Effects of Different Ectomycorrhizal Fungal Inoculates on the Growth of Pinus tabulaeformis Seedlings under Greenhouse Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Seedlings and Fungal Material
2.2. Experimental Design
2.3. Plant Measurements and ECM Colonisation
2.4. Inorganic Nutrient Content
2.5. Soil Sample Preparation and Enzymatic Activity Assays
2.6. Field Test Design and Photosynthetic Data Analysis
2.7. Statistics
3. Results
3.1. ECM Colonisation
3.2. Plant Growth and Biomass
3.3. Nutrient Content
3.4. Enzymatic Activity in the Rhizospheric Soil of Seedlings
3.5. Gas Exchange Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yuan, H.W.; Niu, S.H.; Zhou, X.Q.; Du, Q.P.; Li, Y.; Li, W. Evaluation of seed production in a first-generation seed orchard of chinese pine (Pinus tabuliformis). J. For. Res. 2016, 27, 1003–1008. [Google Scholar] [CrossRef]
- Yuan, H.W.; Niu, S.H.; El-Kassaby, Y.A.; Li, Y.; Li, W. Simple genetic distance-optimized field deployments for clonal seed orchards based on microsatellite markers: As a case of chinese pine seed orchard. PLoS ONE 2016, 11, e157646. [Google Scholar] [CrossRef] [PubMed]
- Marschner, H.; Dell, B. Nutrient uptake in mycorrhizai symbiosis. Plant Soil 1994, 159, 89–102. [Google Scholar]
- Wang, B.; Qiu, Y.L. Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 2006, 16, 299–363. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.H.; Tang, M.; Chen, H.; Zheng, C.L. Effects of inoculation with ectomycorrhizal fungi on microbial biomass and bacterial functional diversity in the rhizosphere of Pinus tabulaeformis seedlings. Eur. J. Soil Biol. 2010, 46, 55–61. [Google Scholar] [CrossRef]
- Rincón, A.; Alvarez, I.F.; Pera, J. Inoculation of containerized Pinus pinea L. seedlings with seven ectomycorrhizal fungi. Mycorrhiza 2001, 11, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Marx, D.H. Variability in ectomycorrhizal development and growth among isolates of Pisolithus tinctorius as affected by source, age, and reisolation. Can. J. For. Res. 1981, 11, 168–174. [Google Scholar] [CrossRef]
