Abstract
Under cool and moist climates, timely implementation of ditch network maintenance (DNM) is crucial for sustaining productivity of drained forests, thus reducing operational costs, while mitigating environmental risks. This underscores the need to understand tree growth responses to DNM. This study evaluated the effects of DNM on tree radial increment in sites with both organic and mineral drained soils, focusing on regionally commercially important species: Scots pine (Pinus sylvestris), Norway spruce (Picea abies), and silver birch (Betula pendula). Responses of relative growth changes over eight years post-DNM to site and tree characteristics were assessed using a linear mixed-effects model. Species- and site-specific growth responses to DNM were indicated by significant interactions between tree species, site type, and distance from the drainage ditch. While growth responses were generally neutral (non-significant), variability among sites and species suggests that both organic and mineral soils might be prone to site-level moisture depletion near drainage infrastructure in the post-DNM period. The effect of stand age and density suggested higher responsiveness of older and less dense stands, hence positive effects of thinning to resilience of stands to DNM. These findings highlight the importance of adapting DNM strategies to local site conditions and stand characteristics to minimize drought-related growth limitations.
1. Introduction
In Northern Europe, particularly during the second half of the 20th century, vast areas of boreal peatlands and wet mineral soils were drained for forestry purposes [1]. Currently, approximately 20–25% of the total forest area in Finland, Sweden, and the Baltic States is drained [2,3]. Considerable drained forest areas also exist in the British Isles, Poland, Germany, European Russia, Belarus, and Ukraine [2]. Due to the evolution of environmental regulations [4,5,6], the construction of new drainage networks has ceased in most regions, shifting the focus to the maintenance [2,7] and modernization of existing drainage networks [8,9]. Properly maintained drainage networks help sustain soil aeration and thus metabolic activity, which typically declines when air-filled porosity falls below 20% and becomes inhibited below 10% [10]. Maintaining groundwater levels at 35–40 cm below the surface is considered optimal for sustaining suitable soil moisture conditions in drained sites managed for commercial forestry in Northern European regions [8,11].
Drained forests have gained renewed attention due to the implementation of the EU Nature Restoration Law (Regulation (EU) 2024/1991) [12]. This legislation prioritizes the restoration of degraded ecosystems with high potential for carbon sequestration, including peatlands and wet forests. Rewetting has been proposed as a climate mitigation measure to reduce greenhouse gas emissions (GHG) from previously drained areas [13]. However, this approach remains controversial, as rewetting may decrease forest productivity and increase tree mortality under prolonged inundation [14,15], potentially leading to carbon losses. Moreover, rewetting may increase anaerobic microbial activity, which can offset climate benefits through elevated methane emissions [16], presenting a challenge in balancing nature restoration and climate mitigation goals.
Over time, drainage ditches may lose their functionality due to collapse of ditch walls, sediment accumulation, vegetation overgrowth, and peat subsidence, all of which reduce drainage efficiency and contribute to rising groundwater tables [2,17]. The severity and rate of drainage network deterioration depend on site properties, including peat characteristics, local climate [18], topography [2] and time elapsed since drainage network establishment [17]. In Northern Europe especially, deterioration is accelerated in flat lowland areas, and is often exacerbated by beaver (Castor sp.) activity [19,20,21,22], which can obstruct water flow, increase flooding, and ultimately lead to suppressed tree growth and dieback [14,23]. Maintaining effective water regulation requires periodic ditch network maintenance (DNM), which includes ditch cleaning, deepening, or complementary ditching [2,7,24]. In Latvian state-owned forests, ditch depths used during DNM typically range from 0.9 to 1.3 m in mineral soils and from 1.2 to 1.5 m in peat soils [25].
While DNM can improve soil moisture conditions and support tree growth, it also can present risks [26]. With rising temperatures and more frequent droughts, DNM may increase the risk of soil over-drying, particularly on well-draining mineral soils [24]. Additionally, maintenance activities can lead to increased nutrient leaching, transport of suspended solids, and the export of dissolved organic carbon into adjacent aquatic systems [26,27,28]. In contrast, by reducing water stagnation, DNM may also lower the potential for mercury (Hg) methylation [29], thereby decreasing the risk of bioavailable Hg leaching in downstream ecosystems [26,30,31].
The response of trees to DNM is highly site-specific and strongly influenced by the pre-maintenance water table depth [2,18]. In Scots pine–dominated peatlands in Finland, the greatest increases in mean annual volume growth were observed at shallow pre-maintenance water table depths (<25–30 cm), whereas sites with deeper water tables (>35–40 cm) often showed little or no response [18]. However, considerable variation in growth outcomes at intermediate water levels (20–35 cm) suggested that relying solely on water table depth is insufficient [18], and additional site-specific indicators are needed to guide DNM decisions. Additional factors include both stand characteristics and local site conditions [32,33].
Stand properties—such as tree species composition, age, and density—affect water use through evapotranspiration [2,17,32,34]. For example, birch with a high transpiration capacity [35], can reduce groundwater levels and serve as a form of biological drainage [9,36,37]. Younger stands, due to their greater growth potential, may show stronger responses to improved soil moisture conditions following DNM [38]. Meanwhile, local site conditions such as soil type, peat depth, and topography affect water retention and drainage effectiveness [2,8,9]. Fine-textured soils and flat terrain may limit drainage efficacy, thereby constraining the impact of DNM [9]. Increased soil aeration in peat promotes humification and compaction, raising bulk density and reducing hydraulic conductivity, which gradually impairs drainage efficiency [2,39]. Hence the estimation of DNM efficiency is likely complex.
