Organic Carbon Accumulation in Topsoil Following Afforestation with Willow: Emphasis on Leaf Litter Decomposition and Soil Organic Matter Quality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites, Management System, and Willow Cultivar
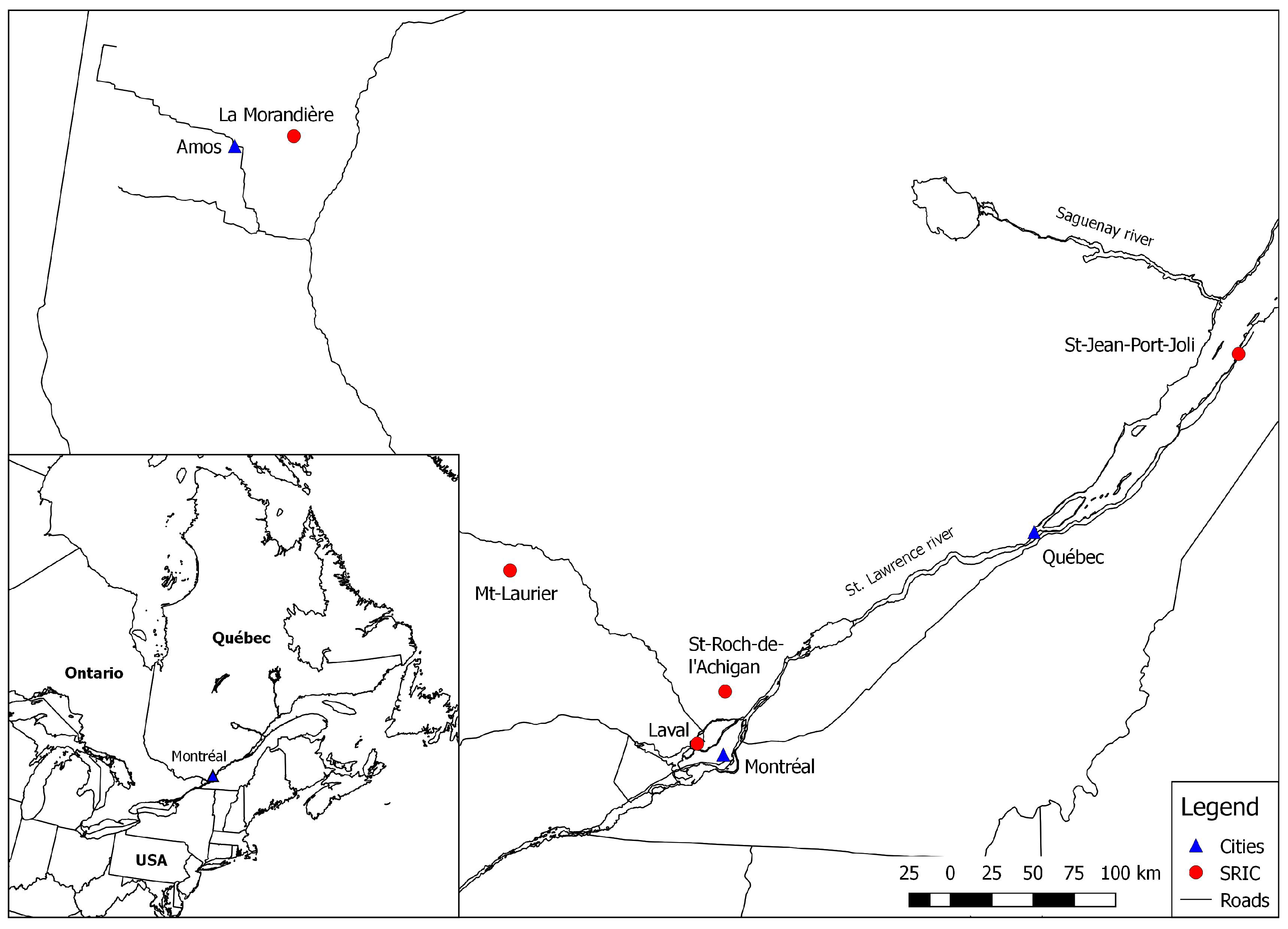
| Site | Growing season precipitation1 (mm) | Growing season average temperature (°C) 2 | Degree-days 1 (>5 °C) | Growing season length1 (days) | Previous land use |
|---|---|---|---|---|---|
| La Morandière (ABI) | 479 | 13.5 | 1376 | 139 | Fallow |
| Laval (LAV) | 630 | 17.5 | 2188 | 204 | Market gardening |
| Mt-Laurier (MTL) | 543 | 15.3 | 1813 | 157 | Mixedwood forest |
| St-Jean-Port-Joli (SJPJ) | 550 | 15.9 | 1419 | 197 | Fallow |
| St-Roch-de-l’ Achigan (STR) | 570 | 16.8 | 2078 | 191 | Market gardening |
2.2. Experimental Design and Sampling Procedure
| Site | Sand (%) | Silt (%) | Clay (%) | Texture 1 | Nitrogen (mg g−1) | Carbon:Nitrogen |
|---|---|---|---|---|---|---|
| ABI | 19.4 (1.4) | 37.5 (2.4) | 43.1 (3.7) | Clay | 1.9 (0.1) | 12.3 (0.3) |
| LAV | 49.6 (0.5) | 39.1 (0.4) | 11.3 (0.9) | Loam | 1.7 (0.1) | 14.4 (0.4) |
| MTL | 74.1 (2.5) | 22.1 (1.4) | 3.7 (1.2) | Loamy sand | 1.1 (0.2) | 16.8 (0.6) |
| SJPJ | 30.7 (4.2) | 46.4 (2.4) | 22.9 (2.1) | Loam | 3.0 (0.2) | 11.4 (0.4) |
| STR | 59.2 (3.1) | 32.1 (2.4) | 8.7 (0.8) | Sandy loam | 1.3 (0.1) | 13.7 (0.3) |
2.3. Laboratory Analyses
2.4. Data Analysis
3. Results
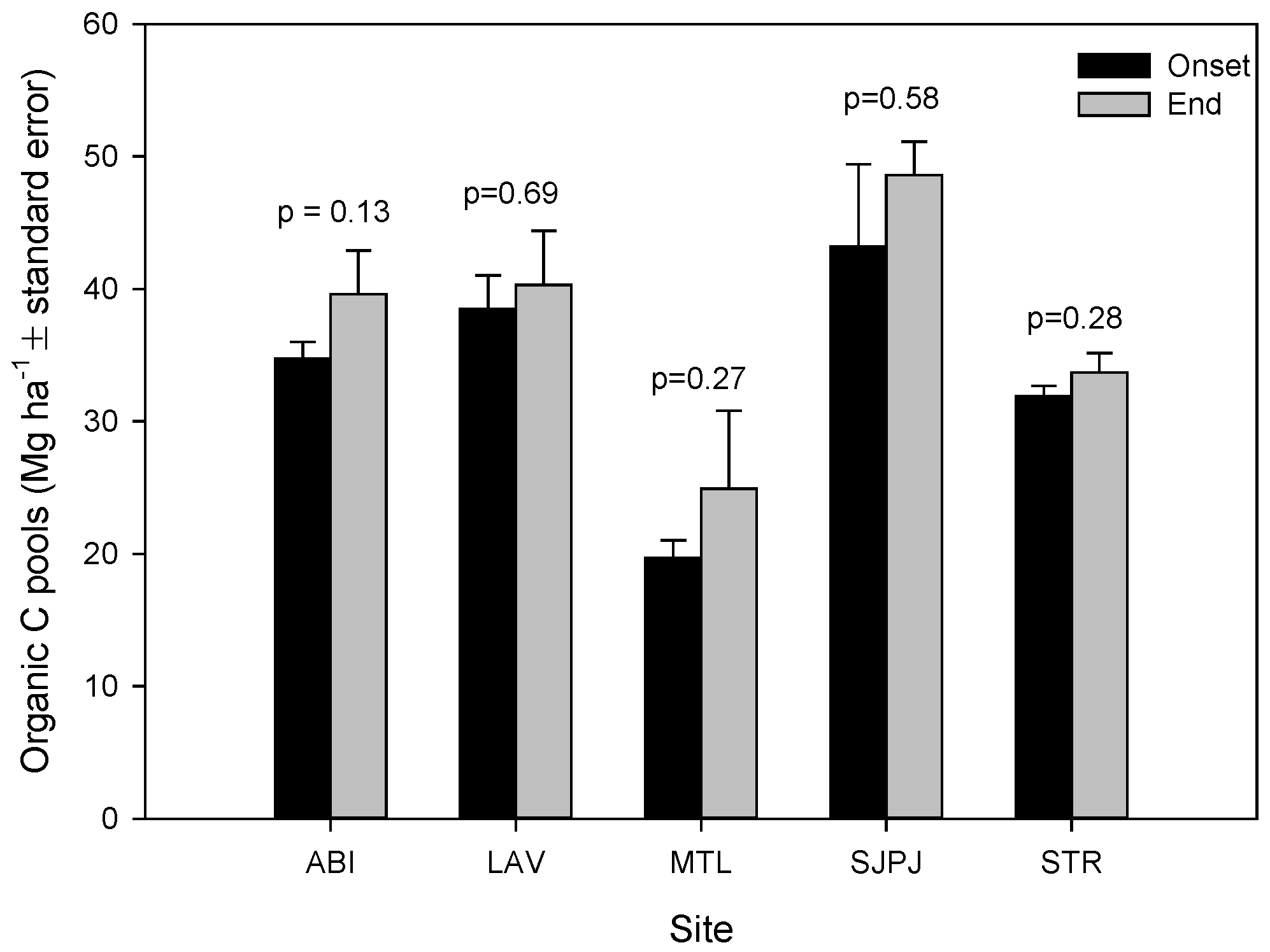
3.1. Carbon Concentrations and Pools in the Topsoil under SRICs and Reference Fields
| Site | Organic C concentration | Organic C pools | ||||
|---|---|---|---|---|---|---|
| SRIC | Reference | Δ 1 (%) | SRIC | Reference | Δ (%) | |
| ABI | 22.8 (0.80) | 22.8 (0.6) | 0.0 | 34.7 (1.3) | 33.4 (1.6) | 3.7 |
| LAV | 24.0 (2.3) | 14.5 (2.3) | 39.6 | 38.5 (2.5) | 16.2 (8.7) | 57.9 |
| MTL | 19.0 (2.7) | 11.8 (1.7) | 37.9 | 19.7 (1.3) | 16.4 (2.8) | 16.8 |
| SJPJ | 34.2 (2.0) | 28.9 (2.0) | 15.5 | 43.2 (6.3) | 39.9 (6.0) | 7.6 |
| STR | 18.0 (0.3) | 11.9 (0.6) | 33.9 | 31.4 (0.8) | 18.8 (0.7) | 40.1 |
| Mean | 23.6 (2.88) | 17.9 (3.39) | 24.2 | 33.5 (3.7) | 24.9 (4.9) | 25.7 |
| t-test | 3.59 | 2.17 | ||||
| p-value | 0.02 | 0.09 | ||||
3.2. Organic Carbon Pool Changes in the Topsoil of SRICs over Two Years of Incubation
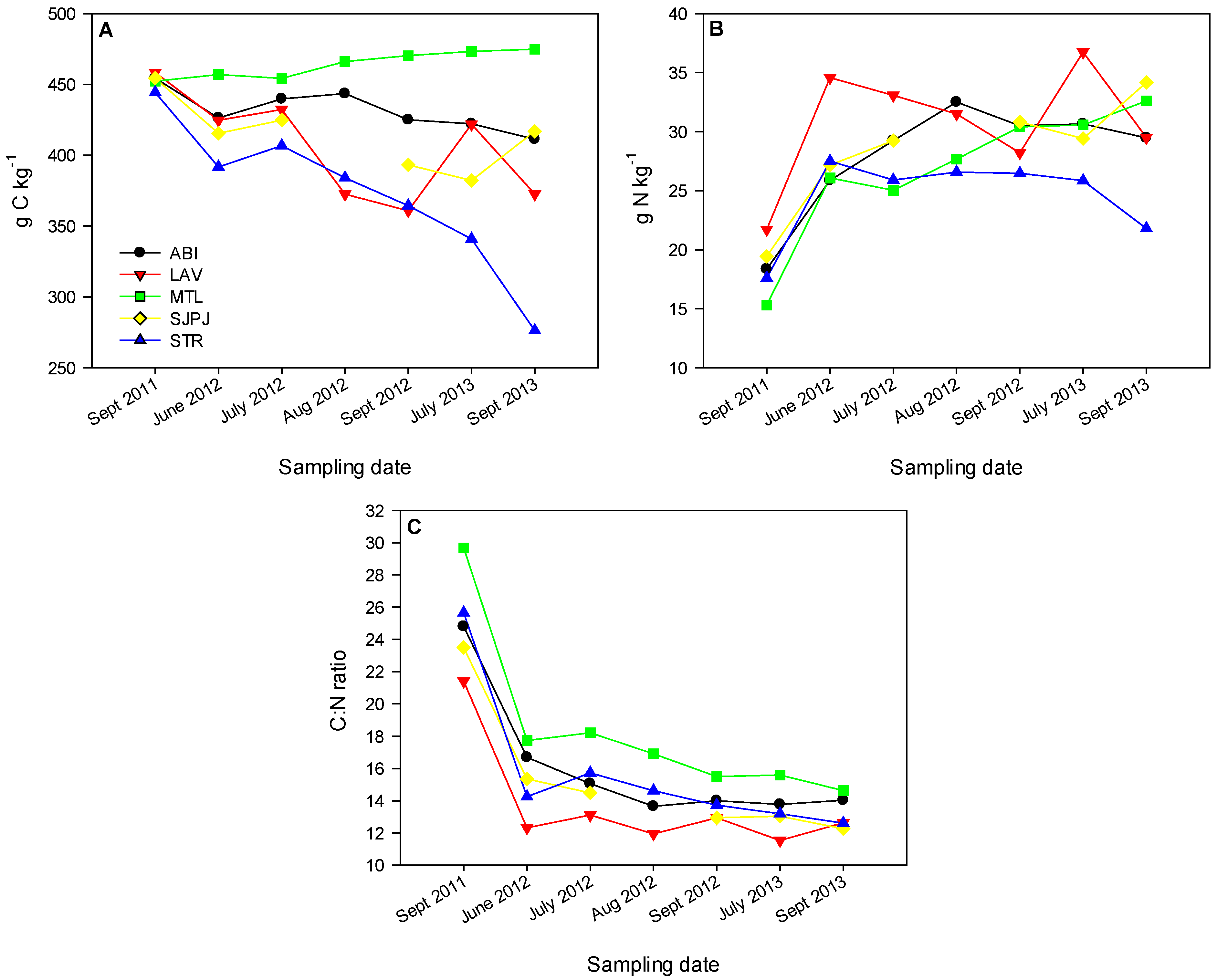
3.3. Organic Matter Chemical Signatures of the Topsoil under SRICs and Reference Fields
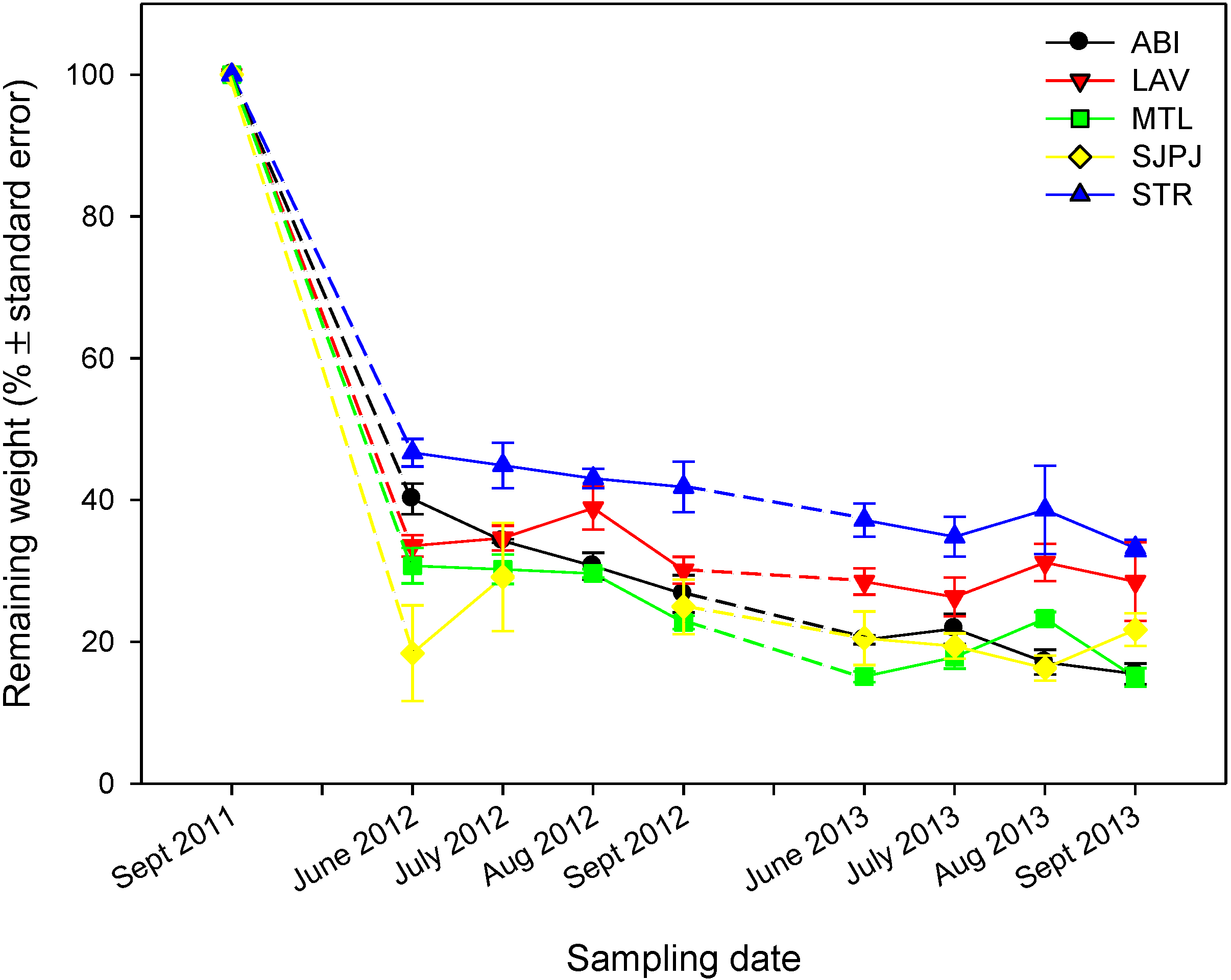
3.4. Willow Leaf Litter Decomposition, Chemical Composition and Litterfall (Leaf Only) Fluxes
| Site | Treatment | Alkyls | O-alkyls | di-O-alkyls | Aromatics | Phenolic | Carboxyl | Aldehydes + ketones | Alkyls / O-alkyls + di-O-alkyls |