- Parladé, J.; Pera, J.; Alvarez, I.F. Inoculation of containerized Pseudotsuga menziesii and Pinus pinaster seedlings with spores of five species of ectomycorrhizal fungi. Mycorrhiza 1996, 6, 237–245. [Google Scholar] [CrossRef]
- Duñabeitia, M.K.; Hormilla, S.; Garcia-Plazaola, J.I.; Txarterina, K.; Arteche, U.; Becerril, J.M. Differential responses of three fungal species to environmental factors and their role in the mycorrhization of Pinus radiata D. Don. Mycorrhiza 2004, 14, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Trappe, J.M. Selection of fungi for ectomycorrhizal inoculation in nurseries. Annu. Rev. Phytopathol. 1977, 15, 203–222. [Google Scholar] [CrossRef]
- Lu, N.; Zhou, X.; Cui, M.; Yu, M.; Zhou, J.X.; Qin, Y.S.; Li, Y. Colonization with arbuscular mycorrhizal fungi promotes the growth of Morus alba L. seedlings under greenhouse conditions. Forests 2015, 6, 734–747. [Google Scholar] [CrossRef]
- Wong, K.K.; Montpetit, D.; Piche, Y.; Lei, J. Root colonization by four closely related genotypes of the ectomycorrhizal basidiomycete laccaria hicolor (maire) orton—Comparative studies using electron microscopy. New Phytol. 1990, 116, 669–679. [Google Scholar] [CrossRef]
- Read, D.J. Mycorrhizas in ecosystems. Experientia 1991, 47, 376–391. [Google Scholar] [CrossRef]
- Huang, Y.; Jiang, X.Y.; Liang, Z.C.; Ji, H.B. Ectomycorrhizal fungi and phosphorus on response of Pinus tabulaeformis plants to saline environment. Ecol. Environ. 2003, 13, 622–625. [Google Scholar]
- Huang, Y.; Jiang, X.Y.; Liang, Z.C.; Li, T. Effect of Ectomycorrhizal Fungi on Growth and Physiology of Pinus tabulaeformis Seedlings Under Saline Stress. J. Agro-Environ. Sci. 2006, 25, 1475–1480. [Google Scholar]
- Wu, B.Y.; Nioh, I. Growth and water relations of P. Tabulaeformis seedlings inoculated with ectomycorrhizal fungi. Microbes Environ. 1997, 12, 69–74. [Google Scholar]
- Yu, M.; Cui, M.; Shen, H.; Huang, J.G. Effect of inoculating with the ectotrophic mycorrhizal fungi on the growth of Pinus tabulaeformis seedlings. J. Sichuan For. Sci. Technol. 2011, 2, 46–48. [Google Scholar]
