Soil CO2 Efflux and Root Productivity in a Switchgrass and Loblolly Pine Intercropping System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description and Experimental Design
2.2. Sampling and Data Collection
2.2.1. In Situ Soil CO2 Efflux
2.2.2. Partitioning of Autotrophic and Heterotrophic Respiration
2.2.3. Root Analysis from Exclusion Cores and Fresh Cores
2.3. Statistical Analysis
3. Results
3.1. Treatment Effects on Soil CO2 Efflux, Soil Temperature and Soil Moisture
3.2. Collective and Individual Treatment Response of Soil CO2 Efflux to Temperature Change
3.3. Soil CO2 Efflux Partitioning
3.4. Root Biomass, Length and Surface Area in Fresh and Exclusion Cores
4. Discussion
4.1. Soil CO2 Efflux
4.2. Partitioning of Soil CO2 Efflux
4.3. Switchgrass Root Productivity and Related RS in SG versus PSG-I
4.4. Soil Moisture and Temperature Influence on Soil CO2 Efflux
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ma, Z.; Wood, C.W.; Bransby, D.I. Carbon dynamics subsequent to establishment of switchgrass. Biomass Bioenergy 2000, 18, 93–104. [Google Scholar] [CrossRef]
- Sanderson, M.A.; Reed, R.L.; McLaughlin, S.B.; Wullschleger, S.D.; Congcr, B.V.; Parrish, D.J.; Wolf, D.D.; Taliaferro, C.; Hopkins, A.A.; Ocumpaugh, W.R.; Hussey, M.A.; Read, J.C.; Tischler, C.R. Switchgrass as a sustainable bioenergy crop. Bioresource Technol. 1996, 56, 83–93. [Google Scholar] [CrossRef]
- Kering, M.K.; Biermacher, J.T.; Butler, T.J.; Mosali, J.; Guretzky, J.A. Biomass yield and nutrient responses of switchgrass to phosphorus application. Bioenergy Res. 2012, 5, 71–78. [Google Scholar] [CrossRef]
- Muir, J.P.; Sanderson, M.A.; Ocumpaugh, W.R.; Jones, R.M.; Reed, R.L. Biomass production of “Alamo” switchgrass in response to nitrogen, phosphorus, and row spacing. Agron. J. 2001, 93, 896–910. [Google Scholar] [CrossRef]
- McLaughlin, S.; Bouton, J.; Bransby, D.; Conger, B.; Ocumpaugh, W.; Parrish, D.; Taliaferro, C.; Vogel, K.; Wullschleger, S. Developing Switchgrass as a Bioenergy Crop; ASHS Press: Alexandria, VA, USA, 1999. [Google Scholar]
- McLaughlin, S.B.; Walsh, M.E. Evaluating environmental consequences of producing herbaceous crops for bioenergy. Biomass Bioenergy 1998, 14, 317–324. [Google Scholar] [CrossRef]
