global vegetation models. Glob. Chang. Biol. 2001, 7, 357–373. [Google Scholar] [CrossRef]
- Kane, E.S.; Pregitzer, K.S.; Burton, A.J. Soil respiration along environmental gradients in olympic national park. Ecosystems 2003, 6, 326–335. [Google Scholar] [CrossRef]
- Li, Y.; Xu, M.; Zou, X.; Xia, Y. Soil CO2 efflux and fungal and bacterial biomass in a plantation and a secondary forest in wet tropics in Puerto Rico. Plant Soil 2005, 268, 151–160. [Google Scholar] [CrossRef]
- Conant, R.T.; Dalla-Betta, P.; Klopatek, C.C.; Klopatek, J.M. Controls on soil respiration in semiarid soils. Soil Biol. Biochem. 2004, 36, 945–951. [Google Scholar] [CrossRef]
- Zimmermann, M.; Meir, P.; Bird, M.I.; Malhi, Y.; Ccahuana, A.J.Q. Climate dependence of heterotrophic soil respiration from a soil-translocation experiment along a 3000 m tropical forest altitudinal gradient. Eur. J. Soil Sci. 2009, 60, 895–906. [Google Scholar] [CrossRef]
- Nottingham, A.T.; Whitaker, J.; Turner, B.L.; Salinas, N.; Zimmermann, M.; Malhi, Y.; Meir, P. Climate warming and soil carbon in tropical forests: Insights from an elevation gradient in the Peruvian Andes. Bioscience 2015, 65, 906–921. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, J.; Taylor, J.A. On the temperature-dependence of soil respiration. Funct. Ecol. 1994, 8, 315–323. [Google Scholar] [CrossRef]
- Kirschbaum, M.U.F. The temperature dependence of soil organic matter decomposition, and the effect of global warming on soil organic C storage. Soil Biol. Biochem. 1995, 27, 753–760. [Google Scholar] [CrossRef]
- Knorr, W.; Prentice, I.C.; House, J.I.; Holland, E.A. Long-term sensitivity of soil carbon turnover to warming. Nature 2005, 433, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Raich, J.W.; Tufekciogul, A. Vegetation and soil respiration: Correlations and controls. Biogeochemistry 2000, 48, 71–90. [Google Scholar] [CrossRef]
- Heneghan, L.; Coleman, D.C.; Zou, X.; Crossley, D.A.; Haines, B.L. Soil microarthropod contributions to decomposition dynamics: Tropical-temperate comparisons of a single substrate. Ecology 1999, 80, 1873–1882. [Google Scholar]
- González, G.; Seastedt, T.R. Soil fauna and plant litter decomposition in tropical and subalpine forests. Ecology 2001, 82, 955–964. [Google Scholar] [CrossRef]
- Dechaine, J.; Ruan, H.; Sánchez-de León, Y.; Zou, X. Correlation between earthworms and plant litter decomposition in a tropical wet forest of Puerto Rico. Pedobiologia 2005, 49, 601–607. [Google Scholar] [CrossRef]
- Sjögersten, S.; Wookey, P.A. Climatic and resource quality controls on soil respiration across a forest-tundra ecotone in Swedish Lapland. Soil Biol. Biochem. 2002, 34, 1633–1646. [Google Scholar] [CrossRef]
- Lawrence, C.R.; Neff, J.C.; Schimel, J.P. Does adding microbial mechanisms of decomposition improve soil organic matter models? A comparison of four models using data from a pulsed rewetting experiment. Soil Biol. Biochem. 2009, 41, 1923–1934. [Google Scholar] [CrossRef]
- Mikan, C.J.; Schimel, J.P.; Doyle, A.P. Temperature controls of microbial respiration in arctic tundra soils above and below freezing. Soil Biol. Biochem. 2002, 34, 1785–1795. [Google Scholar] [CrossRef]
- Xu, L.; Baldocchi, D.D.; Tang, J. How soil moisture, rain pulses, and growth alter the response of ecosystem respiration to temperature. Glob. Biogeochem. Cycles 2004, 18. [Google Scholar] [CrossRef]