- Marx, D.H.; Davey, C.B.; Ruehle, J.L. Infection of Ectomycorrhizal and nonmycorrhizal roots of Shortleaf pine by nematodes and Phytophthora cinnamomi. Phytopathology 1974, 64, 1260–1264. [Google Scholar]
- Brundrett, M.; Bougher, N.; Dell, B.; Grove, T.; Malajczuk, N. Working with Mycorrhizas in Forestry and Agriculture; Australian Centre for International Agricultural Research: Canberra, Australia, 1996. [Google Scholar]
- Gong, M.Q.; Chen, Y.L.; Zhong, C.L. The Research and Application of Mycorrhizas; China Forestry Press: Beijing, China, 1997. [Google Scholar]
- Bradstreet, R.B. The Kjeldahl Method for Organic Nitrogen; Academic Press Inc.: New York, NY, USA, 1965. [Google Scholar]
- Lu, R.K. Soil Analytical Methods of Agronomic Chemica; China Agricultural Science and Technology Press: Beijing, China, 2000. [Google Scholar]
- Page, A.L. Methods of Soil Analysis. Part 2. In Chemical and Microbiological Properties; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1982. [Google Scholar]
- Guan, S.Y. Soil Enzyme and Research Methods; China Agricultural Press: Beijing, China, 1986. [Google Scholar]
- Söderberg, K.H.; Olsson, P.A.; Bååth, E. Structure and activity of the bacterial community in the rhizosphere of different plant species and the effect of arbuscular mycorrhizal colonisation. FEMS Microbiol. Ecol. 2002, 40, 223–231. [Google Scholar] [CrossRef]
- Bever, J.D. Negative feedback within a mutualism: Host-specific growth of mycorrhizal fungi reduces plant benefit. Proc. R. Soc. B Biol. Sci. 2002, 69, 2595–2601. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, A.; Strasser, R.J.; Martins-Loução, M.A. Are mycorrhiza always beneficial? Plant Soil 2006, 279, 65–73. [Google Scholar] [CrossRef]
- Lehto, T.; Zwiazek, J.J. Ectomycorrhizas and water relations of trees: A review. Mycorrhiza 2011, 21, 71–90. [Google Scholar] [CrossRef] [PubMed]
- Jie, W.; Huang, Y.; Jiang, X.Y. Influence of ectomycorrhizal fungi on absorption and balance of essential elements of Pinus tabulaeformis seedlings in saline soil. Pedosphere 2011, 21, 400–406. [Google Scholar]
- Gerdemann, J.W.; Trappe, J.M. Endogonaceae in the Pacific Northwest. Mycol. Mem. 1974, 5, 1–76. [Google Scholar]
