1. Introduction
White spruce (
Picea glauca (Moench) Voss) is widely distributed across North America. Its territory extends from Newfoundland, Labrador, and northern Quebec in the east to west across Canada along the northern tree limit to northwestern Alaska and south to southwestern Alaska, southern British Columbia, southern Alberta, northwestern and southeastern Manitoba, central Minnesota, central Michigan, southern Ontario, northern New York, and Maine [
1]. The wood is valuable for both lumber and pulpwood, and is therefore of vital economic importance. In eastern Canada, substantial artificial regeneration programs as well as tree improvement research and breeding programs have targeted this species. Large-scale reforestation with fast-growing genotypes is likely to shorten rotations in the near future, which may produce an increase in the proportion of juvenile wood (JW; all acronyms used in this text are listed in
Table 1), characterized by progressive changes in fiber and wood properties [
2]. Compared to mature wood (MW), JW is composed of smaller, shorter tracheids with thinner walls, larger microfibril angles, larger spiral grain angles, lower tangential and higher longitudinal shrinkage, lower holocellulose and alpha cellulose content, higher lignin and hemicellulose content, and lower strength properties [
3]. All these characteristics cause problems, including drying issues, warping, and low stiffness, which reduce the utility of JW, especially for the solid wood industry [
3,
4].
Table 1.
Acronyms used in the text and their descriptions.
Table 1.
Acronyms used in the text and their descriptions.
| Acronym | Description |
|---|
| BA | Basal area |
| CA | Cambial age |
| JW | Juvenile wood |
| JWP | Juvenile wood proportion |
| JWV | Juvenile wood volume |
| JWR (JWRh) | Juvenile wood radius (at a given height h) |
| M_Q_L | Maximum-quadratic-linear |
| MW | Mature wood |
| RMSE | Root-mean-squared error |
| TA | Transition age |
| TL | Average ring tracheid length |
| WQA | Wood quality attributes |
JW is formed under the strong regulatory influence of the active living crown, where growth hormones, particularly indole-3-acetic acid (IAA, auxin), are synthesized [
5]. Therefore, trees with a deep crown as well as the top of all trees are generally assumed to be composed entirely of JW [
6,
7]. The first step in characterizing JW is to determine the transition age (TA) from JW to MW. The radial variation patterns of several wood quality attributes (WQA) have been used to distinguish between JW and MW [
3]. However, the estimated TA for a given species depends on the WQA and the analytical method [
8,
9]. Thus, TA varies with WQA and the estimation method. For conifers, reported TA ranges from cambial age (CA) 10 to 20 years [
3]. From an industrial standpoint, TA estimates are useful for determining the appropriate timing for harvesting to ensure optimum processing and end-product quality.
Pith-to-bark variation profiles in WQA such as ring density, fiber length, fibril angle, longitudinal shrinkage, ring width, and latewood proportion are generally described in terms of JW and MW zones, and are used to estimate TA. However, as mentioned above, the estimated TA varies with WQA [
10,
11,
12,
13] and the analytical method [
8,
9]. In addition, for a given species, TA varies with tree height [
14,
15] and across locations [
16].
Several methods to estimate TA have been proposed, including visual examination of pith-to-bark profiles over the tree age [
17], mathematical approaches such as the Gompertz function [
11], and segmented regression techniques [
10,
18,
19,
20,
21]. Other methods include curve-fitting routines that produce a unique polynomial model from each pith-to-bark profile (e.g., [
13,
22]). Iterative and constrained solutions have also been used to determine the JW–MW demarcation in loblolly pine trees [
21]. Visual estimation is a simple and commonly used method to estimate TA [
23] and to verify [
8] the results of other analytical procedures, but it has the drawbacks of arbitrariness and lack of scientific reliability. Segmented models provide more reliable scientific results, but they do not account for the autocorrelation in successive growth rings [
17,
19]. Polynomial regressions generally provide a good fit to many WQA, and they can address autocorrelation with a mixed model [
13]. However, they provide a simplistic approximation that lacks biological significance.
For conifers, the estimated TA is generally similar across methods, usually varying from CA 10 to 20 years, which has been proposed as reasonable for this range [
3]. Only a few studies [
8,
9,
24] have compared analytical methods for determining TA, shedding light on analytical estimates of maturity with a given WQA using certain mathematical models. However, as the end user of the timber supply, the wood industry must understand that maturation is not just a given age determined by a given function: in more practical terms, it is the age at which the supply becomes technologically mature, or qualitatively compatible with the envisioned end product.