- Fox, T.T.; Jokela, E.J.; Allen, H.L. The development of pine plantation silviculture in the southern United States. J. For. 2007, 105, 337–344. [Google Scholar]
- Munsell, J.F.; Fox, T.R. An analysis of the feasibility for increasing woody biomass production from pine plantations in the southern United States. Biomass Bioenergy 2010, 34, 1631–1642. [Google Scholar] [CrossRef]
- Blazier, M. Perfect pair for biofuel: Switchgrass and trees. In Louisiana Agriculture Magazine; Benedict, L.F., Ed.; Louisiana Agricultural Experiment Station: Baton Rouge, LA, USA, 2009; pp. 22–23. [Google Scholar]
- Albaugh, J.M.; Sucre, E.B.; Leggett, Z.H.; Domec, J.C.; King, J.S. Evaluation of intercropped switchgrass establishment under a range of experimental site preparation treatments in a forested setting on the Lower Coastal Plain of North Carolina, U.S.A. Biomass Energy 2012, 46, 673–682. [Google Scholar] [CrossRef]
- Anderson-Teixeira, K.J.; Davis, S.C.; Masters, M.D.; Delucia, E.H. Changes in soil organic carbon under biofuel crops. Glob. Chang. Bioenergy 2009, 1, 75–96. [Google Scholar] [CrossRef]
- Tufekcioglu, A.; Raich, J.W.; Isenhart, T.M.; Schultz, R.C. Fine root dynamics, coarse root biomass, root distribution, and soil respiration in a multispecies riparian buffer in Central Iowa, USA. Agrofor. Syst. 1999, 44, 163–174. [Google Scholar] [CrossRef]
- Gonzalez-Benecke, C.A.; Martin, T.A.; Cropper, W.P., Jr.; Bracho, R. Forest management effects on in situ and ex situ slash pine forest carbon balance. For. Ecol. Manag. 2010, 260, 795–805. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Andrews, J.A. Soil respiration and the global carbon cycle. Biogeochemistry 2000, 48, 7–20. [Google Scholar] [CrossRef]
- Bond-Lamberty, B.; Bronson, D.; Bladyka, E.; Gower, S.T. A comparison of trenched plot techniques for partitioning soil respiration. Soil Biol. Biochem. 2011, 43, 2108–2114. [Google Scholar] [CrossRef]
- Boone, R.D.; Nadelhoffer, K.J.; Canary, J.D.; Kaye, J.P. Roots exert a strong influence on the temperature sensitivity of soil respiration. Nature 1998, 396, 570–572. [Google Scholar] [CrossRef]