- Pessarakli, M. Plant/Crop Physiology and Physiological Aspects of Plant/Crop Production Processes. In Handbook of Plant and Crop Physiology; CRC Press: New York, NY, USA, 2014; pp. 277–278. [Google Scholar]
- Dong, C.; Hu, D.; Fu, Y.; Wang, M.; Liu, H. Analysis and optimization of the effect of light and nutrient solution on wheat growth and development using an inverse system model strategy. Comput. Electron. Agric. 2014, 109, 221–231. [Google Scholar] [CrossRef]
- Martin, T.; Oswald, O.; Graham, I.A. Arabidopsis seedling growth, storage lipid mobilization, and photosynthetic gene expression are regulated by carbon: Nitrogen availability. Plant physiol. 2002, 128, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Sheng, M.; Tang, M.; Chen, H.; Yang, B.; Zhang, F.; Huang, Y. Influence of arbuscular mycorrhizae on photosynthesis and water status of maize plants under salt stress. Mycorrhiza 2008, 18, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Tairo, E.V.; Ndakidemi, P.A. Possible benefits of rhizobial inoculation and phosphorus supplementation on nutrition, growth and economic sustainability in grain legumes. Am. J. Res. Commun. 2013, 1, 532–556. [Google Scholar]
- Chandra, D.; Srivastava, R.; Sharma, A.K. Environment Friendly Phosphorus Biofertilizer as an Alternative to Chemical Fertilizers. Available online: https://www.researchgate.net/publication/291345421 (accessed on 10 January 2016).
- Pacak, A.; Barciszewska-Pacak, M.; Swida-Barteczka, A.; Kruszka, K.; Sega, P.; Milanowska, K.; Jakobsen, I.; Jarmolowski, A.; Szweykowska-Kulinska, Z. Heat stress affects pi-related genes expression and inorganic phosphate deposition/accumulation in barley. Front. Plant Sci. 2016, 7, 926. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E. Mycorrhizal fungi can dominate phosphate supply to plants irrespective of growth responses. Plant. Physiol. 2003, 133, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Al-Karaki, G.N. Growth of mycorrhizal tomato and mineral acquisition under salt stress. Mycorrhiza 2000, 10, 51–54. [Google Scholar] [CrossRef]