To maintain optimal soil moisture for tree growth, DNM should be performed timely; however, determining the optimal timing is not always straightforward [36,40]. Optimal timing of DNM depends on stand characteristics and drainage network condition, with limited effects observed in mature stands where groundwater levels are already low due to high evapotranspiration [2,34]. Seasonal waterlogging and the dominance of moisture-tolerant understory species can indicate the need for DNM [3]. On mineral soils, drainage ditches can remain functional throughout the entire rotation period [3,9]. In contrast, organic soils often require DNM every 20–30 years due to peat’s weak mechanical stability [41,42]. Additionally, organic soils are prone to gradual nutrient depletion over time [3,43], adversely affecting tree growth and productivity [33].
The long-term impact of DNM on forest growth remains a topic of active research. Studies from Finland and Sweden have demonstrated that DNM can significantly enhance tree growth, particularly in drained peatlands [44,45]. However, results vary depending on the length of the post-maintenance evaluation period, as growth responses to DNM are often delayed and may not be fully captured by short-term studies [43]. In Scots pine–dominated peatlands in Finland, reported increases in mean annual volume growth range from 0.5 to 1.8 m3 ha−1 yr−1 over 15–20 years [2]. Despite these findings, most available data are limited to Scots pine-dominated boreal peatlands, with relatively few studies addressing growth responses of Norway spruce, silver birch, or stands on mineral soils.
Quantifying the growth response to DNM is challenging, mainly due to difficulties in defining an appropriate reference growth rate. As groundwater levels gradually rise, growth declines slowly, making the effect of DNM dependent on the degree of prior growth limitation [2]. Experimentally treated stands are often compared to non-treated controls [18], but this approach may be biased by incomplete isolation of treatments in the experimental layout. The alternative approach—comparing growth before and after DNM using analysis of increment allows for a retrospective assessment of stand development without the need for long-term experimental setups and can reveal subtle changes in growth trends that may not be apparent from inventory data [46].
In Latvia, forests with excessive soil moisture are more closely linked to groundwater discharge zones shaped by topography and soil conditions, rather than regional precipitation regimes [3]. These areas, characterized by persistently high soil moisture, often exhibit limited tree growth if unmanaged [37]. Consequently, large-scale drainage projects were implemented between the 1960 s and 1990 s to increase timber yields, which proved to be an effective strategy [3]. Drained forests in Latvia span both organic (12%) and mineral (17%) soils [47]. Scots pine, Norway spruce, and silver birch are among the most economically important tree species in Latvia, with reported post-drainage growth increments increasing two- to threefold [37]. Drained forests dominated by these species represent a substantial share of the national forest area, and maintaining their productivity through targeted drainage practices is essential for the long-term sustainability of commercial forestry and forest management.
This study aimed to evaluate the effect of DNM on tree stem radial increment in hemiboreal forests of Latvia. We hypothesize that DNM has a positive effect on increment, particularly for conifers, but interactions among species, site type, and tree age can influence these responses. We presume that DNM might cause over drainage, particularly facing increasing drought effects.
2. Materials and Methods
2.1. Study Sites
The research was conducted in Latvia, located within the hemiboreal forest zone of the Eastern Baltic region [48]. The climate is characterized as humid continental [49], with a mean annual air temperature of 6.8 °C and an average annual precipitation of 685.6 mm [50]. Study sites were selected across the country at four locations, with nine forest stands chosen at each location (Figure 1). The selection was stratified based on the presence of DNM, stand age (representing the rotation interval of commercial stands), and composition (stands formed by the main economically important species). Accordingly, the dominant species of the stands were Scots pine, Norway spruce, and silver birch, where they comprised at least 60% of the canopy layer (by basal area). DNM was conducted primarily in 2012 (29 stands), while in the remaining seven stands, DNM was carried out in either 2006 or 2010. The study sites were selected to ensure that no commercial thinning or other forestry operations had occurred during the ten years preceding DNM.
Figure 1.
The geographic locations of the 36 studied forest stands distributed across four study locations (red circles).
The stands of each species were represented in three age groups: for pine: (1) 21–50 years, (2) 51–80 years, (3) 81–100 years; for spruce and birch: (1) 21–40 years, (2) 41–60 years, (3) 61–100 years.
2.2. Data Collection
In each forest stand, three circular sample plots, each with an area of 500 m2 (R = 12.62 m), were established along a transect perpendicular to the drainage ditch at distances of 15 m, 45 m, and 75 m (center of sample plot) from the edge of drainage ditch. These distances were selected considering the typical spacing between drainage ditches at the study sites, which ranged from 150 to 200 m. The distance from the ditch to the edge of the stand, as well as the width and direction of the ditch, were measured.
Diameter at breast height (DBH) was measured for all living and dead standing trees that were at least 6.1 cm in DBH. Additional subplots with a radius of 5.64 m (area = 100 m2) were established as a sector (0–90°) at the center of the sample plot, to measure growth parameters for trees with a DBH of 2.1–6.1 cm. Tree height was measured for 10 individuals of the dominant species in the upper canopy layer and for three individuals of each additional species in each canopy layer. For the same trees increment cores were taken using Pressler’s increment corer. The heights of all standing dead trees were measured. Forest site type for each sample plot was noted according to the local classification developed by Bušs [51]. In total, 6143 living trees were measured, and from those, 1036 increment cores were collected (346 for pine, 385 for spruce, and 305 for birch). The studied forest stands varied in age, ranging from young to mature (Table 1). The age of the studied trees ranged from 6 to 120 years, with the stands on organic oligotrophic soils being slightly older than others.

