Water Balance in an Atlantic Forest Remnant: Focus on Representative Tree Species
Abstract
1. Introduction
2. Materials and Methods
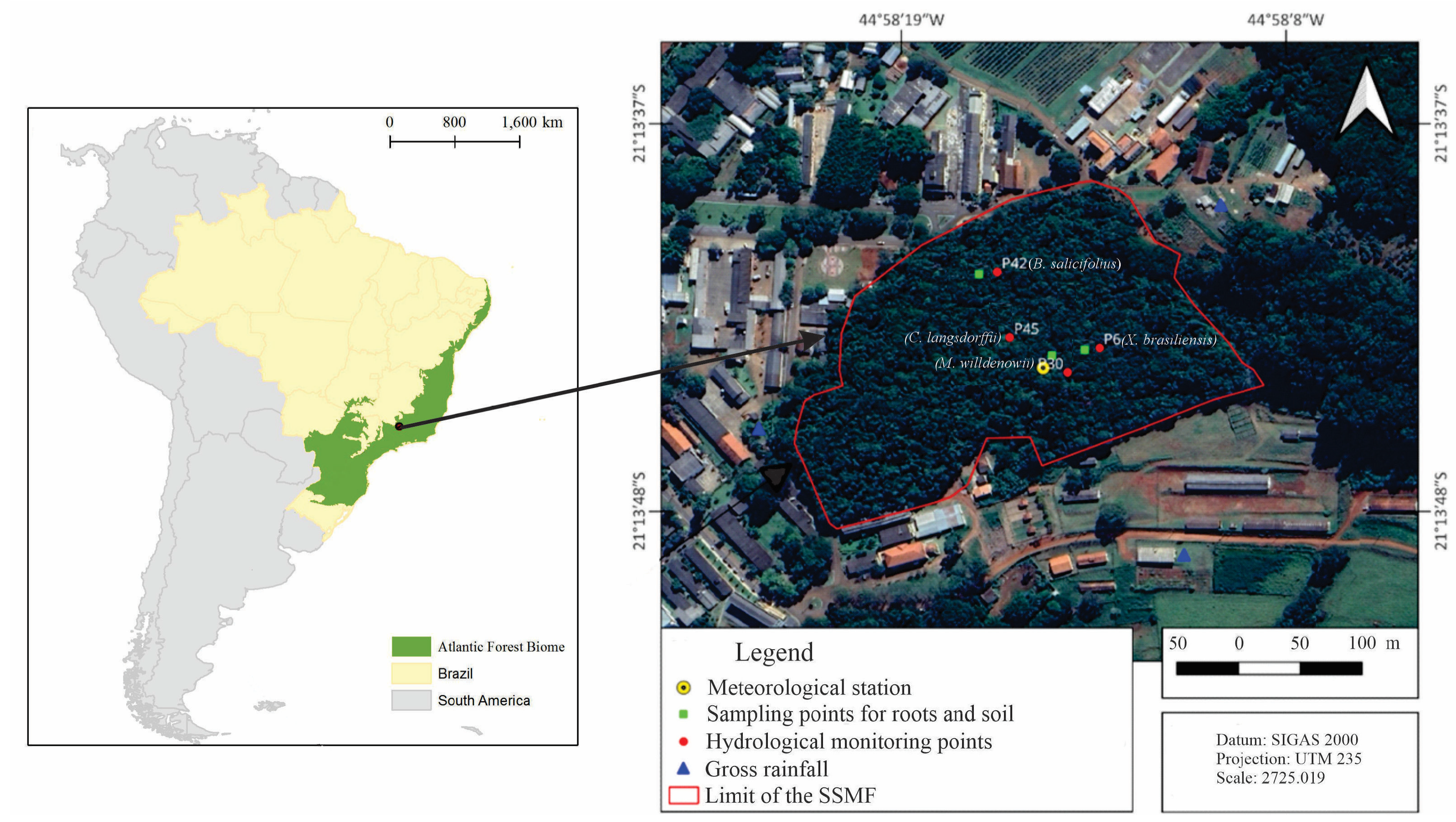
2.1. Location and General Aspects of the Study Area
2.2. Hydrological Monitoring Sets for This Study
2.3. Soil Moisture, Soil Water Storage, and Water Balance