Problems in interpreting biological patterns as well as the multiplicity of TA models can generate confusion. To respond to these issues, Cown [
25] proposed basing TA estimation on technological thresholds for WQA. One such threshold has been used for wood density [
25], a property closely related to solid wood physical and mechanical properties and fiber product yield. Tracheid length (TL) is an important WQA for paper products because it has a significant impact on the quality of pulp and paper and fiber-based products such as wood-plastic composites and fiberboards. In addition, the TL radial pattern of variation clearly defines the JW and MW zones. A TL threshold could be useful for both optimizing fiber product properties and for determining the JW–MW demarcation. However, because TL requirements change with the end product, it is difficult to define these thresholds in raw material. A two millimeter threshold has been suggested [
26,
27,
28] for good paper tear strength, but this would require much longer tracheids in the raw material, as the length diminishes drastically during pulping. A reduction rate ranging from 8% to 45%, depending on the pulp refining method, was reported for black spruce (
Picea mariana (Mill.) B.S.P) thermomechanical pulp [
29]. Although JW can be acceptable or even preferable for writing paper, tissue, and newsprint [
30], it is unsuitable for paper that requires high tear strength, and its proportion must be carefully controlled in both Kraft [
31] and high-yield [
31] pulping.
Previous studies have determined TA with two or more models, using maximum ring density and ring area [
24], microfibril angle [
8], and modulus of elasticity [
9]. However, no study to date has compared the analytical results of TA obtained with TL. Although TA can be reliably estimated using most WQA, the use of TL provides certain advantages. TL radial variation is more consistent and less sensitive to growth rate variation than other WQA, such as specific gravity [
32]. On the other hand, measuring TL is time-consuming and expensive [
33]. The available data on estimated TA using TL for various species are presented in
Table 2.
The objective of this study was to estimate tracheid length (TL) juvenile wood (JW) to mature wood (MW) transition age (TA) and the JW proportion (JWP) in white spruce (Picea glauca (Moench) Voss) using three different approaches.
Table 2.
Reported transition age (TA) with tracheid length (TL).
Table 2.
Reported transition age (TA) with tracheid length (TL).
| Main Transition Method | Transition Age Value (Years) | Species | Source |
|---|
| Piecewise | 18 | Lodgepole pine | Mansfield,
et al. [34] |
| Piecewise | 14 | Jack pine | Fujiwaraand Yang [35] |
| Piecewise | 20 | Balsam fir | Fujiwaraand Yang [35] |
| Piecewise | 12–19 | White spruce | Fujiwaraand Yang [35], Yang [36] |
| Piecewise | 11–21 | Black spruce | Fujiwaraand Yang [35], Yang [36], Yangand Hazenberg [37] |
| Piecewise | 10–18 | Loblolly pine | Bendtsenand Senft [4], Loo, Tauerand McNew [10] |
| Visual | 16–18 | Scots pine | Fries, Ericssonand Morling [33] |
| Visual | 18–20 | Norway spruce | Kučera [15] |
| Visual | 15 | Douglas-fir | Ericksonand Harrison [38] |
4. Discussion
The entire Canadian wood sector is coping with major issues [
49], including timber shortages, rising material costs, and the low competitiveness of traditional products. To meet the growing demand for forest products and alleviate shortages, industries must now rely on improved trees from managed forests [
3,
7]. Thus, as the juvenile wood proportion (JWP) continues to increase in the timber supply, industries must learn how to deal with juvenile wood (JW). A deeper understanding of the transition age from JW to mature wood (MW) in white spruce, from both an analytical and a technological standpoint, will benefit both wood industries and researchers. As a first step, pinpointing TA, or the age at which the material is suitable for processing a given end product in the radial direction, can help delineate the tree parts and allocate them for the best end uses.
Because TA is based on traits that directly impact the wood as well as end-product properties and processing, several wood quality attributes (WQA) are used to estimate TA. For instance, tracheid length (TL) is a critical WQA for fiber products, including pulp and paper, fiber boards, and fiber-based composites. In addition, the variation in TL with age clearly defines the JW and MW zones. Thus, TL is an appropriate attribute for determining TA.
4.1. Tracheid Length Radial and Longitudinal Variation
The greater variation observed in the TL of JW compared to MW can be explained by the distance of the tracheid from the active living crown during xylogenesis [
7,
50]. The xylem synthesized by the cambium at a given height is located farther from the living crown as the tree ages. Due to the strong regulatory influence of growth hormones, particularly indole-3-acetic acid (IAA auxin), which is synthesized in the living crown [
7,
51], the wood formed near the pith is JW, characterized by greater variations and shorter tracheids than wood formed during maturity (MW) [
3].