- Raich, J.W.; Schlesinger, W.H. The global carbon dioxide flux in soil respiration and its relationship to vegetation and climate. Tellus 1992, 44, 81–99. [Google Scholar] [CrossRef]
- Bond-Lamberty, B.; Wang, C.; Gower, S.T. A global relationship between the heterotrophic and autotrophic components of soil respiration. Glob. Chang. Biol. 2004, 10, 1756–1766. [Google Scholar] [CrossRef]
- Hanson, P.J.; Edwards, N.T.; Garten, C.T.; Andrews, J.A. Separating root and soil microbial contributions to soil respiration: A review of methods and observations. Biogeochemistry 2000, 48, 115–146. [Google Scholar] [CrossRef]
- Hogberg, P.; Nordgren, A.; Buchmann, N.; Taylor, A.F.S.; Ekbald, A.; Hogberg, M.N.; Nyberg, G.; Lofvenius, M.O.; Read, D.J. Large scale forest girdling shows that current photosynthesis drives soil respiration. Nature 2001, 411, 789–791. [Google Scholar] [CrossRef] [PubMed]
- Heim, B.R.; Seiler, J.R.; Strahm, B.D. Root nonstructural carbohydrates and their relationship with autotrophic respiration of loblolly pine (Pinus taeda L.). Commun. Soil Sci. Plant Anal. 2015, 46, 888–896. [Google Scholar] [CrossRef]
- Vogel, J.G.; Valentine, D.W. Small root exclusion collars provide reasonable estimates of root respiration when measured during the growing season of installation. Can. J. For. Res. 2005, 35, 2112–2117. [Google Scholar] [CrossRef]
- Walsh, M.E. The Cost of Producing Switchgrass as Dedicated Energy Crop; Oak Ridge National Laboratory Working Paper: Washington, DC, USA, 1994; pp. 35–36. [Google Scholar]
- Doran, F.S.; Dangerfield, C.W.; Cubbage, F.W.; Johnson, J.E.; Pease, J.E.; Johnson, L.A.; Hopper, G.M. Tree Crops for Marginal Farmland: Loblolly Pine. Available online: https://pubs.ext.vt.edu/446/446-604/446-604_pdf.pdf (accessed on 15 July 2016).
- Minick, K.J.; Strahm, B.D.; Fox, T.R.; Sucre, E.B.; Leggett, Z.H. Microbial nitrogen cycling response to forest-based bioenergy production. Ecol. Appl. 2015, 25, 2366–2381. [Google Scholar] [CrossRef] [PubMed]
- U.S. Climate Data. Available online: http//www.usclimatedata.com/climate/north-carolina/united-states/3203 (accessed on 15 July 2013).