2.4. Quantification of Root Weight Distribution in the Soil Profile
2.5. Estimating Aboveground Biomass
2.6. Characterization of the Saturated Soil Hydraulic Conductivity (Ksat)
2.7. Data Analysis
3. Results and Discussion
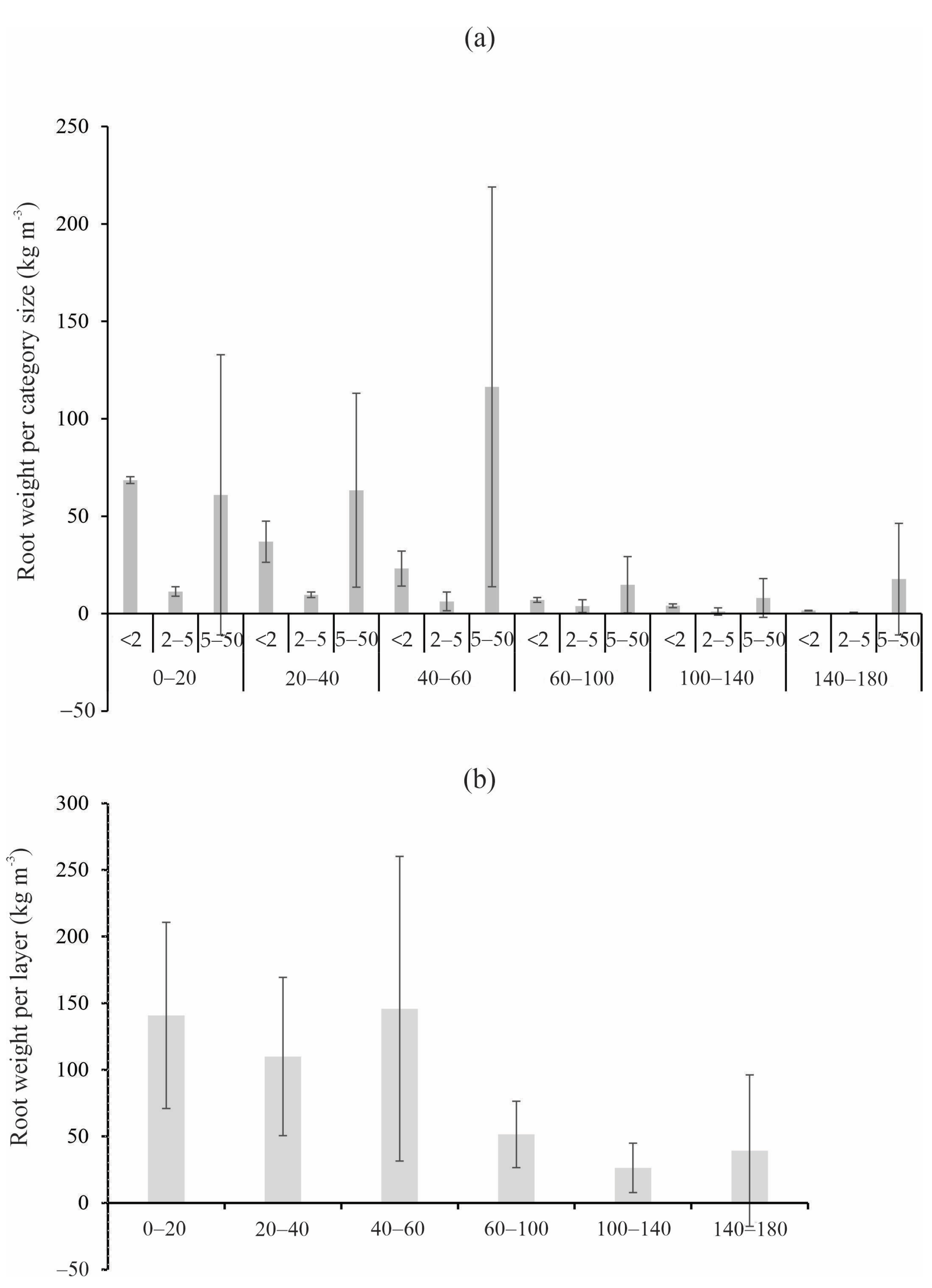
3.1. Distribution of the Fresh Root Biomass
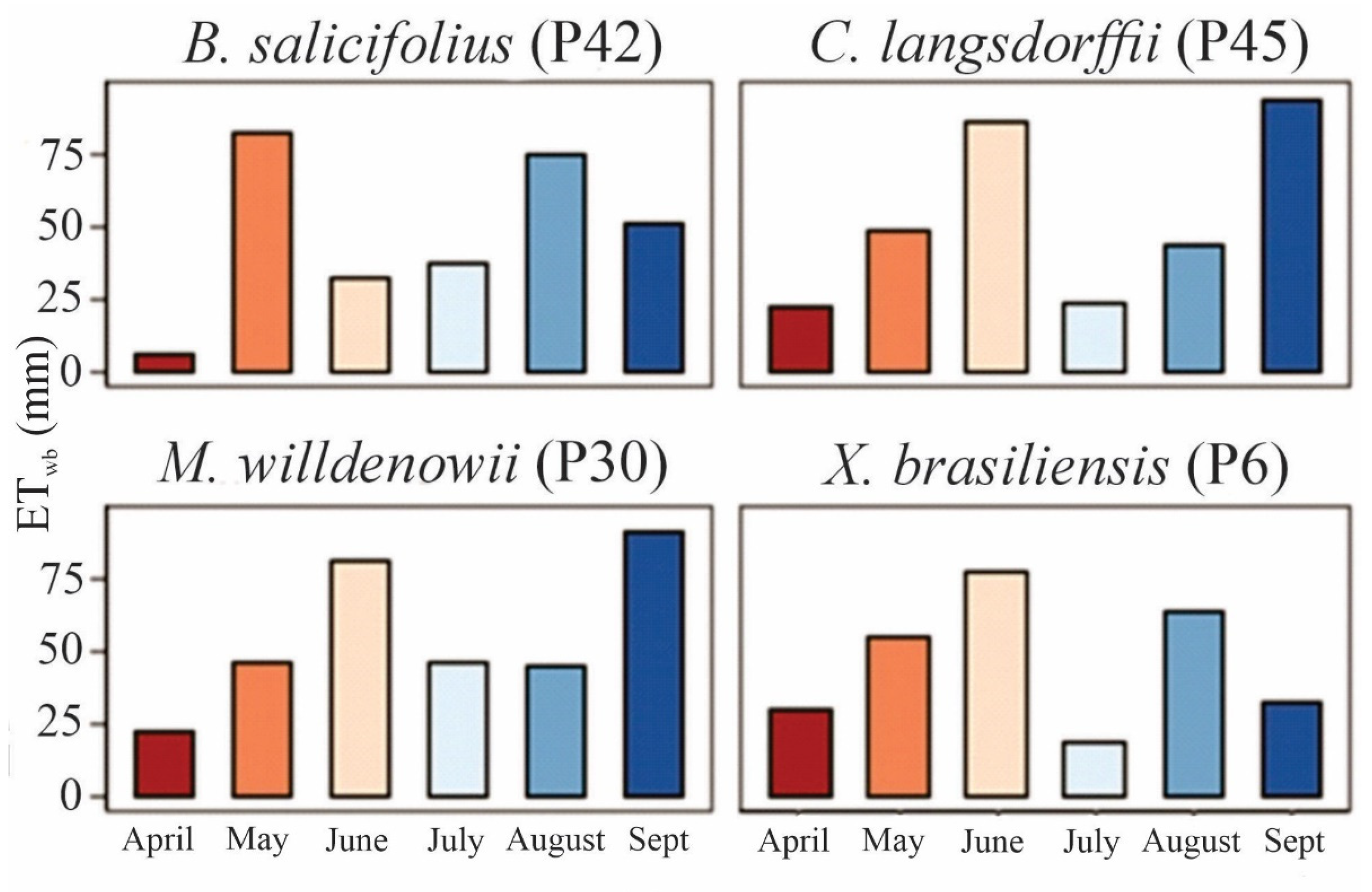