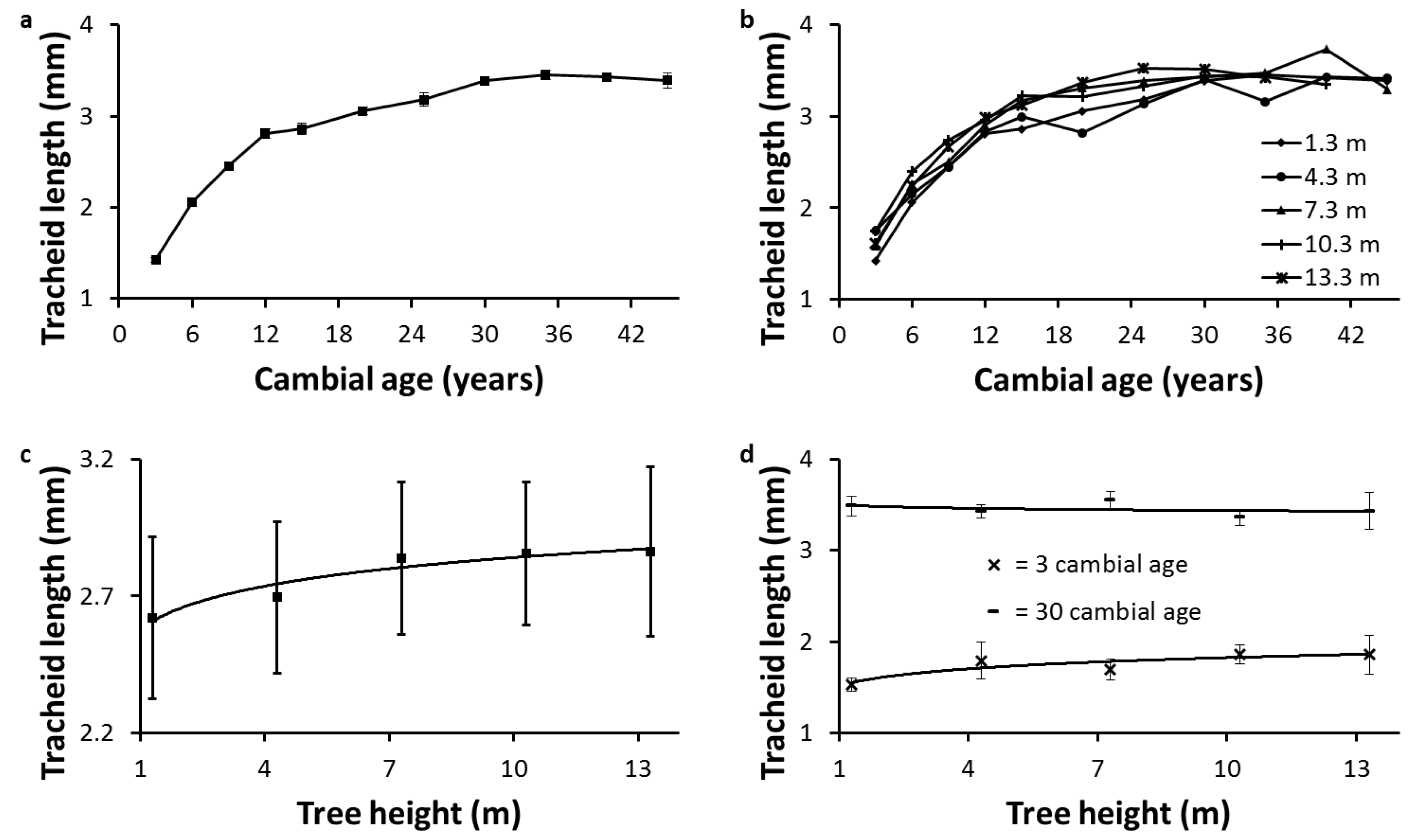
The large standard errors found for average TL (
Figure 1c) can be explained by the influence of the living crown on anatomical properties.
Table 3 shows that tree height, crown length, and length of the clear bole differed across the sampled trees. Consequently, due to differences in auxin regulation [
7], the proportion of earlywood to latewood and JW to MW differ at a given height between the trees. This is supported by the large differences in JWP observed across the trees (
Table 7).
The increase in average TL with tree height is due to many interrelated factors. The lower circumferential and radial growth rate, the lower frequency of anticlinal divisions in the cambium, and the greater cambium maturation with tree height favor longer tracheids [
51]. Additionally, longer tracheids at upper heights are believed to be an adaptation to sustain higher mechanical stresses [
52]. These results concur with previous studies on white spruce [
53,
54] and eastern white cedar [
55].
