Effects of Buffering Key Habitat for Terrestrial Salamanders: Implications for the Management of the Federally Threatened Red Hills Salamander (Phaeognathus hubrichti) and Other Imperiled Plethodontids
Abstract
:1. Introduction
2. Experimental Section
2.1. Field Data Collection
| Site | County | Buffered | Burrow density (burrows/m2) | Total number of transects analyzed | Total # of transects analyzed |
|---|---|---|---|---|---|
| Hwy 21 | Monroe | N | 0.344 | 80 | 6 |
| McKenzie 14 | Butler | N | 0.295 | 87 | 6 |
| Pigeon Creek 15 | Butler | N | 0.675 | 79 | 7 |
| Pigeon Creek 21E | Butler | N | 0.377 | 141 | 6 |
| Skinnerton E | Conecuh | N | 0.249 | 37 | 6 |
| Vredenburg 2 | Monroe | N | 0.310 | 131 | 5 |
| WH 1 | Wilcox | N | 0.495 | 38 | 5 |
| WH Conecuh | Conecuh | N | 0.754 | 76 | 6 |
| Forever Wild 1 | Monroe | Y | 0.747 | 245 | 9 |
| Forever Wild 2 | Monroe | Y | 0.500 | 75 | 6 |
| Forever Wild 3 | Monroe | Y | 0.730 | 100 | 7 |
| Haines Island | Monroe | Y | 0.896 | 328 | 12 |
| Sepulga River | Conecuh | Y | 0.292 | 76 | 14 |
| WH 2 | Wilcox | Y | 0.362 | 44 | 4 |
