Scots Pine at Its Southern Range in Siberia: A Combined Drought and Fire Influence on Tree Vigor, Growth, and Regeneration
Abstract
1. Introduction
- Warming-driven air temperature increase stimulates the growth of Pinus sylvestris in conditions of sufficient moisture supply;
- Warming-driven increase in burning rate is a threat to the Pinus sylvestris habitat within its southern range in Siberia.
- How do changing hydrothermal regimes influence the growth index of pine trees?
- How does the changing burning rate influence pine stands and pine regeneration?
2. Materials and Methods
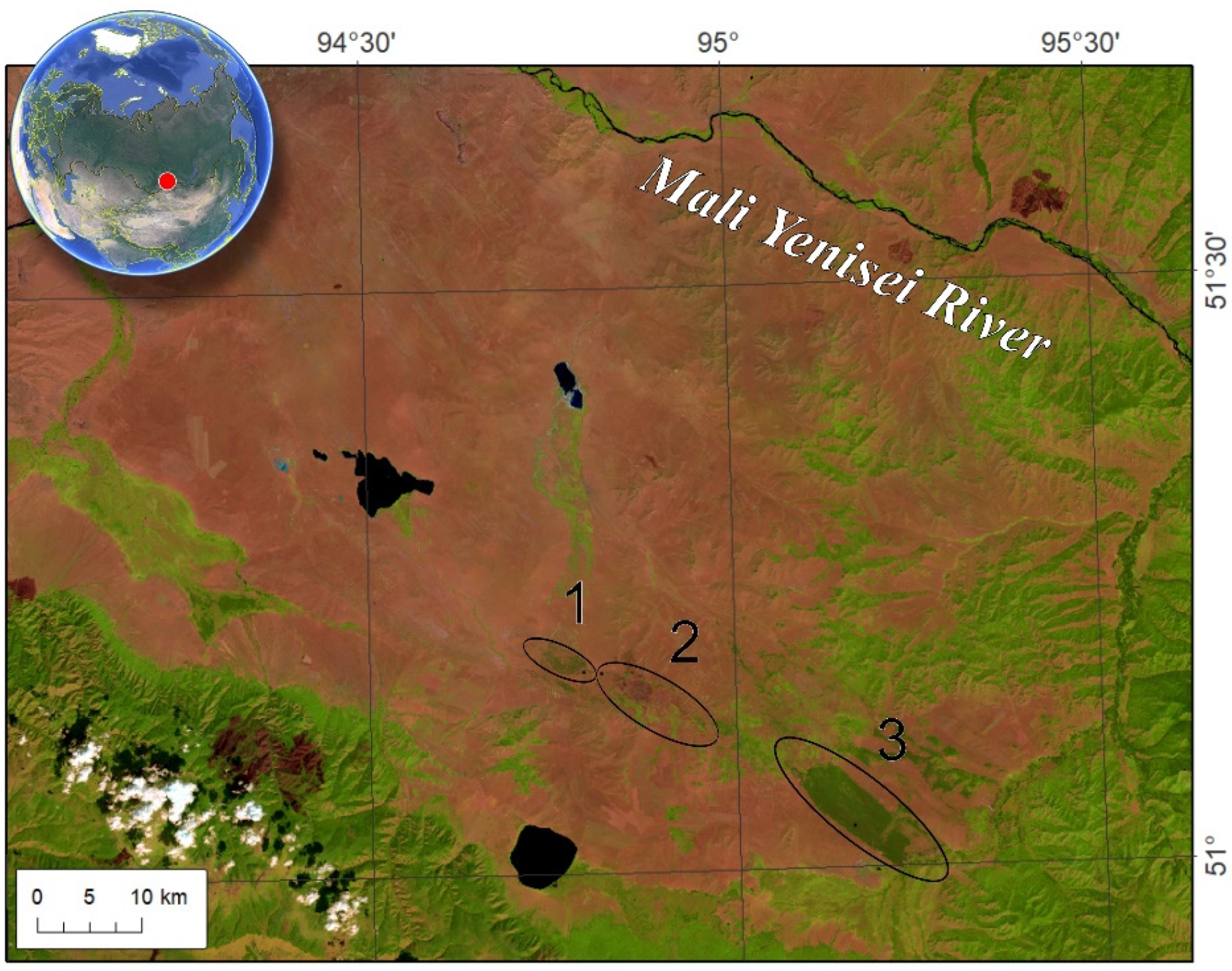
2.1. Study Area
2.2. Ground Survey Data
2.3. Dendrochronological Analysis
2.4. The Burning-Rate Analysis
2.5. Vegetation Productivity and Index Data
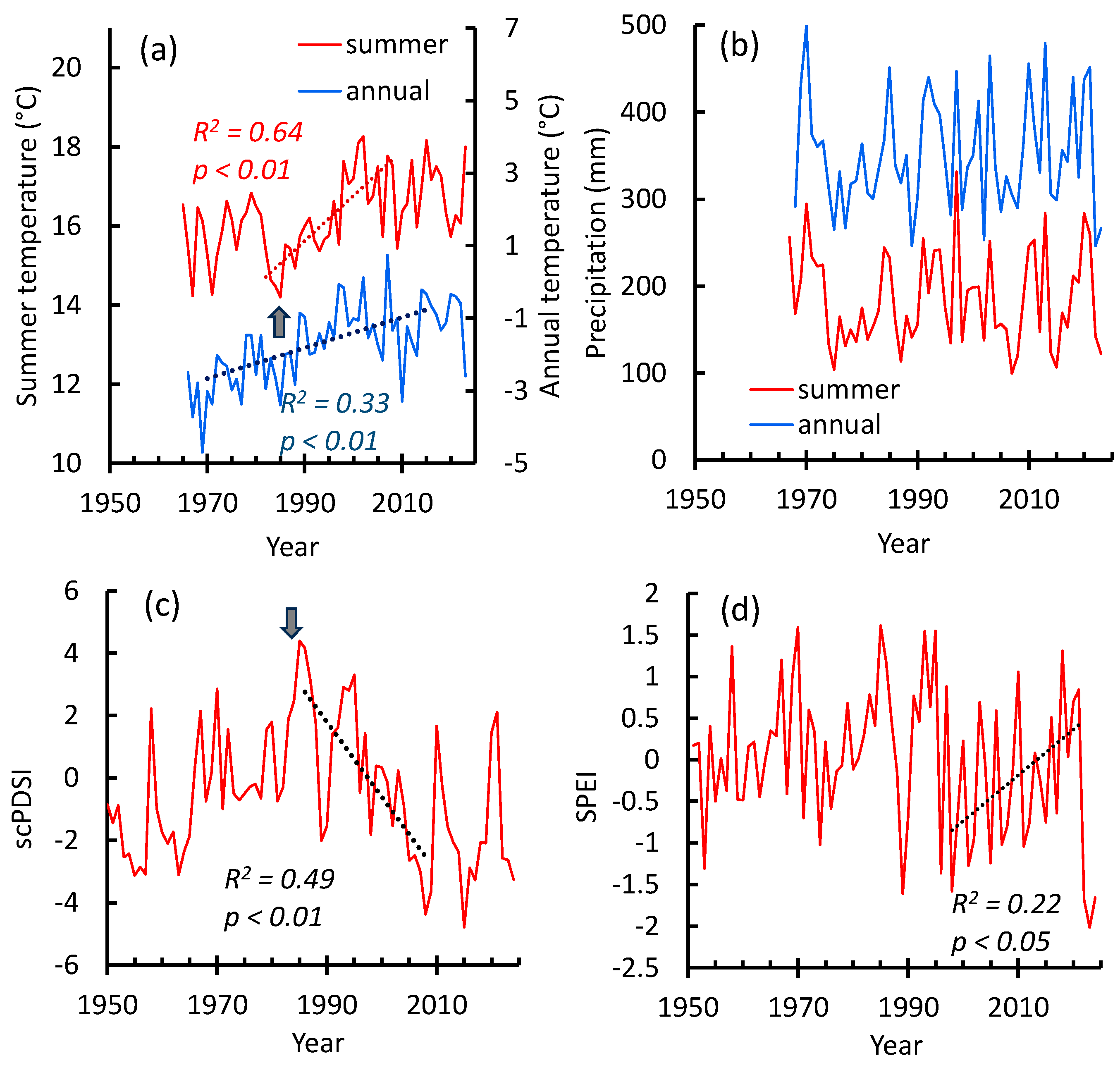
2.6. Climate Data
2.7. Statistical Analysis
3. Results
3.1. Field Data
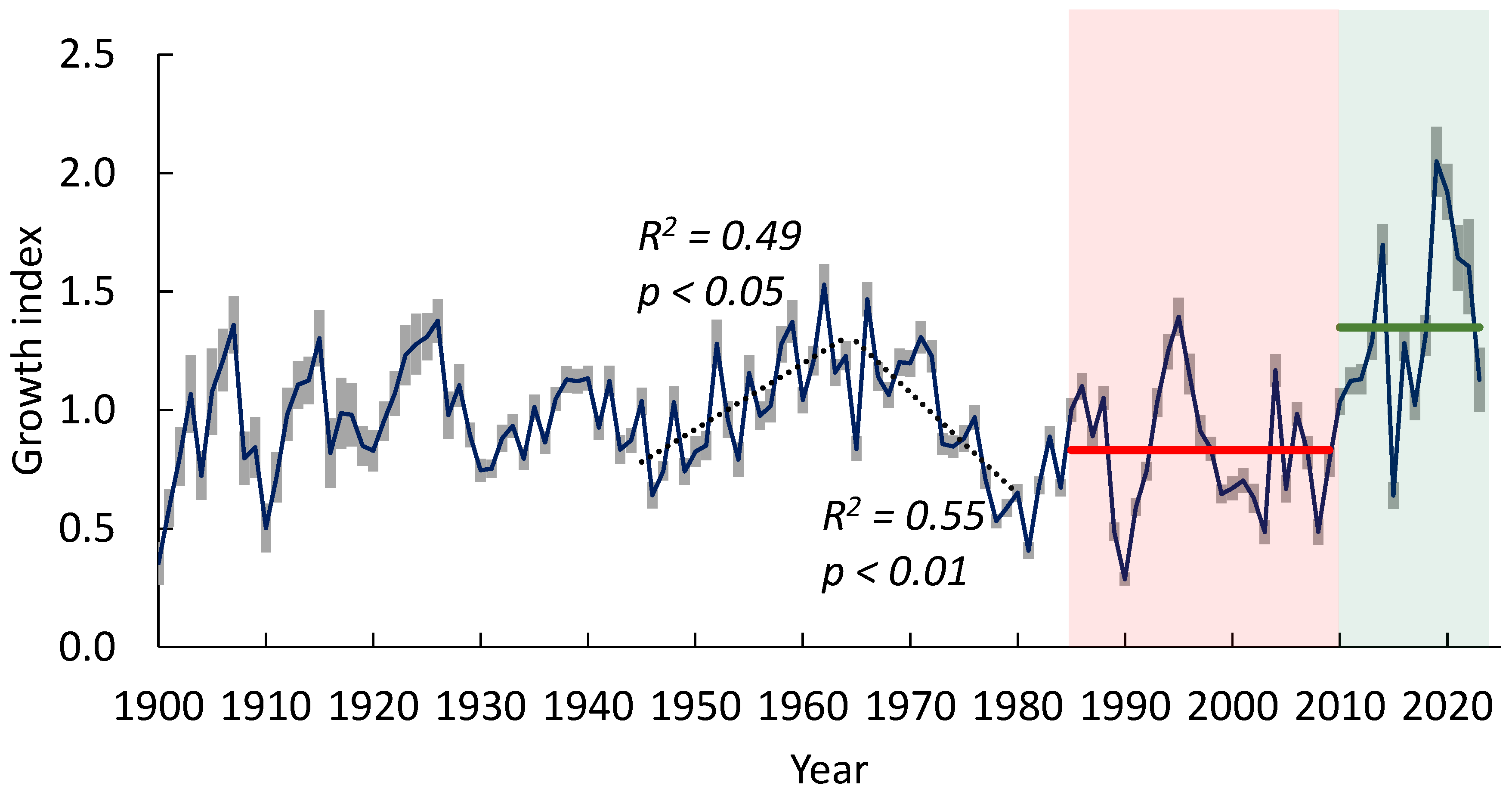
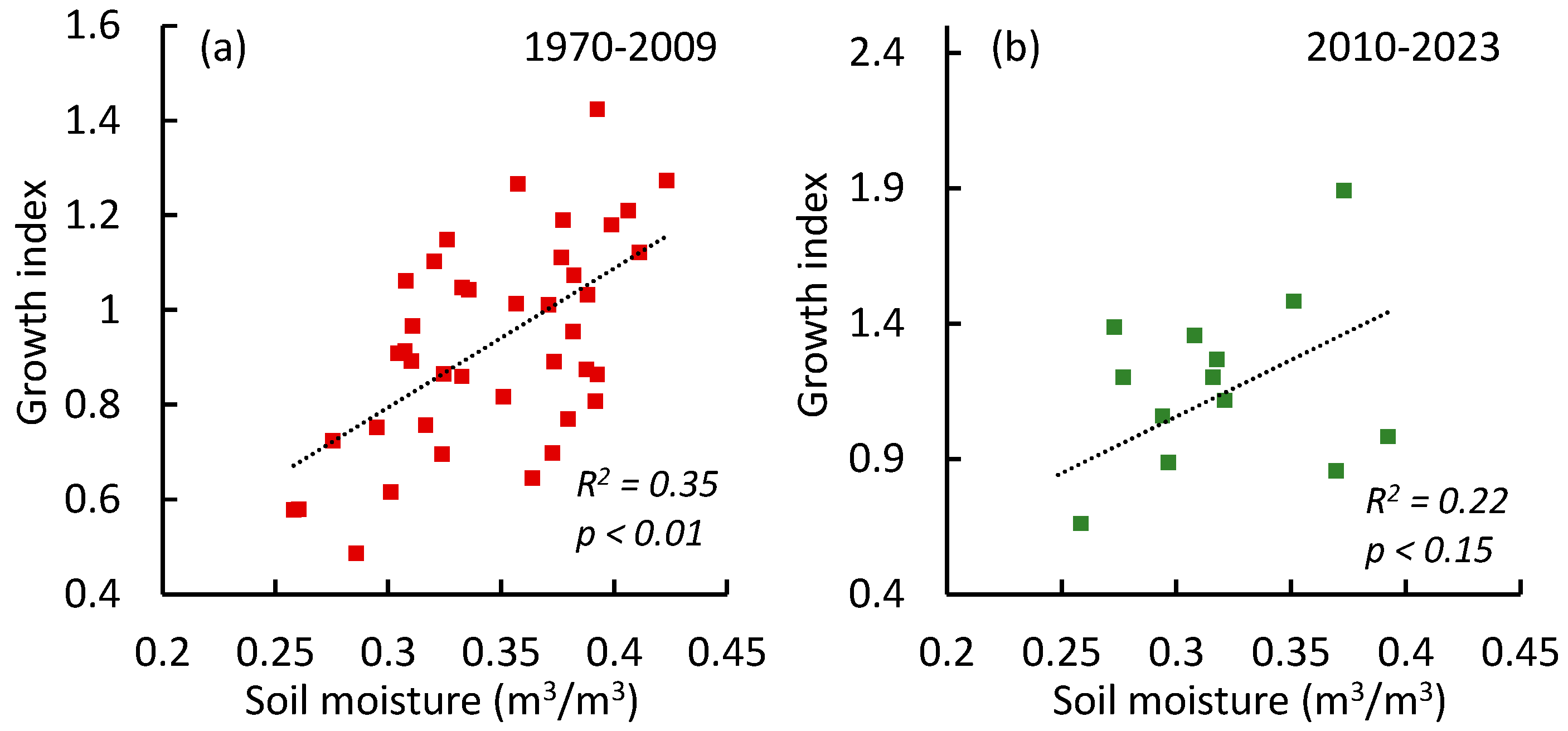