The radial pattern of TL at breast height was consistent with a previous study in five Canadian conifers, including white spruce [
35]. This radial pattern is also due to many interrelated factors, the most influential being the distance of the tracheid from the active living crown [
7,
50] and the age of the cambium during xylogenesis [
3].
4.2. Transition Age, Juvenile Wood Proportion, and Tracheid Length at the Time of Transition at Breast Height
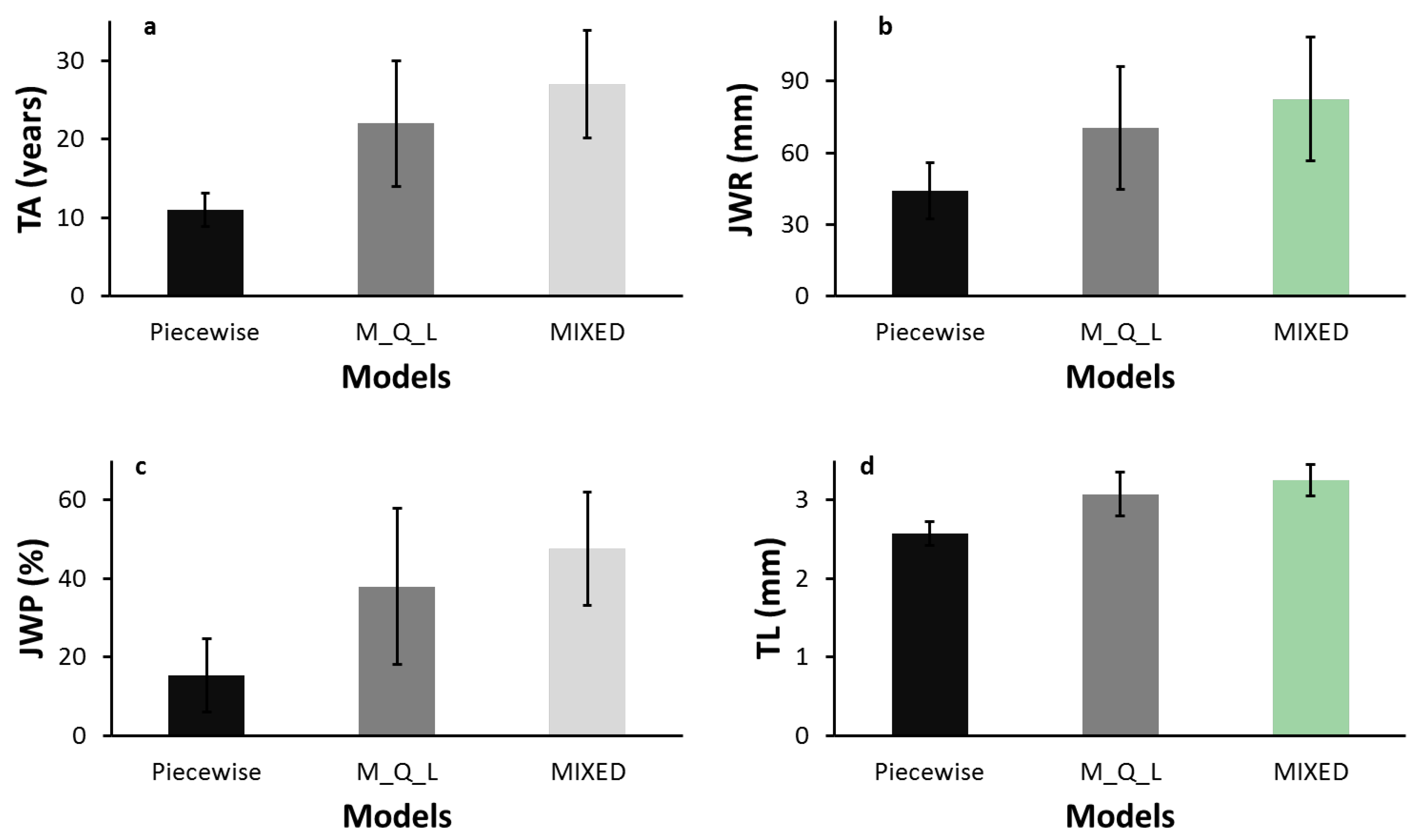
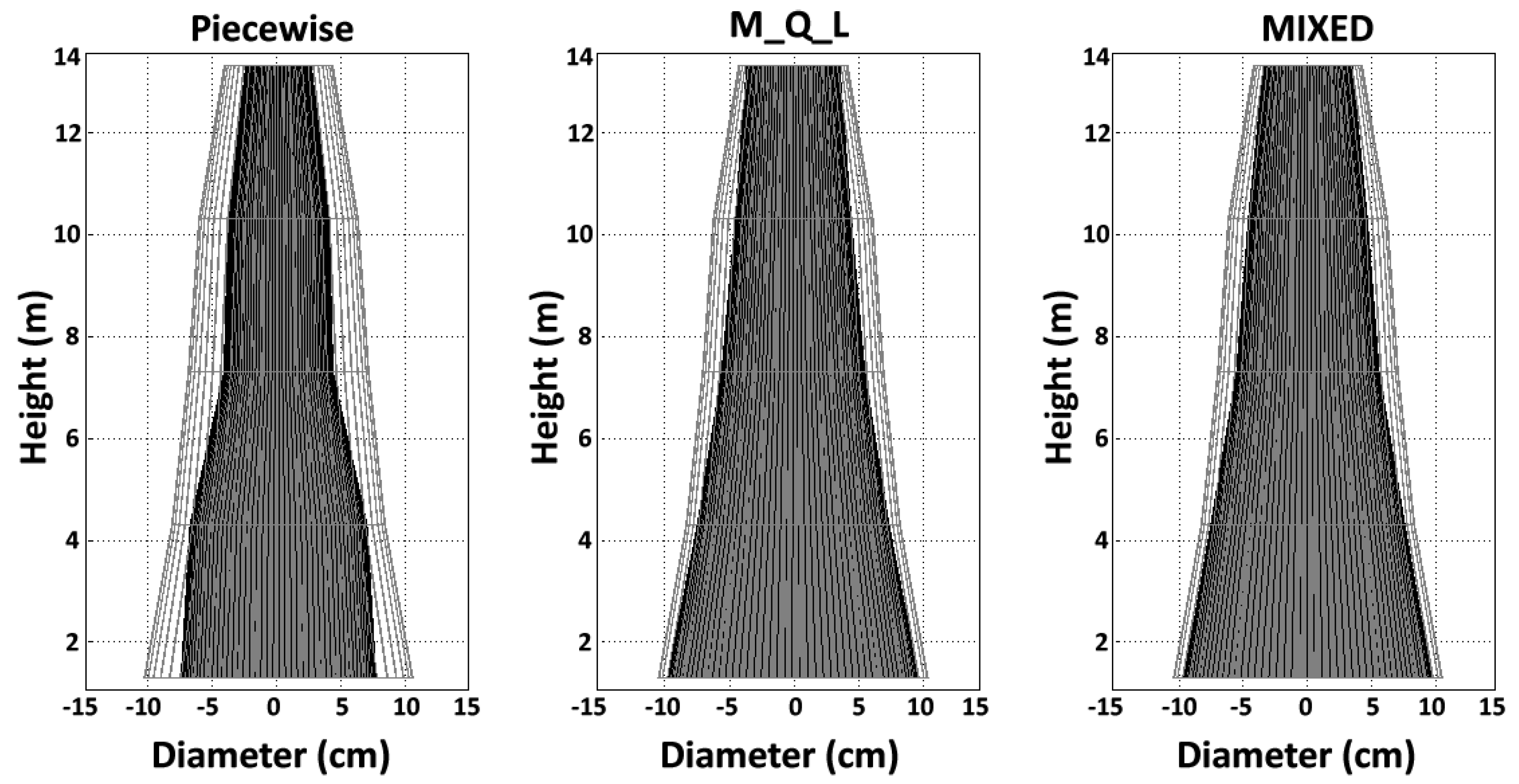
In this study, we found that the piecewise model, the maximum-quadratic-linear (M_Q_L) model, and the third-order polynomial model with the repeated structure age obtained different estimates of TA. The MIXED polynomial model provided a better fit than the segmented models. Segmented models are based on the assumption of a linear pattern in at least one segment, whereas linear polynomials are known to provide a very close fit to TL data [
56]. TA assessed from TL data corresponds to the age at which the fitted functions reached a maximum for the linear polynomial model [
13] and the M_Q_L model [
8]. Because the polynomial is third order for the MIXED model and second order for the M_Q_L model, the MIXED model estimates were higher than those for the M_Q_L model. In contrast, the piecewise model estimated TA as the point of abrupt change in the slope of the property [
35,
36,
37]. This explains why the mean TA, TL, JWR, and JWP obtained with the linear polynomial model and the M_Q_L model, although statistically different, were similar, whereas the means estimated with the piecewise model differed substantially from those of the other two models (
Table 5 and
Figure 2). The discrepancies in terms of differences were even greater.
