Effects of Biochar Application on Nitrogen Fixation and Water Use Efficiency of Understorey Acacia Species as well as Soil Carbon and Nitrogen Pools in a Subtropical Native Forest
Abstract
1. Introduction
2. Materials and Methods
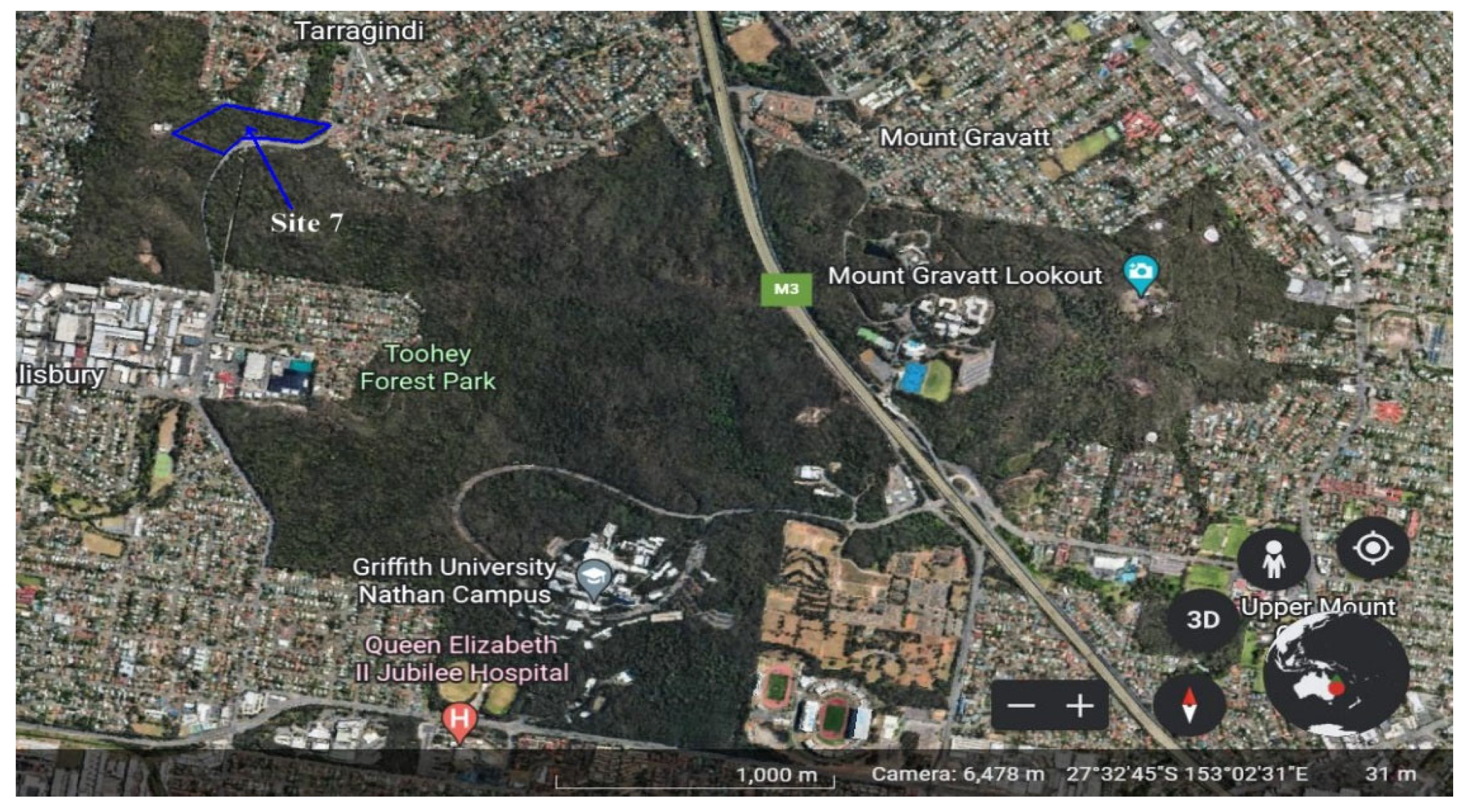
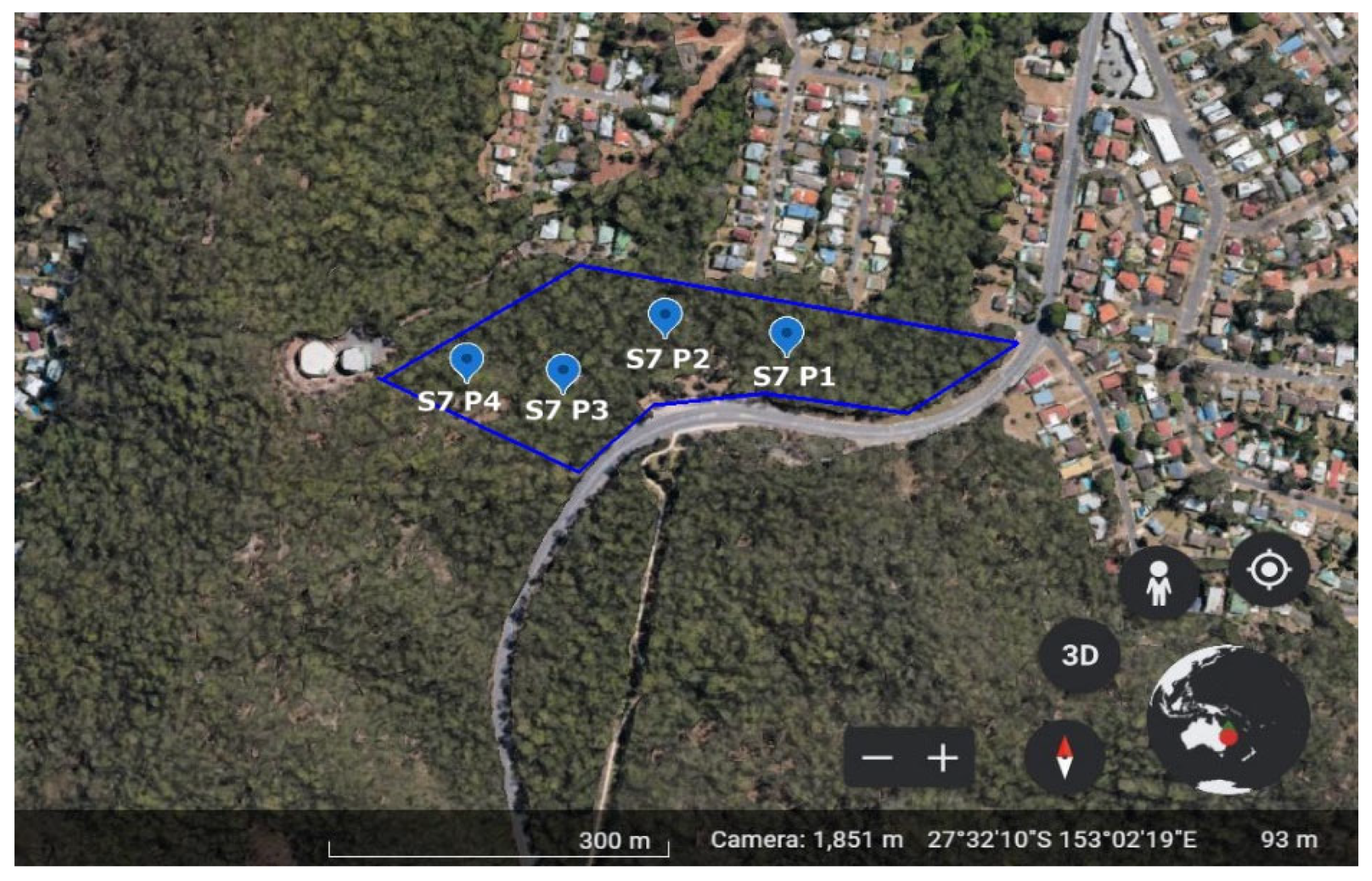
2.1. Site Description
2.2. Biochar Production and Characterisation
2.3. Experimental Design and Treatments
2.4. Sample Collection and Analysis
2.4.1. Foliar Sample Collection and Analysis
- Rsample represents the ratio of 15N/14N and 13C/12C of the samples.
- Rstd represents the ratio of 15N/14N of the international standard (atmospheric N2).
- RVPDB represents the ratio of 13C/12C of the international standard (Vienna Peedee Belemnite (VPDB)).
2.4.2. Acacia Species Measurement
2.4.3. Soil Sample Collection and Analysis
2.5. Statistical Analysis
3. Results
3.1. Foliar C and N as well as BNF of Acacia Species
3.2. Plant Growth
3.3. Soil C and N Pools
3.3.1. 0–5 cm Depth
3.3.2. 5–10 cm Depth
3.3.3. 10–20 cm Depth
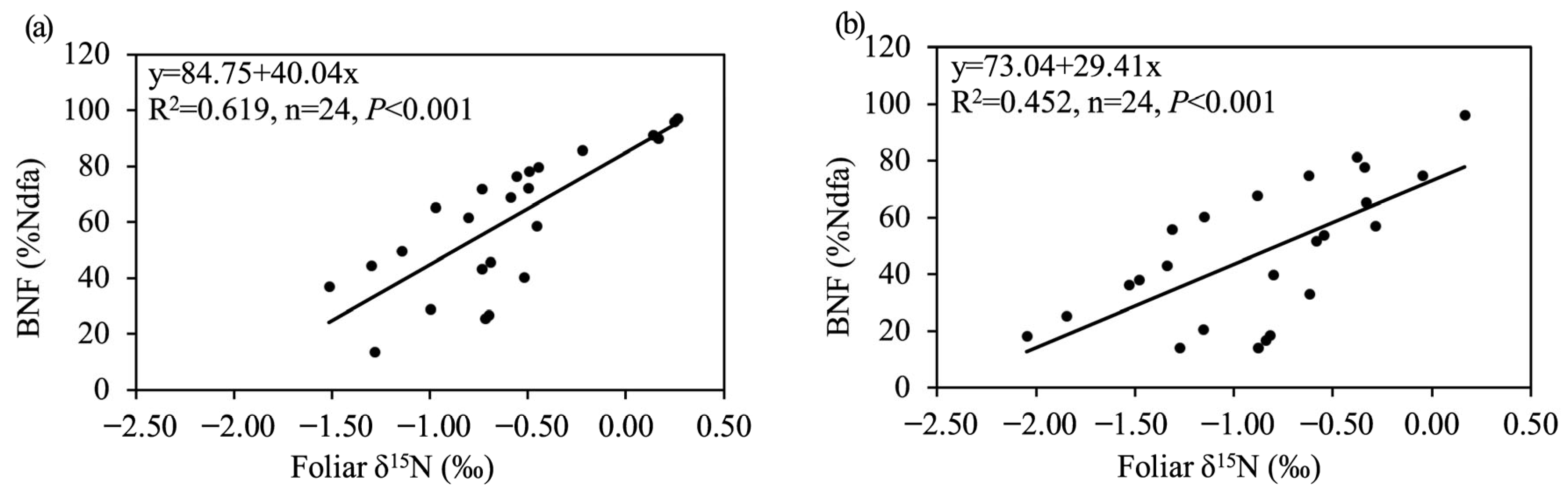
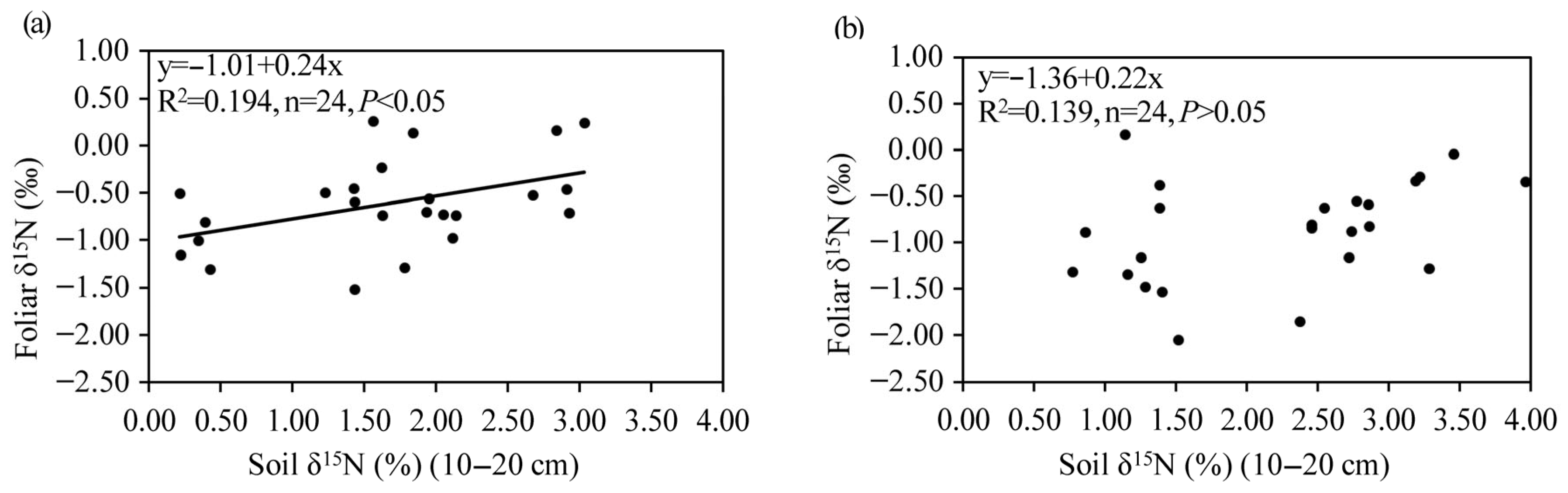
3.4. Relationships Among Foliar Properties, BNF, and Soil Properties
4. Discussion
4.1. Biochar Effects on BNF and WUE of Acacia Species
4.2. Acacia Species Differences in Foliar δ15N and BNF
4.3. Acacia Species Effects on Soil N Pools
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Succarie, A.; Xu, Z.; Wang, W.; Liu, T.; Zhang, X.; Cao, X. Effects of climate change on tree water use efficiency, nitrogen availability and growth in boreal forest of northern China. J. Soils Sediments 2020, 20, 3607–3614. [Google Scholar] [CrossRef]
- Wang, D.; Abdullah, K.M.; Tahmasbian, I.; Xu, Z.; Wang, W. Impacts of prescribed burnings on litter production, nitrogen concentration, δ13C and δ15N in a suburban eucalypt natural forest of subtropical Australia. J. Soils Sediments 2020, 20, 3148–3157. [Google Scholar] [CrossRef]
- Cui, J.; Zheng, M.; Bian, Z.; Pan, N.; Tian, H.; Zhang, X.; Qiu, Z.; Xu, J.; Gu, B. Elevated CO2 levels promote both carbon and nitrogen cycling in global forests. Nat. Clim. Change 2024, 14, 511–517. [Google Scholar] [CrossRef]
- Bai, S.H.; Sun, F.; Xu, Z.; Blumfield, T.J. Ecophysiological status of different growth stage of understorey Acacia leiocalyx and Acacia disparrima in an Australian dry sclerophyll forest subjected to prescribed burning. J. Soils Sediments 2013, 13, 1378–1385. [Google Scholar] [CrossRef]
- Butler, O.M.; Rashti, M.R.; Lewis, T.; Elser, J.J.; Chen, C. High-frequency fire alters soil and plant chemistry but does not lead to nitrogen-limited growth of Eucalyptus pilularis seedlings. Plant Soil 2018, 432, 191–205. [Google Scholar] [CrossRef]
- Muqaddas, B.; Lewis, T.; Esfandbod, M.; Chen, C. Responses of labile soil organic carbon and nitrogen pools to long-term prescribed burning regimes in a wet sclerophyll forest of southeast Queensland, Australia. Sci. Total Environ. 2019, 647, 110–120. [Google Scholar] [CrossRef]
- Taresh, S.; Bai, S.H.; Kichamu-Wachira, E.; Xu, Z. Impact of biochar addition on water use efficiency, biological nitrogen fixation and growth of understory Acacia leiocalyx and Acacia disparimma in a suburban native forest of subtropical Australia. J. Soils Sediments 2025. early access. [Google Scholar] [CrossRef]
- Taresh, S.; Bai, S.H.; Abdullah, K.M.; Zalucki, J.; Nessa, A.; Omidvar, N.; Wang, D.; Zhan, J.; Wang, F.; Yang, J.; et al. Long-term impact of prescribed burning on water use efficiency, biological nitrogen fixation, and tree growth of understory acacia species in a suburban forest ecosystem of subtropical Australia. J. Soils Sediments 2021, 21, 3620–3631. [Google Scholar] [CrossRef]
