Forest Management Influences Aboveground Carbon and Tree Species Diversity in Myanmar’s Mixed Deciduous Forests
Abstract
:1. Introduction
2. Materials and Methods
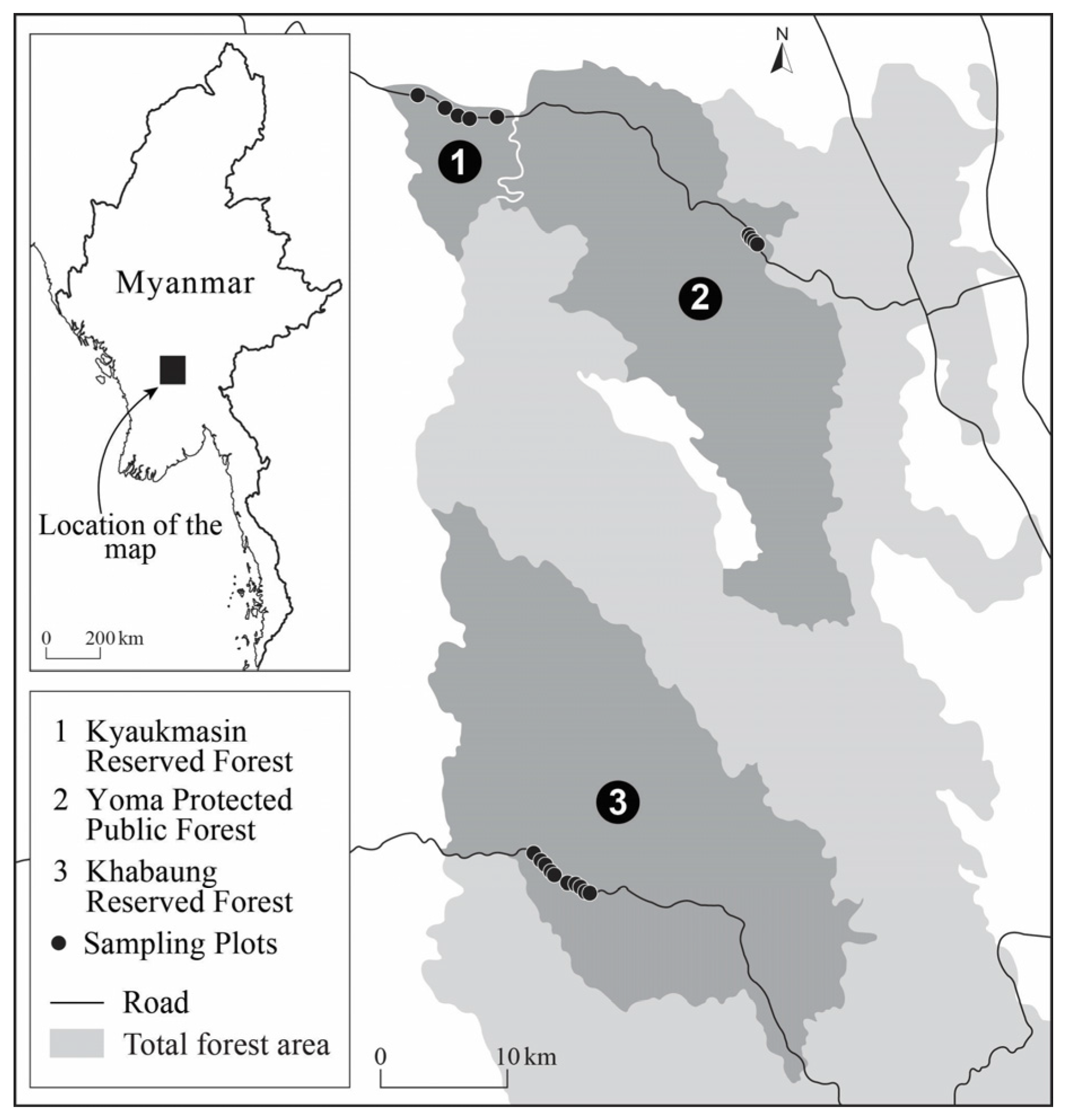
2.1. Study Area
2.2. Plot Establishment
2.3. Tree Biomass and Carbon Content
2.4. Tree Species Composition and Diversity
3. Results
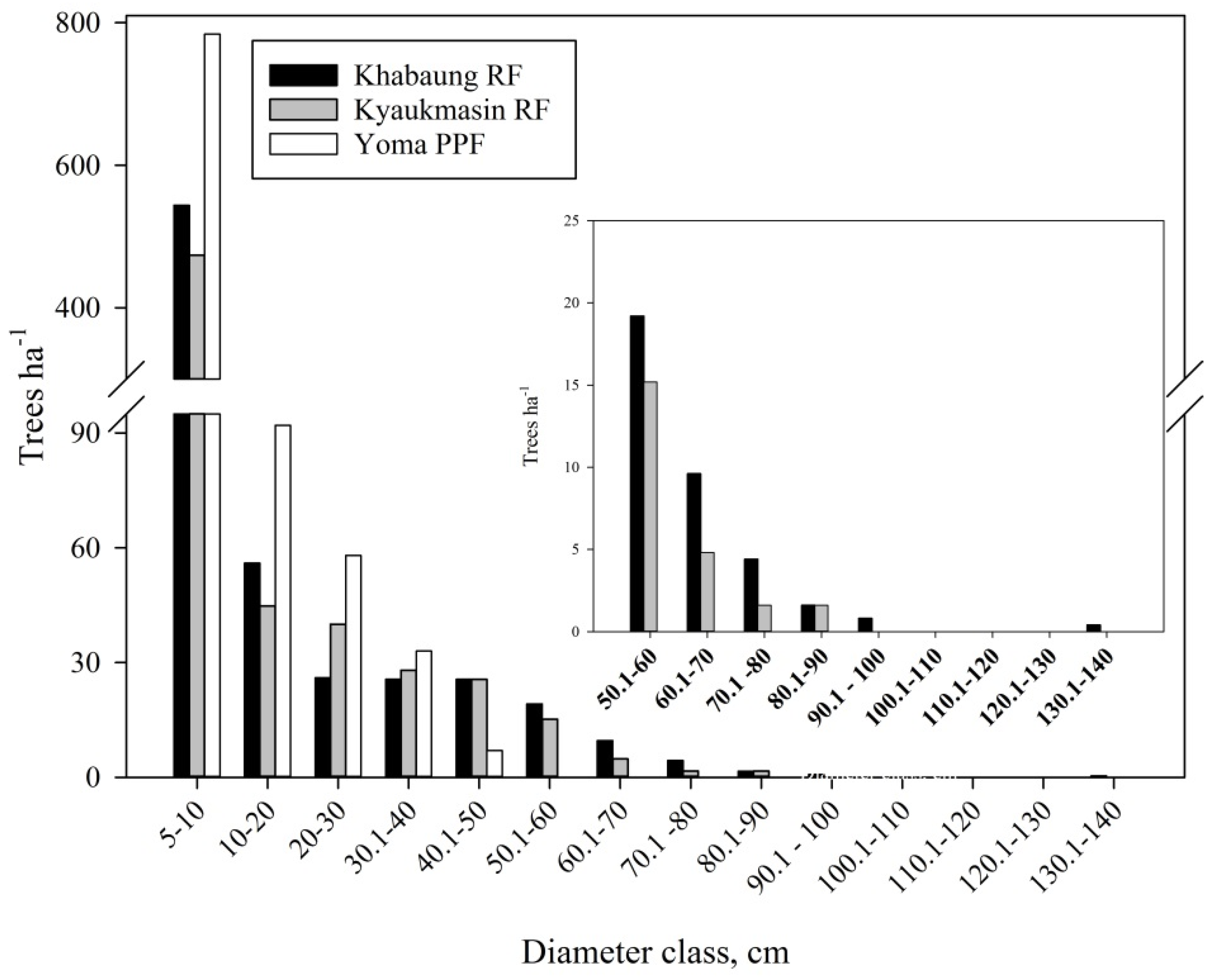
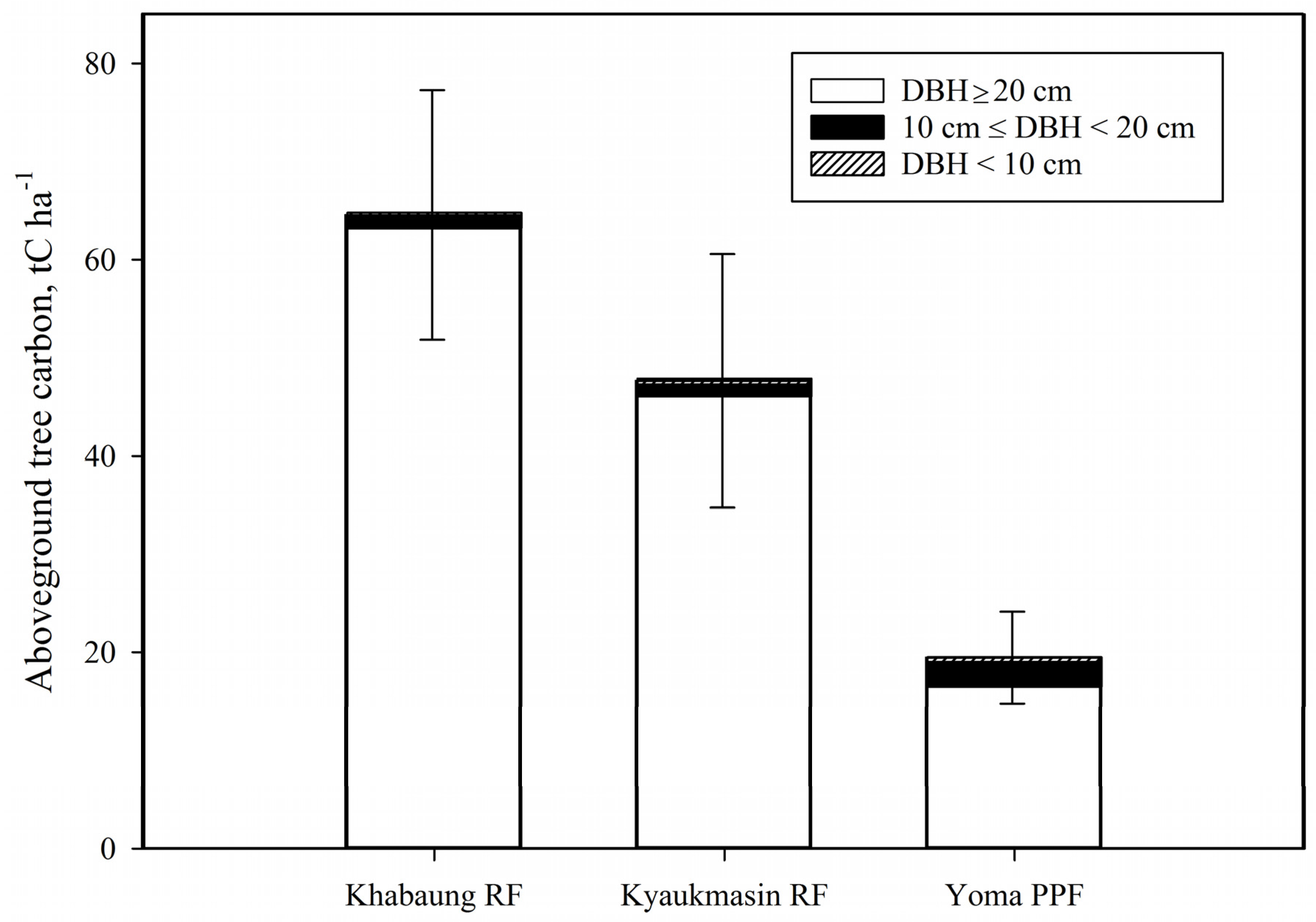
3.1. Forest Structure and Carbon Storage
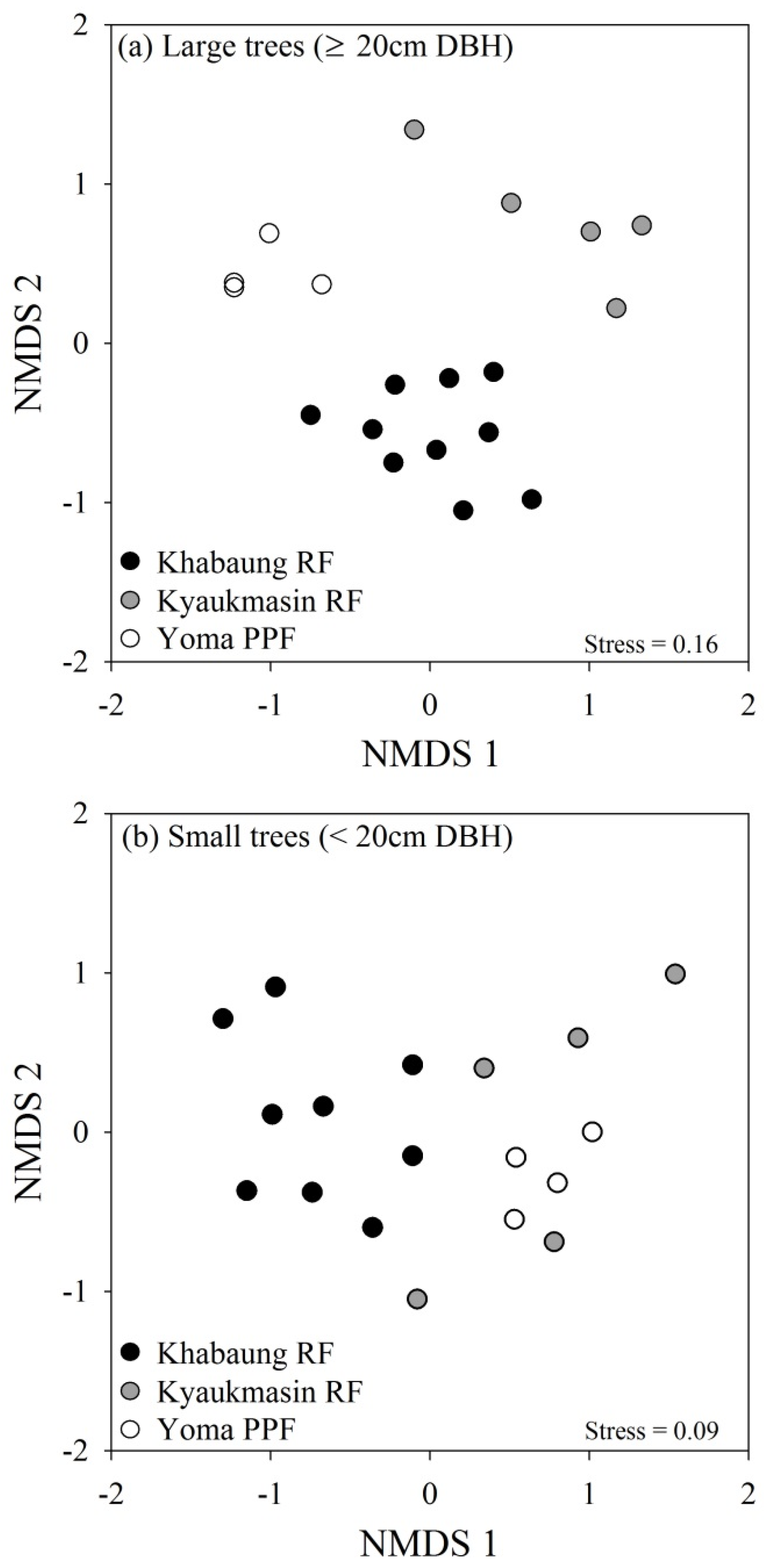
3.2. Tree Species Richness, Diversity, and Composition
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Goklany, I.M. Climate Change: The 21st Century’s Most Urgent Environmental Problem or