| Wilmon | Monroe | Y | 0.686 | 110 | 8 |
2.2. Genetic Analyses
2.3. Transect Data Analyses
2.4. Comparison of Buffered and Unbuffered Habitats
2.5. General Patterns
3. Results and Discussion
3.1. Genetic Diversity
| Site | Buffered | Tissue samples | Fixation index (FIS) | Ho population mean | Ho population standard deviation | Total number of alleles |
|---|---|---|---|---|---|---|
| Hwy 21 | N | 12 | 0.198 | 0.53 | 0.19 | 30 |
| Pigeon Creek | N | 12 | 0.041 | 0.45 | 0.32 | 16 |
| Vredenburg 2 | N | 8 | 0.229 | 0.45 | 0.32 | 30 |
| WH 1 | N | 10 | 0.13 | 0.55 | 0.22 | 32 |
| WH Conecuh | N | 14 | 0.075 | 0.59 | 0.21 | 28 |
| Forever Wild 1 | Y | 11 | 0.024 | 0.59 | 0.28 | 31 |
| Forever Wild 2 | Y | 12 | 0.029 | 0.59 | 0.26 | 39 |
| Haines Island | Y | 11 | 0.027 | 0.55 | 0.21 | 49 |
| Sepulga River | Y | 10 | 0.023 | 0.54 | 0.21 | 35 |
| Wilmon | Y | 10 | 0.105 | 0.54 | 0.22 | 37 |
3.2. Transect Data
3.3. Comparison of Buffered and Unbuffered Habitats
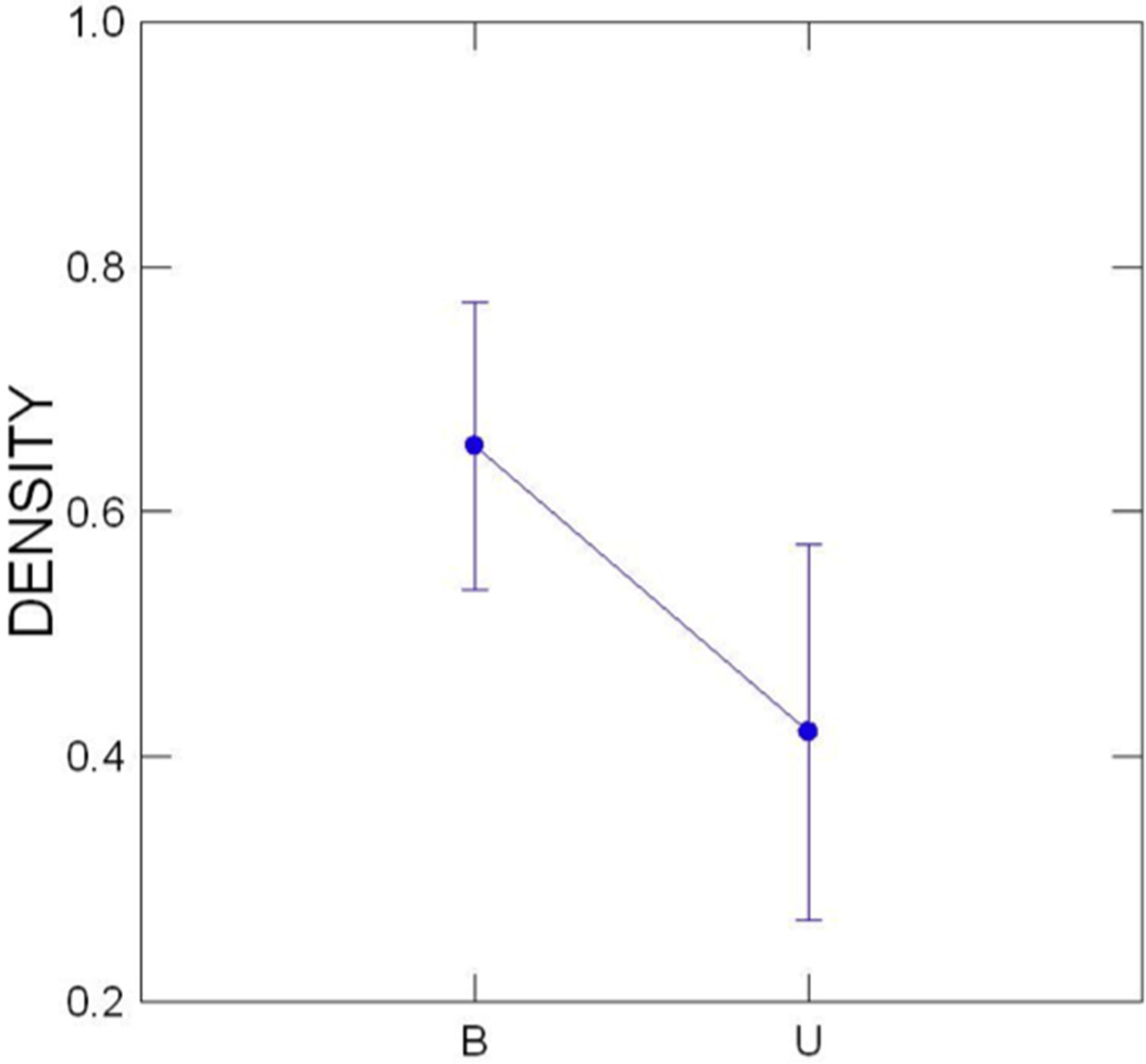
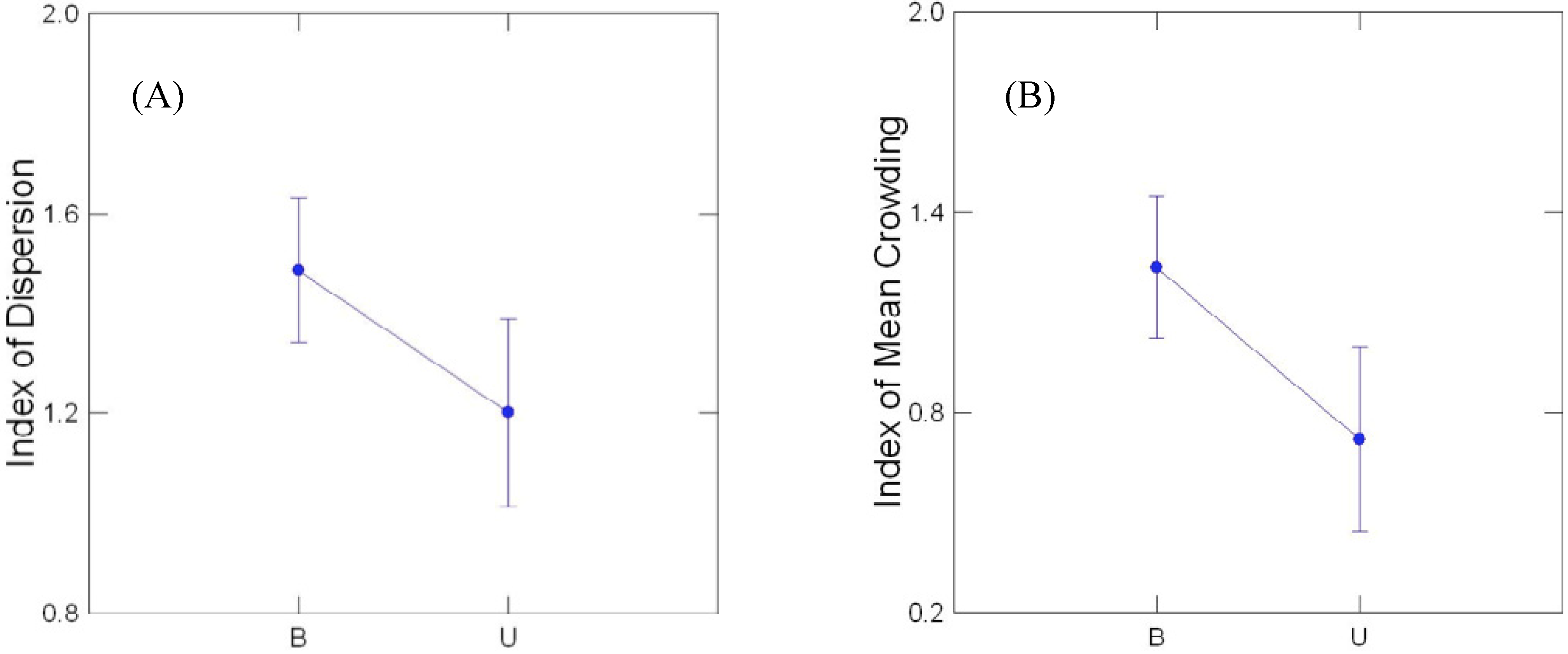