Table 1.
Mean values (± SE) of stand age, diameter at breast height (DBH), tree height (H) and number of trees for categories (N) across site types and species.
Tree-ring width (TRW) was measured with an accuracy of 0.01 mm using a LINTAB6 measuring system (RinnTech, Heidelberg, Germany). TRW data were cross-dated both visually and statistically using the COFECHA (version 6.06P) software to ensure accuracy [52]. TRW was used as the proxy of growth. To evaluate responses of trees to DNM, relative radial increment was calculated as the ratio between the mean TRW 8 years before vs. 8 years after DNM. The 8-year post-treatment period was selected as it represented the longest consistent timeframe available across all study sites, allowing for standardized comparison of growth responses. A linear mixed-effects model (LMM) was used to analyze the factors influencing relative radial increment. Both tree-level and stand-level variables were tested, including numeric and categorical variables. The model included three tree species (pine, spruce, birch) across four forest site types, representing two soil types (organic and mineral) and two fertility levels (oligotrophic and mesotrophic). Tree slenderness index, calculated as the ratio of tree height (H) to DBH, was tested as an implicit proxy of stand density [34]. The effect of DNM was represented in the model by the distance of each individual tree from the ditch. Model selection was performed using backward elimination, starting with all variables and sequentially removing non-significant variables and interactions. Interaction terms between species, site type, and distance from the ditch were also evaluated to assess species and site-specific responses to DNM. A stand and sample plot identifiers were included as random effects.
The model was fitted using the ‘lmer’ function from the lme4 [53] package in R version 4.4.1. [54]. Model fitting was performed using restricted maximum likelihood (REML). Model performance was evaluated using Type II Wald chi-square tests for fixed effects, marginal and conditional R-squared values for explained variance, and variance inflation factors (VIFs) to assess multicollinearity. Diagnostic checks included residual analysis using QQ plots and histograms to confirm assumptions of normality and homoscedasticity. The significance of random effects was evaluated using likelihood ratio tests with the ‘ranova’ function from the ‘LmerTest’ package (version 3.1-3) [55].
3. Results and Discussion
The DNM had a significant, yet complex effect on the relative radial increment of tress, as indicated by a significant interaction between species-site type and distance to the drainage ditch (Table 2, Supplementary Material Table S1). The conditional R2 of the model was 0.36, while the marginal R2 was only 0.09, indicating that most of the variation in relative radial increment after DNM was explained by random effects, specifically—forest stand (p < 0.001) and sample plot (p = 0.044). This suggests that local site-specific conditions have a greater influence on tree response than individual tree characteristics, likely due to soil properties [33] and microrelief [32,56].

Table 2.
Type-III analysis-of-variance table for the model assessing the response of relative radial increment to the distance from the ditch, tree age, slenderness index, tree species, site type, and their interactions (set of the fixed effects) and variance and significance of the random effects (sample plot, forest stand, year of maintenance) (Χ2—chi-square statistic, Df—degrees of freedom).
In most cases, tree stem radial increment did not respond to DNM, as indicated by the confidence intervals of the slope coefficients, which contained zero (Figure 2A). Nevertheless the radial increment was not suppressed (Supplementary Material Table S2) This indicated that overall soil moisture conditions prior to DNM were not a major constraint on tree growth [18]. However, in two specific cases—pine on mineral oligotrophic sites and spruce on organic oligotrophic sites—the relative radial increment increased significantly with increasing distance from the ditch [57]. This indicates a growth limitation for trees growing closer to the ditch, likely due to water shortage after DNM [56,58,59]. Conifer responses to DNM are likely influenced by species-specific adaptations to environmental changes [60].
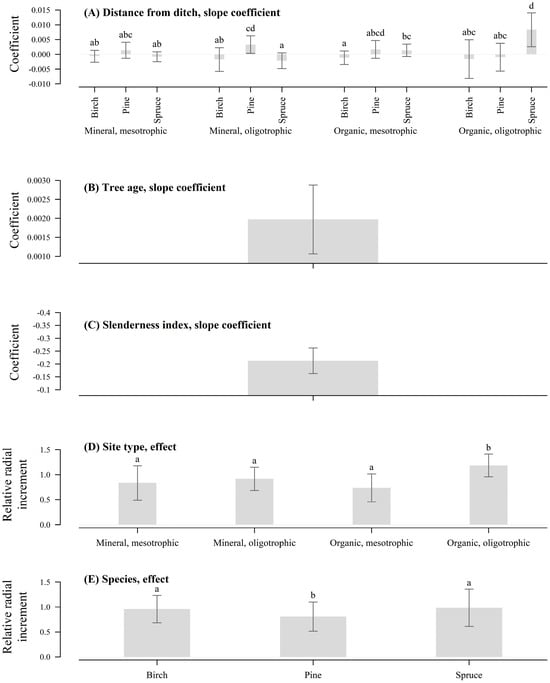
Figure 2.
Tree species and age, site type, distance from the drainage ditch, and slenderness index effects (gray bars) on relative radial increment after DNM (Bars sharing the same lowercase letter are not significantly different at α = 0.05).
Responses of conifers may be rooted in differing drought tolerance strategies, with spruce and pine both showing sensitivity to post-DNM soil drying, yet on different site types (Figure 2A). While spruce is more vulnerable in peat-based environments that become too dry, pine may be more affected in nutrient-poor mineral soils that offer limited buffering against drought once drainage is enhanced.