Proverbial Last Straw? In Adapt or Die: the Science, Politics and Economics of Climate Change; Okonski, K., Ed.; Profile Books: London, UK, 2003; pp. 56–74. [Google Scholar]
- Pachauri, R.K.; Allen, M.R.; Barros, V.R.; Broome, J.; Cramer, W.; Christ, R.; Church, J.A.; Clarke, L.; Dahe, Q.; Dasgupta, P.; et al. Synthesis Report. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel. In Climate Change; IPCC.: Geneva, Switzerland, 2007; p. 104. [Google Scholar]
- Van der Werf, G.R.; Morton, D.C.; DeFries, R.S.; Olivier, J.G.J.; Kasibhatla, P.S.; Jackson, R.B.; Collatz, G.J.; Randerson, J.T. CO2 emissions from forest loss. Nature Geosci. 2009, 2, 737–738. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Franklin, J.F.; Fischer, J. General management principles and a checklist of strategies to guide forest biodiversity conservation. Biol. Conserv. 2006, 131, 433–445. [Google Scholar] [CrossRef]
- Sala, O.E.; Chapin, F.S.; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A.; et al. Biodiversity—Global biodiversity scenarios for the year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef] [PubMed]
- Van Vuuren, D.P.; Sala, O.E.; Pereira, H.M. The future of vascular plant diversity under four global scenarios. Ecol. Soc. 2006, 11, 19. [Google Scholar]
- Pereira, H.M.; Leadley, P.W.; Proenca, V.; Alkemade, R.; Scharlemann, J.P.W.; Fernandez-Manjarres, J.F.; Araujo, M.B.; Balvanera, P.; Biggs, R.; Cheung, W.W.L.; et