|---|---|---|---|---|---|---|---|---|---|
| ABI | SRIC | 22 | 45 | 9 | 12 | 5 | 7 | 0 | 0.41 |
| Reference | 18 | 42 | 9 | 14 | 6 | 8 | 1 | 0.35 | |
| LAV | SRIC | 25 | 36 | 8 | 15 | 7 | 8 | 1 | 0.57 |
| Reference | 25 | 38 | 8 | 14 | 6 | 7 | 1 | 0.54 | |
| MTL | SRIC | 23 | 39 | 9 | 16 | 7 | 6 | 0 | 0.48 |
| Reference | 22 | 41 | 9 | 14 | 7 | 6 | 0 | 0.44 | |
| SJPJ | SRIC | 29 | 40 | 8 | 11 | 4 | 7 | 0 | 0.60 |
| Reference | 25 | 40 | 8 | 12 | 5 | 8 | 2 | 0.52 | |
| STR | SRIC | 31 | 36 | 7 | 10 | 5 | 8 | 3 | 0.72 |
| Reference | 25 | 37 | 7 | 11 | 6 | 10 | 3 | 0.56 | |
| Mean | SRIC | 26.0 (1.7) | 39.2 (1.7) | 8.2 (0.4) | 12.8 (1.2) | 5.6 (0.6) | 7.2 (0.4) | 0.8 (0.6) | 0.56 (0.05) |
| Reference | 23.0 (1.4) | 39.6 (0.9) | 8.2 (0.4) | 13.0 (0.7) | 6.0 (0.3) | 7.8 (0.7) | 1.4 (0.5) | 0.48 (0.03) | |
| t-test | 2.73 | –0.43 | 0.00 | –0.27 | –1.00 | –1.18 | –1.50 | 3.13 | |
| p-value | 0.05 | 0.68 | 1.00 | 0.80 | 0.37 | 0.30 | 0.21 | 0.03 |
| Site | K 1 | DLV 2 | A 3 |
|---|---|---|---|
| ABI | 1.7 | 84.5 | 0.15 |
| LAV | 2.8 | 71.5 | 0.28 |
| MTL | 1.9 | 85.0 | 0.15 |
| SJPJ | 2.0 | 78.3 | 0.22 |
| STR | 2.0 | 66.8 | 0.33 |
| Mean (±SD) | 2.1 (0.4) | 77.2 (8.0) | 0.23 (0.08) |
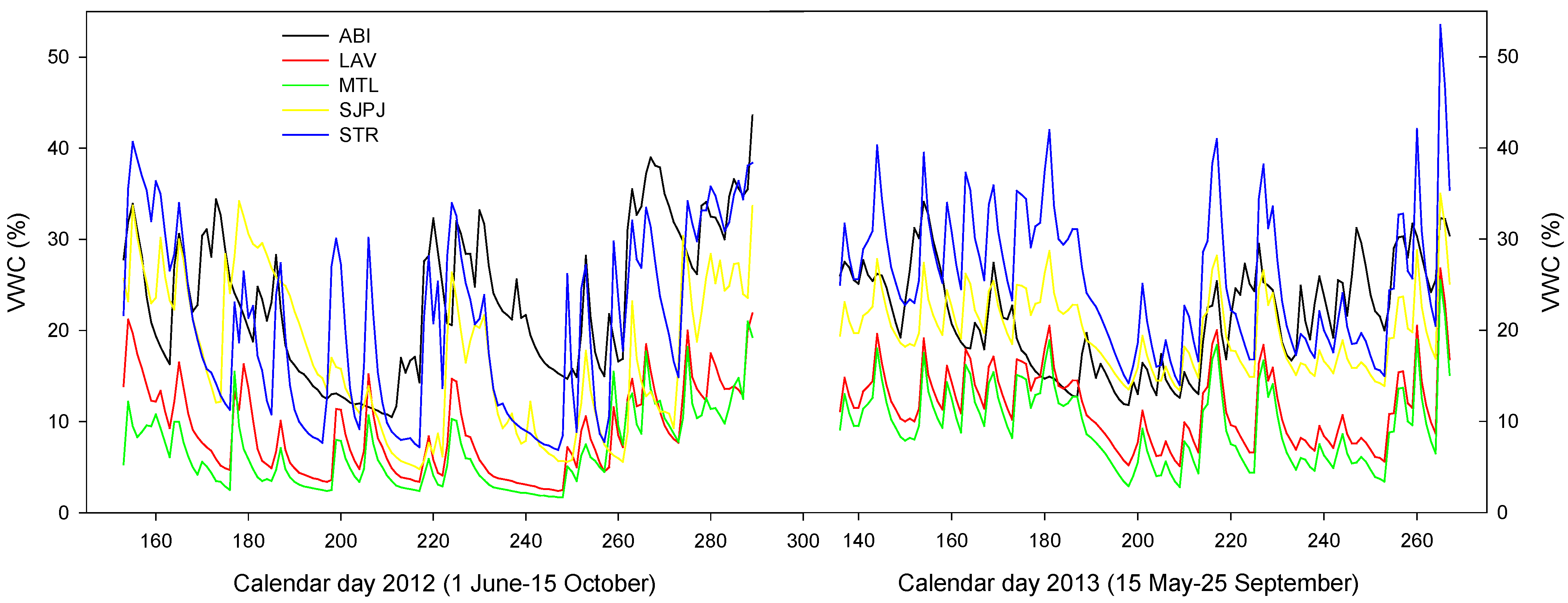
3.5. Microclimate of the Topsoil of the Five SRICs
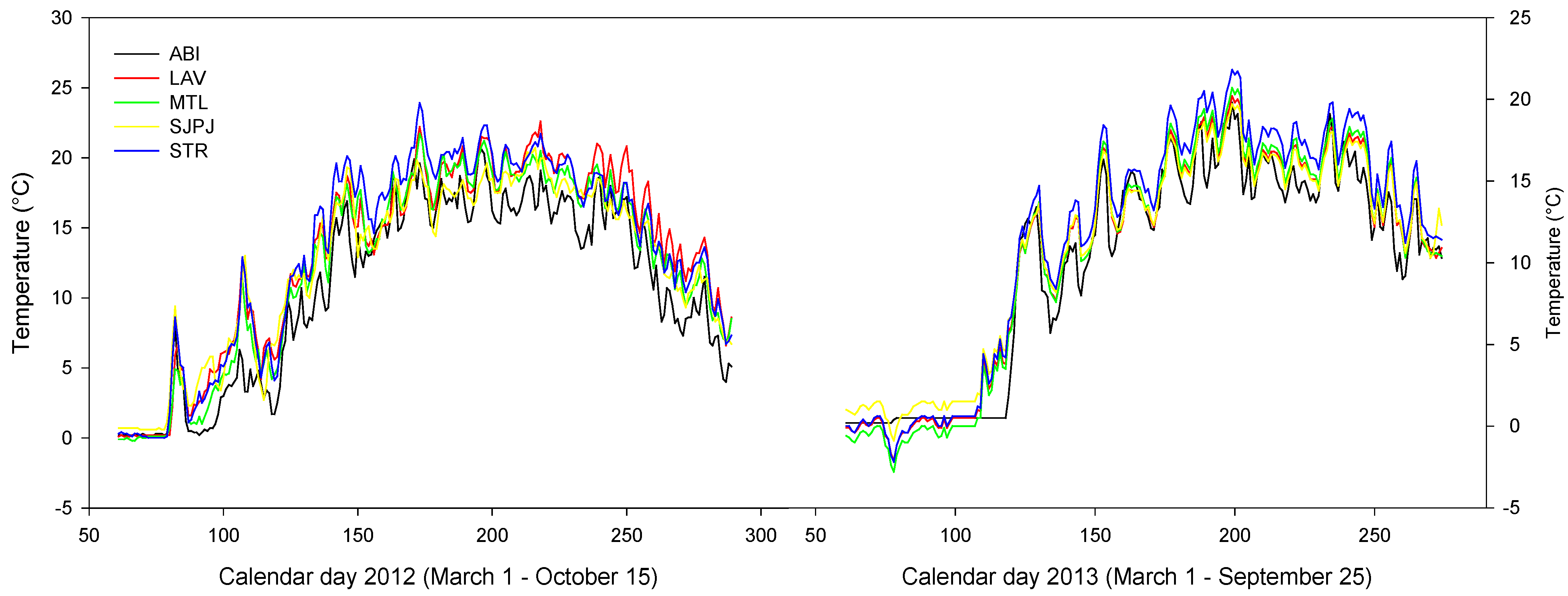
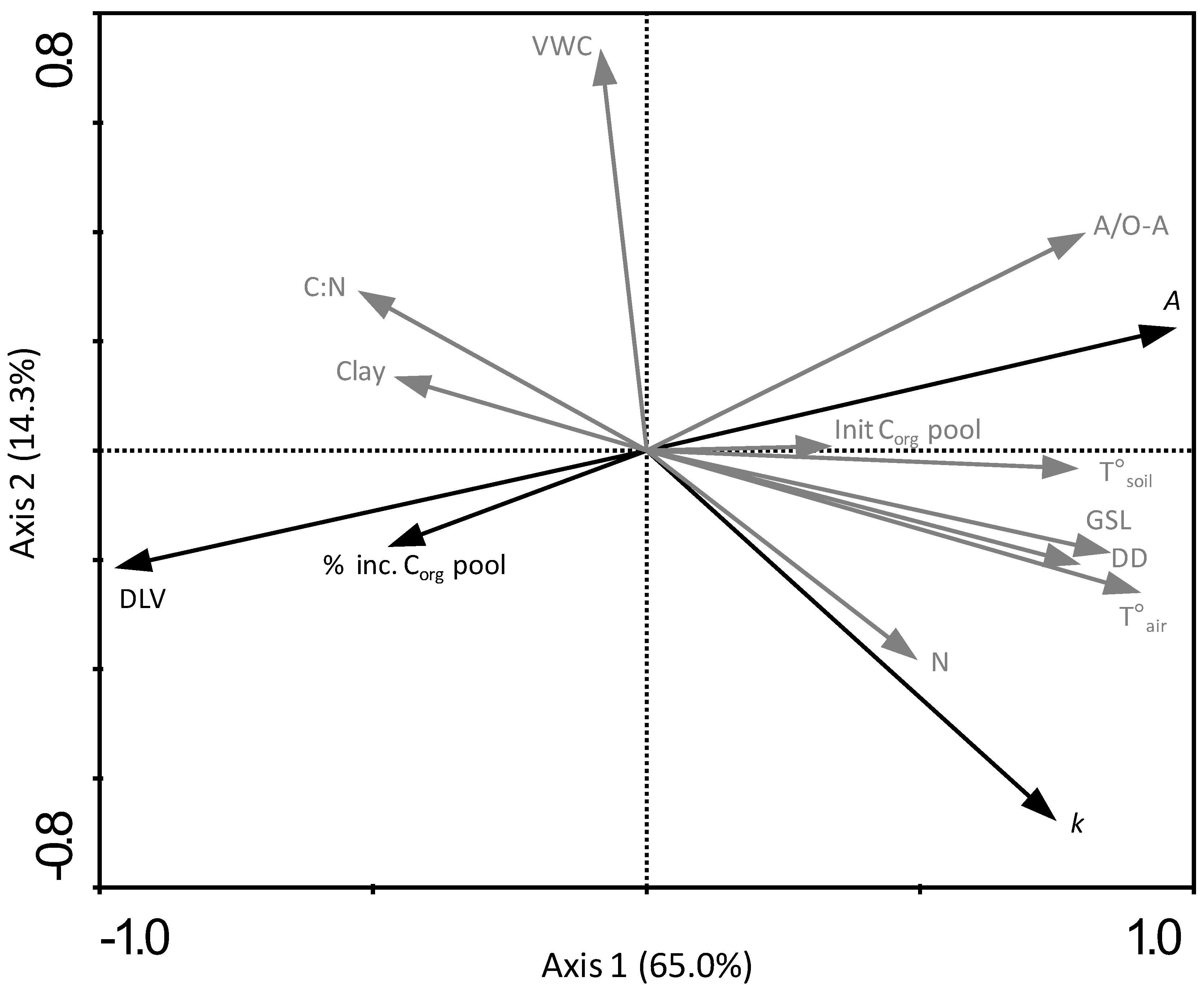
3.6. Relating Leaf Litter Decomposition and Organic Carbon Pool Increases in the Topsoil to Climatic Conditions, Leaf Chemistry and Organic Matter Quality
4. Discussion
4.1. Organic Carbon Accumulation Following Afforestation with Willow
4.2. Willow Leaf Litter Decomposition
4.3. Soil Organic Matter Quality
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Graham, R.L.; Wright, L.L.; Turhollow, A.F. The potential for short-rotation woody crops to reduce U.S. CO2 emissions. Clim. Chang. 1992, 22, 223–238. [Google Scholar] [CrossRef]
- Bonin, C.; Lal, R. Bioethanol potentials and life cycle assessments of biofuel feedstocks. Crit. Rev. Plant Sci. 2012, 31, 271–289. [Google Scholar] [CrossRef]
- Amichev, B.Y.; Kurz, W.A.; Smyth, C.; van Rees, K.C.J. The carbon implications of large-scale afforestation of agriculturally marginal land with short-rotation willow in Saskatchewan. GCB Bioener. 2012, 4, 70–87. [Google Scholar] [CrossRef]
- Guidi, W.; Pitre, F.E.; Labrecque, M. Short-rotation coppice of willows for the production of biomass in eastern Canada. In Biomass Now—Sustainable Growth and Use; Matovic, M.D., Ed.; InTech: Rijeka, Croatia, 2013; pp. 421–448. [Google Scholar]
- Ens, J.A.; Farrell, R.E.; Bélanger, N. Rapid biomass estimation using optical stem density of willow (Salix spp.) grown in short rotation. Biom. Bioener. 2009, 33, 174–179. [Google Scholar] [CrossRef]