Peat humification and the resulting subsidence are typically more pronounced adjacent to drainage ditches, where the depression of the groundwater table is greatest [61], potentially leading to increased soil drying following DNM [34]. However, the absence of data on drainage network condition prior to DNM constrained the ability to assess pre-maintenance soil moisture condition and determine whether the drainage network was functioning poorly or sub-optimally. The maintenance was carried out at the discretion of forest planners, likely based on observed ditch degradation or declining tree growth, although specific justifications were not recorded. Presumably, under high soil moisture conditions prior to DNM, trees may have developed shallower root systems [62]. Following DNM, the resulting drop in ground water level could expose tree roots to increased drought stress, especially during dry periods [2,63]. The pronounced response of conifers at greater distances from the ditch may reflect more favorable post-DNM soil moisture regimes, where soil water conditions tend to be more stable [56].
A contrasting response was observed for spruce in mineral oligotrophic sites (Figure 2A), where the relative radial increment showed marginally significant declined with increasing distance from the ditch. The proximity to the drainage ditch likely provided better soil aeration, which is favorable for spruce, as it prefers well-aerated soil conditions [64]. This suggests that soil moisture prior to DNM may have been suboptimal for spruce, which is particularly sensitive to excessive moisture [64]. This dual pattern observed in spruce highlights its sensitivity to both water excess and water deficit, emphasizing the importance of local/site conditions. This supports the relevance of considering both species traits and site hydrology when evaluating possible impacts of DNM.
Birch response to DNM showed no significant increase or decrease in relation to the interaction between site type and proximity to the drainage ditch (Figure 2A), indicating greater plasticity to altered edaphic conditions. Additionally, birch has high evapotranspiration potential, thus, it is likely that sub-optimal drainage conditions before DNM were compensated by evapotranspiration [18,44]. This suggests that birch is more tolerant of hydrological changes and is a more suitable species in drained sites [65].
Tree age had a uniform effect on relative radial increment across all ages, as indicated by the non-interacted effect, despite the high variation between stand ages (Table 2, Figure 2B). Even though the studied species differed by biological age, the estimated uniformed response suggests similar growth response under drained conditions. Age showed a positive influence on relative radial increment [44], indicating that older trees tend to exhibit higher relative growth following DNM, likely due to their larger canopy and greater ability to assimilate carbon [66]. While younger stands, those without yet undergone non-commercial thinning, may still be competing for light, which can limit their ability to exploit improved edaphic conditions fully [67].
The effect of stand density on tree responses to DNM [18] was implicitly supported by the significant effect of slenderness index (Table 2, Figure 2C), as greater relative increment was observed in trees with smaller slenderness index values. Dense stands can lower the water table more effectively; however, after DNM, improved drainage conditions in combination with pronounced evapotranspiration may lead to excessive soil drying as suggested by observed negative relationship between increment and distance from the ditch (Figure 2A). Therefore, forest management strategies in drained stands should carefully consider stand density [34,36] when evaluating the necessity and timing for DNM in conjunction with thinning to optimize tree soil moisture conditions and mitigate drought stress following DNM.
The non-interacted effect of site type indicated that relative radial increment was significant only in organic oligotrophic soils, whereas other site types showed only minor or negligible increases following DNM (Table 2, Figure 2D). This pattern likely reflects the fact that organic oligotrophic soils—characterized by higher water content due to their greater retention capacity—exhibit more favorable moisture conditions following DNM than mineral soils. DNM likely improved the aeration of the root zone, leading to enhanced topsoil metabolic rates and, consequently, increased nutrient availability, which had a notable effect on tree growth. In contrast, mineral soils—characterized by lower water retention and faster drying—may have already been sufficiently drained or responded less noticeably to DNM [18].
The independent effect of tree species showed that all species tended to have a positive relative radial increment following DNM (Table 2, Figure 2E), although this may be underestimated due to the increment comparison method, which does not account for the natural decline in growth expected in untreated stands [68]. Spruce and birch showed similar responses, with significantly greater relative growth change (p < 0.05) compared to pine. Birch, in particular, showed a stable response across different site conditions, highlighting its ecological plasticity in adapting to altered soil moisture regimes [69]. Suitability of birch in drained stands is further supported by its high wind resistance compared to conifers, making it a more reliable species in peatland forests prone to windthrow [70]. In contrast, pine’s relatively lower growth response may reflect its adaptation to drier conditions and a more conservative growth strategy [63] under improved soil aeration, which limits its responsiveness to changes induced by DNM Similar results have been reported from Finland [59], where Scots pine in drained peatlands grew better under shallow water tables, contradicting the view that a deep water table is necessary to support growth, thus supporting the idea that DNM may not always have positive effects on pine growth.
The hypothesis was partly supported as the anticipated positive effect of DNM, particularly for conifers, was not observed. However, interactions among species, site type, and tree age had a significant effect on relative radial increment. The assumption that DNM can lead to over-drainage was supported in two cases—pine on mineral oligotrophic sites and spruce on organic oligotrophic sites—where relative radial increment was significantly greater at increasing distances from the ditch, indicating reduced growth in trees located closer to the ditch.