- Reverchon, F.; Kadum, M.A.; Bai, S.H.; Villafán, E.; Blumfield, T.J.; Patel, B.; Xu, Z. Biological nitrogen fixation by two Acacia species and associated root-nodule bacteria in a suburban Australian forest subjected to prescribed burning. J. Soils Sediments 2019, 20, 122–132. [Google Scholar] [CrossRef]
- West, J.B.; HilleRisLambers, J.; Lee, T.D.; Hobbie, S.E.; Reich, P.B. Legume species identity and soil nitrogen supply determine symbiotic nitrogen-fixation responses to elevated atmospheric [CO2]. New Phytol. 2005, 167, 523–530. [Google Scholar] [CrossRef]
- Bai, S.H.; Sun, F.; Xu, Z.; Blumfield, J.; Chen, C.R.; Wild, C. Appraisal of 15N enrichment and 15N natural abundance methods for estimating N2 fixation by understorey Acacia leiocalyx and A. disparimma in a native forest of subtropical Australia. J. Soils Sediments 2012, 12, 653–662. [Google Scholar] [CrossRef]
- Jesus, J.G.d.; Tenreiro, R.; Máguas, C.; Trindade, H. Acacia longifolia: A Host of Many Guests Even After Fire. Diversity 2020, 12, 250. [Google Scholar] [CrossRef]
- Fernández-Fernández, M.; Rütting, T.; González-Prieto, S. Effects of a high-severity wildfire and post-fire straw mulching on gross nitrogen dynamics in Mediterranean shrubland soil. Geoderma 2017, 305, 328–335. [Google Scholar] [CrossRef]
- Ramlow, M.; Rhoades, C.C.; Cotrufo, M.F. Promoting revegetation and soil carbon sequestration on decommissioned forest roads in Colorado, USA: A comparative assessment of organic soil amendments. For. Ecol. Manag. 2018, 427, 230–241. [Google Scholar] [CrossRef]
- Rhoades, C.C.; Battaglia, M.A.; Rocca, M.E.; Ryan, M.G. Short- and medium-term effects of fuel reduction mulch treatments on soil nitrogen availability in Colorado conifer forests. For. Ecol. Manag. 2012, 276, 231–238. [Google Scholar] [CrossRef]
- Laiho, R.; Prescott, C.E. Decay and nutrient dynamics of coarse woody debris in northern coniferous forests: A synthesis. Canadian J. For. Res. 2004, 34, 763–777. [Google Scholar] [CrossRef]
- Bai, S.H.; Reverchon, F.; Xu, C.Y.; Xu, Z.; Blumfield, T.J.; Zhao, H.; Van Zwieten, L.; Wallace, H.M. Wood biochar increases nitrogen retention in field settings mainly through abiotic processes. Soil Biol. Biochem. 2015, 90, 232–240. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Noack, A.G. Black carbon in soils and sediments: Analysis, distribution, implications, and current challenges. Glob. Biogeochem. Cycl. 2000, 14, 777–793. [Google Scholar] [CrossRef]
- Downie, A.; Crosky, A.; Munroe, P. Physical properties of biochar. In Biochar for Environmental Management, 1st ed.; Lehmann, J., Joseph, S., Eds.; Routledge: London, UK, 2012; pp. 45–64. [Google Scholar]
- Nguyen, T.T.N.; Xu, C.Y.; Tahmasbian, I.; Che, R.; Xu, Z.; Zhou, X.; Wallace, H.M.; Bai, S.H. Effects of biochar on soil available inorganic nitrogen: A review and meta-analysis. Geoderma 2017, 288, 79–96. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Bahar, M.M.; Sarkar, B.; Donne, S.W.; Ok, Y.S.; Palansooriya, K.N.; Kirkham, M.B.; Chowdhury, S.; Bolan, N. Biochar and its importance on nutrient dynamics in soil and plant. Biochar 2020, 2, 379–420. [Google Scholar] [CrossRef]
- Asadyar, L.; Xu, C.Y.; Wallace, H.M.; Xu, Z.; Reverchon, F.; Bai, S.H. Soil-plant nitrogen isotope composition and nitrogen cycling after biochar applications. Environ. Sci. Pollut. Res. 2021, 28, 6684–6690. [Google Scholar] [CrossRef]
- Zackrisson, O.; DeLuca, T.H.; Nilsson, M.C.; Sellstedt, A.; Berglund, L.M. Nitrogen Fixation Increases with Successional Age in Boreal Forests. Ecology 2004, 85, 3327–3334. [Google Scholar] [CrossRef]