al. Scenarios for Global Biodiversity in the 21st Century. Science 2010, 330, 1496–1501. [Google Scholar] [CrossRef] [PubMed]
- Sloan, S.; Sayer, J.A. Forest resources assessment of 2015 shows positive global trends but forest loss and degradation persist in poor tropical countries. For. Ecol. Manag. 2015, 352, 134–145. [Google Scholar] [CrossRef]
- Laurance, W.F.; Sayer, J.; Cassman, K.G. Agricultural expansion and its impacts on tropical nature. Trends Ecol. Evol. 2014, 29, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Houghton, R. Aboveground forest biomass and the global carbon balance. Glob. Chan. Biol. 2005, 11, 945–958. [Google Scholar] [CrossRef]
- Gibson, L.; Lee, T.M.; Koh, L.P.; Brook, B.W.; Gardner, T.A.; Barlow, J.; Peres, C.A.; Bradshaw, C.J.A.; Laurance, W.F.; Lovejoy, T.E.; et al. Primary forests are irreplaceable for sustaining tropical biodiversity. Nature 2011, 478, 378. [Google Scholar] [CrossRef] [PubMed]
- State of World’s Forests; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2011; pp. 113–123.
- FAO. Global Forest Resources Assessment 2015: Desk Reference; Food and Agricultural Organization of the United Nations: Rome, Italy, 2015; p. 253. [Google Scholar]
- Lynam, A.J. A National Tiger Action Plan for the Union of Myanmar; Myanmar Forest Department, Ministry of Forestry: Nay Pyi Taw, Myanmar, 2003.