Table 6 shows that the differences between the paired models MIXED
vs. piecewise and M_Q_L
vs. piecewise are threefold greater and twofold greater, respectively, than those between the paired models MIXED
vs. M_Q_L. Thus, the mathematical interpretation used to delineate TA determines the estimates one can expect. Therefore, even when TA is estimated on the same trees and using the same WQA, large differences may be obtained when different mathematical interpretations of maturation are used, and comparisons must be made accordingly. The TA estimation with the piecewise model using TL was consistent with those of previous studies in black spruce and white spruce [
35,
36,
37] using the same model. Similar rankings between models have been obtained for black spruce TA (MIXED > piecewise) using maximum density and ring area [
24] and for lodgepole pine TA (M_Q_L > piecewise) using microfibrils angle [
8] and modulus of elasticity [
9]. In the present study, estimates of JWR and JWP with the MIXED model were also more conservative than with the M_Q_L model, and both were more conservative than with the piecewise model. These results were expected, because JWR, JWP, and TL at the time of transition normally increase or decrease with the number of rings required to reach maturity.
Zobel and Sprague’s [
3] definition of maturity is that, “
The juvenile wood is the area of rapid change in properties near the pith; mature wood is more uniform towards the bark”. Accordingly, the MIXED estimates, and to a lesser extent the M_Q_L estimates, represented real mature wood.
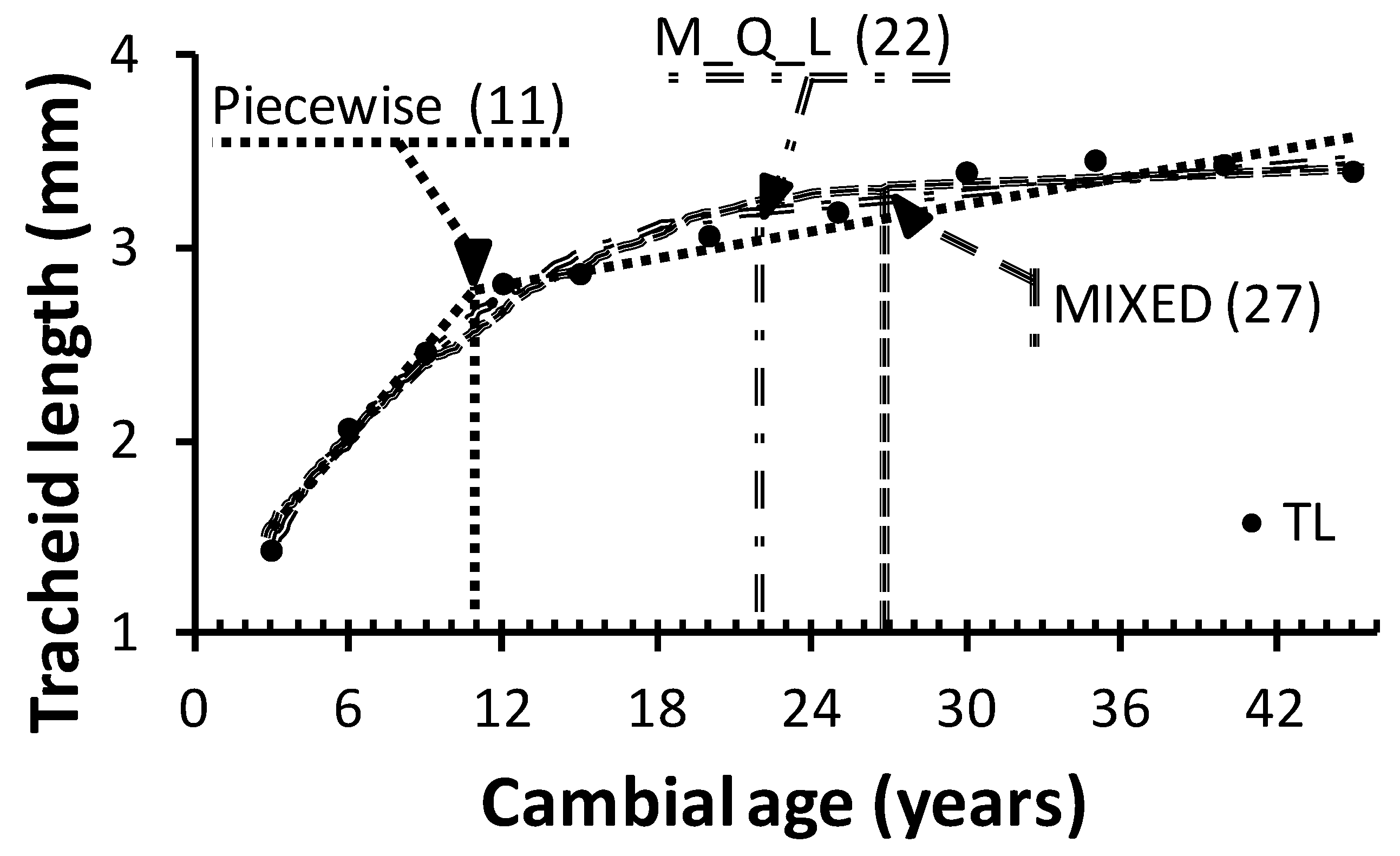
Figure 3 shows that the average TA estimated with the MIXED model coincided with the beginning of the plateau phase. In contrast, TA estimated with the piecewise model falls into the area commonly referred to as “transition wood,” where the WQA exhibit JW and MW characteristics.