- DeLuca, T.H.; Zackrisson, O.; Gentili, F.; Sellstedt, A.; Nilsson, M.C. Ecosystem controls on nitrogen fixation in boreal feather moss communities. Oecologia 2007, 152, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh-Toosi, A.; Clough, T.J.; Sherlock, R.R.; Condron, L.M. A wood based low-temperature biochar captures NH3-N generated from ruminant urine-N, retaining its bioavailability. Plant Soil 2011, 353, 73–84. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.K.; Thomas, B.W.; Sachdeva, V.; Deng, H. Physico-chemical properties and microbial responses in biochar-amended soils: Mechanisms and future directions. Agric. Ecosyst. Environ. 2015, 206, 46–59. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.K. Biochemical cycling of nitrogen and phosphorus in biochar-amended soils. Soil Biol. Biochem. 2016, 103, 1–15. [Google Scholar] [CrossRef]
- Razzaghi, F.; Obour, P.B.; Arthur, E. Does biochar improve soil water retention? A systematic review and meta-analysis. Geoderma 2020, 361, 114055. [Google Scholar] [CrossRef]
- Xiang, Y.; Deng, Q.; Duan, H.; Guo, Y. Effects of biochar application on root traits: A meta-analysis. GCB Bioenergy 2017, 9, 1563–1572. [Google Scholar] [CrossRef]
- Xiu, L.; Zhang, W.; Wu, D.; Sun, Y.; Zhang, H.; Gu, W.; Wang, Y.; Meng, J.; Chen, W. Biochar can improve biological nitrogen fixation by altering the root growth strategy of soybean in Albic soil. Sci. Total Environ. 2021, 773, 144564. [Google Scholar] [CrossRef]
- Li, Y.; Hu, S.; Chen, J.; Müller, K.; Li, Y.; Fu, W.; Lin, Z.; Wang, H. Effects of biochar application in forest ecosystems on soil properties and greenhouse gas emissions: A review. J. Soils Sediments 2017, 18, 546–563. [Google Scholar] [CrossRef]
- Rondon, M.; Lehmann, J.; Ramírez, J.; Hurtado, M. Biological nitrogen fixation by common beans (Phaseolus vulgaris L.) increases with bio-char additions. Biol. Ferti. Soils 2006, 43, 699–708. [Google Scholar] [CrossRef]
- Mia, S.; van Groenigen, J.W.; van de Voorde, T.F.J.; Orama, N.J.; Bezemer, T.M.; Mommer, L.; Jeffery, S. Biochar application rate affects biological nitrogen fixation in red clover conditional on potassium availability. Agric. Ecosyst. Environ. 2014, 191, 83–91. [Google Scholar] [CrossRef]
- Horel, A.; Gelybo, G.; Potyo, I.; Pokovai, K.; Bakacsi, Z. Soil Nutrient Dynamics and Nitrogen Fixation Rate Changes over Plant Growth in Temperate Soil. Agronomy 2019, 9, 179. [Google Scholar] [CrossRef]
- Farhangi-Abriz, S.; Ghassemi-Golezani, K.; Torabian, S.; Qin, R. A meta-analysis to estimate the potential of biochar in improving nitrogen fixation and plant biomass of legumes. Biom. Conv. Bioref. 2022, 14, 3293–3303. [Google Scholar] [CrossRef]
- Premalatha, R.P.; Poorna Bindu, J.; Nivetha, E.; Malarvizhi, P.; Manorama, K.; Parameswari, E.; Davamani, V. A review on biochar’s effect on soil properties and crop growth. Front. Energy Res. 2023, 11, 1092637. [Google Scholar] [CrossRef]
- Nelson, J.A.; Morgan, J.A.; LeCain, D.R.; Mosier, A.R.; Milchunas, D.G.; Parton, B.A. Elevated CO2 increases soil moisture and enhances plant water relations in a long-term field study in semiarid shortgrass steppe of Colorado. Plant Soil 2004, 259, 169–179. [Google Scholar] [CrossRef]
- Liu, T.; Xu, Y.; Xu, Z.; Deng, H. Effects of climate change and local environmental factors on long-term tree water-use efficiency and growth of Pseudolarix amabilis and Cryptomeria japonica in subtropical China. J. Soils Sediments 2021, 21, 869–880. [Google Scholar] [CrossRef]