3.2. Chronology of Pine Tree Growth Index
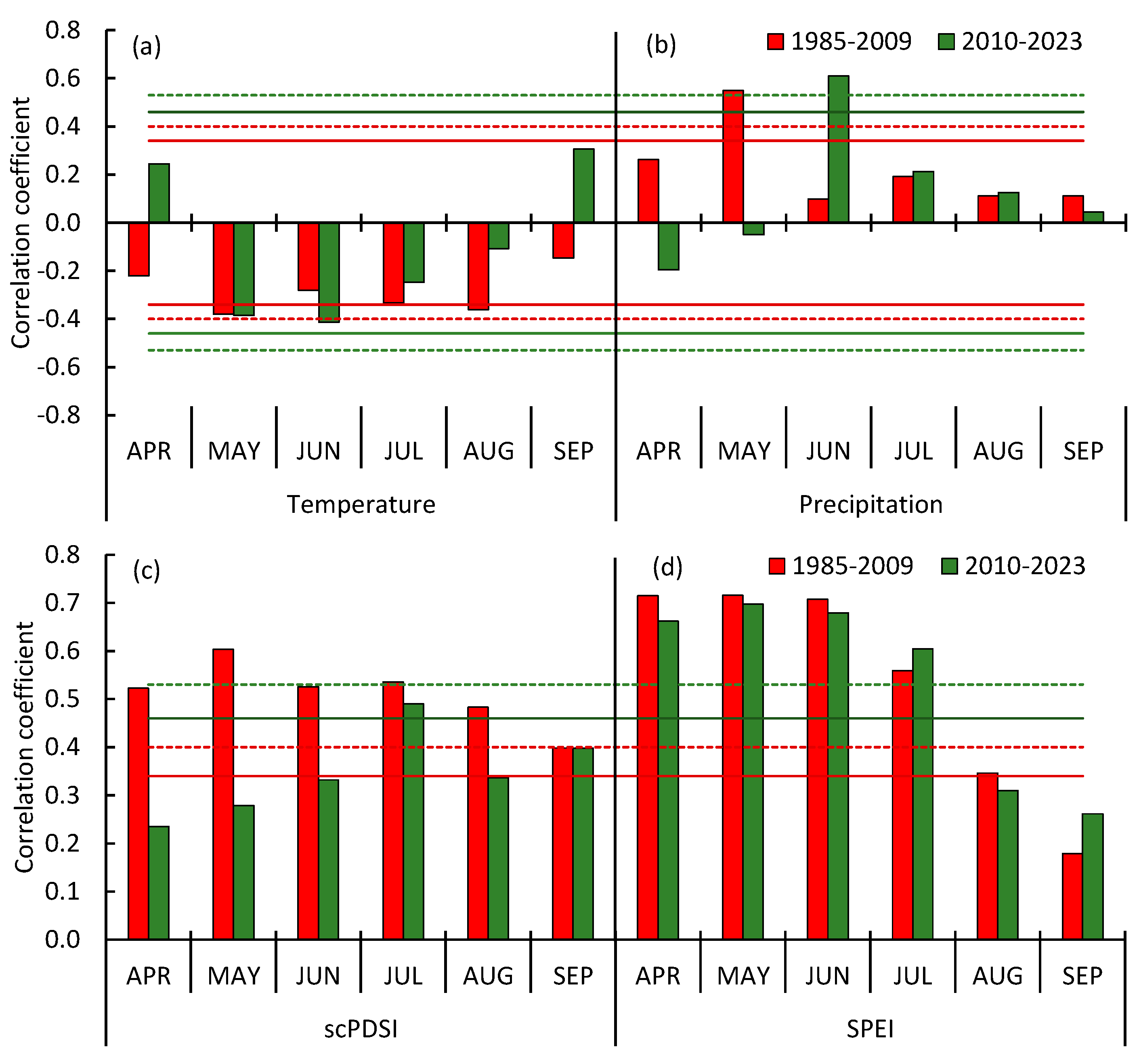
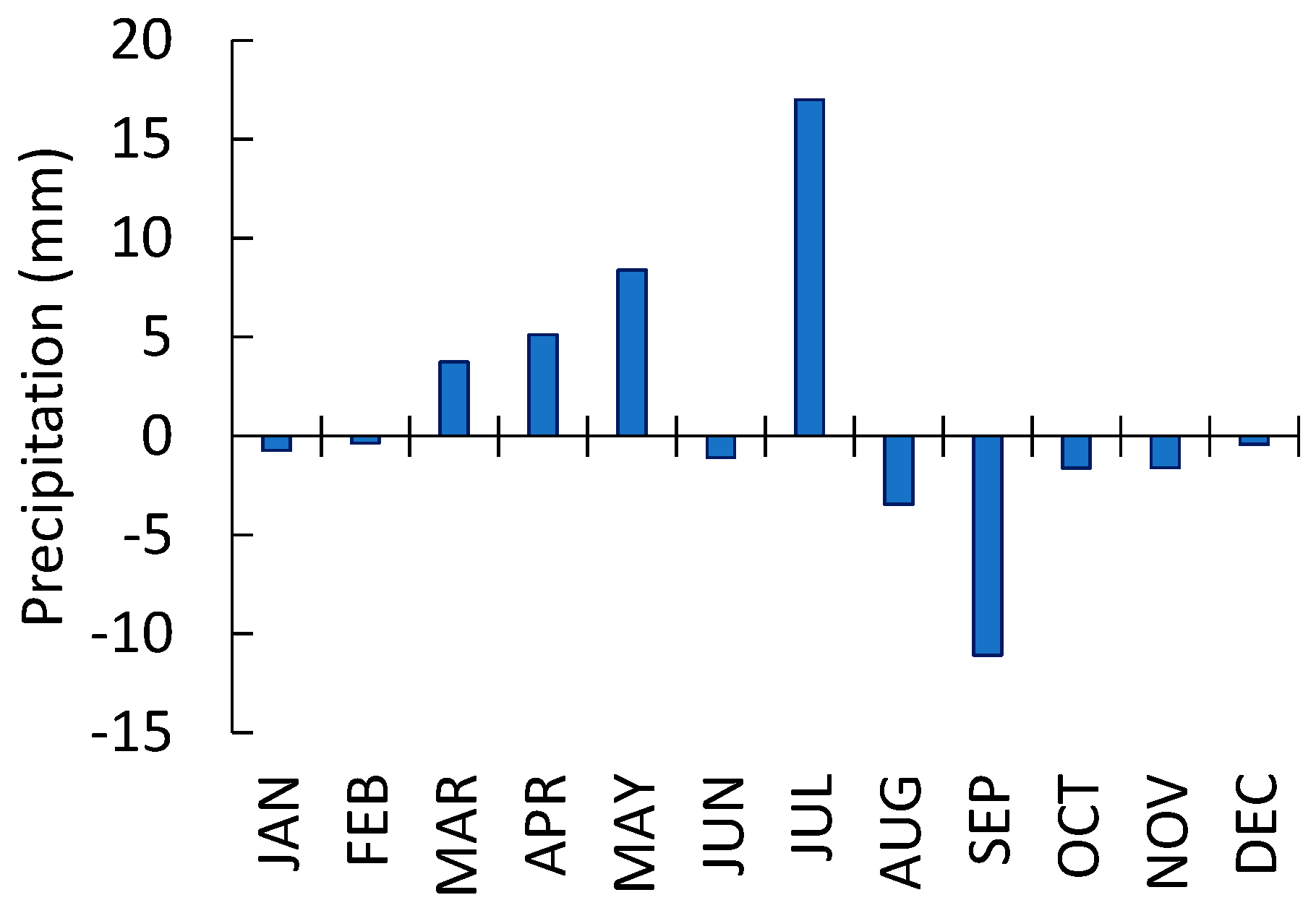
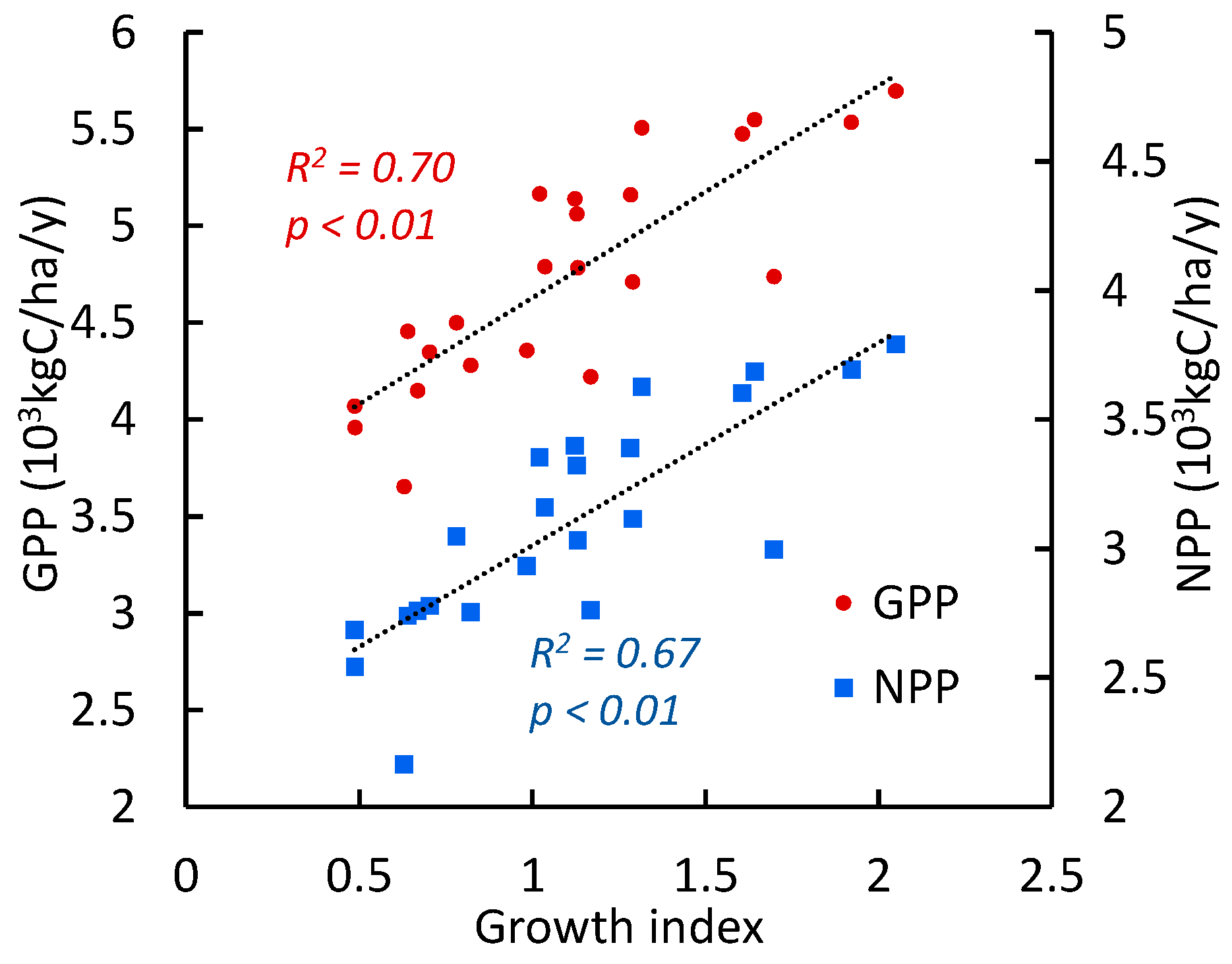
3.3. Pine Tree Growth Index: Relationship with Climate Variables
Correlations Between the Growth Index and Climate Variables
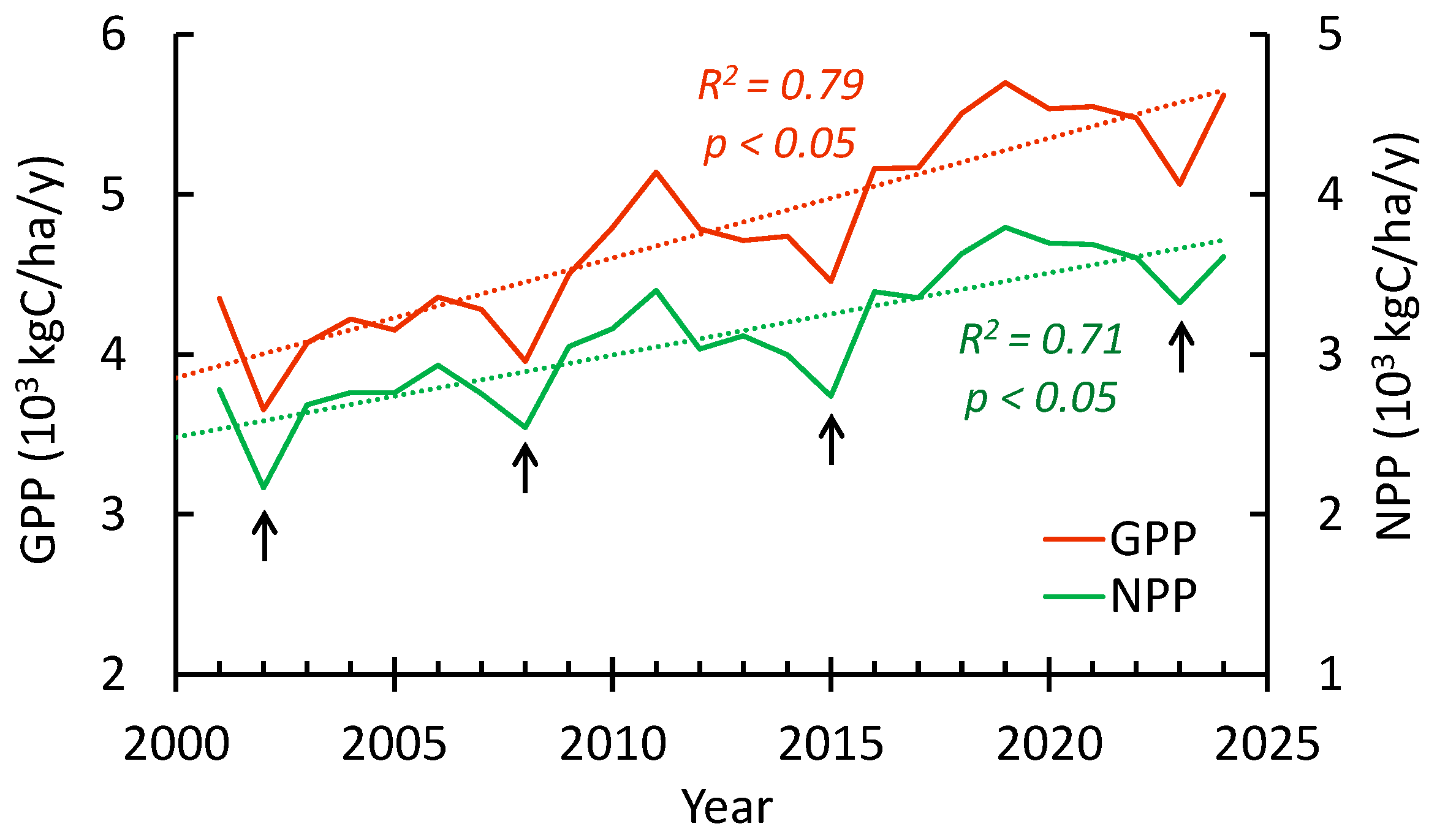
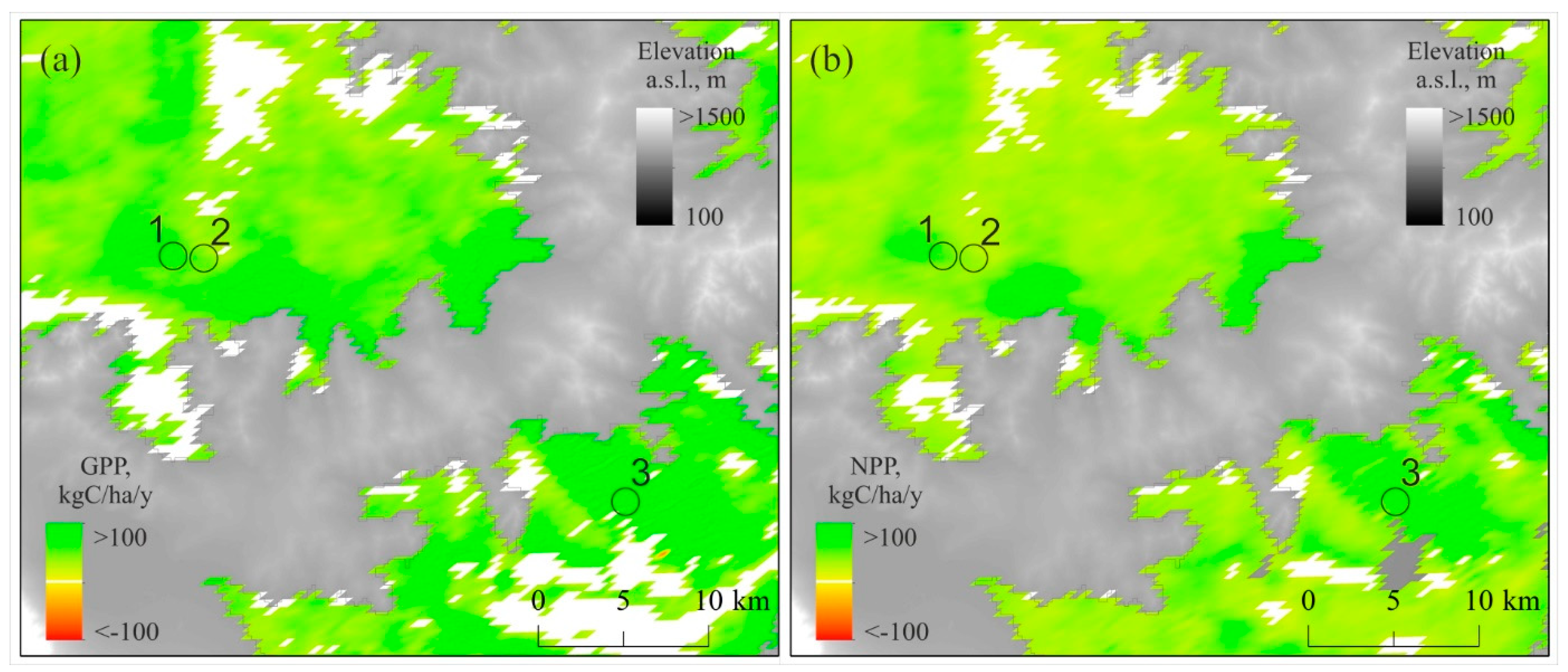
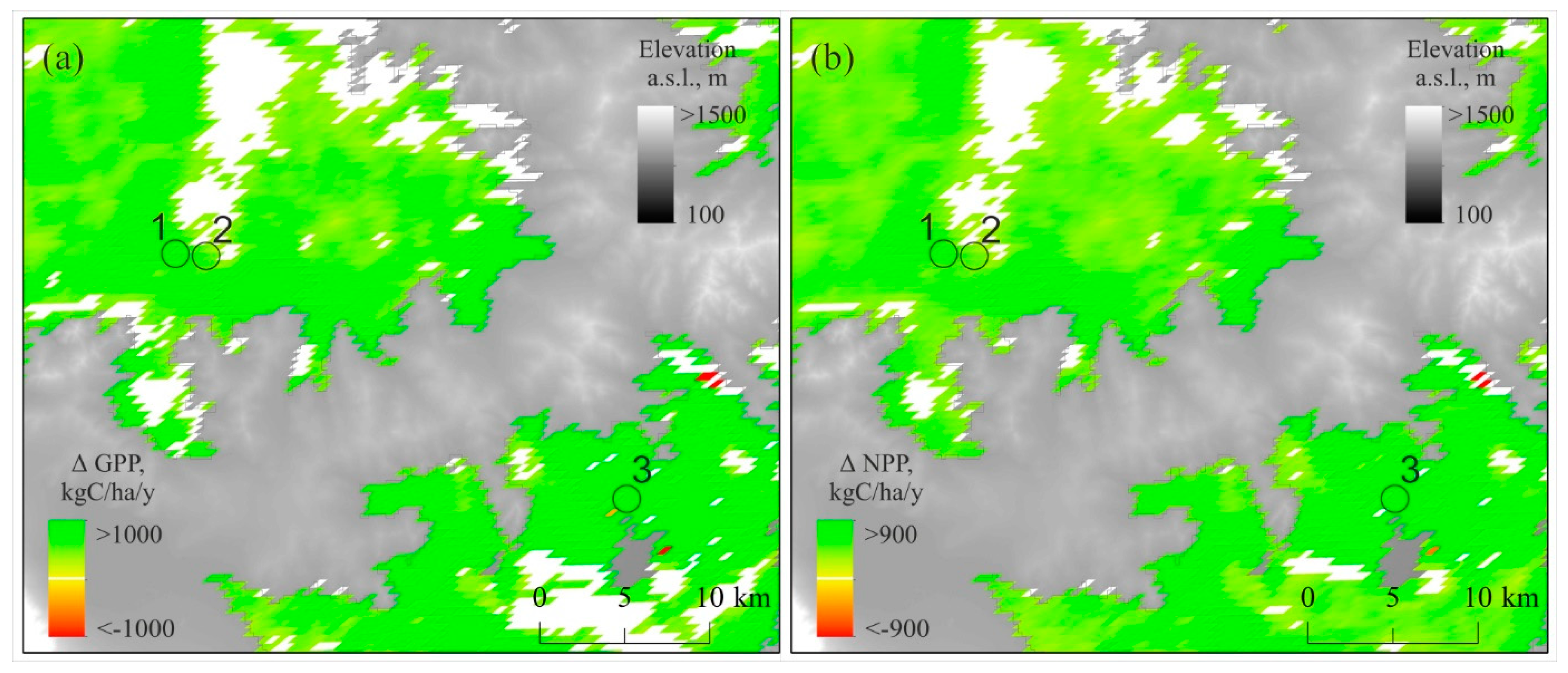
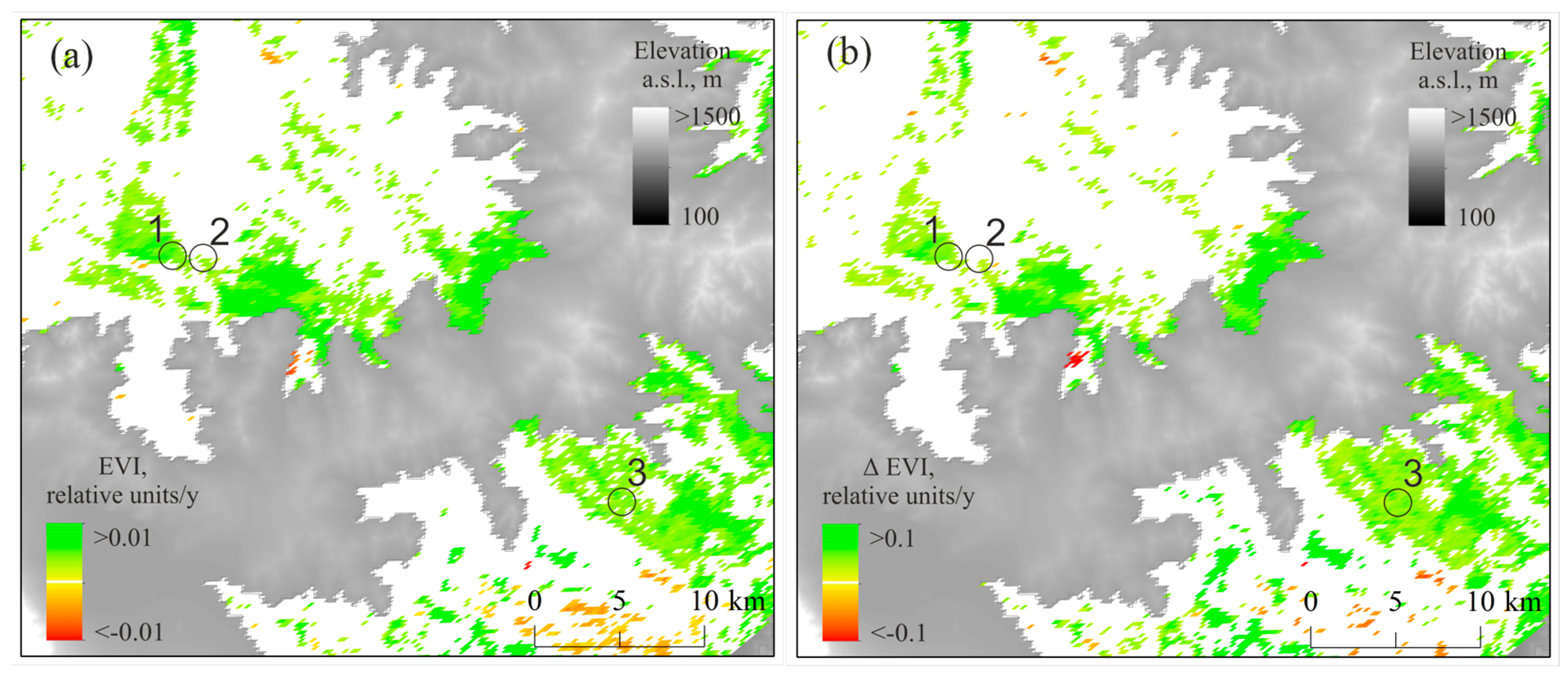
3.4. GPP, NPP, and EVI Trends Within the Study Sites
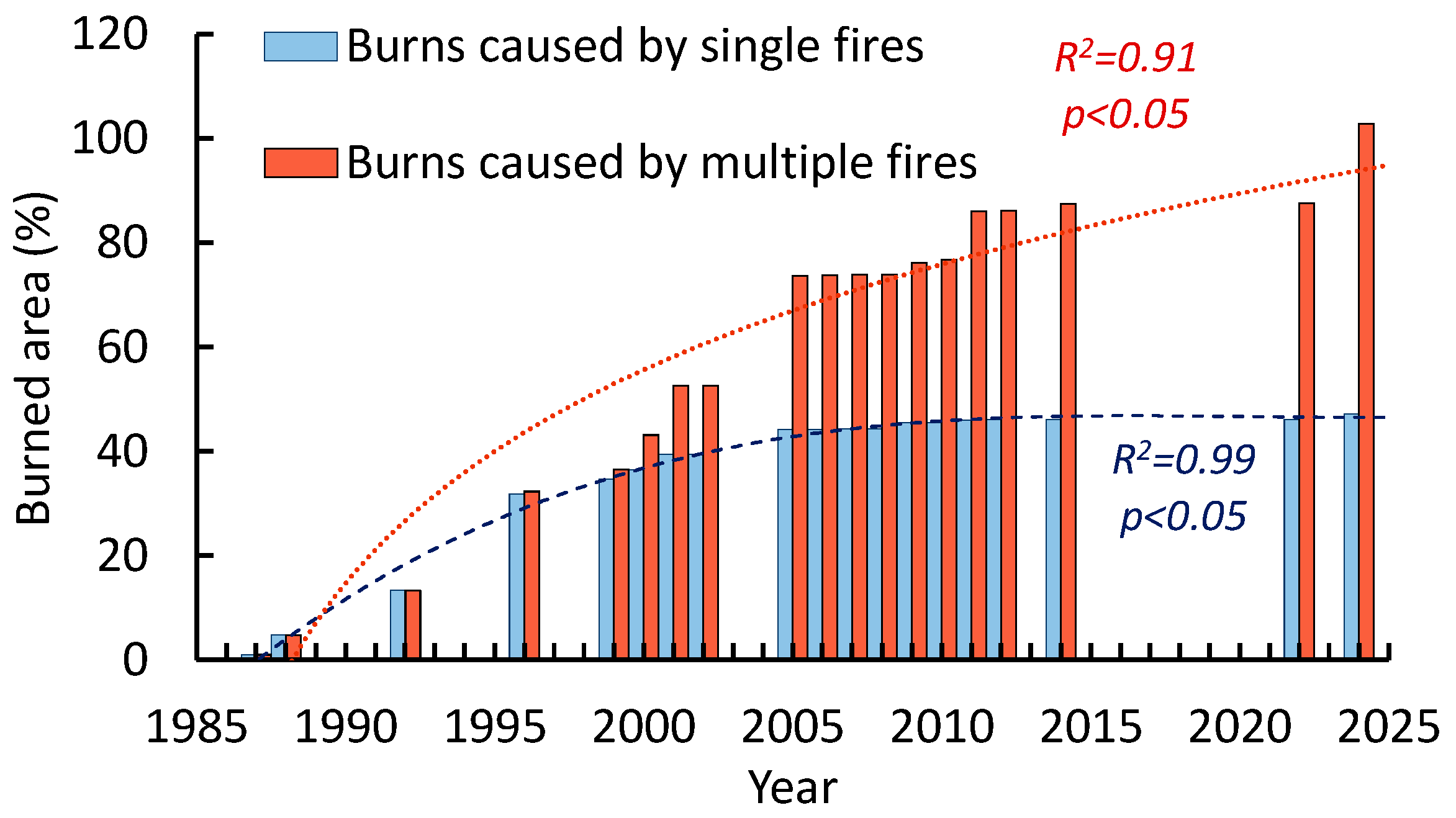
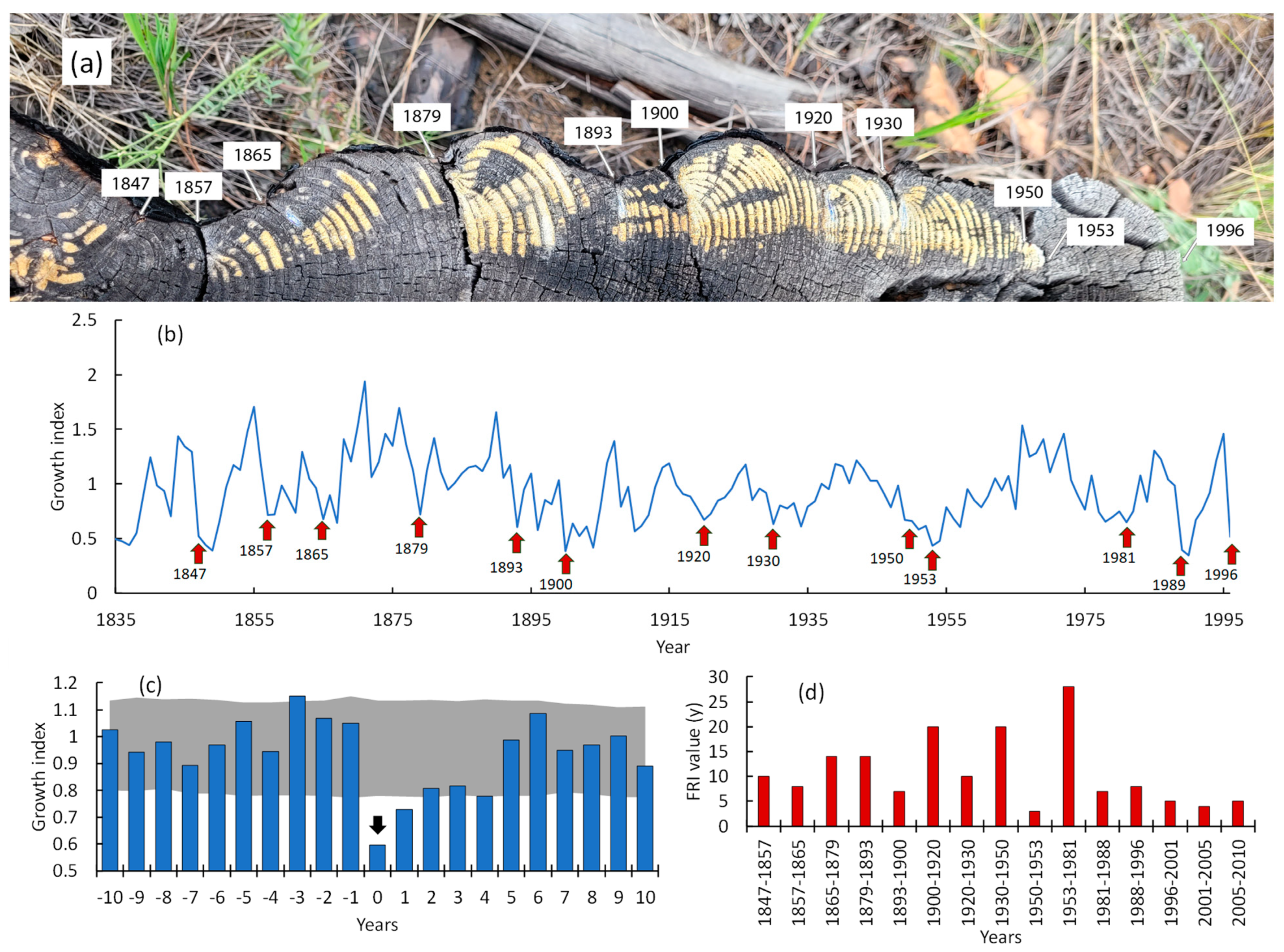
3.5. Fire Dynamics
3.6. Post-Fire Regeneration
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| FRI | Fire-return interval |
| GI | Growth index |
| GPP | Gross primary production |
| NPP | Net primary production |
| PET | Potential evapotranspiration |
| SSP | Shared Socioeconomic Pathway |
| scPDSI | Self-Calibrated Palmer Drought Severity Index |
| SPEI | Standardized Precipitation Evaporation Index |
| TP | Test plot |