- Schroeder, M.S.; Janos, D.P. Plant growth, phosphorus nutrition, and root morphological responses to arbuscular mycorrhizas, phosphorus fertilization, and intraspecific density. Mycorrhiza 2005, 15, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Klose, S.; Acosta-Martínez, V.; Ajwa, H.A. Microbial community composition and enzyme activities in a sandy loam soil after fumigation with methyl bromide or alternative biocides. Soil Biol. Biochem. 2006, 38, 1243–1254. [Google Scholar] [CrossRef]
- Pritsch, K.; Garbaye, J. Enzyme secretion by ECM fungi and exploitation of mineral nutrients from soil organic matter. Ann. For. Sci. 2011, 68, 25–32. [Google Scholar] [CrossRef]
- Joner, E.J.; Johansen, A. Phosphatase activity of external hyphae of two arbuscular mycorrhizal fungi. Mycol. Res. 2000, 104, 81–86. [Google Scholar] [CrossRef]
- Brzostek, E.R.; Greco, A.; Drake, J.E.; Finzi, A.C. Root carbon inputs to the rhizosphere stimulate extracellular enzyme activity and increase nitrogen availability in temperate forest soils. Biogeochemistry 2013, 115, 65–76. [Google Scholar] [CrossRef]
- Wang, F.; Lin, X.; Yin, R.; Wu, L. Effects of arbuscular mycorrhizal inoculation on the growth of Elsholtzia splendens and Zea mays and the activities of phosphatase and urease in a multi-metal-contaminated soil under unsterilized conditions. Appl. Soil Ecol. 2006, 31, 110–119. [Google Scholar] [CrossRef]
- Schwacke, R.; Hager, A. Fungal elicitors induce a transient release of active oxygen species from cultured spruce cells that is dependent on Ca(2+) and protein-kinase activity. Planta 1992, 187, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Dosskey, M.G.; Linderman, R.G.; Boersma, L. Carbon-sink stimulation of photosynthesis in Douglas Fir seedlings by some ectomycorrhizas. New Phytol. 1990, 115, 269–274. [Google Scholar] [CrossRef]
- Reid, C.P.P.; Kidd, F.A.; Ekwebelam, S.A. Nitrogen nutrition, photosynthesis and carbon allocation in ectomycorrhizal pine. Plant Soil 1983, 71, 415–431. [Google Scholar] [CrossRef]
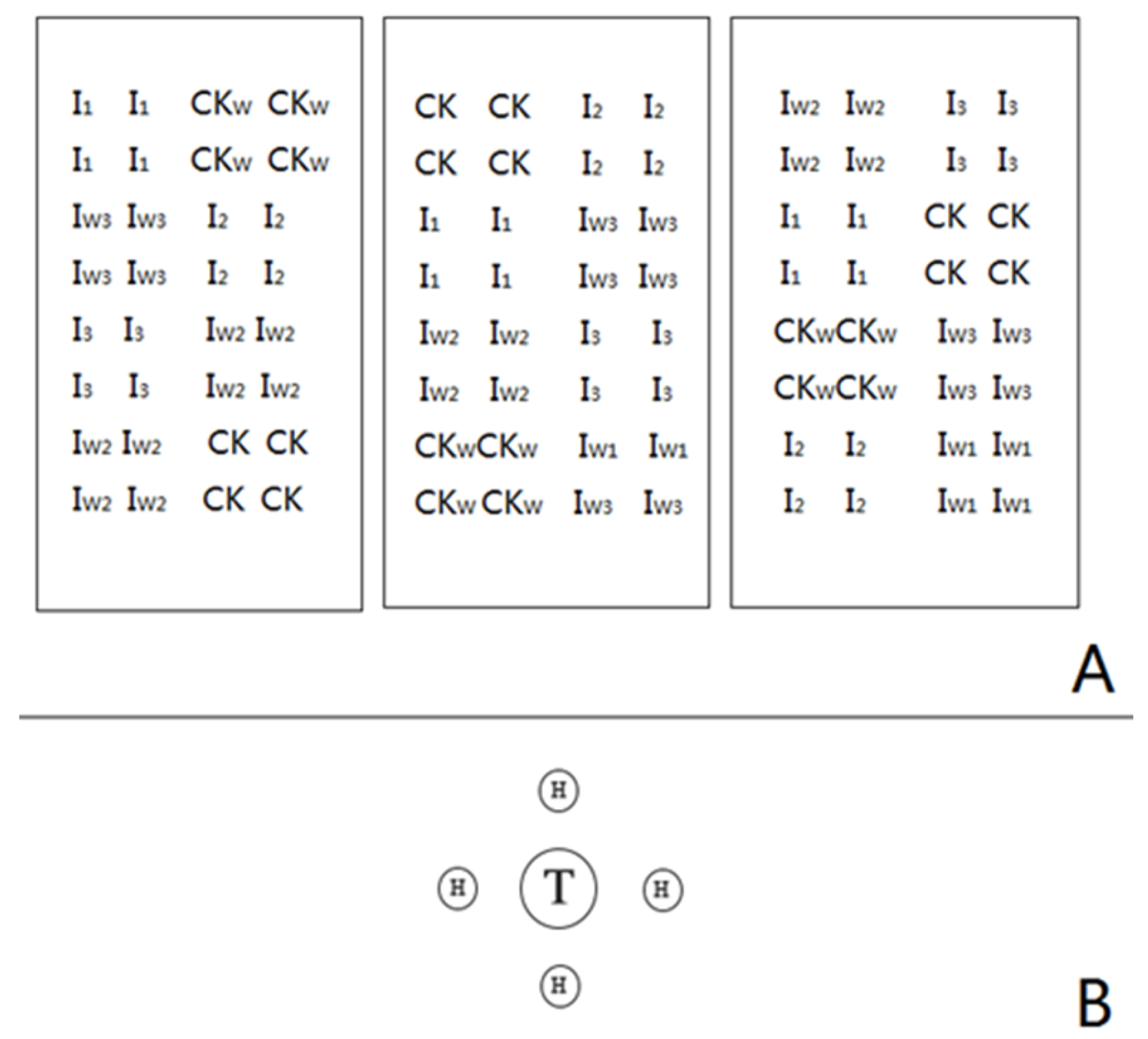
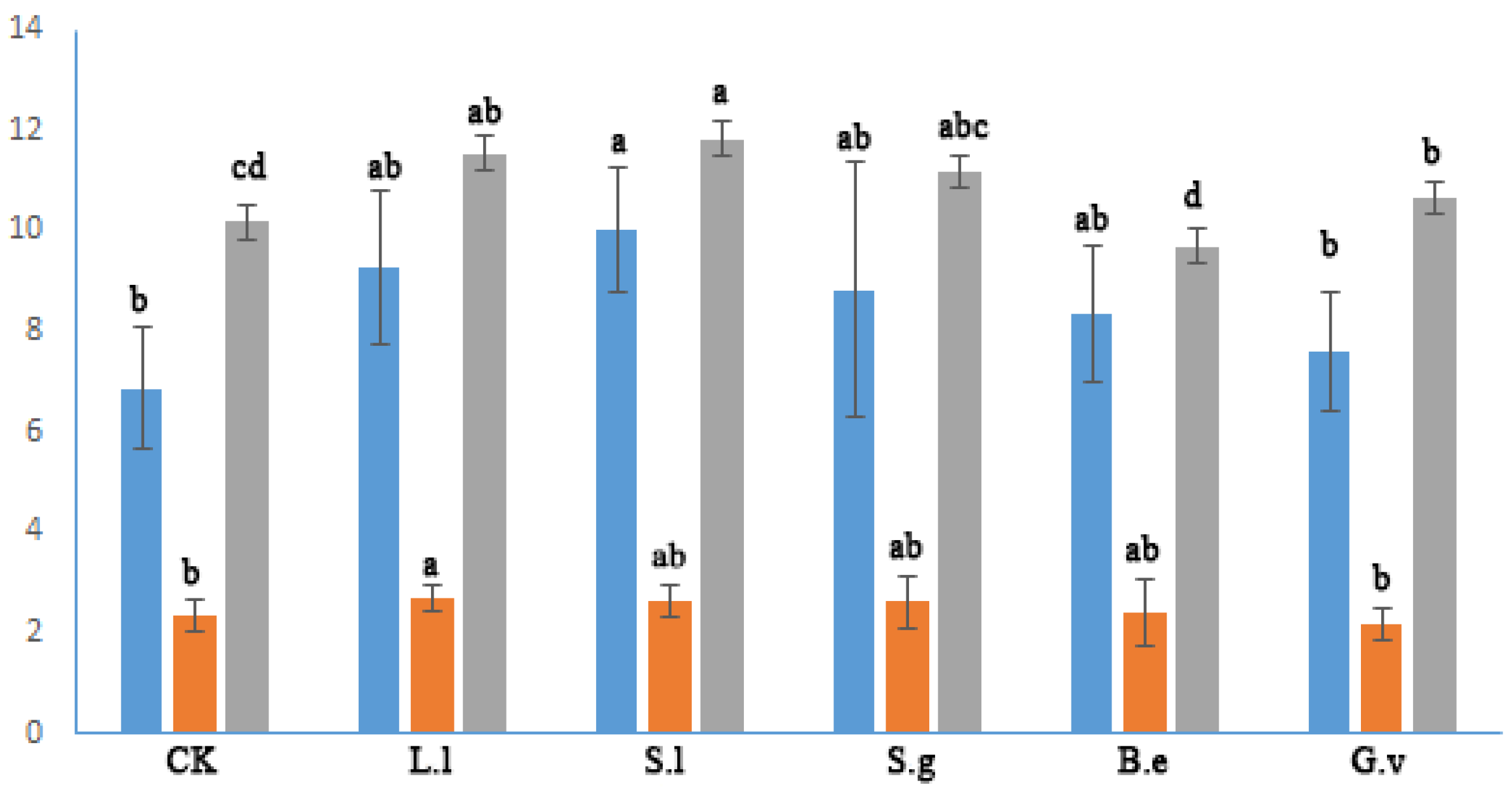