- Xu, Z.; Saffigna, P.G.; Farquhar, G.D.; Simpson, J.A.; Haines, R.J.; Walker, S.; Osborne, D.O.; Guinto, D. Carbon isotope discrimination and oxygen isotope composition in clones of the F1 hybrid between slash pine and Caribbean pine in relation to tree growth, water-use efficiency and foliar nutrient concentration. Tree Physiol. 2000, 20, 1209–1217. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Richards, R.A. Isotopic composition of plant carbon correlates with water-use efficiency in wheat genotypes. Aust. J. Plant Physiol. 1984, 11, 539–552. [Google Scholar] [CrossRef]
- Gao, Y.; Shao, G.; Lu, J.; Zhang, K.; Wu, S.; Wang, Z. Effects of biochar application on crop water use efficiency depend on experimental conditions: A meta-analysis. Field Crops Res. 2020, 249, 107763. [Google Scholar] [CrossRef]
- Hussain, R.; Bordoloi, S.; Gupta, P.; Garg, A.; Ravi, K.; Sreedeep, S.; Sahoo, L. Effect of biochar type on infiltration, water retention and desiccation crack potential of a silty sand. Biochar 2020, 2, 465–478. [Google Scholar] [CrossRef]
- Novak, J.M.; Lima, I.; Xing, B.; Gaskin, J.W.; Steiner, C.; Das, K.C.; Ahmedna, M.; Rehrah, D.; Watts, D.W.; Busscher, W.J.; et al. Characterization of designer biochar produced at different temperatures and their effects on a loamy sand. pdf. Ann. Environ. Sci. 2009, 3, 195–206. [Google Scholar]
- Streubel, J.D.; Collins, H.P.; Garcia-Perez, M.; Tarara, J.; Granatstein, D.; Kruger, C.E. Influence of Contrasting Biochar Types on Five Soils at Increasing Rates of Application. Soil Sci. Soc. Am. J. 2011, 75, 1402–1413. [Google Scholar] [CrossRef]
- Abel, S.; Peters, A.; Trinks, S.; Schonsky, H.; Facklam, M.; Wessolek, G. Impact of biochar and hydrochar addition on water retention and water repellency of sandy soil. Geoderma 2013, 202–203, 183–191. [Google Scholar] [CrossRef]
- Rogovska, N.; Laird, D.A.; Rathke, S.J.; Karlen, D.L. Biochar impact on Midwestern Mollisols and maize nutrient availability. Geoderma 2014, 230–231, 340–347. [Google Scholar] [CrossRef]
- Qian, Z.; Tang, L.; Zhuang, S.; Zou, Y.; Fu, D.; Chen, X. Effects of biochar amendments on soil water retention characteristics of red soil at south China. Biochar 2020, 2, 479–488. [Google Scholar] [CrossRef]
- Artiola, J.F.; Rasmussen, C.; Freitas, R. Effects of a Biochar-Amended Alkaline Soil on the Growth of Romaine Lettuce and Bermudagrass. Soil Sci. 2012, 177, 561–570. [Google Scholar] [CrossRef]
- Aller, D.; Rathke, S.; Laird, D.; Cruse, R.; Hatfield, J. Impacts of fresh and aged biochars on plant available water and water use efficiency. Geoderma 2017, 307, 114–121. [Google Scholar] [CrossRef]
- Reyes-Cabrera, J.; Leon, R.G.; Erickson, J.E.; Rowland, D.L.; Silveira, M.L.; Morgan, K.T. Differences in biomass and water dynamics between a cotton-peanut rotation and a sweet sorghum bioenergy crop with and without biochar and vinasse as soil amendments. Field Crops Res. 2017, 214, 123–130. [Google Scholar] [CrossRef]
- Licht, J.; Smith, N. The influence of lignocellulose and hemicellulose biochar on photosynthesis and water use efficiency in seedlings from a Northeastern U.S. pine-oak ecosystem. J. Sustain. For. 2017, 37, 25–37. [Google Scholar] [CrossRef]
- Langeroodi, A.R.S.; Campiglia, E.; Mancinelli, R.; Radicetti, E. Can biochar improve pumpkin productivity and its physiological characteristics under reduced irrigation regimes? Sci. Horti. 2019, 247, 195–204. [Google Scholar] [CrossRef]
- Kumar, A.; Bhattacharya, T.; Mukherjee, S.; Sarkar, B. A perspective on biochar for repairing damages in the soil–plant system caused by climate change-driven extreme weather events. Biochar 2022, 4, 22. [Google Scholar] [CrossRef]