- Labrecque, M.; Teodorescu, T.I. Biomass yield and nutrient uptake of Salix clones after two 3-year coppice rotations on southern Quebec, Canada. Biom. Bioener. 2003, 25, 135–146. [Google Scholar] [CrossRef]
- Hammar, T.; Ericsson, N.; Sundberg, C.; Hansson, P.-A. Climate impact of willow grown for bioenergy in Sweden. Bioener. Res. 2014. [Google Scholar] [CrossRef]
- Caputo, J.; Balogh, S.B.; Volk, T.A.; Johnson, L.; Puettmann, M.; Lippke, B.; Oneil, E. Incorporating uncertainty into a life cycle assessment (LCA) model of short-rotation willow biomass (Salix spp.) crops. Bioener. Res. 2009, 7, 48–59. [Google Scholar] [CrossRef]
- Jug, A.; Hofmann-Schielle, C.; Makeschin, F.; Rehfuess, K.E. Short-rotation plantations of balsam poplars, aspen and willows on former arable land in the Federal Republic of Germany III. Soil ecological effects. For. Ecol. Manag. 1999, 121, 85–99. [Google Scholar] [CrossRef]
- Kahle, P.; Hildebrand, E.; Baum, C.; Boelcke, B. Long-term effects of short rotation forestry with willows and poplar on soil properties. Arch. Agron. Soil Sci. 2007, 53, 673–682. [Google Scholar] [CrossRef]
- Pacaldo, R.S.; Volk, T.A.; Briggs, R.D. No significant differences in soil organic carbon contents along a chronosequence of shrub willow biomass crop fields. Biom. Bioener. 2013, 6, 252–262. [Google Scholar]
- Walter, K.; Don, A.; Flessa, H. No general soil carbon sequestration under Central European short rotation coppices. GCB Bioener. 2014. [Google Scholar] [CrossRef]
- Ens, J.; Farrell, R.E.; Bélanger, N. Early effects of afforestation with willow (Salix purpurea, “Hotel”) on soil carbon and nutrient availability. Forests 2013, 4, 137–154. [Google Scholar] [CrossRef]
- Zan, C.S.; Fyles, J.W.; Girourard, P.; Samson, R.A. Carbon sequestration in perennial bioenergy, annual corn and uncultivated systems in southern Quebec. Agric. Ecosyst. Environ. 2001, 86, 135–144. [Google Scholar] [CrossRef]
- Lockwell, J.; Guidi, W.; Labrecque, M. Soil carbon sequestration potential of willows in short-rotation copice established on abandoned farm lands. Plant Soil 2012, 360, 299–318. [Google Scholar] [CrossRef]
- Lal, R. Soil carbon sequestration impacts on global climate change and food security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef] [PubMed]
- Laganière, J.; Angers, D.A.; Paré, D. Carbon accumulation in agriculture soils after afforestation: A meta-analysis. Glob. Change Biol. 2010, 16, 439–453. [Google Scholar] [CrossRef]
- Heywood, P.; Turpin, S. Variation in soil carbon stocks with texture and previous landuse in north-western NSW, Australia. Sust. Agric. Res. 2013, 2, 124–133. [Google Scholar]