- Qi, Y.; Xu, M.; Wu, J. Temperature sensitivity of soil respiration and its effects on ecosystem carbon budget: Nonlinearity begets surprises. Ecol. Model. 2002, 153, 131–142. [Google Scholar] [CrossRef]
- Rastetter, E.B.; Ryan, M.G.; Shaver, G.R.; Melillo, J.M.; Nadelhoffer, K.J.; Hobbie, J.E.; Aber, J.D. A general biogeochemical model describing the responses of the C-cycle and N-cycle in terrestrial ecosystems to changes in CO2, climate, and N-deposition. Tree Physiol. 1991, 9, 101–126. [Google Scholar] [CrossRef] [PubMed]
- Gifford, R. The global carbon cycle: A viewpoint on the missing sink. Funct. Plant Biol. 1994, 21, 1–15. [Google Scholar] [CrossRef]
- Li, Y.; Xu, M.; Zou, X. Heterotrophic soil respiration in relation to environmental factors and microbial biomass in two wet tropical forests. Plant Soil 2006, 281, 193–201. [Google Scholar] [CrossRef]
- Wang, W.J.; Dalal, R.C.; Moody, P.W.; Smith, C.J. Relationships of soil respiration to microbial biomass, substrate availability and clay content. Soil Biol. Biochem. 2003, 35, 273–284. [Google Scholar] [CrossRef]
- Vance, E.D.; Chapin Iii, F.S. Substrate limitations to microbial activity in taiga forest floors. Soil Biol. Biochem. 2001, 33, 173–188. [Google Scholar] [CrossRef]
- Blagodatsky, S.; Blagodatskaya, E.; Yuyukina, T.; Kuzyakov, Y. Model of apparent and real priming effects: Linking microbial activity with soil organic matter decomposition. Soil Biol. Biochem. 2010, 42, 1275–1283. [Google Scholar] [CrossRef]
- Smith, D.L.; Johnson, L. Vegetation-mediated changes in microclimate reduce soil respiration as woodlands expand into grasslands. Ecology 2004, 85, 3348–3361. [Google Scholar] [CrossRef]
- Kleb, H.R.; Wilson, S.D. Vegetation effects on soil resource heterogeneity in prairie and forest. Am. Nat. 1997, 150, 283–298. [Google Scholar] [PubMed]
- Bruijnzeel, L.A.; Veneklaas, E.J. Climatic conditions and tropical montane forest productivity: The fog has not lifted yet. Ecology 1998, 79, 3–9. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Givnish, T.J. Altitudinal gradients in tropical forest composition, structure, and diversity in the sierra de manantlán. J. Ecol. 1998, 86, 999–1020. [Google Scholar]
- Gould, W.A.; González, G.; Carrero, R.G. Structure and composition of vegetation along an elevational gradient in Puerto Rico. J. Veg. Sci. 2006, 17, 653–664. [Google Scholar] [CrossRef]
- Barone, J.A.; Thomlinson, J.; Cordero, P.A.; Zimmerman, J.K. Metacommunity structure of tropical forest along an elevation gradient in Puerto Rico. J. Trop. Ecol. 2008, 24, 525–534. [Google Scholar] [CrossRef]
- González, G.; García, E.; Cruz, V.; Borges, S.; Zalamea, M.; Rivera, M.M. Earthworm communities along an elevation gradient in northeastern Puerto Rico. Eur. J. Soil Biol. 2007, 43, S24–S32. [Google Scholar] [CrossRef]
- Zimmermann, M.; Meir, P.; Bird, M.I.; Malhi, Y.; Ccahuana, A.J.Q. Temporal variation and climate dependence of soil respiration and its components along a 3000 m altitudinal tropical forest gradient. Glob. Biogeochem. Cycles 2010, 24. [Google Scholar] [CrossRef]
- Garcia-Martinó, A.G.; Warner, G.S.; Scatena, F.N.; Civco, D.L. Rainfall, runoff and elevation relationships in the Luquillo mountains of Puerto Rico. Caribb. J. Sci. 1996, 32, 413–424. [Google Scholar]