- Page-Dumroese, D.S.; Busse, M.D.; Archuleta, J.G.; McAvoy, D.; Roussel, E. Methods to Reduce Forest Residue Volume After Timber Harvesting and Produce Black Carbon. Scientifica 2017, 2017, 2745764. [Google Scholar] [CrossRef]
- Berglund, L.; DeLuca, T.H.; Zackrisson, O. Activated carbon amendments to soil alters nitrification rates in Scots pine forests. Soil Biol. Biochem. 2004, 36, 2067–2073. [Google Scholar] [CrossRef]
- DeLuca, T.H.; MacKenzie, M.D.; Gundale, M.J.; Holben, W.E. Wildfire-Produced Charcoal Directly Influences Nitrogen Cycling in Ponderosa Pine Forests. Soil Sci. Soc. Am. J. 2006, 70, 448–453. [Google Scholar] [CrossRef]
- Ball, P.N.; MacKenzie, M.D.; DeLuca, T.H.; Montana, W.E.H. Wildfire and charcoal enhance nitrification and ammonium-oxidizing bacterial abundance in dry montane forest soils. J. Environ. Qual. 2010, 39, 1243–1253. [Google Scholar] [CrossRef]
- Stavi, I. Biochar use in forestry and tree-based agro-ecosystems for increasing climate change mitigation and adaptation. Int. J. Sustain. Develop. World Ecol. 2013, 20, 166–181. [Google Scholar] [CrossRef]
- Thomas, S.C.; Gale, N. Biochar and forest restoration: A review and meta-analysis of tree growth responses. New For. 2015, 46, 931–946. [Google Scholar] [CrossRef]
- Palviainen, M.; Berninger, F.; Bruckman, V.J.; Köster, K.; deAssumpção, C.R.M.; Aaltonen, H.; Makita, N.; Mishra, A.; Kulmala, L.; Adamczyk, B.; et al. Effects of biochar on carbon and nitrogen fluxes in boreal forest soil. Plant Soil 2018, 425, 71–85. [Google Scholar] [CrossRef]
- Gundale, M.J.; Nilsson, M.C.; Pluchon, N.; Wardle, D.A. The effect of biochar management on soil and plant community properties in a boreal forest. GCB Bioenergy 2015, 8, 777–789. [Google Scholar] [CrossRef]
- Luo, Y.; Yu, Z.; Zhang, K.; Xu, J.; Brookes, P.C. The properties and functions of biochars in forest ecosystems. J. Soils Sediments 2016, 16, 2005–2020. [Google Scholar] [CrossRef]
- Shan, S.; Coleman, M.D. Biochar influences nitrogen availability in Andisols of north Idaho forests. SN App. Sci. 2020, 2, 362. [Google Scholar] [CrossRef]
- Ohtsuka, T.; Tomotsune, M.; Ando, M.; Tsukimori, Y.; Koizumi, H.; Yoshitake, S. Effects of the Application of Biochar to Plant Growth and Net Primary Production in an Oak Forest. Forests 2021, 12, 152. [Google Scholar] [CrossRef]
- Yu, M.; Liang, S.; Dai, Z.; Li, Y.; Luo, Y.; Tang, C.; Xu, J. Plant material and its biochar differ in their effects on nitrogen mineralization and nitrification in a subtropical forest soil. Sci. Total Environ. 2021, 763, 143048. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.Z.; Xu, Z.; Fu, L. Impacts of prescribed burning on soil greenhouse gas fluxes in a suburban native forest of south-eastern Queensland, Australia. Biogeosciences 2015, 12, 6279–6290. [Google Scholar] [CrossRef]
- Catterall, C.; Piper, S.; Bunn, S.E.; Arthur, J.M. Flora and fauna assemblages vary with local topography in a subtropical eucalypt forest. Austral. Ecol. 2001, 26, 56–69. [Google Scholar] [CrossRef]
- Yang, J.; Zhan, J.; Taresh, S.; Nessa, A.; Sun, W.; Wu, Q.; Xu, Z. Short-term responses of soil carbon and nitrogen pools as well as their isotopic compositions to biochar applications in a suburban forest in subtropical Australia subjected to prescribed burning. J. Soils Sediments 2023, 23, 1473–1484. [Google Scholar] [CrossRef]
- Nessa, A.; Bai, S.H.; Wang, D.; Karim, Z.; Omidvar, N.; Zhan, J.; Xu, Z. Soil nitrification and nitrogen mineralization responded non-linearly to the addition of wood biochar produced under different pyrolysis temperatures. J. Soils Sediments 2021, 21, 3813–3824. [Google Scholar] [CrossRef]