3.4. General Patterns
4. Discussion
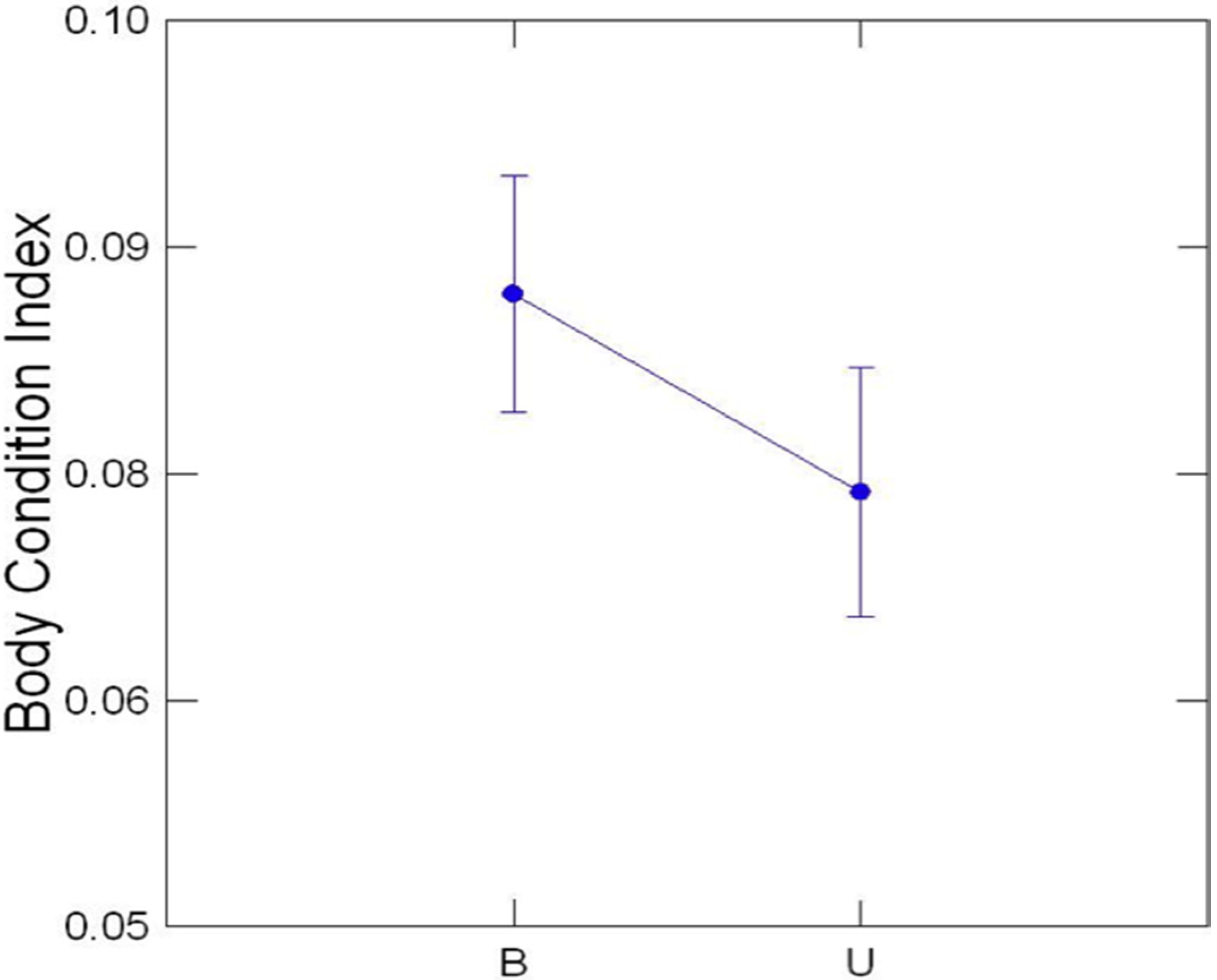
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bury, R.B. Differences in amphibian populations in logged and old growth redwood forest. Northwest Sci. 1983, 57, 167–178. [Google Scholar]
- Harpole, D.N.; Haas, C.A. Effects of seven silvicultural treatments on terrestrial salamanders. For. Ecol. Manag. 1999, 114, 349–356. [Google Scholar] [CrossRef]
- Means, D.B.; Palis, J.G.; Baggett, M. Effects of slash pine silviculture on a Florida population of flatwoods salamander. Conserv. Biol. 1996, 10, 426–437. [Google Scholar] [CrossRef]
- Petranka, J.W.; Brannon, M.P.; Hopey, M.E.; Smith, C.K. Effects of timber harvesting on low elevation populations of southern Appalachian salamanders. For. Ecol. Manag. 1994, 67, 135–147. [Google Scholar] [CrossRef]
- Putz, F.E.; Sist, P.; Fredericksen, T.; Dykstra, D. Reduced-impact logging: Challenges and opportunities. For. Ecol. Manag. 2008, 256, 1427–1433. [Google Scholar] [CrossRef]