| Treatment | ECM Colonisation Rate (%) | Colonisation Level |
|---|---|---|
| L. laccata | 85.9 ± 4.8 b | 5 |
| S. luteus | 93.2 ± 2.7 a | 5 |
| S. grevillei | 78.7 ± 4.5 c | 5 |
| B. edulis | 66.0 ± 6.3 d | 5 |
| G. viscidus | 71.0 ± 5.2 d | 5 |
| CK | 4.2 ± 0.8 e | 0 |
| Treatments | Biomass (Dry)/g | Aboveground (Dry)/g | Underground (Dry)/g | Root:Shoot Ratio |
|---|---|---|---|---|
| Nonmycorrhizal | 1.3 ± 0.3 b | 0.8 ± 0.1 b | 0.5 ± 0.1 b | 0.6 ± 0.1 ab |
| L. laccata | 1.5 ± 0.1 ab | 0.9 ± 0.1 ab | 0.6 ± 0.1 a | 0.7 ± 0.1 a |
| S. luteus | 1.7 ± 0.2 a | 1.0 ± 0.1 a | 0.6 ± 0.1 a | 0.6 ± 0.1 ab |
| S. grevillei | 1.5 ± 0.2 ab | 0.9 ± 0.1 ab | 0.6 ± 0.3 ab | 0.7 ± 0.1 a |
| B. edulis | 1.3 ± 0.3 bc | 0.8 ± 0.1 ab | 0.4 ± 0.1 b | 0.5 ± 0.1 b |
| G. viscidus | 1.0 ± 0.1 c | 0.6 ± 0.1 c | 0.4 ± 0.1 b | 0.7 ± 0.1 a |
| Treatments | N | P | K | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Leaf | Stem | Root | Leaf | Stem | Root | Leaf | Stem | Root | |
| CK | 16.6 ± 1.7 c | 17.7 ± 3.0 b | 22.6 ± 2.9 b | 6.1 ± 0.1 c | 10.9 ± 1.2 c | 13.7 ± 3.0 d | 10.6 ± 1.9 a | 10.1 ± 2.6 ab | 11.5 ± 3.0 ab |
| L. laccata | 23.0 ± 2.8 abc | 28.2 ± 3.9 a | 35.3 ± 6.5 a | 8.8 ± 0.2 c | 30.1 ± 3.8 a | 35.3 ± 9.4 ab | 9.7 ± 1.3 ab | 13.1 ± 3.2 ab | 16.0 ± 4.7 ab |
| S. luteus | 30.9 ± 1.4 ab | 23.1 ± 5.1 ab | 33.2 ± 2.3 a | 25.2 ± 7.8 a | 27.0 ± 5.1 ab | 21.1 ± 3.8 cd | 13.0 ± 2.2 a | 14.4 ± 3.4 a | 16.7 ± 3.5 a |
| S. grevillei | 25.1 ± 3.3 abc | 28.2 ± 4.7 a | 34.1 ± 3.7 a | 10.5 ± 1.3 bc | 24.8 ± 2.9 ab | 43.5 ± 5.2 a | 11.4 ± 2.0 a | 12.3 ± 3.7 ab | 14.6 ± 2.4 ab |
| B. edulis | 33.1 ± 14.4 a | 27.4 ± 7.0 a | 21.0 ± 1.0 b | 7.3 ± 1.8 c | 28.4 ± 3.9 a | 24.5 ± 2.6 c | 10.3 ± 2.6 a | 11.1 ± 1.8 ab | 11.1 ± 2.6 ab |
| G. viscidus | 20.7 ± 1.1 bc | 14.7 ± 2.8 b | 21.9 ± 1.4 b | 15.5 ± 2.2 b | 20.9 ± 2.7 b | 28.4 ± 2.6 bc | 6.7 ± 0.6 b | 8.1 ± 1.3 b | 10.7 ± 1.4 b |
| Inoculum Dosage | p-Values (Two-Way ANOVA) | ||||||
|---|---|---|---|---|---|---|---|
| 0 mL | 200 mL | 400 mL | 600 mL | Humidity | Dosage | Humidity and Dosage | |
| Pn | |||||||
| Dry Inoculum | 2.14 ± 0.06 c | 3.25 ± 0.10 a | 2.84 ± 0.08 b | 2.90 ± 0.05 b | <0.01 | <0.01 | <0.01 |