- Yang, L.; Liu, N.; Ren, H.; Wang, J. Facilitation by two exotic Acacia: Acacia auriculiformis and Acacia mangium as nurse plants in South China. For. Ecol. Manag. 2009, 257, 1786–1793. [Google Scholar] [CrossRef]
- Witt, G.B.; English, N.B.; Balanzategui, D.; Hua, Q.; Gadd, P.; Heijnis, H.; Bird, M.I. The climate reconstruction potential of Acacia cambagei (gidgee) for semi-arid regions of Australia using stable isotopes and elemental abundances. J. Arid Environ. 2017, 136, 19–27. [Google Scholar] [CrossRef]
- Page-Dumroese, D.S.; Coleman, M.D.; Thomas, S.C. Opportunities and Uses of Biochar on Forest Sites in North America. In Biochar—A Regional Supply Chain Approach in View of Climate Change Mitigation, 1st ed.; Bruckman, V.J., Varol, E.A., Uzun, B.B., Liu, J., Eds.; Cambridge University Press: Cambridge, UK, 2016; pp. 315–335. [Google Scholar] [CrossRef]
- Bruckman, V.J.; Klinglmüller, M.; Milenković, M. Biochar in the View of Climate Change Mitigation: The FOREBIOM Experience. In Biochar—A Regional Supply Chain Approach in View of Climate Change Mitigation, 1st ed.; Bruckman, V.J., Varol, E.A., Uzun, B.B., Liu, J., Eds.; Cambridge University Press: Cambridge, UK, 2016; pp. 1–22. [Google Scholar] [CrossRef]
- Williams, M.M.; Arnott, J.C. A comparison of variable economic costs associated with two proposed biochar application methods. Ann. Environ. Sci. 2010, 4, 23–30. [Google Scholar]
- Boddey, R.M.; Peoples, M.B.; Palmer, B.; Dart, P.J. Use of the 15N natural abundance technique to quantify biological nitrogen fixation by woody perennials. Nutr. Cycl. Agroecosyst. 2000, 57, 235–270. [Google Scholar] [CrossRef]
- Unkovich, M.J.; Pate, J.S. An appraisal of recent field measurements of symbiotic N2 fixation by annual legumes. Field Crops Res. 2000, 65, 211–228. [Google Scholar] [CrossRef]
- Gehring, C.; Vlek, P.L.G. Limitations of the 15N natural abundance method for estimating biological nitrogen fixation in Amazonian forest legumes. Basic Appl. Ecol. 2004, 5, 567–580. [Google Scholar] [CrossRef]
- Li, Y.; Sun, W.; Xu, Z.; Bai, Y.; Bai, S.H. Long-term effects of biochar application on biological nitrogen fixation of acacia species and soil carbon and nitrogen pools in an Australian subtropical native forest. J. Soils Sediments 2024, 24, 1956–1968. [Google Scholar] [CrossRef]
- Shearer, G.; Kohl, D.H. N2-fixation in field settings: Estimations based on natural 15N abundance. Aust. J. Plant Physiol. 1986, 13, 699–756. [Google Scholar]
- Chen, C.R.; Xu, Z. Soil carbon and nitrogen pools and microbial properties in a 6-year-old slash pine plantation of subtropical Australia: Impacts of harvest residue management. For. Ecol. Manag. 2005, 206, 237–247. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Y.; Liu, B.; Amonette, J.E.; Lin, Z.; Liu, G.; Ambus, P.; Xie, Z. How does biochar influence soil N cycle? A meta-analysis. Plant Soil 2018, 426, 211–225. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Q.; Li, X.; Ai, W.; Liu, D.; Qi, W.; Zhang, M.; Yang, C.; Liao, H. Characterization of Genetic Basis on Synergistic Interactions Between Root Architecture and Biological Nitrogen Fixation in Soybean. Front. Plant Sci. 2017, 8, 1466. [Google Scholar] [CrossRef]
- Poole, P.; Ramachandran, V.; Terpolilli, J. Rhizobia: From saprophytes to endosymbionts. Nat. Rev. Microbiol. 2018, 16, 291–303. [Google Scholar] [CrossRef]
- Quilliam, R.S.; DeLuca, T.H.; Jones, D.L. Biochar application reduces nodulation but increases nitrogenase activity in clover. Plant Soil 2012, 366, 83–92. [Google Scholar] [CrossRef]