| WMO | World Meteorological Organization |
Appendix A


References
- Anderegg, W.R.L.; Wu, C.; Acil, N.; Carvalhais, N.; Pugh, T.A.M.; Sadler, J.P.; Seidl, R.A. Climate risk analysis of Earth’s forests in the 21st century. Science 2022, 377, 1099–1103. [Google Scholar] [CrossRef] [PubMed]
- Boonman, C.C.F.; Serra-Diaz, J.M.; Hoeks, S.; Guo, W.-Y.; Enquist, B.J.; Maitner, B.; Malhi, Y.; Merow, C.; Buitenwerf, R.; Svenning, J.-C. More than 17,000 tree species are at risk from rapid global change. Nat. Commun. 2024, 15, 166. [Google Scholar] [CrossRef] [PubMed]
- Millar, C.I.; Stephenson, N.L. Temperate forest health in an era of emerging megadisturbance. Science 2015, 349, 823–826. [Google Scholar] [CrossRef]
- Coogan, S.C.P.; Robinne, F.-N.; Jain, P.; Flannigan, M.D. Scientists’ warning on wildfire—A Canadian perspective. Can. J. For. Res. 2019, 49, 1015–1023. [Google Scholar] [CrossRef]
- Tymstra, C.; Stocks, B.; Cai, X.; Flannigan, M. Wildfire management in Canada: Review, challenges and opportunities. Prog. Disaster Sci. 2020, 5, 100045. [Google Scholar] [CrossRef]
- Neumann, M.; Mues, V.; Moreno, A.; Hasenauer, H.; Seidl, R. Climate variability drives recent tree mortality in Europe. Glob. Change Biol. 2017, 23, 4788–4797. [Google Scholar] [CrossRef]
- Goulden, M.L.; Bales, R.C. California forest die-off linked to multi-year deep soil drying in 2012–2015 drought. Nat. Geosci. 2019, 12, 632–637. [Google Scholar] [CrossRef]
- Harvey, J.E.; Batllori, E.; Lloret, F.; Aakala, T.; Anderegg, W.R.L.; Aynekulu, E.; Bendixsen, D.P.; Bentouati, A.; Bigler, C.; Burk, C.J.; et al. Forest and woodland replacement patterns following drought-related mortality. Proc. Natl. Acad. Sci. USA 2020, 117, 29720–29729. [Google Scholar]
- Davis, F.W.; Parkinson, A.-M.; Moritz, M.A.; Isaac, W.; Park, C.M.; D’Antonio, C.M. Increasing vulnerability of an endemic Mediterranean-climate conifer to changing climate and fire regime. Front. For. Glob. Change 2025, 8, 1516623. [Google Scholar] [CrossRef]