- Dang, Y.; Ren, W.; Tao, B.; Chen, G.; Lu, C.; Yang, J.; Pan, S.; Wang, G.; Li, S.; Tian, H. Climate and land use controls on soil organic carbon in the Loess Plateau region of China. PLoS ONE 2014, 9, e95548. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.W.I.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kögel-Knabner, I.; Lehmann, J.; Manning, DA.A.C.; Nannipieri, P.; Rasse, D.P.; Weiner, S.; Trumbore, S.E. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Grogan, P.; Matthews, R. Review of the Potential for Soil Carbon Sequestration under Bioenergy Crops in the U.K; MAFF report on contract NF0418; Institute of Water and Environment, Cranfield University: Silsoe Bedfordshire, UK, 2001; p. 38. [Google Scholar]
- Routhier, M.; Lafleur, B.; Bélanger, N. Accumulation des stocks de carbone dans les sols sous des cultures bioénergétiques de Populus spp., Salix spp., et Panicum virgatum. VertigO 2014. [Google Scholar] [CrossRef]
- Lützov, M.V.; Kögel-Knaber, I.; Ekschmitt, K.; Matzner, E.; Guggenberger, G.; Marschner, B.; Flessa, H. Stabilization of organic matter intemperate soils: Mechanisms and their relevance under different soil conditions—A review. Eur. J. Soil Sci. 2006, 57, 426–445. [Google Scholar] [CrossRef]
- Zinn, Y.L.; Resck, D.V.S.; da Silva, J.E. Soil organic carbon as affected by afforestation with Eucapyptus and Pinus in the Cerrado region of Brazil. For. Ecol. Manag. 2002, 166, 285–294. [Google Scholar] [CrossRef]
- Skjemstad, J.O.; Clarke, P.; Golchin, A.; Oades, J.M. Characterisation of soil organic matter by solid-state 13C NMR spectrometry. In Driven by Nature: Plant Litter Quality and Decomposition; Cadisch, G., Giller, G.E., Eds.; CAB International: Wallingford, UK, 1997; pp. 253–271. [Google Scholar]
- Labrecque, M.; Teodorescu, T.I. Field performance and biomass production of 12 willow and poplars in short-rotation coppice in southern Quebec (Canada). Biom. Bioener. 2005, 29, 1–9. [Google Scholar] [CrossRef]
- Fontana, M.J.; Lafleur, B.; Labrecque, M.; Courchesne, F.; Bélanger, N. Productivity of Salix miyabeana SX67 in southern Quebec: Stool age, coppicing and site effects. 2015; submitted for publication. [Google Scholar]
- Soil Classification Working Group. The Canadian System of Soil Classification, 3rd ed.; Research Branch, Agricultural and Agri-Food Canada, Publication 1646; NRC Research Press: Ottawa, ON, Canada, 1998. [Google Scholar]
- Régnière, J.; St-Amant, R. BioSim 9 User’s Manual; Information Report LAU-X-134; Natural Resources Canada, Canadian Forest Service, Laurentian Forestry Centre: Quebec, QC, Canada, 2008. [Google Scholar]