4. Conclusions
In most cases, DNM had a generally neutral effect on tree growth, as the drainage network remained functional and maintained optimal soil moisture conditions. Although delayed DNM may lead to growth decline, high stand density (implicitly suggested by the effect of slenderness index) could partially compensate for elevated moisture levels. Although drought risk following DNM was not pronounced, some early warning signs were observed. Overly intensive DNM may reduce soil water availability, increasing the risk of drought stress in both drained peat and mineral sites. Although the applied DNM was largely balanced, to mitigate drought risk more effectively, future DNM planning should be more targeted, considering both tree species, stand density and site type. Furthermore, controlled experiments on drainage intensity are needed to provide valuable insights into optimal soil moisture conditions for sustaining tree growth.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f16081318/s1. Table S1: Fixed-effect parameter estimates from the model assessing the response of relative radial increment to distance from the ditch, tree age, slenderness index, tree species, site type, and their interactions. Table S2: Estimated marginal mean tree-ring width (±95% of confidence interval) of trees before and after drainage ditch maintenance in stands differing by soil type (mineral and organic) in Latvia. The estimates are made accounting for the spatial distribution (spatial dependence of sites), dominant species and stand age.
Author Contributions
Conceptualization, R.M. and K.B.; methodology, J.D., G.Š., Ā.J. and R.M.; software, R.M.; validation, K.B., Ā.J., J.D. and G.Š.; formal analysis, K.B. and R.M.; investigation, R.M. and K.B.; resources, Ā.J. and R.M.; data curation, K.B., I.J., D.J., J.D. and G.Š.; writing—original draft preparation, K.B.; writing—review and editing, R.M., J.D., Ā.J., G.Š. and D.J.; visualization, R.M.; supervision, R.M.; project administration, R.M. and Ā.J.; funding acquisition, Ā.J. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded by Latvia Council of Science national research program project: “Forest4LV—Innovation in Forest Management and Value Chain for Latvia’s Growth: New Forest Services, Products and Technologies” (No.: VPP-ZM-VRIIILA-2024/2-0002) and Research program supported by the JSC Latvian State Forests “Effect of climate change on forestry and associated risks” (Agreement No. 5-5.9.1_007p_101_21_78).
Data Availability Statement
Data available on request from the corresponding author (The data set contains potentially sensitive commercial information, e.g., location of monitoring sampling plots).
Acknowledgments
We thank the technical staff, especially Andis Adamovičs, for assistance with fieldwork, and Endijs Bāders for creating the graphical illustration of the research map. During the preparation of this manuscript, the authors used ChatGPT (GPT-4o) and Grammarly Pro to assist with language editing, paraphrasing, and grammar improvement. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest. Funding sources are listed above.
References
- Laine, J.; Laiho, R.; Minkkinen, K.; Vasander, H. Forestry and Boreal Peatlands. In Forestry and Boreal Peatlands; Springer: Berlin/Heidelberg, Germany, 2006; pp. 331–357. [Google Scholar]
- Sikström, U.; Hökkä, H. Interactions between Soil Water Conditions and Forest Stands in Boreal Forests with Implications for Ditch Network Maintenance. Silva Fenn. 2016, 50, 1416. [Google Scholar] [CrossRef]
- Paavilainen, E.; Päivänen, J. Peatland Forestry; Springer: Berlin/Heidelberg, Germany, 1995; Volume 111. [Google Scholar]
- FSC Sweden. FSC-STD-SWE-02-04-2010 Sweden Natural, Plantations and SLIMF EN; Forest Stewardship Council A.C.: Bonn, Germany, 2010. [Google Scholar]
- Finnish Forest Stewardship Council. FSC Standard for Finnish Peatlands [Draft]; Forest Stewardship Council Finland: Helsinki, Finland, 2012.
- European Parliament and Council. Directive 2000/60/EC of the European Parliament and of the Council Establishing a Framework for Community Action in the Field of Water Policy; European Union: Brussels, Belgium, 2000. [Google Scholar]
- Ahti, E.; Kojola, S.; Nieminen, M.; Penttilä, T.; Sarkkola, S. The Effect of Ditch Cleaning and Complementary Ditching on the Development of Drained Scots Pine-Dominated Peatland Forests in Finland. Peatl. For. 2008, 1, 457–459. [Google Scholar]
- Hökkä, H.; Laurén, A.; Stenberg, L.; Launiainen, S.; Leppä, K.; Nieminen, M. Defining Guidelines for Ditch Depth in Drained Scots Pine Dominated Peatland Forests. Silva Fenn. 2021, 55, 10494. [Google Scholar] [CrossRef]
- Finér, L.; Čiuldienė, D.; Lībieté, Z.; Lode, E.; Nieminen, M.; Pierzgalski, E.; Ring, E.; Strand, L.; Sikström, U. WAMBAF—Good Practices for Ditch Network Maintenance to Protect Water Quality in the Baltic Sea Region. In Natural Resources and Bioeconomy Studies 25/2018; Natural Resources Institute Finland: Helsinki, Finland, 2018; p. 35. [Google Scholar]
- Gliński, J.; Stępniewski, W. Soil Aeration and Its Role for Plants; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Zālītis, P. Mežs Un Ūdens; 2012; ISBN 9789934801662. Available online: https://www.silava.lv/images/Produkti/2012-Monografija-Zalitis-Mezs-un-udens.pdf (accessed on 9 July 2025).
- European Parliament and Council of the European Union. Regulation (EU) 2024/1991 of the European Parliament and of the Council of 24 June 2024 on Nature Restoration and Amending Regulation (EU) 2022/869; European Union: Brussels, Belgium, 2024.