3.2. Water Balance
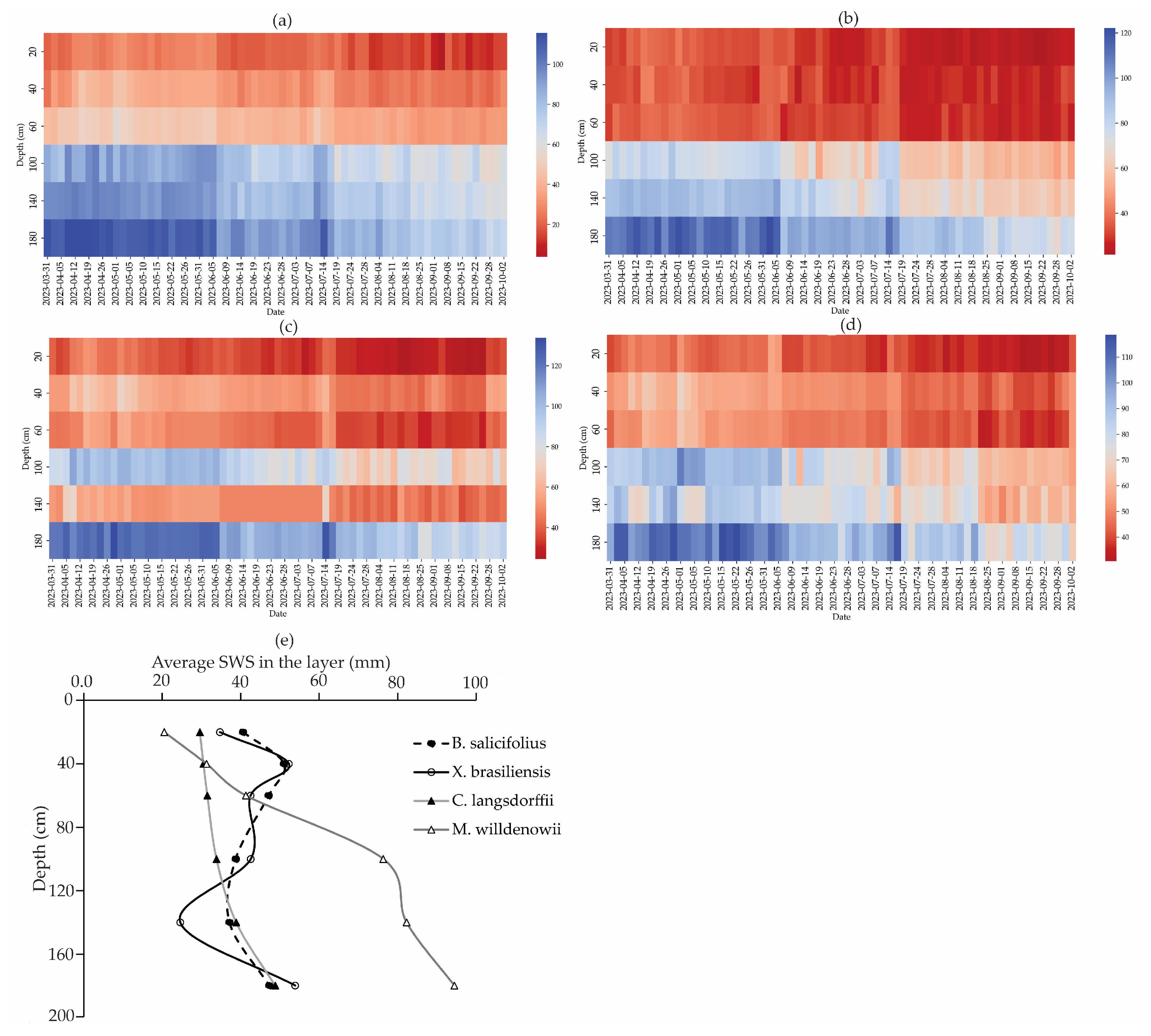
3.3. Connections Between SWS, Root Distribution, and Ksat
3.4. Brief Discussion of Mechanisms
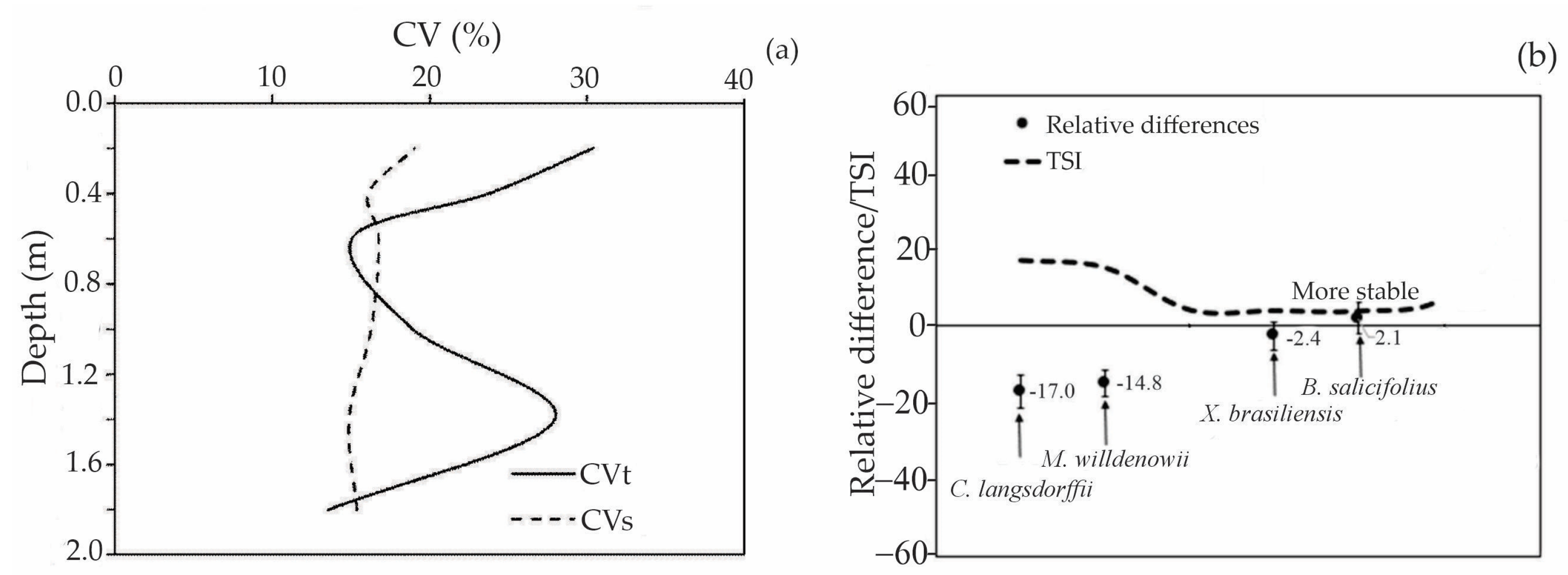
3.5. Temporal Variability of Soil Moisture in the Soil Profile at MSSF
4. Conclusions
- The rainfall pattern influenced the ET, EP, and CI in the monitored species during the dry period of 2023. This fact is associated with the canopy architecture, canopy density, and morphological characteristics of the species.
- We observed that the higher the AGB, the higher the CI; this relationship is inverse between AGB and ET in the trees assessed.
- X. brasiliensis and M. willdenowii showed greater variation in EP and lower values of SWS, with M. willdenowii presenting the highest SWS values.
- The species’ roots are mainly concentrated in the surface layers (<60 cm), associated with the availability of nutrients and water, with a non-uniform distribution in the soil profile.