- Cobo-Diaz, J.F.; Fernandez-Gonzalez, A.J.; Villadas, P.J.; Robles, A.B.; Toro, N.; Fernandez-Lopez, M. Metagenomic assessment of the potential microbial nitrogen pathways in the rhizosphere of a mediterranean forest after a wildfire. Microb. Ecol. 2015, 69, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Van Zwieten, L.; Rose, T.; Herridge, D.; Kimber, S.; Rust, J.; Cowie, A.; Morris, S. Enhanced biological N2 fixation and yield of faba bean (Vicia faba L.) in an acid soil following biochar addition: Dissection of causal mechanisms. Plant Soil 2015, 395, 7–20. [Google Scholar] [CrossRef]
- Reverchon, F.; Yang, H.; Ho, T.Y.; Yan, G.; Wang, J.; Xu, Z.; Chen, C.; Zhang, D. A preliminary assessment of the potential of using an acacia-biochar system for spent mine site rehabilitation. Environ. Sci. Pollut. Res. Int. 2015, 22, 2138–2144. [Google Scholar] [CrossRef]
- Rocci, K.S.; Fonte, S.J.; Von Fischer, J.C.; Cotrufo, M.F. Nitrogen Dynamics in an Established Alfalfa Field Under Low Biochar Application Rates. Soil Syst. 2019, 3, 77. [Google Scholar] [CrossRef]
- Mukome, F.N.D.; Zhang, X.M.; Silva, L.C.R.; Six, J.; Parikh, S.J. Use of chemical and physical characteristics to investigate trends in biochar feedstocks. J. Agric. Food Chem. 2013, 61, 2196–2204. [Google Scholar] [CrossRef]
- Chan, K.Y.; Xu, Z. Biochar: Nutrient Properties and Their Enhancement. In Biochar for Environmental Management, 1st ed.; Lehmann, J., Joseph, S., Eds.; Routledge: London, UK, 2012; pp. 67–84. [Google Scholar]
- Agbna, G.H.D.; She, D.L.; Liu, Z.P.; Elshaikh, N.A.; Shao, G.C.; Timm, L.C. Effects of deficit irrigation and biochar addition on the growth, yield, and quality of tomato. Sci. Hortic. 2017, 222, 90–101. [Google Scholar] [CrossRef]
- Abideen, Z.; Koyro, H.W.; Huchzermeyer, B.; Ansari, R.; Zulfiqar, F.; Gul, B. Ameliorating effects of biochar on photosynthetic efficiency and antioxidant defence of Phragmites karka under drought stress. Plant Biol. 2019, 22, 259–266. [Google Scholar] [CrossRef]
- Abubaker, B.A.; Yan, H.F.; Li, H.; Wu, Y.Y.; Elshaikh, N.A.; Hussein, G.; Pandab, S.; Hassan, S. Enhancement of depleted loam soil as well as cucumber productivity utilizing biochar under water stress. Commun. Soil Sci. Plant Anal. 2019, 50, 49–64. [Google Scholar] [CrossRef]
- Bai, S.H.; Xu, Z.; Blumfield, T.J.; Reverchon, F. Human footprints in urban forests: Implication of nitrogen deposition for nitrogen and carbon storage. J. Soils Sediments 2015, 15, 1927–1936. [Google Scholar] [CrossRef]
- Reverchon, F.; Flicker, R.C.; Yang, H.; Yan, G.; Xu, Z.; Chen, C.; Bai, S.H.; Zhang, D. Changes in δ15N in a soil–plant system under different biochar feedstocks and application rates. Biol. Fertil. Soils 2013, 50, 275–283. [Google Scholar] [CrossRef]
- Nadelhoffer, K.J.; Fry, B. Nitrogen Isotope Studies in Forest Ecosystems. In Stable Isotopes in Ecology and Environmental Science, 1st ed.; Lajtha, K., Michener, R.H., Eds.; Blackwell Scientific Publications: Oxford, UK, 1994; pp. 22–44. ISBN 9780632031542. [Google Scholar]
- Hogberg, P. Tansley Review No. 95 15N natural abundance in soil-plant systems. New Phytol. 1997, 137, 179–203. [Google Scholar] [CrossRef]
- Cheng, S.L.; Fang, H.J.; Yu, G.R.; Zhu, T.H.; Zheng, J.J. Foliar and soil 15N natural abundances provide field evidence on nitrogen dynamics in temperate and boreal forest ecosystems. Plant Soil 2010, 337, 285–297. [Google Scholar] [CrossRef]
| Treatments | TC (%) | TN (%) | δ13C (‰) | δ15N (‰) | BNF (%) |
|---|---|---|---|---|---|
| Biochar rates (t ha−1) | |||||
| 0 | 48.90 a * | 2.37 a | −32.25 a | −0.66 a | 56.43 a |
| 5 | 49.07 a | 2.31 a | −31.78 a | −0.84 a | 49.65 a |
| 10 | 49.26 a | 2.20 a | −32.50 a | −0.73 a | 55.43 a |
| Acacia species | |||||
| Acacia leiocalyx | 48.36 b | 2.51 a | −32.28 a | −0.61 a | 60.35 a |