- Tyree, M.C.; Seiler, J.R.; Aust, M.W.; Sampson, D.A.; Fox, T.R. Long-term effects of site preparation and fertilization on total soil CO2 efflux and heterotrophic respiration in a 33-year-old Pinus taeda L. plantation on the wet flats of the Virginia Lower Coastal Plain. For. Ecol. Manag. 2006, 234, 363–369. [Google Scholar] [CrossRef]
- Warembourg, F.R.; Paul, E.A. The use of 14CO2 canopy techniques for measuring carbon transfer through the plant-soil system. Plant Soil 1973, 38, 331–345. [Google Scholar] [CrossRef]
- Knoepp, J.D.; Coleman, D.C.; Crossley, D.A.; Clark, J.S. Biological indices of soil quality, an ecosystem case study of their use. For. Ecol. Manag. 2000, 138, 357–368. [Google Scholar] [CrossRef]
- Raich, J.W.; Nadelhoffer, K.J. Belowground carbon allocation in forest ecosystems, global trends. Ecology 1989, 70, 1346–1354. [Google Scholar] [CrossRef]
- Schlesinger, W.H. Biogeochemistry, an Analysis of Global Change; Elsevier: San Diego, CA, USA, 1997. [Google Scholar]
- Maier, C.A.; Kress, L.W. Soil CO2 evolution and root respiration in 11 year-old loblolly pine (Pinus taeda) plantations as affected by moisture and nutrient availability. Can. J. For. Res. 2000, 30, 347–359. [Google Scholar] [CrossRef]
- Paul, E.A. Soil Microbiology, Ecology and Biochemistry, 4th ed.; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Gough, C.M.; Seiler, J.R.; Wiseman, P.E.; Maier, C.A. Soil CO2 efflux in loblolly pine (Pinus taeda L.) plantations on the Virginia piedmont and South Carolina coastal plain over a rotation-length chronosequence. Biogeochemistry 2005, 73, 127–147. [Google Scholar] [CrossRef]
- Wiseman, E.P.; Seiler, J.R. Soil CO2 efflux across four age classes of plantation loblolly pine (Pinus taeda L.) on the Virginia Piedmont. For. Ecol. Manag. 2004, 192, 297–311. [Google Scholar] [CrossRef]
- Liski, J.; Perruchoud, D.; Karjalainen, T. Increasing carbon stocks in the forest soils of western Europe. For. Ecol. Manag. 2002, 169, 159–175. [Google Scholar] [CrossRef]
- Valentini, R.; Matteucci, G.; Dolman, A.J.; Schulze, E.D.; Rebmann, C.; Moors, E.J.; Granier, A.; Gross, P.; Jensen, N.O.; Pilegaard, K.; et al. Respiration as the main determinant of carbon balance in European forests. Nature 2000, 404, 861–864. [Google Scholar] [CrossRef] [PubMed]