- Weaver, P.L. Environmental gradients affect forest structure in Puerto Rico's Luquillo mountains. Interciencia 2000, 25, 254–259. [Google Scholar]
- Weaver, P.L.; Gould, W.A. Forest vegetation along environmental gradients in northeastern Puerto Rico. Ecol. Bull. 2013, 54, 43–65. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- Bond-Lamberty, B.; Bolton, H.; Fansler, S.; Heredia-Langner, A.; Liu, C.; McCue, L.A.; Smith, J.; Bailey, V. Soil respiration and bacterial structure and function after 17 years of a reciprocal soil transplant experiment. PLoS ONE 2016, 11, e0150599. [Google Scholar] [CrossRef] [PubMed]
- Kirschbaum, M.U.F. The temperature dependence of organic-matter decomposition—Still a topic of debate. Soil Biol. Biochem. 2006, 38, 2510–2518. [Google Scholar] [CrossRef]
- Sayer, E.J.; Heard, M.S.; Grant, H.K.; Marthews, T.R.; Tanner, E.V.J. Soil carbon release enhanced by increased tropical forest litterfall. Nat. Clim. Chang. 2011, 1, 304–307. [Google Scholar] [CrossRef]
- Müller, T.; Höper, H. Soil organic matter turnover as a function of the soil clay content: Consequences for model applications. Soil Biol. Biochem. 2004, 36, 877–888. [Google Scholar] [CrossRef]
- Ruan, H.H.; Zou, X.M.; Scatena, E.; Zimmerman, J.K. Asynchronous fluctuation of soil microbial biomass and plant litterfall in a tropical wet forest. Plant Soil 2004, 260, 147–154. [Google Scholar] [CrossRef]
- Wang, S.; Ruan, H.; Wang, B. Effects of soil microarthropods on plant litter decomposition across an elevation gradient in the Wuyi mountains. Soil Biol. Biochem. 2009, 41, 891–897. [Google Scholar] [CrossRef]
- Malhi, Y.; Grace, J. Tropical forests and atmospheric carbon dioxide. Trends Ecol. Evol. 2000, 15, 332–337. [Google Scholar] [CrossRef]
- Clark, D.A. Sources or sinks? The responses of tropical forests to current and future climate and atmospheric composition. Philos. Trans. R. Soc. B Biol. Sci. 2004, 359, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Plante, A.F.; Conant, R.T.; Carlson, J.; Greenwood, R.; Shulman, J.M.; Haddix, M.L.; Paul, E.A. Decomposition temperature sensitivity of isolated soil organic matter fractions. Soil Biol. Biochem. 2010, 42, 1991–1996. [Google Scholar] [CrossRef]
- Conant, R.T.; Drijber, R.A.; Haddix, M.L.; Parton, W.J.; Paul, E.A.; Plante, A.F.; Six, J.; Steinweg, J.M. Sensitivity of organic matter decomposition to warming varies with its quality. Glob. Chang. Biol. 2008, 14, 868–877. [Google Scholar] [CrossRef]
- Von Lützow, M.; Kögel-Knabner, I. Temperature sensitivity of soil organic matter decomposition—What do we know? Biol. Fertil. Soils 2009, 46, 1–15. [Google Scholar] [CrossRef]
- Davidson, E.A.; Janssens, I.A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Conant, R.T.; Ryan, M.G.; Ågren, G.I.; Birge, H.E.; Davidson, E.A.; Eliasson, P.E.; Evans, S.E.; Frey, S.D.; Giardina, C.P.; Hopkins, F.; et al. Temperature and soil organic matter decomposition rates—Synthesis of current knowledge and a way forward. Glob. Chang. Biol. 2011, 17, 3392–3404. [Google Scholar] [CrossRef]
- Balser, T.C.; Wixon, D.L. Investigating biological control over soil carbon temperature sensitivity. Glob. Chang. Biol. 2009, 15, 2935–2949. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).