- Polglase, P.J.; Paul, K.I.; Khanna, P.K.; Gwinyai Nyakuengama, J.; O’Connell, A.M.; Grove, T.S.; Battaglia, M. Change in Soil Carbon Following Afforestation or Reforestation, National Carbon Accounting System; Technical report no. 20; Australian Greenhouse Office: Canberra, Australia, 2000. [Google Scholar]
- Lal, R. Forest soils and carbon sequestration. For. Ecol. Manag. 2005, 220, 242–258. [Google Scholar] [CrossRef]
- Don, A.; Schumacher, J.; Scherer-Lorenzen, M.; Scholten, T.; Schulze, E.-D. Spatial and vertical variation of soil carbon at two grassland sites—Implications for measuring soil carbon stocks. Geoderma 2007, 141, 272–283. [Google Scholar] [CrossRef]
- Hobbie, S.E.; Eddy, W.C.; Buyarski, C.R.; Adair, E.C.; Ogdahl, M.L.; Weisenhorn, P. Response of decomposing litter and its microbial community to multiple forms of nitrogen enrichment. Ecol. Monogr. 2012, 82, 389–405. [Google Scholar] [CrossRef]
- Hangs, R.D.; Schoneau, J.J.; van Rees, K.C.J.; Bélanger, N.; Volk, T. Leaf litter decomposition and nutrient-release characteristics of several willow varieties within short-rotation coppice plantations in Saskatchewan, Canada. Bioener. Res. 2014, 7, 1074–1090. [Google Scholar] [CrossRef]
- Maroufpoor, I.; Emamgholizadeh, S.; Torabi, H.; Behzadinasab, M. Impact of soil texture on the calibration of TDR for water content measurement. J. Appl. Sci. 2009, 9, 2933–2940. [Google Scholar] [CrossRef]
- Slapokas, T.; Granhall, U. Decomposition of litter in fertilized short-rotation forests on a low-humified peat bog. For. Ecol. Manag. 1991, 41, 143–165. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Knicker, H.; Hatcher, P.G.; Kögel-Knabner, I. Improvement of 13C and 15N CPMAS NMR spectra of bulk soils, particle size fractions and organic material by treatment with 10% hydrofluoric acid. Eur. J. Soil Sci. 1997, 48, 319–328. [Google Scholar] [CrossRef]
- Bennet, A.E.; Rienstra, C.M.; Auger, M.; Lakshmi, K.A.; Griffin, R.G. Heteronuclear decoupling in rotating solids. J. Chem. Phys. 1995, 103, 6951–6958. [Google Scholar] [CrossRef]
- Morcombe, C.R.; Zilm, K.W. Chemical sshift referencing in MAS solid state NMR. J. Magn. Reson. 2003, 162, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Thiffault, E.; Hannam, K.D.; Quideau, S.A.; Paré, D.; Bélanger, N.; Oh, S.-W.; Munson, A.D. Chemical composition of forest floor and consequences for nutrient availability after wildfire and harvesting in the boreal forest. Plant Soil 2008, 308, 37–53. [Google Scholar] [CrossRef]
- SAS. JMP version 10. SAS Institute Inc: Cary, NC, USA, 2012. [Google Scholar]
- Ter Braak, C.J.F.; Smilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’S Guide: Software for Canonical Community Ordination (version 4.5); Microcomputer Power: MIthaca, NY, USA, 2002. [Google Scholar]