- Bowden, R.D.; Davidson, E.; Savage, K.; Arabia, C.; Steudler, P. Chronic nitrogen additions reduce total soil respiration and microbial respiration in temperature forest soils at the Harvard forest. For. Ecol. Manag. 2004, 196, 43–56. [Google Scholar] [CrossRef]
- Hogberg, P.; Singh, B.; Lofvenius, M.O.; Nordgren, A. Partitioning of soil respiration into its autotrophic and heterotrophic components by means of tree-girdling in old boreal spruce forest. For. Ecol. Manag. 2009, 257, 1764–1767. [Google Scholar] [CrossRef]
- Díaz-Pinés, E.; Schindlbacher, A.; Pfeffer, M.; Jandl, R.; Zechmeister-Boltenstern, S.; Rubio, A. Root trenching: A useful tool to estimate autotrophic soil respiration? A case study in an Austrian mountain forest. Eur. J. For. Res. 2010, 129, 101–109. [Google Scholar] [CrossRef]
- Scott-Denton, L.E.; Rosenstiel, T.N.; Monson, R.K. Differential controls by climate and substrate over the heterotrophic and rhizospheric components of soil respiration. Glob. Chang. Biol. 2006, 12, 205–216. [Google Scholar] [CrossRef]
- Ekbald, A.; Nordgren, A. Is growth of soil microorganisms in boreal forests limited by carbon or nitrogen availability? Plant Soil 2002, 242, 115–122. [Google Scholar] [CrossRef]
- Albaugh, J.M.; Albaugh, T.J.; Heiderman, R.R.; Leggett, Z.H.; Stape, J.L.; King, K.; O’Neil, K.P.; King, J.S. Evaluating changes in switchgrass physiology, biomass, and light-use efficiency under artificial shade to estimate yields if intercropped with Pinus taeda L. Agrofor. Syst. 2014, 88, 489–503. [Google Scholar] [CrossRef]
- Xu, B.C.; Li, F.M.; Shan, L. Switchgrass and milkvetch intercropping under 2,1 row-replacement in semiarid region, northwest China, aboveground biomass and water use efficiency. Eur. J. Agron. 2008, 28, 485–492. [Google Scholar] [CrossRef]
- Samuelson, L.J.; Mathew, R.; Stokes, T.; Feng, Y.; Aubrey, D.; Coleman, M. Soil and microbial respiration in a loblolly pine plantation in response to seven years of irrigation and fertilization. For. Ecol. Manag. 2009, 258, 2431–2438. [Google Scholar] [CrossRef]
- Raich, J.W.; Potter, C.S. Global patterns of carbon dioxide emissions from soils. Glob. Biogeochem. Cycles 1995, 9, 23–26. [Google Scholar] [CrossRef]