In order to prevent the confusion that can arise from analytical methods, it was proposed [
25] to base TA estimation on technological thresholds of WQA. Moreover, earlier studies [
26,
27,
28] found that tracheids longer than two millimeters could be safely used to produce paper with good tear strength. However, to obtain refined pulp with this TL, the raw material must contain much longer tracheids, because the pulping process reduces the length [
29]. Therefore, even if a technological threshold were defined for the production of paper with good tear strength, it would be safer to estimate TA with the MIXED or M_Q_L model than the piecewise model. TL at the time of transition were 3.3 mm, 3.1 mm, and 2.6 mm with the MIXED, M_Q_L, and piecewise model, respectively (
Table 5). However, it must be kept in mind that the technological threshold used in the present study applies specifically to paper tear strength. Other TL safe points will be defined according to various criteria for end-product quality used in the paper industry. Similarly, WQA other than TL may be more appropriate for solid wood products and for other bioprocessing industries.
4.3. Juvenile Wood Volume, Proportion, and Shape
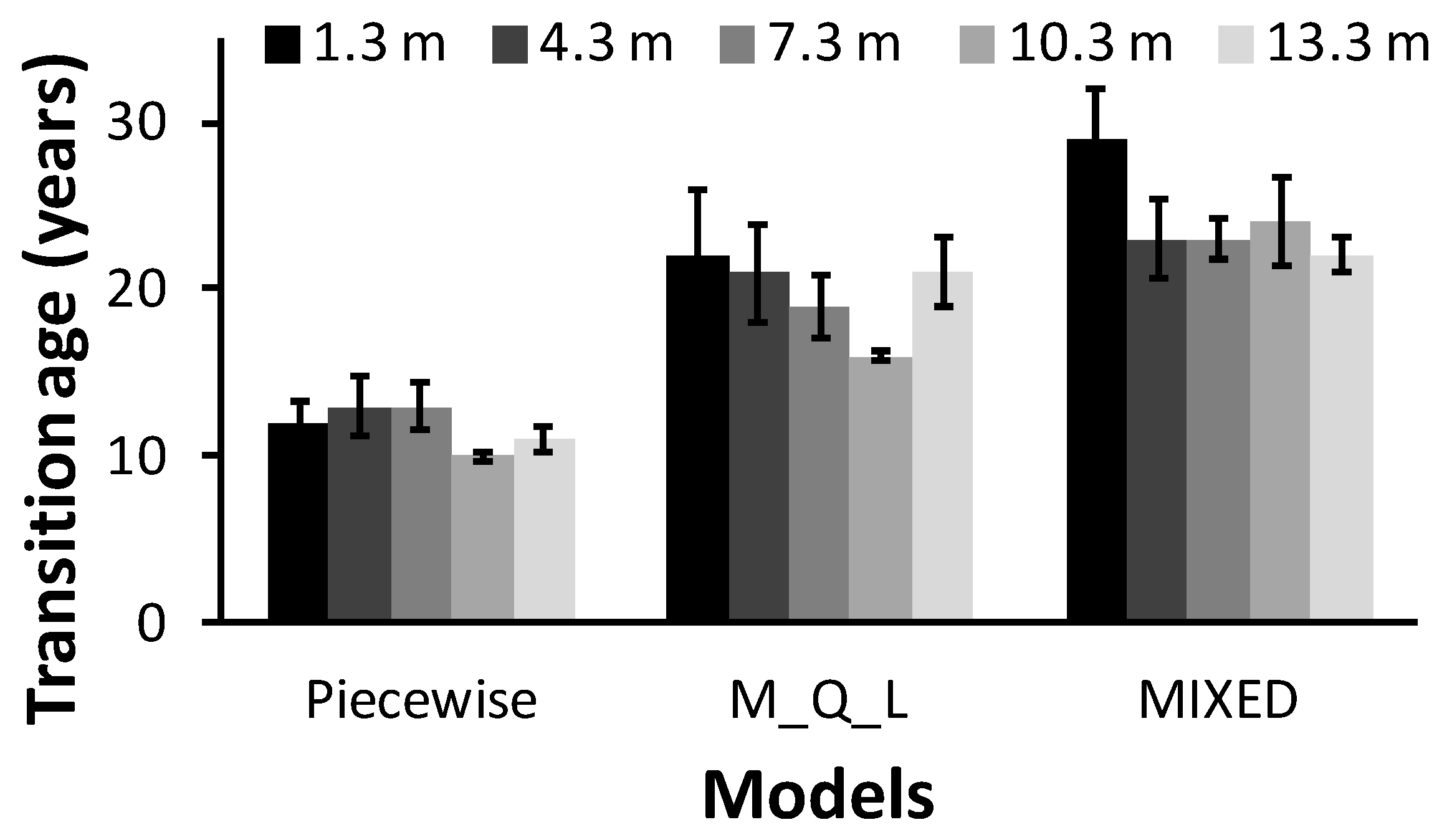
Overall, the JW proportion below the living crown was less than 50% in these 72-year-old trees according to the piecewise model, whereas the opposite was true for the MIXED model. The JWV and JWP estimates for a given tree increased according to the method: the piecewise model yielded the lowest estimates, with intermediate estimates for the M_Q_L model and the highest estimates for the MIXED model. This trend was expected, because the juvenile wood area, JWV, and JWP mirror the variation in TA. We found higher TA for models using the maximum TL (MIXED and M_Q_L) compared to the piecewise model, which considered TA as the change in the TL slope. However,
Figure 5 and
Table 7 show that the discrepancies between the MIXED and M_Q_L models were far smaller than the differences between each of these models and the piecewise model. The M_Q_L and piecewise results concur with those of recent studies that estimated lodgepole pine TA based on microfibril angle [
8] and modulus of elasticity [
9]. The decreasing pattern of TA with increasing tree height is due to tracheids having characteristics of MW earlier at the top portions of trees than at lower heights [
27,
28,
57]. Many interrelated factors, particularly the lower circumferential and radial growth rate, the lower frequency of anticlinal division of cambial initials, along with their greater age at upper height, favor longer tracheids [
51]. In fact, the cambial initials, when considered as tissue, are as old as the tree [
51], and their length was found to increase with age, even for long-living trees [