- Aung, M.M. State of forests and forest genetic resources in Myanmar. In Proceedings of the Southeast Asian Moving Workshop on Conservation, Management and Utilization of Forest Genetic Resources, Bangkok, Thailand, 25 Febuary–10 March 2001; Koskela, J., Appanah, S., Pedersen, A.P., Markopoulos, M.D., Eds.; FORSPA: Bangkok, Tailand, 2001; Volume 31. [Google Scholar]
- Mon, M.S.; Mizoue, N.; Htun, N.Z.; Kajisa, T.; Yoshida, S. Factors affecting deforestation and forest degradation in selectively logged production forest: A case study in Myanmar. For. Ecol. Manag. 2012, 267, 190–198. [Google Scholar] [CrossRef]
- Bryant, R.L. The Political Ecology of Forestry in Burma: 1824–1994; University of Hawaii Press: Hawaii, HI, USA, 1997. [Google Scholar]
- Flint, E.P.; Richards, J.F. Historical-analysis of changes in land-use and carbon stock of vegetation in South and Southeast-Asia. Can. J. For. Res. 1991, 21, 91–110. [Google Scholar] [CrossRef]
- Leimgruber, P.; Kelly, D.S.; Steininger, M.K.; Brunner, J.; Müller, T.; Songer, M. Forest cover change patterns in Myanmar (Burma) 1990–2000. Environ. Conserv. 2005, 32, 356–364. [Google Scholar] [CrossRef]
- Blanford, H.R. Highlights of one hundred years of forestry in Burma. Emp. For. Rev. 1958, 37, 33–42. [Google Scholar]
- Joppa, L.N.; Pfaff, A. Global protected area impacts. Proc. R. Soc. Lond. B: Biol. Sci. 2011, 278, 1633–1638. [Google Scholar] [CrossRef] [PubMed]
- Robinson, B.E.; Holland, M.B.; Naughton-Treves, L. Does secure land tenure save forests? A meta-analysis of the relationship between land tenure and tropical deforestation. Glob. Environ. Chan. 2014, 29, 281–293. [Google Scholar] [CrossRef]
- UN-REDD. 2015 Annual Report of the UN-REDD Programme Fund. Executive Summary. Available online: http://www.unredd.net/index.php?view=list&slug=2015-annual-report&option=com_docman&Itemid=134 (accessed on 20 July 2016).
- Taungoo District Forestry Working Plan (2006–2016); Forest Department, Ministry of Environmental Conservation and Forestry: Taungoo, Myanmar, 2013.
- Kress, W.; DeFilipps, R.; Farr, E.; Daw, Y.Y.K. A Checklist of the trees, shrubs, herbs and climbers of Myanmar (Revised from the original works by JH Lace and HG Hundley). Contrib. USA Herb. 2003, 45, 1–590. [Google Scholar]
- Siregar, C.A. Develop Forest Carbon Standard and Carbon Accounting System for Small-scale Plantation Based on Local Experiences. RED-PD 2011, 7, 09. [Google Scholar]
- IPCC. Good Practice Guidance for Land Use, Land-Use Change and Forestry; Penman, J., Gytarsky, M., Hiraishi, T., Krug, T., Kruger, D., Pipatti, R., Buendia, L., Miwa, K., Ngara, T., Tanabe, K., et al., Eds.; Institute for Global Environmental Strategies (IGES) for the Intergovernmental Panel on Climate Change (IPCC): Kanagawa, Japan, 2003; pp. 3.11–13.22. [Google Scholar]
- Clarke, K.R.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, 2nd ed.; PRIMER-E: Plymouth, UK, 2001. [Google Scholar]
- Jost, L. Entropy and diversity. Oikos 2006, 113, 363–375. [Google Scholar] [CrossRef]