- Lin, G.; Ehleringer, J.R.; Gygiewicz, P.T.; Johnson, M.G.; Tingey, D.T. Elevated CO2 and temperature impacts on different components of soil CO2 efflux in Doughlas-fir terracosms. Glob. Chang. Biol. 1999, 5, 157–168. [Google Scholar] [CrossRef]
- Pang, X.; Bao, W.; Zhu, B.; Cheng, W. Responses of soil respiration and its temperature sensitivity to thinning in a pine plantation. Agric. For. Meteorol. 2013, 171, 57–64. [Google Scholar] [CrossRef]
- Maier, C.A.; Albaugh, T.J.; Allen, H.L.; Dougherty, P.M. Respiratory carbon use and carbon storage in mid-rotation loblolly pine (Pinus taeda L.) plantations, the effect of site resources on the stand carbon balance. Glob. Chang. Biol. 2004, 10, 1335–1350. [Google Scholar] [CrossRef]
- Davidson, E.A.; Belk, E.; Boone, R.D. Soil water content and temperature as independent or confounded factors controlling soil respiration in a temperate mixed hardwood forest. Glob. Chang. Biol. 1998, 4, 217–227. [Google Scholar] [CrossRef]
- Jenkins, M.E.; Adams, M.A. Respiratory quotients and Q10 of soil respiration in sub-alpine Australia reflect influences of vegetation types. Soil Biol. Biochem. 2011, 43, 1266–1274. [Google Scholar] [CrossRef]
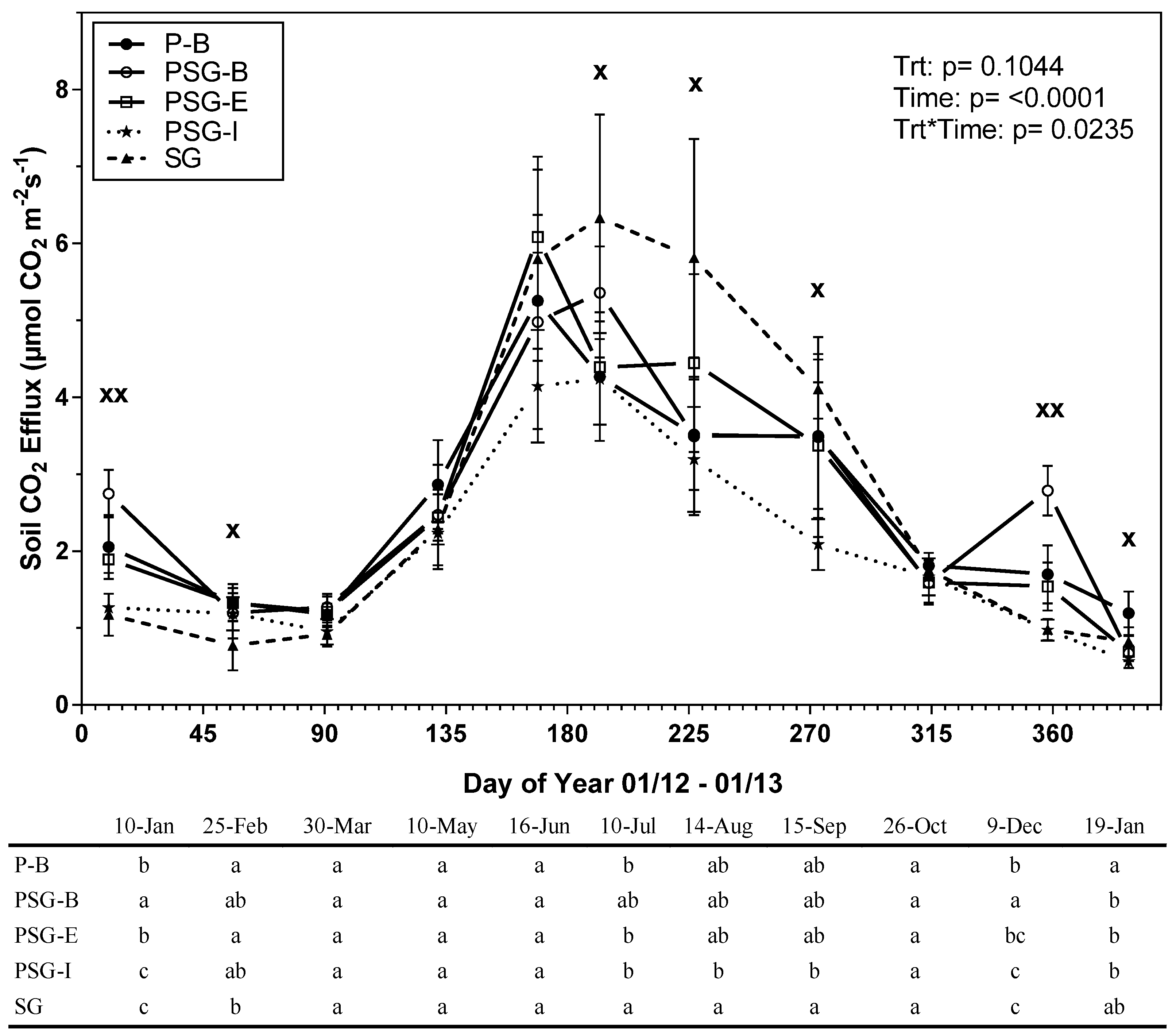
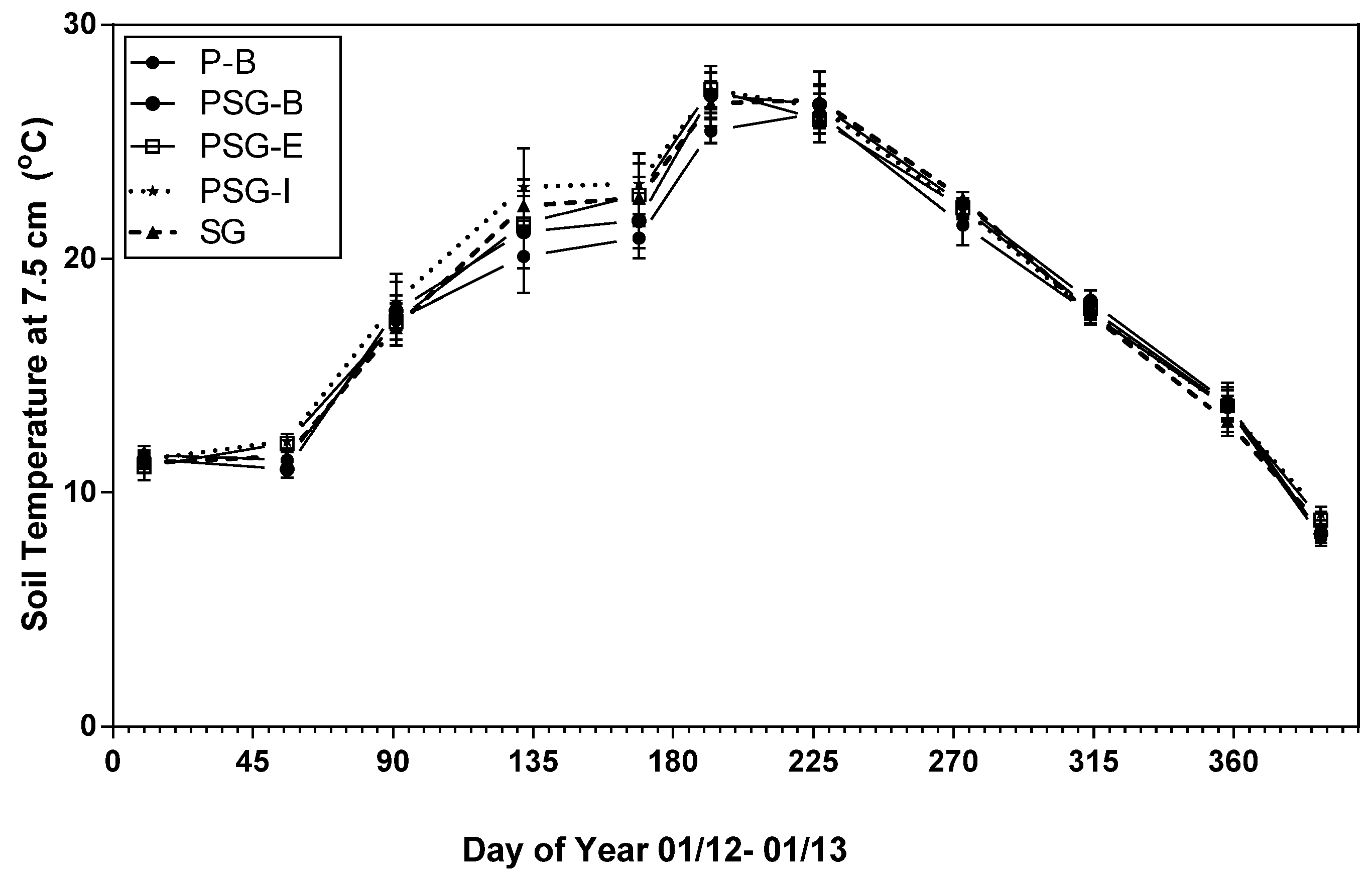
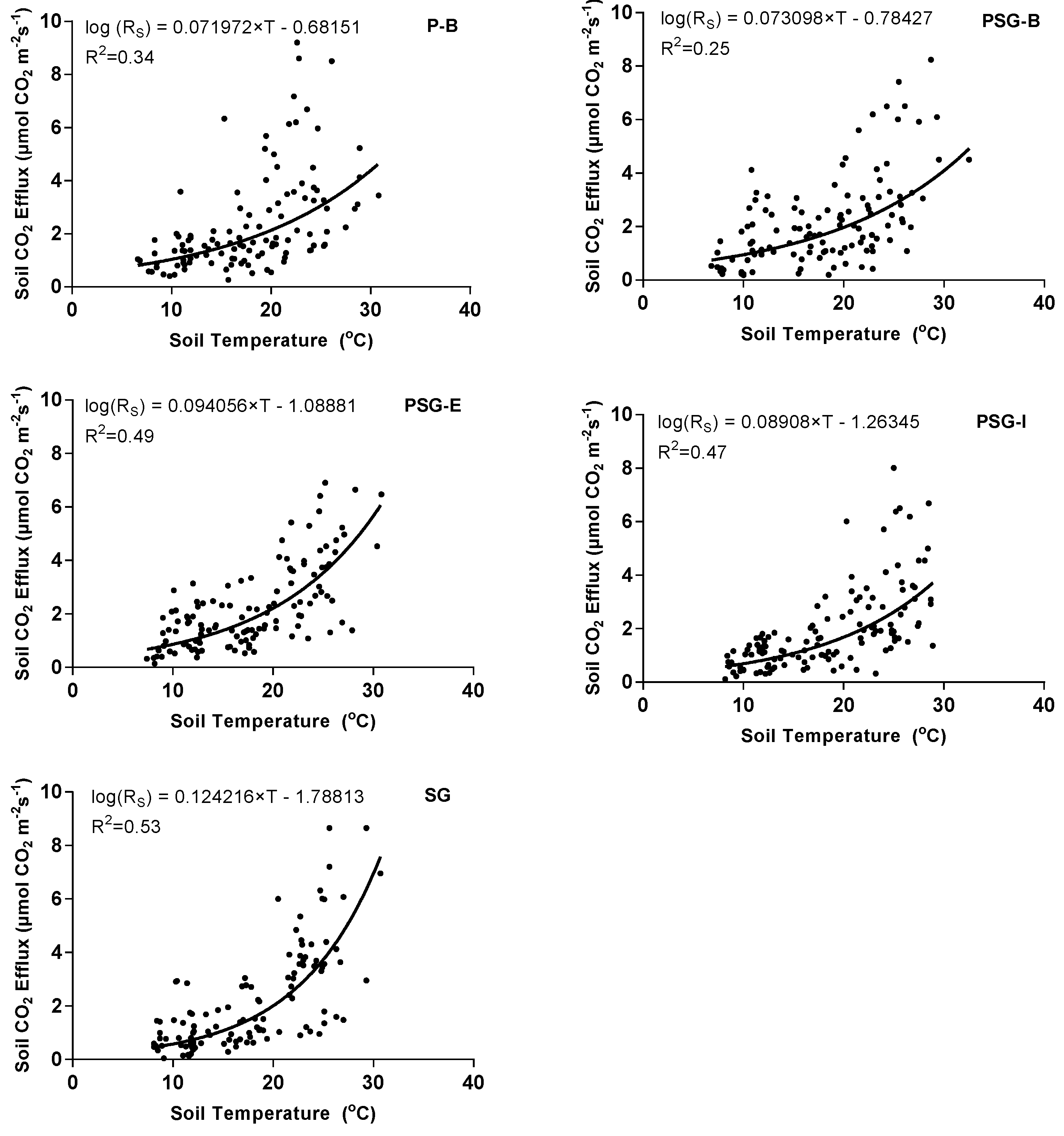
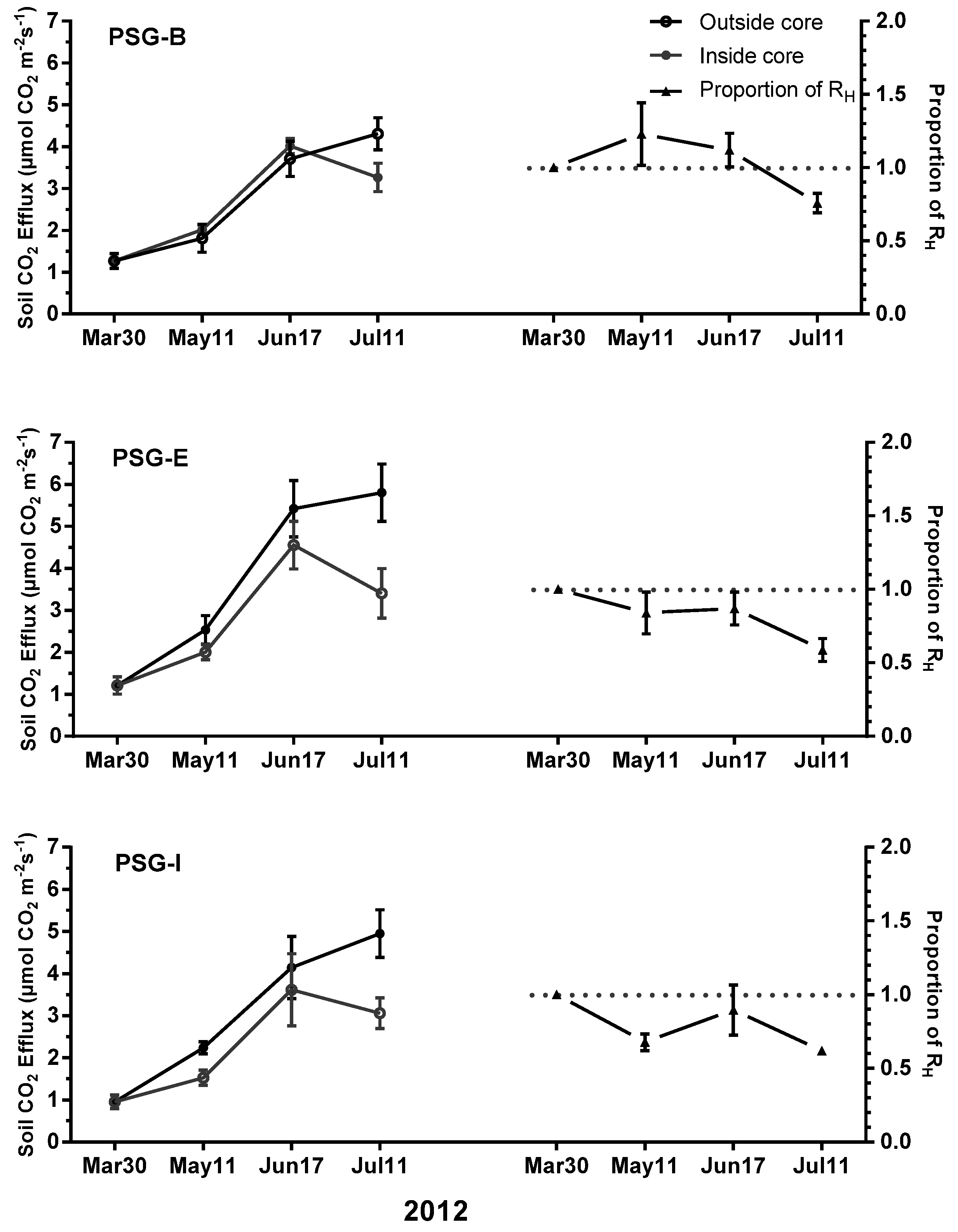
| Treatment 1 | P-B | PSG-B | PSG-E | PSG-I | SG |
|---|---|---|---|---|---|
| Differences between slopes | |||||
| P-B | - | 0.9368 | 0.1228 | 0.2251 | 0.0002 |
| PSG-B | - | 0.1322 | 0.2436 | 0.0002 | |
| PSG-E | - | 0.7181 | 0.0305 | ||
| PSG-I | - | 0.0106 | |||
| SG | - | ||||
| Differences between intercepts | |||||
| P-B | - | 0.6972 | 0.1278 | 0.0295 | 0.00004 |
| PSG-B | - | 0.2460 | 0.0678 | 0.0001 | |
| PSG-E | - | 0.5102 | 0.0085 | ||
| PSG-I | - | 0.0477 | |||
| SG | - | ||||
| Measurement Date | 1 PSG-B | PSG-E | PSG-I | |||
|---|---|---|---|---|---|---|
| Inside Core | Outside Core | Inside Core | Outside Core | Inside Core | Outside Core | |
| May11 | 22.3a (3.2) | 14.8b (2.0) | 38.9a (3.7) | 26.3b (1.0) | 35.9a (2.4) | 26.8b (1.6) |
| Jul11 | 20.8a (3.6) | 9.9b (1.2) | 35.1a (4.7) | 17.6b (3.6) | 29.8a (1.9) | 25.6b (0.3) |