- Mander, Ü.; Espenberg, M.; Melling, L.; Kull, A. Peatland Restoration Pathways to Mitigate Greenhouse Gas Emissions and Retain Peat Carbon. Biogeochemistry 2024, 167, 523–543. [Google Scholar] [CrossRef] [PubMed]
- Gackis, M. Coniferous forest annual growth under impact of beaver-made inundations in Dobele forestry, Latvia. Acta Biol. Univ. Daugavp. 2011, 11, 96–100. [Google Scholar]
- Ojanen, P.; Minkkinen, K. Rewetting Offers Rapid Climate Benefits for Tropical and Agricultural Peatlands But Not for Forestry-Drained Peatlands. Glob. Biogeochem. Cycles 2020, 34, 1–16. [Google Scholar] [CrossRef]
- Abdalla, M.; Hastings, A.; Truu, J.; Espenberg, M.; Mander, Ü.; Smith, P. Emissions of Methane from Northern Peatlands: A Review of Management Impacts and Implications for Future Management Options. Ecol. Evol. 2016, 6, 7080–7102. [Google Scholar] [CrossRef]
- Zālītis, P.; Zālītis, T.; Lībiete-Zālīte, Z. Kokaudzes Ražības Izmaiņas Saistībā Ar Grāvju Deformēšanos. Mežzinātne 2010, 22, 103–115. [Google Scholar]
- Sarkkola, S.; Hökkä, H.; Ahti, E.; Koivusalo, H.; Nieminen, M. Depth of Water Table Prior to Ditch Network Maintenance Is a Key Factor for Tree Growth Response. Scand. J. For. Res. 2012, 27, 649–658. [Google Scholar] [CrossRef]
- Lamsodis, R.; Ulevicius, A. Geomorphological Effects of Beaver Activities in Lowland Drainage Ditches. Z. Fur. Geomorphol. 2012, 56, 435–458. [Google Scholar] [CrossRef]
- Kalvīte, Z.; Lībiete, Z.; Kļaviņš, I.; Bārdule, A.; Bičkovskis, K. The Impact of Beaver Dam Removal on the Chemical Properties of Water in Drainage Ditches in Peatland Forests. Scand. J. For. Res. 2020, 36, 1–14. [Google Scholar] [CrossRef]
- Hartman, G. Habitat Selection by European Beaver (Castor Fiber) Colonizing a Boreal Landscape. J. Zool. 1996, 240, 317–325. [Google Scholar] [CrossRef]
- Gackis, M. The Aspects of Beaver Castor Fiber L. Population in Drained Forests. In Summary of Academic Dissertation; Latvia University of Agriculture: Jelgava, Latvia, 2013. [Google Scholar]
- Rosell, F.; Campbell-Palmer, R. Beavers: Ecology, Behaviour, Conservation, and Management; Oxford University Press: Oxford, UK, 2022; ISBN 978-0-19-883504-2. [Google Scholar]
- Koivusalo, H.; Ahti, E.; Laurén, A.; Kokkonen, T.; Karvonen, T.; Nevalainen, R.; Finér, L. Impacts of Ditch Cleaning on Hydrological Processes in a Drained Peatland Forest. Hydrol. Earth Syst. Sci. 2008, 12, 1211–1227. [Google Scholar] [CrossRef]
- JSC “Latvian State Forests”. Technical Regulations for the Design of Forest Infrastructure Facilities; JSC “Latvian State Forests”: Riga, Latvia, 2021.
- Laudon, H.; Mosquera, V.; Eklöf, K.; Järveoja, J.; Karimi, S.; Krasnova, A.; Peichl, M.; Pinkwart, A.; Tong, C.H.M.; Wallin, M.B.; et al. Consequences of Rewetting and Ditch Cleaning on Hydrology, Water Quality and Greenhouse Gas Balance in a Drained Northern Landscape. Sci. Rep. 2023, 13, 20218. [Google Scholar] [CrossRef] [PubMed]
- Nieminen, M.; Palviainen, M.; Sarkkola, S.; Laurén, A.; Marttila, H.; Finér, L. A Synthesis of the Impacts of Ditch Network Maintenance on the Quantity and Quality of Runoff from Drained Boreal Peatland Forests. Ambio 2018, 47, 523–534. [Google Scholar] [CrossRef]
- Nieminen, M.; Ahti, E.; Koivusalo, H.; Mattsson, T.; Sarkkola, S.; Laurén, A. Export of Suspended Solids and Dissolved Elements from Peatland Areas after Ditch Network Maintenance in South-Central Finland. Silva Fenn. 2010, 44, 39–49. [Google Scholar] [CrossRef]
- Kļaviņš, I.; Bārdule, A.; Kļaviņa, Z.; Lībiete, Z. Increased Hg Methylation Risks in Management-Induced Terrain Depressions in Forests with Organic-Matter-Rich Soils. Hydrology 2024, 11, 26. [Google Scholar] [CrossRef]
- Bitenieks, K.; Bārdule, A.; Eklöf, K.; Espenberg, M.; Ruņģis, D.E.; Kļaviņa, Z.; Kļaviņš, I.; Hu, H.; Lībiete, Z. The Influence of the Degree of Forest Management on Methylmercury and the Composition of Microbial Communities in the Sediments of Boreal Drainage Ditches. Microorganisms 2022, 10, 1981. [Google Scholar] [CrossRef]
- Bārdule, A.; Gerra-Inohosa, L.; Kļaviņš, I.; Kļaviņa, Z.; Bitenieks, K.; Butlers, A.; Lazdiņš, A.; Lībiete, Z. Variation in the Mercury Concentrations and Greenhouse Gas Emissions of Pristine and Managed Hemiboreal Peatlands. Land 2022, 11, 1414. [Google Scholar] [CrossRef]