| Moist Inoculum | 2.18 ± 0.07 d | 6.23 ± 0.08 a | 3.94 ± 0.07 c | 4.90 ± 0.06 b | |||
| Gs | |||||||
| Dry Inoculum | 0.028 ± 0.002 c | 0.037 ± 0.001 b | 0.041 ± 0.001 b | 0.052 ± 0.002 a | <0.01 | <0.01 | <0.01 |
| Moist Inoculum | 0.027 ± 0.002 d | 0.066 ± 0.004 a | 0.047 ± 0.001 c | 0.052 ± 0.002 b | |||
| Tr | |||||||
| Dry Inoculum | 0.25 ± 0.01 b | 0.25 ± 0.02 b | 0.26 ± 0.02 b | 0.29 ± 0.01 a | <0.01 | <0.01 | <0.01 |
| Moist Inoculum | 0.24 ± 0.01 d | 0.62 ± 0.02 a | 0.34 ± 0.02 c | 0.41 ± 0.03 b | |||
| Ci | |||||||
| Dry Inoculum | 176.33 ± 11.50 c | 264.33 ± 8.14 ab | 253.00 ± 9.17 b | 274.00 ± 11.53 a | <0.01 | <0.01 | <0.01 |
| Moist Inoculum | 178.0 ± 10.44 c | 213.33 ± 22.30 b | 243.67 ± 20.74 a | 215.00 ± 14.00 b | |||
| WUE | |||||||
| Dry Inoculum | 8.58 ± 0.47 c | 12.86 ± 1.13 a | 10.83 ± 0.88 ab | 9.89 ± 0.54 b | <0.01 | <0.01 | ns |
| Moist Inoculum | 9.20 ± 0.39 c | 10.05 ± 0.20 b | 11.72 ± 0.82 a | 11.89 ± 0.76 a | |||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, N.; Yu, M.; Cui, M.; Luo, Z.; Feng, Y.; Cao, S.; Sun, Y.; Li, Y. Effects of Different Ectomycorrhizal Fungal Inoculates on the Growth of Pinus tabulaeformis Seedlings under Greenhouse Conditions. Forests 2016, 7, 316. https://doi.org/10.3390/f7120316
Lu N, Yu M, Cui M, Luo Z, Feng Y, Cao S, Sun Y, Li Y. Effects of Different Ectomycorrhizal Fungal Inoculates on the Growth of Pinus tabulaeformis Seedlings under Greenhouse Conditions. Forests. 2016; 7(12):316. https://doi.org/10.3390/f7120316
Chicago/Turabian StyleLu, Nan, Meng Yu, Ming Cui, Zijing Luo, Yue Feng, Sen Cao, Yuhan Sun, and Yun Li. 2016. "Effects of Different Ectomycorrhizal Fungal Inoculates on the Growth of Pinus tabulaeformis Seedlings under Greenhouse Conditions" Forests 7, no. 12: 316. https://doi.org/10.3390/f7120316
APA StyleLu, N., Yu, M., Cui, M., Luo, Z., Feng, Y., Cao, S., Sun, Y., & Li, Y. (2016). Effects of Different Ectomycorrhizal Fungal Inoculates on the Growth of Pinus tabulaeformis Seedlings under Greenhouse Conditions. Forests, 7(12), 316. https://doi.org/10.3390/f7120316