| Root Variable | 1 Treatment | Pine | Switchgrass | ||||
|---|---|---|---|---|---|---|---|
| 0–15 cm | 15–35 cm | Total | 0–15 cm | 15–35 cm | Total | ||
| Biomass (g·m−3) | P-B | 1080a (217.7) | 2182a (519.6) | 3262a (501.1) | NA | NA | NA |
| PSG-B | 996.8a (290.2) | 2838a (644.4) | 3835a (628.7) | 116.9c (22.37) | 65.94b (30.59) | 182.8b (51.36) | |
| PSG-E | 1161a (292.0) | 505.2b (176.3) | 1666b (197.0) | 736.7b (195.1) | 510.0ab (302.6) | 1247b (424.4) | |
| PSG-I | 241.6ab (61.59) | 84.39b (25.70) | 325.9c (56.28) | 1308ab (405.6) | 173.1b (17.37) | 1481b (418.7) | |
| SG | 138.5b (95.20) | 272.5b (89.09) | 411.0c (178.2) | 3620a (1358) | 1739a (524.6) | 5359a (1842) | |
| Length (cm·dm−3) | P-B | 1297a (252.7) | 1038a (303.0) | 2335a (532.0) | NA | NA | NA |
| PSG-B | 630.7a (105.5) | 414.0ab (78.23) | 1045b (170.7) | 864.59c (238.5) | 285.0a (115.6) | 1150c (330.1) | |
| PSG-E | 721.2a (93.42) | 122.9bc (41.29) | 844.1bc (60.38) | 2568b (533.4) | 373.0a (132.4) | 2941bc (565.7) | |
| PSG-I | 258.6ab (57.44) | 53.41c (9.668) | 312.0cd (56.65) | 4029ab (990.7) | 473.3a (190.3) | 4503ab (1163) | |
| SG | 80.07b (67.52) | 78.32bc (13.33) | 158.4d (76.94) | 6581a (905.58) | 1084a (300.3) | 7665a (640.9) | |
| Surface Area (cm2·dm−3) | P-B | 16.76a (2.619) | 19.10a (4.884) | 35.76a (7.287) | NA | NA | NA |
| PSG-B | 10.25ab (1.938) | 9.252ab (1.616) | 19.50ab (3.457) | 7.662c (2.048) | 2.808b (0.9621) | 10.47c (2.787) | |
| PSG-E | 13.32ab (2.392) | 2.708bc (0.9730) | 16.03bc (1.712) | 26.35bc (5.689) | 5.150b (1.467) | 31.50bc (6.147) | |
| PSG-I | 5.035bc (1.295) | 1.153c (0.1758) | 6.188cd (1.239) | 36.67b (8.667) | 5.352b (1.252) | 42.02b (9.801) | |
| SG | 1.236c (0.989) | 1.664bc (0.3909) | 2.900d (1.270) | 71.53a ( 4.495) | 18.89a (6.992) | 90.41a (4.807) | |
| Root Variable | 1 Treatment | Pine | Switchgrass | ||||
|---|---|---|---|---|---|---|---|
| 0–15 cm | 15–35 cm | Total | 0–15 cm | 15–35 cm | Total | ||
| Biomass (g·m−3) | PSG-B | 439.48ab (140.9) | 971.71a (390.4) | 1411.19a (460) | na | na | na |
| PSG-E | 597.86a (56.2) | 512.94a (195.3) | 1110.80ab (215) | 393.43a (124.2) | 94.26a (36.9) | 487.69a (112.4) | |