- Semlitsch, R.D.; Todd, B.D.; Blomquist, S.M.; Calhoun, A.J.K.; Gibbons, J.W.; Gibbs, J.P.; Graeter, G.J.; Harper, E.B.; Hocking, D.J.; Hunter, M.L., Jr.; et al. Effects of timber harvest on amphibian populations: understanding mechanisms from forest experiments. BioScience 2009, 59, 853–862. [Google Scholar] [CrossRef]
- Spence, J.R. The new boreal forestry: Adjusting timber management to accommodate biodiversity. Trends Ecol. Evol. 2001, 16, 591–593. [Google Scholar] [CrossRef]
- Veysey, J.S.; Babbitt, K.J.; Cooper, A. An experimental assessment of buffer width: Implications for salamander migratory behavior. Biol. Conserv. 2009, 142, 2227–2239. [Google Scholar] [CrossRef]
- Trenham, P.C.; Shaffer, H.B. Amphibian upland habitat use and its consequences for population viability. Ecol. Appl. 2005, 15, 1158–1168. [Google Scholar] [CrossRef]
- International Union for Conservation of Nature and Natural Resources (IUCN). The IUCN Red List of Threatened Species, version 3.1. 2011. Available online: http://www.iucnredlist.org/technical-documents/categories-and-criteria/2001-categories-criteria (accessed on 25 May 2012).