- Petsri, S.; Pumijumnong, N.; Wachrinrat, C.; Thoranisorn, S. Aboveground carbon content in mixed deciduous forest and teak plantations. Environ. Nat. Resour. J. 2007, 5, 1–10. [Google Scholar]
- Salunkhe, O.; Khare, P.K.; Sahu, T.R.; Singh, S. Estimation of tree biomass reserves in tropical deciduous forests of Central India by non-destructive approach. Trop. Ecol. 2016, 57, 153–161. [Google Scholar]
- FAO. Best Practices for Improving Law Compliance in the Forest Sector; Food and Agricultural Organization: Rome, Italy, 2005. [Google Scholar]
- Whitworth, A.; Downie, R.; von May, R.; Villacampa, J.; MacLeod, R. How much potential biodiversity and conservation value can a regenerating rainforest provide? A ‘best-case scenario’ approach from the Peruvian Amazon. Trop. Conserv. Sci. 2016, 9, 224–245. [Google Scholar]
- Oo, T.N.; Lee, D.K. Species composition and stand structure of natural forest, timber-harvested forest and degraded forest in the Bago Yoma region of Myanmar. J. Korean For. Soc. 2007, 96, 572–579. [Google Scholar]
- Onyekwelu, J.C.; Mosandl, R.; Stimm, B. Tree species diversity and soil status of primary and degraded tropical rainforest ecosystems in south-western Nigeria. J. Trop. For. Sci. 2008, 20, 193–204. [Google Scholar]
- Fibich, P.; Leps, J.; Novotny, V.; Klimes, P.; Tesitel, J.; Molem, K.; Damas, K.; Weiblen, G.D. Spatial patterns of tree species distribution in New Guinea primary and secondary lowland rain forest. J. Veg. Sci. 2016, 27, 328–339. [Google Scholar] [CrossRef]
- Rubin, B.D.; Manion, P.D.; Faber-Langendoen, D. Diameter distributions and structural sustainability in forests. For. Ecol. Manag. 2006, 222, 427–438. [Google Scholar] [CrossRef]
- Meyer, H.A.; Stevenson, D.D. The structure and growth of virgin beech-birch-maple-hemlock forests in northern Pennsylvania. J. Agric. Res. 1943, 67, 465–484. [Google Scholar]
- Kadambi, K. Silviculture and Management of Teak; Bulletin 24 School of Forestry Stephen F; Austin State University Nacogdoches: Nacogdoches, TX, USA, 1972. [Google Scholar]
- Krishnapillai, B. Silviculture and management of teak plantations. FAO Unasylva, No. 201, Teak Volume 51, 2000/2. Available online: http://www.fao.org/DOCREP/X4565E/x4565e04.htm#P0_0.2000 (accessed on 10 June 2016).

| Study Site | *Dominant Species (% of Basal Area/ha) | No. of Species | No. of Genera | No. of Families | No. Stems (ha−1) | Basal Area (m2 ha−1) | Av Height (m) | Av DBH (cm) |
|---|---|---|---|---|---|---|---|---|
| Khabaung Reserved Forest | ||||||||
| Plot 1 | T(13), TT(17), YD(17), P(13) | 10 | 9 | 8 | 96 | 37.7 | 21.8 | 39.3 |
| Plot 2 | T(36), PK(9), P(9), TK(9) | 13 | 13 | 8 | 132 | 61.0 | 26.3 | 46.2 |
| Plot 3 | T(22), TK(28), K(9), TW(6) | 14 | 13 | 7 | 128 | 55.9 | 22.3 | 43.7 |
| Plot 4 | T(33), Y(9), TW (6), A(6) | 15 | 14 | 9 | 132 | 59.7 | 23.7 | 45.2 |
| Plot 5 | T(54), P(17), YD(8), TK(8) | 7 | 6 | 6 | 96 | 38.9 | 19.0 | 40.5 |
| Plot 6 | T(8), P(14), B(14), TP(8) | 16 | 14 | 10 | 144 | 60.6 | 21.8 | 42.1 |