58]. Therefore, even if tracheids in the apex of a 72-year-old tree (with cambial initial tissue that is 72 years old) are located in the JW zone (there is only one ring at the apex, and the tracheids are therefore in the first cambial ring) [
3], they will be longer than tracheids in the first cambial ring at ground level (where the cambial initial tissue was one-year-old at the time of tracheid formation) [
51]. However, even if tracheids located in the apex of a mature tree are longer than tracheids at lower height, they are in the JW zone due to the influence of the active living crown [
7]. These results concur with previous studies [
14,
59]. The use of Newton’s formula for section scaling accurately captured the intra-tree variation in the JW shape: in the present study, we found a conical shape. Therefore, although the notion of a juvenile cylinder core within the tree trunk offers a plausible explanation for JW formation, it is more appropriately represented as a cone shape, in agreement with previous reports [
14,
59].
5. Conclusions
Tracheid length (TL) in white spruce followed Sanio’s law, increasing from pith to bark and from breast height to upper heights. TL was also shorter in juvenile wood (JW) compared to mature wood (MW). Transition age (TA) was estimated with three different statistical models: two two-segment regression models (a piecewise model and a maximum-quadratic-linear model–M_Q_L) and one third-order polynomial regression model (MIXED model). Average TA and the associated JW proportion (JWP) at breast height occurred at 11 years (15.3%), 22 years (37.9%), and 27 years (47.5%) for the piecewise, M_Q_L, and MIXED models, respectively. We found a conical shape for JW using Newton’s formula, regardless of the model investigated. The MIXED model was more conservative than the M_Q_L model, and both were more conservative than the piecewise model in estimating TA, JW radius (JWR), and JWP. Estimates of TA, JWP, and TL at the time of transition differed significantly between models. Model fit diagnostics using the root-mean-squared error indicated that the MIXED model provided the best fit to the data, followed by the M_Q_L, with weaker fit provided by the piecewise model. Maturation age appeared to depend on the mathematical interpretation of TA, suggesting that the confusion surrounding TA in the literature could be lessened if results were compared using not only the same species and wood quality attributes (WQA), but in particular the same mathematical interpretation of the JW–MW transition. From a practical standpoint, TA estimated with the MIXED and M_Q_L models appear to be more appropriate for producing paper with good tear strength. Because each end product has specific criteria for the determination of good raw material, the use of technological thresholds combined with analytical determination of TA, which are relevant to both the scientific and industrial communities, should be promoted in futures studies.