| PSG-I | 182.07b (49.3) | 46.16a (16.3) | 228.23b (41) | 549.33a (91.5) | 146.96a (34.3) | 696.29a (112) | |
| Length (cm·dm−3) | PSG-B | 36.26ab (9.65) | 43.90a (7.66) | 80.17a (16.82) | na | na | na |
| PSG-E | 55.21a (9.42) | 18.43b (4.16) | 73.64a (12.5) | 98.20a (22.25) | 32.9a (6.66) | 131.14a (25.75) | |
| PSG-I | 9.86b (1.32) | 5.55b (0.69) | 15.4b (1.6) | 103.33a (33.42) | 21.50a (1.10) | 124.82a (33.78) | |
| Surface Area (cm2·dm−3) | PSG-B | 0.66ab (0.14) | 1.26a (0.33) | 1.92a (0.46) | na | na | na |
| PSG-E | 1.03a (0.17) | 0.34b (0.08) | 1.36ab (0.24) | 1.09a (0.22) | 0.4a (0.09) | 1.5a (0.28) | |
| PSG-I | 0.19b (0.01) | 0.13b (0.02) | 0.3b (0.02) | 1.25a (0.39) | 0.39a (0.07) | 1.64a (0.45) | |
| RS | RA | |
|---|---|---|
| Pine biomass | 0.1395 (0.4242) | −0.2408 (0.0416**) |
| Grass biomass | 0.0591 (0.7359) | 0.3707 (0.0013**) |
| Total root biomass | 0.2938 (0.0867*) | −0.0521 (0.6640) |
| Fine root surface area | 0.1824 (0.2944) | 0.2645 (0.0248xx) |
| Medium root surface area | 0.0381 (0.8280) | 0.1991 (0.0936x) |
| Fine root length | 0.2515 (0.1449) | 0.0388 (0.7463) |
| Medium root length | 0.0588 (0.7371) | −0.0913 (0.4457) |
| Coarse woody debris | 0.0958 (0.5841) | −0.0985 (0.4104) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shrestha, P.; Seiler, J.R.; Strahm, B.D.; Sucre, E.B.; Leggett, Z.H. Soil CO2 Efflux and Root Productivity in a Switchgrass and Loblolly Pine Intercropping System. Forests 2016, 7, 221. https://doi.org/10.3390/f7100221
Shrestha P, Seiler JR, Strahm BD, Sucre EB, Leggett ZH. Soil CO2 Efflux and Root Productivity in a Switchgrass and Loblolly Pine Intercropping System. Forests. 2016; 7(10):221. https://doi.org/10.3390/f7100221
Chicago/Turabian StyleShrestha, Paliza, John R. Seiler, Brian D. Strahm, Eric B. Sucre, and Zakiya H. Leggett. 2016. "Soil CO2 Efflux and Root Productivity in a Switchgrass and Loblolly Pine Intercropping System" Forests 7, no. 10: 221. https://doi.org/10.3390/f7100221
APA StyleShrestha, P., Seiler, J. R., Strahm, B. D., Sucre, E. B., & Leggett, Z. H. (2016). Soil CO2 Efflux and Root Productivity in a Switchgrass and Loblolly Pine Intercropping System. Forests, 7(10), 221. https://doi.org/10.3390/f7100221