- Grogan, P; Matthews, R. A modeling analysis of the potential for soil carbon sequestration under short rotation coppice willow bioenergy plantations. Soil Use Manag. 2002, 18, 175–183. [Google Scholar] [CrossRef]
- Rytter, R.-M. The potential of willow and poplar plantations as carbon sinks in Sweden. Biom. Bioener. 2012, 36, 86–95. [Google Scholar] [CrossRef]
- Sartori, F.; Lal, R.; Ebinger, M.H.; Parrish, D.J. Potential soil carbon sequestration and CO2 offset by dedicated energy crops in the USA. Crit. Rev. Plant Sci. 2006, 25, 441–472. [Google Scholar] [CrossRef]
- Rytter, M.R. Fine-root production and turnover in a willow plantation estimated by different calculation methods. Scand. J. For. Res. 1999, 14, 526–537. [Google Scholar] [CrossRef]
- Tremblay, G. Séquestration de carbone atmosphérique dans la biomasse racinaire de plantations de saules. Master’s Thesis, Master’s ThesisDépartement de géographie, Université de Montréal, Montréal, Canada, 2014. [Google Scholar]
- Six, J.; Elliott, E.T.; Paustian, K. Aggregate and soil organic matter dynamics under conventional and no-tillage systems. Soil Sci. Soc. Am. J. 1999, 63, 1350–1358. [Google Scholar] [CrossRef]
- VandenBygaart, A.J.; Gregorich, E.G.; Angers, D.A. Influence of agricultural management on soil organic carbon: A compendium and assessment of Canadian studies. Can. J. Soil Sci. 2003, 83, 363–380. [Google Scholar]
- Lal, R. Soil carbon sequestration to mitigate climate change. Geoderma 2004, 123, 1–22. [Google Scholar] [CrossRef]
- Martens, D.A. Plant residue biochemistry regulates soil carbon cycling and carbon sequestration. Soil Biol. Biochem. 2000, 32, 361–369. [Google Scholar] [CrossRef]
- Stockmann, U.; Adams, M.A.; Crawford, J.W.; Field, D.J.; Henakaarchchi, N.; Jenkins, M.; Minasny, B.; McBratney, A.B.; de Courcelles, V.R.; Singh, K.; et al. The knows, known unknowns and unknowns of sequestration of soil organic carbon. Agric. Ecosyst. Environ. 2013, 164, 80–99. [Google Scholar] [CrossRef]
- Coûteaux, M.M.; Bottner, P.; Berg, B. Litter decomposition, climate, and litter quality. Trends Ecol. Evol. 1995, 10, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Aerts, R. Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: A triangular relationship. Oikos 1997, 79, 439–449. [Google Scholar] [CrossRef]
- Berg, B.; McClaugherty, C.A. Plant Litter, 2nd ed.; Springer: Berlin, Germany, 2008. [Google Scholar]
- Fisher, R.F.; Binkley, D. Ecology and Management of Forest Soils; Wiley & Sons: New York, NY, USA, 2000; p. 489. [Google Scholar]
- Linn, D.M.; Doran, J.W. Effect of water-filled pore space on carbon dioxide and nitrous oxide production in tilled and non-tilled soils. Soil Sci. Soc. Am. J. 1984, 48, 1267–1272. [Google Scholar] [CrossRef]