| Plot 7 | T (55), M (15), YZ (10), B (10) | 6 | 5 | 4 | 80 | 33.6 | 24.1 | 42.0 |
| Plot 8 | T(33), D(13), H(8), N(4) | 14 | 13 | 10 | 96 | 44.4 | 28.1 | 46.3 |
| Plot 9 | T(31), TK(22), Y(6), TW(6) | 14 | 14 | 11 | 128 | 53.2 | 20.0 | 41.6 |
| Plot 10 | T(28), TD(12), P(8), N(8) | 12 | 10 | 9 | 100 | 45.8 | 24.0 | 45.8 |
| Average ± 95% CI | 12 | 11 | 8 | 113 ± 16 | 49.1 ± 7.4 | 23.1 ± 1.9 | 43.3 ± 1.8 | |
| Kyaukmasin Reserved Forest | ||||||||
| Plot 1 | T(13), PK(29), N(13), S(8) | 12 | 12 | 10 | 96 | 36.4 | 12.6 | 38.0 |
| Plot 2 | T(12), TK(23), G(19), PT(8) | 12 | 12 | 12 | 104 | 36.6 | 14.6 | 35.2 |
| Plot 3 | T(33), G(21), N(6), K(6) | 14 | 14 | 11 | 132 | 52.0 | 23.8 | 39.4 |
| Plot 4 | T(22), TY(19), G(8), TT(8) | 17 | 15 | 12 | 148 | 56.2 | 18.5 | 38.0 |
| Plot 5 | T(19), TY(19), A(8), DN(8) | 14 | 13 | 14 | 104 | 42.0 | 19.2 | 40.3 |
| Average ± 95% CI | 14 | 13 | 12 | 117 ± 28 | 44.7 ± 11 | 17.7 ± 5.4 | 38.2 ± 2.4 | |
| Yoma Protected Public Forest | ||||||||
| Plot 1 | T(93), PD(7) | 2 | 2 | 2 | 112 | 31.5 | 17.6 | 28.0 |
| Plot 2 | T(78), PD(22) | 2 | 2 | 2 | 92 | 24.3 | 18.8 | 26.3 |
| Plot 3 | T(94), N(6) | 2 | 2 | 2 | 72 | 22.6 | 17.0 | 31.3 |
| Plot 4 | T(52), PK(38), PD(10) | 3 | 3 | 3 | 116 | 35.7 | 18.0 | 30.8 |
| Average ± 95% CI | 2 | 2 | 2 | 98 ± 32 | 28.5 ± 9.8 | 17.8 ± 1.2 | 29.1 ± 3.8 | |
| Biodiversity Metric | Site | ||
|---|---|---|---|
| Khabaung | Kyaukmasin | Yoma | |
| Species richness (large trees) | 12.0 ± 2.4 | 13.4 ± 3.2 | 2.3 ± 0.8 |
| Species richness (small trees) | 4.9 ± 2.2 | 5.0 ± 5.2 | 7.8 ± 5.3 |
| Diversity (large trees) | 8.6 ± 2.0 | 9.9 ± 2.2 | 1.7 ± 1.0 |
| Diversity (small trees) | 4.1 ± 1.8 | 4.7 ± 4.7 | 5.8 ± 5.1 |
| Contrast | Effect size (R) |
|---|---|
| Large trees (≥20 cm DBH) | |
| Khabaung vs. Kyaukmasin | 0.87 |
| Khabaung vs. Yoma | 0.89 |
| Kyaukmasin vs. Yoma | 0.78 |
| Small trees (<20 cm DBH) | |
| Khabaung vs. Kyaukmasin | 0.51 |
| Khabaung vs. Yoma | 0.61 |
| Kyaukmasin vs. Yoma | 0.26 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sein Win Tun, K.; Di Stefano, J.; Volkova, L. Forest Management Influences Aboveground Carbon and Tree Species Diversity in Myanmar’s Mixed Deciduous Forests. Forests 2016, 7, 217. https://doi.org/10.3390/f7100217
Sein Win Tun K, Di Stefano J, Volkova L. Forest Management Influences Aboveground Carbon and Tree Species Diversity in Myanmar’s Mixed Deciduous Forests. Forests. 2016; 7(10):217. https://doi.org/10.3390/f7100217
Chicago/Turabian StyleSein Win Tun, Kyaw, Julian Di Stefano, and Liubov Volkova. 2016. "Forest Management Influences Aboveground Carbon and Tree Species Diversity in Myanmar’s Mixed Deciduous Forests" Forests 7, no. 10: 217. https://doi.org/10.3390/f7100217
APA StyleSein Win Tun, K., Di Stefano, J., & Volkova, L. (2016). Forest Management Influences Aboveground Carbon and Tree Species Diversity in Myanmar’s Mixed Deciduous Forests. Forests, 7(10), 217. https://doi.org/10.3390/f7100217