- Stenberg, L.; Haahti, K.; Hökkä, H.; Launiainen, S.; Nieminen, M.; Laurén, A.; Koivusalo, H. Hydrology of Drained Peatland Forest: Numerical Experiment on the Role of Tree Stand Heterogeneity and Management. Forests 2018, 9, 645. [Google Scholar] [CrossRef]
- Laurén, A.; Palviainen, M.; Launiainen, S.; Leppä, K.; Stenberg, L.; Urzainki, I.; Nieminen, M.; Laiho, R.; Hökkä, H. Drainage and Stand Growth Response in Peatland Forests—Description, Testing, and Application of Mechanistic Peatland Simulator SUSI. Forests 2021, 12, 293. [Google Scholar] [CrossRef]
- Sarkkola, S.; Nieminen, M.; Ahti, E.; Hökkä, H.; Koivusalo, H.; Päivänen, J.; Laine, J. Role of Tree Stand Evapotranspiration in Maintaining Satisfactory Drainage Conditions in Drained Peatlands. Can. J. For. Res. 2010, 40, 1485–1496. [Google Scholar] [CrossRef]
- Oltchev, A.; Cermak, J.; Nadezhdina, N.; Tatarinov, F.; Tishenko, A.; Ibrom, A.; Gravenhorst, G. Transpiration of a Mixed Forest Stand: Field Measurements and Simulation Using SVAT Models. Boreal Environ. Res. 2002, 389–397. [Google Scholar]
- Sarkkola, S.; Nieminen, M.; Koivusalo, H.; Laurén, A.; Ahti, E.; Launiainen, S.; Nikinmaa, E.; Marttila, H.; Laine, J.; Hökkä, H. Domination of Growing-Season Evapotranspiration over Runoff Makes Ditch Network Maintenance in Mature Peatland Forests Questionable. Mires Peat 2013, 11, 2. [Google Scholar]
- Zālītis, P.; Jansons, J.; Indriksons, A. Mežaudžu Parametri Hidrotehniski Meliorētajos Mežos Pēdējos Piecdesmit Gados. Mežzinātne 2013, 27, 36–66. [Google Scholar]
- Payandeh, B. Analyses of a Forest Drainage Experiment in Northern Ontario. I: Growth Analysis. Can. J. For. Res. 1973, 3, 387–398. [Google Scholar] [CrossRef]
- Turunen, J.; Anttila, J.; Laine, A.M.; Ovaskainen, J.; Laatikainen, M.; Alm, J.; Larmola, T. Impacts of Forestry Drainage on Surface Peat Stoichiometry and Physical Properties in Boreal Peatlands in Finland. Biogeochemistry 2024, 167, 589–608. [Google Scholar] [CrossRef]
- Kojola, S.; Ahtikoski, A.; Hökkä, H.; Penttilä, T. Profitability of Alternative Management Regimes in Scots Pine Stands on Drained Peatlands. Eur. J. For. Res. 2012, 131, 413–426. [Google Scholar] [CrossRef]
- Dubra, S.; Samariks, V.; Līcīte, I.; Butlers, A.; Purviņa, D.; Lupiķis, A.; Jansons, Ā. Effects of Drainage on Carbon Stock in Hemiboreal Forests: Insights from a 54-Year Study. Sustainability 2023, 15, 16622. [Google Scholar] [CrossRef]
- Lazdiņš, A.; Lupiķis, A.; Polmanis, K.; Bārdule, A.; Butlers, A.; Kalēja, S. Carbon Stock Changes of Drained Nutrient-Rich Organic Forest Soils in Latvia. Silva Fenn. 2024, 58, 22017. [Google Scholar] [CrossRef]
- Lauhanen, R.; Ahti, E. Effects of Maintaining Ditch Networks on the Development of Scots Pine Stands. SUO 2001, 52, 29–38. [Google Scholar]
- Sikström, U.; Jansson, G.; Pettersson, F. Growth Responses of Pinus Sylvestris and Picea Abies after Ditch Cleaning—A Survey in Sweden. Scand. J. For. Res. 2020, 35, 69–84. [Google Scholar] [CrossRef]
- Hökkä, H.; Salminen, H.; Ahtikoski, A.; Kojola, S.; Launiainen, S.; Lehtonen, M. Long-Term Impact of Ditch Network Maintenance on Timber Production, Profitability and Environmental Loads at Regional Level in Finland: A Simulation Study. For. Int. J. For. Res. For. 2017, 90, 234–246. [Google Scholar] [CrossRef]
- Smiljanić, M.; Seo, J.W.; Läänelaid, A.; van der Maaten-Theunissen, M.; Stajić, B.; Wilmking, M. Peatland Pines as a Proxy for Water Table Fluctuations: Disentangling Tree Growth, Hydrology and Possible Human Influence. Sci. Total Environ. 2014, 500–501, 52–63. [Google Scholar] [CrossRef]
- Latvian State Forest Research Institute “Silava”. Meža Resursu Monitoringa Rezultāti (Results of National Forest Inventory); Latvian State Forest Research Institute “Silava”: Salaspils, Latvia, 2024. [Google Scholar]
- Ahti, T.; Hämet-Ahti, L.; Jalas, J. Vegetation Zones and Their Sections in Northwestern Europe. Ann. Bot. Fenn. 1968, 5, 169–211. [Google Scholar]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated World Map of the Köppen-Geiger Climate Classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Harris, I.; Jones, P.D.; Osborn, T.J.; Lister, D.H. Updated High-Resolution Grids of Monthly Climatic Observations—The CRU TS3.10 Dataset. Int. J. Climatol. 2014, 34, 623–642. [Google Scholar] [CrossRef]