| Acacia disparrima | 49.79 a | 2.08 b | −32.08 a | −0.87 b | 47.34 b |
| Treatments | Height (cm) | DGL (cm) | BA (cm2) | Volume (cm3) |
|---|---|---|---|---|
| Biochar rates (t ha−1) | ||||
| 0 | 119.56 a * | 3.29 a | 9.34 a | 435.40 a |
| 5 | 119.31 a | 3.36 a | 9.61 a | 434.18 a |
| 10 | 137.06 a | 3.80 a | 12.58 a | 639.89 a |
| Acacia species | ||||
| Acacia leiocalyx | 119.17 a | 3.38 a | 10.05 a | 467.29 a |
| Acacia disparrima | 131.46 a | 3.58 a | 10.97 a | 539.02 a |
| Treatments | TC (%) | TN (%) | δ13C (‰) | δ15N (‰) | NH4+-N (µgNg−1) | δ15N of NH4+-N (‰) |
|---|---|---|---|---|---|---|
| 0–5 cm | ||||||
| Biochar rates (t ha−1) | ||||||
| 0 | 7.89 a * | 0.290 a | −26.49 a | 0.434 a | 6.39 a | 1038.40 a |
| 5 | 8.37 a | 0.316 a | −26.51 a | 0.289 a | 4.38 b | 1050.88 a |
| 10 | 8.52 a | 0.320 a | −26.39 a | 0.282 a | 4.24 b | 1045.04 a |
| Acacia species | ||||||
| Acacia leiocalyx | 8.29 a | 0.311 a | −26.54 a | 0.242 a | 4.53 a | 1045.77 a |
| Acacia disparrima | 8.23 a | 0.306 a | −26.38 a | 0.428 a | 5.48 a | 1043.75 a |
| 5–10 cm | ||||||
| Biochar rates (t ha−1) | ||||||
| 0 | 5.03 b | 0.189 a | −25.95 a | 0.784 a | 9.34 a | 1018.84 a |
| 5 | 5.58 a | 0.208 a | −25.97 a | 0.571 a | 7.67 a | 1019.23 a |
| 10 | 5.57 a | 0.211 a | −25.96 a | 0.588 a | 8.11 a | 1017.99 a |
| Acacia species | ||||||
| Acacia leiocalyx | 5.49 a | 0.210 a | −26.13 b | 0.522 b | 8.83 a | 1020.11 a |
| Acacia disparrima | 5.27 a | 0.195 a | −25.79 a | 0.773 a | 7.91 a | 1017.27 a |
| 10–20 cm | ||||||
| Biochar rates (t ha−1) | ||||||
| 0 | 3.71 a | 0.133 a | −25.46 b | 1.96 a | 6.93 a | 1041.37 a |
| 5 | 3.60 a | 0.137 a | −25.54 b | 1.81 a | 5.98 b | 1034.63 a |
| 10 | 3.75 a | 0.150 a | −25.18 a | 2.05 a | 6.86 a | 1039.33 a |
| Acacia species | ||||||
| Acacia leiocalyx | 3.55 a | 0.139 a | −25.50 b | 1.67 b | 7.09 a | 1034.44 a |
| Acacia disparrima | 3.82 a | 0.140 a | −25.29 a | 2.21 a | 6.09 b | 1042.45 a |
| Treatments | HWEOC (µg g−1) | HWETN (µg g−1) | WEOC (µg g−1) | WETN (µg g−1) |
|---|---|---|---|---|
| Biochar rates (t ha−1) | ||||
| 0 | 688.54 a * | 74.71 a | 294.76 a | 15.36 a |
| 5 | 610.13 a | 38.01 a | 226.40 a | 12.98 a |
| 10 | 790.67 a | 45.59 a | 254.54 a | 12.09 a |
| Acacia species | ||||
| Acacia leiocalyx | 697.97 a | 42.92 a | 237.98 a | 12.41 a |
| Acacia disparrima | 694.92 a | 62.62 a | 279.14 a | 14.54 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nessa, A.; Bai, S.H.; Karim, Z.; Yang, J.; Xu, Z. Effects of Biochar Application on Nitrogen Fixation and Water Use Efficiency of Understorey Acacia Species as well as Soil Carbon and Nitrogen Pools in a Subtropical Native Forest. Forests 2025, 16, 1350. https://doi.org/10.3390/f16081350
Nessa A, Bai SH, Karim Z, Yang J, Xu Z. Effects of Biochar Application on Nitrogen Fixation and Water Use Efficiency of Understorey Acacia Species as well as Soil Carbon and Nitrogen Pools in a Subtropical Native Forest. Forests. 2025; 16(8):1350. https://doi.org/10.3390/f16081350
Chicago/Turabian StyleNessa, Ashrafun, Shahla Hosseini Bai, Zakaria Karim, Jiaping Yang, and Zhihong Xu. 2025. "Effects of Biochar Application on Nitrogen Fixation and Water Use Efficiency of Understorey Acacia Species as well as Soil Carbon and Nitrogen Pools in a Subtropical Native Forest" Forests 16, no. 8: 1350. https://doi.org/10.3390/f16081350
APA StyleNessa, A., Bai, S. H., Karim, Z., Yang, J., & Xu, Z. (2025). Effects of Biochar Application on Nitrogen Fixation and Water Use Efficiency of Understorey Acacia Species as well as Soil Carbon and Nitrogen Pools in a Subtropical Native Forest. Forests, 16(8), 1350. https://doi.org/10.3390/f16081350