- Berg, B.; Ekbohm, G. Litter mass-loss rates and decomposition patterns in some needle and leaf litter types. Long-term decomposition in a Scots pine forest. VII. Can. J. Bot. 1991, 69, 1449–1456. [Google Scholar] [CrossRef]
- Berg, B. Litter decomposition and organic matter turnover in northern forest soils. For. Ecol. Manag. 2000, 133, 13–22. [Google Scholar] [CrossRef]
- Aweto, A.O. Secondary succession and soil fertility restoration in south-western Nigeria. II. Soil fertility restoration. J. Ecol. 1981, 69, 609–614. [Google Scholar] [CrossRef]
- Hättenschwiler, S.; Vitousek, P.M. The role of polyphenols in terrestrial ecosystems nutrient cycling. Trends Ecol. Evol. 2000, 15, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Prescott, C.E.; Vesterdal, L.; Preston, C.M.; Simard, S.W. Influence of initial chemistry on decomposition of foliar litter in contrasting forest types in British Columbia. Can. J. For. Res. 2004, 34, 1714–1729. [Google Scholar] [CrossRef]
- Rahman, M.M.; Moitur, R.M. Quantitative chemical defense traits, litter decomposition and forest ecosystem functioning. In Forest Ecosystems—More than Just Trees; Blanco, J.A., Lo, Y.-H., Eds.; InTech: Rijeka, Croatia, 2012; pp. 295–314. [Google Scholar]
- Zak, D.R.; Grigal, D.F.; Gleeson, S.; Tilman, D. Carbon and nitrogen cycling during olf-field succession: Constraints on plant and microbial biomass. Biogeochemistry 1990, 11, 111–129. [Google Scholar] [CrossRef]
- Schofield, J.A.; Hagerman, A.E.; Harold, A. Loss of tannins and other phenolics from willow leaf litter. J. Chem. Ecol. 1998, 24, 1409–1421. [Google Scholar] [CrossRef]
- Van Veen, J.A.; Kuikman, P.J. Soil structural aspects of decomposition of organic matter by microorganisms. Biogeochemistry 1990, 11, 213–233. [Google Scholar] [CrossRef]
- Foote, R.L.; Grogan, P. Soil carbon accumulation during temperate forest succession on abandoned low productivity agricultural lands. Ecosystems 2010, 13, 795–812. [Google Scholar] [CrossRef]
- Sanborn, P.; Lamontagne, P.; Hendershot, W. Podzolic soils of Canada: Genesis, distribution, and classification. Can. J. Soil Sci. 2011, 91, 843–880. [Google Scholar] [CrossRef]
- Baldock, J.A.; Skjemstad, O.J. Role of the soil matrix and minerals in protecting natural organic materials against biologic attack. Org. Geochem. 2000, 31, 697–710. [Google Scholar] [CrossRef]
- Prescott, C.E. Decomposition and mineralization of nutrients from litter and humus. In Nutrient Acquisition by Plants; BassiriRad, H., Ed.; Springer: New York, NY, USA, 2005; pp. 15–41. [Google Scholar]