- Bušs, K. Forest Ecosystem Classification in Latvia. Proc. Latv. Acad. Sci. 1997, 51, 204–218. [Google Scholar]
- Grissino-Mayer, H.D. Evaluating Crossdating Accuracy: A Manual and Tutorial for the Computer Program COFECHA. Tree-Ring Res. 2001, 57, 205–221, ISSN 0735-9453. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.M.; Walker, S.C. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. LmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Haahti, K.; Koivusalo, H.; Hökkä, H.; Nieminen, M. Factors Affecting the Spatial Variability of Water Table Depth within a Drained Peatland Forest Stand in Northern Finland. SUO 2012, 63, 107–121. [Google Scholar]
- Jutras, S.; Plamondon, A.P. Water Table Rise after Harvesting in a Treed Fen Previously Drained for Forestry. SUO 2005, 56, 95–100. [Google Scholar]
- Harvey, J.E.; Smiljanić, M.; Scharnweber, T.; Buras, A.; Cedro, A.; Cruz-García, R.; Drobyshev, I.; Janecka, K.; Jansons, Ā.; Kaczka, R.; et al. Tree Growth Influenced by Warming Winter Climate and Summer Moisture Availability in Northern Temperate Forests. Glob. Chang. Biol. 2020, 26, 2505–2518. [Google Scholar] [CrossRef]
- Hökkä, H.; Palviainen, M.; Stenberg, L.; Heikkinen, J.; Laurén, A. Changing Role of Water Table and Weather Conditions in Diameter Growth of Scots Pine in Drained Peatlands. Can. J. For. Res. 2025, 55, 1–12. [Google Scholar] [CrossRef]
- Lévesque, M.; Saurer, M.; Siegwolf, R.; Eilmann, B.; Brang, P.; Bugmann, H.; Rigling, A. Drought Response of Five Conifer Species under Contrasting Water Availability Suggests High Vulnerability of Norway Spruce and European Larch. Glob. Chang. Biol. 2013, 19, 3184–3199. [Google Scholar] [CrossRef] [PubMed]
- Oleszczuk, R.; Zając, E.; Urbański, J.; Jadczyszyn, J. Rate of Fen-Peat Soil Subsidence near Drainage Ditches (Central Poland). Land 2021, 10, 1287. [Google Scholar] [CrossRef]
- Glenz, C.; Schlaepfer, R.; Iorgulescu, I.; Kienast, F. Flooding Tolerance of Central European Tree and Shrub Species. For. Ecol. Manag. 2006, 235, 1–13. [Google Scholar] [CrossRef]
- Matisons, R.; Jansone, D.; Bāders, E.; Dubra, S.; Zeltiņš, P.; Schneck, V.; Jansons, Ā. Weather–Growth Responses Show Differing Adaptability of Scots Pine Provenances in the South-Eastern Parts of Baltic Sea Region. Forests 2021, 12, 1641. [Google Scholar] [CrossRef]
- Puhe, J. Growth and Development of the Root System of Norway Spruce (Picea Abies) in Forest Stands—A Review. For. Ecol. Manag. 2003, 175, 253–273. [Google Scholar] [CrossRef]
- Dubois, H.; Verkasalo, E.; Claessens, H. Potential of Birch (Betula Pendula Roth and b. Pubescens Ehrh.) for Forestry and Forest-Based Industry Sector within the Changing Climatic and Socio-Economic Context Ofwestern Europe. Forests 2020, 11, 336. [Google Scholar] [CrossRef]
- Garfì, V.; Garfì, G. Differential Tree Growth Response to Management History and Climate in Multi-Aged Stands of Pinus pinea L. Plants 2023, 13, 61. [Google Scholar] [CrossRef]
- Bickovskis, K.; Samariks, V.; Jansons, A. Effect of Forest Stand Thinning on Tree Biomass Carbon Stock. In Proceedings of the 23rd International Scientific Conference Engineering for Rural Development, Jelgava, Latvia, 22–24 May 2024; Volume 23, pp. 36–41. [Google Scholar]
- Hökkä, H.; Repola, J.; Laine, J. Quantifying the Interrelationship between Tree Stand Growth Rate and Water Table Level in Drained Peatland Sites within Central Finland. Can. J. For. Res. 2008, 38, 1775–1783. [Google Scholar] [CrossRef]
- Matisons, R.; Jansone, D.; Elferts, D.; Schneck, V.; Kowalczyk, J.; Wojda, T.; Jansons, Ā. Silver Birch Shows Nonlinear Responses to Moisture Availability and Temperature in the Eastern Baltic Sea Region. Dendrochronologia 2022, 76, 3. [Google Scholar] [CrossRef]
- Krišāns, O.; Matisons, R.; Kitenberga, M.; Donis, J.; Rust, S.; Elferts, D.; Jansons, Ā. Wind Resistance of Eastern Baltic Silver Birch (Betula Pendula Roth.) Suggests Its Suitability for Periodically Waterlogged Sites. Forests 2021, 12, 21. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).