- Verhoeven, D.; de Boer, W.F.; Henkens, R.J.H.G.; Sass-Klaassena, U.G.W. Water availability as driver of birch mortality in Hustai National Park, Mongolia. Dendrochronologia 2018, 49, 127–133. [Google Scholar] [CrossRef]
- Boyd, M.A.; Berner, L.T.; Doak, P.; Goetz, S.J.; Rogers, B.M.; Wagner, D.; Walker, X.J.; Mack, M.C. Impacts of climate and insect herbivory on productivity and physiology of trembling aspen (Populus tremuloides) in Alaskan boreal forests. Environ. Res. Lett. 2019, 14, 085010. [Google Scholar] [CrossRef]
- Kharuk, V.I.; Im, S.T.; Petrov, I.A.; Dvinskaya, M.L.; Shushpanov, A.S.; Golyukov, A.S. Climate-driven conifer mortality in Siberia. Glob. Ecol. Biogeogr. 2021, 30, 543–556. [Google Scholar] [CrossRef]
- Dial, R.J.; Maher, C.T.; Hewitt, R.E.; Sullivan, P.F. Sufficient conditions for rapid range expansion of a boreal conifer. Nature 2022, 608, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Kharuk, V.I.; Petrov, I.A.; Im, S.T.; Golyukov, A.S.; Dvinskaya, M.L.; Shushpanov, A.S. Tree