- Dodd, C.K. The status of the Red Hills salamander Phaeognathus hubrichti, Alabama, USA, 1976–1988. Biol. Conserv. 1991, 55, 57–75. [Google Scholar] [CrossRef]
- Jordan, R.J.; Mount, R.H. The status of the Red Hills salamander, Phaeognathus hubrichti Highton. J. Herpetol. 1975, 9, 211–215. [Google Scholar] [CrossRef]
- Bakkegard, K.A. Activity patterns of Red Hills salamanders (Phaeognathus hubrichti) at their burrow entrances. Copeia 2002, 3, 851–856. [Google Scholar] [CrossRef]
- Means, D.B. Notes on the reproductive biology of the Alabama Red Hills Salamander (Phaeognathus hubrichti). Contemp. Herpetol. 2003, 3, 1–5. [Google Scholar]
- Apodaca, J.J. Prioritizing Regions for the Conservation of Amphibians with Special Emphasis on the Red Hills Salamander (Phaeognathus Hubrichti). Ph.D. Thesis, The University of Alabama, Tuscaloosa, AL, USA, 2010. [Google Scholar]
- Apodaca, J.J.; Rissler, L.J.; Godwin, J.C. Population structure and gene flow in a heavily disturbed habitat: implications for the management of the imperilled Red Hills salamander (Phaeognathus hubrichti). Conserv. Genet. 2012, 13, 913–923. [Google Scholar] [CrossRef]
- Godwin, J.C. Red Hills salamander habitat delineation, breeding bird surveys, and habitat restoration recommendations on commercial timberlands. Unpublished report prepared for The Alabama Department of Conservation and Natural Resources. 2008. Available online: http://www.alnhp.org/reports/Red_Hills_SWG_Final_Report.pdf (accessed on 4 October 2010).
- Schwaner, T.D.; Mount, R.H. Notes on the distribution, habits, and ecology of the salamander Phaeognathus hubrichti Highton. Copeia 1970, 3, 571–573. [Google Scholar] [CrossRef]
- Harris, R.N. Body condition and order of arrival affect cooperative nesting behaviour in four-toed salamanders Hemidactylium scutatum. Anim. Behav. 2008, 75, 229–233. [Google Scholar] [CrossRef]
- Lowe, W.H.; Likens, G.E.; Cosentino, B.J. Self-organization in streams: the relationship between movement behaviour and body condition in a headwater salamander. Freshw. Biol. 2006, 51, 2052–2062. [Google Scholar] [CrossRef]
- Lowe, W.H.; McPeek, M.A.; Likens, G.E.; Cosentino, B.J. Linking movement behaviour to dispersal and divergence in plethodontid salamanders. Mol. Ecol. 2008, 17, 4459–4469. [Google Scholar] [CrossRef] [PubMed]
- Semlitsch, R.D. Differentiating migration and dispersal processes for pond-breeding amphibians. J. Wildl. Manag. 2008, 72, 260–267. [Google Scholar] [CrossRef]
- Mount, R.H.; Schwaner, T.D. A technique for collecting the plethodontid salamander Phaeognathus hubrichti. Copeia 1970, 1, 205–206. [Google Scholar] [CrossRef]
- Karraker, N.E.; Welsh, H.H. Long-term impacts of even-aged timber management on abundance and body condition of terrestrial amphibians in northwestern California. Biol. Conserv. 2006, 131, 132–140. [Google Scholar] [CrossRef]
- Lance, S.L.; Hagen, C.; Glenn, T.C.; Apodaca, J.J.; Rissler, L.J. Development and characterization of twelve polymorphic microsatellite loci in the threatened Red Hills salamander, Phaeognathus hubrichti. Conserv. Genet. Resour. 2009, 10, 1919–1921. [Google Scholar] [CrossRef]
- Van Oosterhout, C.; Hutchinson, W.F.; Wills, D.P.M.; Shipley, P. MICRO-CHECKER: Software for identifying and correcting genotyping errors in microsatellite data. Mol. Ecol. Notes 2004, 4, 535–538. [Google Scholar] [CrossRef]
- Goudet, J. FSTAT, a program to estimate and test gene diversities and fixation indices (Version 2.9.3). 2001. Available online: http://www.unil.ch/izea/software/fstat.html (accessed on 12 December 2010).