- Preston, C.M.; Axelson, D.E.; Lévesque, M.; Mathur, S.P.; Dinal, H.; Dudley, R.L. Carbon-13 NMR and chemical characterization of particle-size separates of peats differing in degree of decomposition. Org. Geochem. 1989, 14, 393–403. [Google Scholar] [CrossRef]
- Trofymow, J.A.; Preston, C.M.; Prescott, C.E. Litter quality and its potential effect on decay rates of materials from Canadian forests. Water Air Soil Poll. 1995, 82, 215–226. [Google Scholar] [CrossRef]
- Kögel-Knabner, I. 13C and 15N NMR spectrometry as a tool in soil organic matter studies. Geoderma 1997, 80, 243–270. [Google Scholar] [CrossRef]
- Webster, E.A.; Chudes, J.A.; Hopknis, D.W. Carbon transformations during decomposition of different components of plant leaves in soil. Soil Biol. Biochem. 2000, 32, 301–314. [Google Scholar] [CrossRef]
- Baldock, J.A.; Preston, C.M. Chemistry of carbon decomposition in forests as revealed by solid state carbon-13 nuclear magnetic resonance. In Carbon Forms and Function in Forest Soils; McFee, W.W., Kelly, J.M., Eds.; Soil Science Society of America: Madison, WI, USA, 1995; pp. 89–117. [Google Scholar]
- Baldock, J.A.; Oades, J.M.; Nelson, P.N.; Skene, T.M.; Golchin, A.; Clarke, P. Assessing the extent of decomposition of natural organic materials using solid-state 13C NMR spectroscopy. Aust. J. Soil Res. 1997, 35, 1061–1083. [Google Scholar] [CrossRef]
- Cornwell, W.K.; Cornelissen, J.H.C.; Amatangelo, K.; Dorrepaal, E.; Eviner, V.T.; Gogod, O.; Hobbie, S.E.; Hoorens, B.; Kurokawa, H.; Pérez-Harguindeguy, N.; et al. Plant species traits are the dominant control on litter decomposition rates within biomes worldwide. Ecol. Lett. 2008, 11, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lafleur, B.; Labrecque, M.; Arnold, A.A.; Bélanger, N. Organic Carbon Accumulation in Topsoil Following Afforestation with Willow: Emphasis on Leaf Litter Decomposition and Soil Organic Matter Quality. Forests 2015, 6, 769-793. https://doi.org/10.3390/f6030769
Lafleur B, Labrecque M, Arnold AA, Bélanger N. Organic Carbon Accumulation in Topsoil Following Afforestation with Willow: Emphasis on Leaf Litter Decomposition and Soil Organic Matter Quality. Forests. 2015; 6(3):769-793. https://doi.org/10.3390/f6030769
Chicago/Turabian StyleLafleur, Benoit, Michel Labrecque, Alexandre A. Arnold, and Nicolas Bélanger. 2015. "Organic Carbon Accumulation in Topsoil Following Afforestation with Willow: Emphasis on Leaf Litter Decomposition and Soil Organic Matter Quality" Forests 6, no. 3: 769-793. https://doi.org/10.3390/f6030769
APA StyleLafleur, B., Labrecque, M., Arnold, A. A., & Bélanger, N. (2015). Organic Carbon Accumulation in Topsoil Following Afforestation with Willow: Emphasis on Leaf Litter Decomposition and Soil Organic Matter Quality. Forests, 6(3), 769-793. https://doi.org/10.3390/f6030769