Clusters Migration into Alpine Tundra, Siberia. J. Mt. Sci. 2022, 19, 3426–3440. [Google Scholar] [CrossRef]
- Buryak, L.V.; Sukhinin, A.I.; Kalenskaya, O.P.; Ponomarev, E.I. Effects of fires in ribbon-like pine forests of southern Siberia. Contemp. Probl. Ecol. 2011, 4, 248–253. [Google Scholar] [CrossRef]
- Buryak, L.V.; Kalenskaya, O.P.; Kukavskaya, E.A.; Luzganov, A.G. Zonal and Geographical Features of the Impact of Fires on Forest Formation of Light Coniferous Stands in the South of Siberia; Nauka: Novosibirsk, Russia, 2022. (In Russian) [Google Scholar]
- Ivanova, G.A.; Ivanov, V.A.; Kukavskaya, E.A. Periodicity of fires in the forests of the Republic of Tuva. Conifers Boreal Zone 2015, XXXIII, 204–209. (In Russian) [Google Scholar]
- Wang, Z.; Huang, J.G.; Ryzhkova, N.; Li, J.; Kryshen, A.; Voronin, V.; Li, R.; Bergeron, Y.; Drobyshev, I. 352 years long fire history of a Siberian boreal forest and its primary driving factor. Glob. Planet. Change 2021, 207, 103653. [Google Scholar] [CrossRef]
- Mamet, S.D.; Carissa, D.B.; Andrew, J.T.; Colin, P.L. Shifting global Larix distributions: Northern expansion and southern retraction as species respond to changing climate. J. Biogeogr. 2018, 46, 30–44. [Google Scholar] [CrossRef]
- Kharuk, V.I.; Ranson, K.J.; Petrov, I.A.; Dvinskaya, M.L.; Im, S.T.; Golyukov, A.S. Larch (Larix dahurica Turcz) Growth Response to Climate Change in the Siberian Permafrost Zone. Reg. Environ. Change 2019, 19, 233–243. [Google Scholar] [CrossRef]
- Kharuk, V.I.; Shvetsov, E.G.; Buryak, L.V.; Golyukov, A.S.; Dvinskaya, M.L.; Petrov, I.A. Wildfires in the Larch Range within Permafrost, Siberia. Fire 2023, 6, 301. [Google Scholar] [CrossRef]
- NOAA National Centers for Environmental Information. Climate at a Glance: Global Time Series. 2025. Available online: https://www.ncei.noaa.gov/access/monitoring/climate-at-a-glance/global/time-series (accessed on 21 February 2025).