- Lloyd, M. Mean crowding. J. Anim. Ecol. 1967, 36, 1–30. [Google Scholar] [CrossRef]
- Pielou, E.C. An Introduction to Mathematical Ecology; John Wiley & Sons: New York, NY, USA, 1969. [Google Scholar]
- Rosenberg, M.S.; Anderson, C.D. PASSaGE: Pattern analysis, spatial statistics and geographic exegesis. Version 2. Methods Ecol. Evol. 2011, 2, 229–232. [Google Scholar] [CrossRef]
- Bakkegard, K.A.; Guyer, C. Sexual size dimorphism in the Red Hills salamander, Phaeognathus hubrichti (Caudata: Plethodontidae: Desmognathinae). J. Herpetol. 2004, 38, 8–15. [Google Scholar] [CrossRef]
- Jakob, E.M.; Marshall, S.D.; Uetz, G.W. Estimating fitness: A comparison of body condition indices. Oikos 1996, 77, 61–67. [Google Scholar] [CrossRef]
- Frankham, R.; Ballou, J.D.; Briscoe, D.A. Introduction to Conservation Genetics; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Homyack, J.A.; Haas, C.A.; Hopkins, W.A. Energetics of surface-active terrestrial salamanders in experimentally harvested forest. J. Wildl. Manag. 2011, 75, 1267–1278. [Google Scholar] [CrossRef]
- Semlitsch, R.D.; Bodie, J.R. Biological criteria for buffer zones around wetlands and riparian habitats for amphibians and reptiles. Conserv. Biol. 2003, 17, 1219–1228. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apodaca, J.J.; Godwin, J.C. Effects of Buffering Key Habitat for Terrestrial Salamanders: Implications for the Management of the Federally Threatened Red Hills Salamander (Phaeognathus hubrichti) and Other Imperiled Plethodontids. Forests 2015, 6, 827-838. https://doi.org/10.3390/f6030827
Apodaca JJ, Godwin JC. Effects of Buffering Key Habitat for Terrestrial Salamanders: Implications for the Management of the Federally Threatened Red Hills Salamander (Phaeognathus hubrichti) and Other Imperiled Plethodontids. Forests. 2015; 6(3):827-838. https://doi.org/10.3390/f6030827
Chicago/Turabian StyleApodaca, Joseph J., and James C. Godwin. 2015. "Effects of Buffering Key Habitat for Terrestrial Salamanders: Implications for the Management of the Federally Threatened Red Hills Salamander (Phaeognathus hubrichti) and Other Imperiled Plethodontids" Forests 6, no. 3: 827-838. https://doi.org/10.3390/f6030827
APA StyleApodaca, J. J., & Godwin, J. C. (2015). Effects of Buffering Key Habitat for Terrestrial Salamanders: Implications for the Management of the Federally Threatened Red Hills Salamander (Phaeognathus hubrichti) and Other Imperiled Plethodontids. Forests, 6(3), 827-838. https://doi.org/10.3390/f6030827