- Holmes, R.L. Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bull. 1983, 44, 69–75. [Google Scholar]
- Rinn, F.; Tsap, V. 3.6 Reference Manual: Computer Program for Tree-Ring Analysis and Presentation; Frank Rinn Distribution: Heidelberg, Germany, 1996. [Google Scholar]
- Speer, J.H. Fundamentals of Tree-Ring Research; University of Arizona Press: Tucson, AZ, USA, 2010. [Google Scholar]
- Richards, J.A. Remote Sensing Digital Image Analysis, 6th ed.; Springer: Cham, Switzerland, 2022. [Google Scholar]
- Running, S.W.; Zhao, M. User’s Guide Daily GPP and Annual NPP (MOD17A2H/A3H) and Year-End Gap-Filled (MOD17A2HGF/A3HGF) Products NASA Earth Observing System MODIS Land Algorithm (For Collection 6.1). 2021. Available online: https://lpdaac.usgs.gov/documents/972/MOD17_User_Guide_V61.pdf (accessed on 21 March 2025).
- Didan, K.; Munoz, A.B. MODIS Vegetation Index User’s Guide (MOD13 Series). Version 3.10 (Collection 6.1); Vegetation Index and Phenology Lab; The University of Arizona: Tucson, AZ, USA, 2019. Available online: https://lpdaac.usgs.gov/documents/621/MOD13_User_Guide_V61.pdf (accessed on 20 March 2024).
- Conover, W.J. Practical nonparametric statistics. Wiley series in probability and mathematical statistics. In Applied Probability and Statistics; Wiley: Chichester, UK, 1999. [Google Scholar]
- Specialized Arrays for Climate Research. All-Russia Research Institute of Hydrometeorological Information—World Data Center. Available online: http://aisori-m.meteo.ru/waisori/index.xhtml?idata=2 (accessed on 21 March 2025). (In Russian).
- Liu, Q.; Yang, S.; Li, S.; Zhang, H.; Zhang, J.; Fan, H. The optimal applications of scPDSI and SPEI in characterizing meteorological drought, agricultural drought and terrestrial water availability on a global scale. Sci. Total Environ. 2024, 952, 175933. [Google Scholar] [CrossRef] [PubMed]
- Wells, N.; Goddard, S.; Hayes, M.J. A self-calibrating Palmer Drought Severity Index. J. Clim. 2004, 17, 2335–2351. [Google Scholar] [CrossRef]
- Muñoz Sabater, J. ERA5-Land Monthly Averaged Data from 1950 to Present. Copernicus Climate Change Service (C3S) Climate Data Store (CDS). 2019. Available online: https://cds.climate.copernicus.eu/datasets/reanalysis-era5-land-monthly-means?tab=overview (accessed on 21 February 2025).
- ERA5-Land: Data Documentation. Available online: https://confluence.ecmwf.int/display/CKB/ERA5-Land (accessed on 11 May 2025).
- Beguería, S.; Vicente-Serrano, S.M. SPEI: Calculation of the Standardized Precipitation-Evapotranspiration Index. 2023. Available online: https://github.com/sbegueria/SPEI (accessed on 21 March 2025).
- Cailleret, M.; Jansen, S.; Robert, E.M.R.; Desoto, L.; Aakala, T.; Antos, J.A.; Beikircher, B.; Bigler, C.; Bugmann, H.; Caccianiga, M.; et al. A synthesis of radial growth patterns preceding tree mortality. Glob. Change Biol. 2017, 23, 1675–1690. [Google Scholar] [CrossRef]
- Petrov, I.A.; Shushpanov, A.S.; Golyukov, A.S.; Dvinskaya, M.L.; Kharuk, V.I. Wildfire Dynamics in Pine Forests of Central Siberia in a Changing Climate. Contemp. Probl. Ecol. 2023, 16, 36–46. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Jones, R.G.; Narisma, G.T.; Alves, L.M.; Amjad, M.; Gorodetskaya, I.V.; Grose, M.; Klutse, N.A.B.; Krakovska, S.; Li, J.; et al. Atlas. In Climate Change 2021: The Physical Science Basis; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Chen, Y., Godfarb, L., Gomis, M.L., Matthews, J.B.R., Berger, S., et al., Eds.; Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021; Interactive Atlas; Available online: http://interactive-atlas.ipcc.ch/ (accessed on 21 March 2025).
- IPCC. Climate Change 2023: Synthesis Report; Core Writing Team, Lee, H., Romero, J., Eds.; Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2023; Available online: https://www.ipcc.ch/report/ar6/syr/downloads/report/IPCC_AR6_SYR_FullVolume.pdf (accessed on 21 March 2025).


| Sites | Coordinates | Number of Trees, n/ha | Age, y | Height, m | Diameter, cm | Seedlings, Thousands/ha | Crown Closure |
|---|---|---|---|---|---|---|---|
| Balgazyn | 51° 02′/95° 09′ | 950 | 106 ± 6 | 14 | 30 | 7 | 0.6 |
| Ulug-Hady | 51° 10′/94° 49′ | 150 | 119 ± 25 | 13 | 33 | 8 | 0.3 |
| Biche-Hady | 51° 10′/94° 47′ | 150 | 94 ± 7 | 18 | 38 | 10 | 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kharuk, V.I.; Petrov, I.A.; Shushpanov, A.S.; Im, S.T.; Ondar, S.O. Scots Pine at Its Southern Range in Siberia: A Combined Drought and Fire Influence on Tree Vigor, Growth, and Regeneration. Forests 2025, 16, 819. https://doi.org/10.3390/f16050819
Kharuk VI, Petrov IA, Shushpanov AS, Im ST, Ondar SO. Scots Pine at Its Southern Range in Siberia: A Combined Drought and Fire Influence on Tree Vigor, Growth, and Regeneration. Forests. 2025; 16(5):819. https://doi.org/10.3390/f16050819
Chicago/Turabian StyleKharuk, Viacheslav I., Il’ya A. Petrov, Alexander S. Shushpanov, Sergei T. Im, and Sergei O. Ondar. 2025. "Scots Pine at Its Southern Range in Siberia: A Combined Drought and Fire Influence on Tree Vigor, Growth, and Regeneration" Forests 16, no. 5: 819. https://doi.org/10.3390/f16050819
APA StyleKharuk, V. I., Petrov, I. A., Shushpanov, A. S., Im, S. T., & Ondar, S. O. (2025). Scots Pine at Its Southern Range in Siberia: A Combined Drought and Fire Influence on Tree Vigor, Growth, and Regeneration. Forests, 16(5), 819. https://doi.org/10.3390/f16050819