- The root concentration throughout the soil profile contributes to soil moisture variability, especially in the first layers; we observed an inverse relationship between SWS and root concentration in M. willdenowii and C. langsdorffii, indicating that higher root concentration results in lower soil moisture in layers < 100 cm.
- Blefarocalice salicifolius and Xylopia brasiliensis are the trees more adapted to MSSF under drier conditions; such information can be used to establish recovery planning for degraded areas originally covered by the Atlantic Forest in Dark Red Oxisol.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Han, Q.; Sun, K.; Wang, H.; Pei, Z.; Chen, H.; Yang, J.; Sun, X. Water Balance Characteristics of the Salix Shelterbelt in the Kubuqi Desert. Forests 2024, 15, 278. [Google Scholar] [CrossRef]
- Pizarro, R.; Valdés-Pineda, R.; Garcia-Chevesich, P.A.; Ibáñez, A.; Pino, J.; Scott, D.F.; Ubilla, P. The large-scale effect of forest cover on long-term streamflow variations in mediterranean catchments of central Chile. Sustainability 2022, 14, 4443. [Google Scholar] [CrossRef]
- Joly, C.A.; Metzger, J.P.; Tabarelli, M. Experiences from the Brazilian Atlantic Forest: Ecological findings and conservation initiatives. New Phytol. 2014, 204, 459–473. [Google Scholar] [CrossRef]
- Junqueira Junior, J.A.; Mello, C.R.; Mello, J.M.; Scolforo, H.F.; Beskow, S.; McCarter, J. Rainfall partitioning measurement and rainfall interception modelling in a tropical semi-deciduous Atlantic forest remnant. Agric. For. Meteorol. 2019, 275, 170–183. [Google Scholar] [CrossRef]
- Salemi, L.F.; Groppo, J.D.; Trevisan, R.; de Moraes, J.M.; Ferraz, S.F.d.B.; Villani, J.P.; Duarte-Neto, P.J.; Martinelli, L.A. Land-use change in the Atlantic rainforest region: Consequences for the hydrology of small catchments. J. Hydrol. 2013, 499, 100–109. [Google Scholar] [CrossRef]
- Guauque-Mellado, D.; Rodrigues, A.; Terra, M.; Mantovani, V.; Yanagi, S.; Diotto, A.; Mello, C.R. Evapotranspiration under Drought Conditions: The Case Study of a Seasonally Dry Atlantic Forest. Atmosphere 2022, 13, 871. [Google Scholar] [CrossRef]
- Junqueira Junior, J.J.; Mello, C.R.; Owens, P.R.; Mello, J.M.; Curi, N.; Alves, G.J. Time-stability of soil water content (SWC) in an Atlantic Forest-Latosol site. Geoderma 2017, 288, 64–78. [Google Scholar] [CrossRef]
- Oliveira, V.A.; Rodrigues, A.F.; Morais, M.A.V.; Terra, M.D.C.N.S.; Guo, L.; Mello, C.R. Spatiotemporal modelling of soil moisture in an Atlantic Forest through machine learning algorithms. Eur. J. Soil Sci. 2021, 72, 1969–1987. [Google Scholar] [CrossRef]
- Macinnis-ng, C.M.O.; Flores, E.E.; Müller, H.; Schwendenmann, L. Throughfall and stemflow vary seasonally in different land-use types in a lower montane tropical region of Panama. Hydrol. Process. 2014, 28, 2174–2184. [Google Scholar] [CrossRef]
- Helman, D.; Lensky, I.M.; Yakir, D.; Osem, Y. Forests growing under dry conditions have higher hydrological resilience to drought than do more humid forests. Glob. Change Biol. 2017, 23, 2801–2817. [Google Scholar] [CrossRef]
- Lima, J.D.S.; Villela, D.M.; Calderano Filho, B.; Pérez, D.V. Biomassa radicular fina em fragmentos da Mata Atlântica fluminense. Floresta 2011, 41, 27–38. [Google Scholar] [CrossRef]
- Choat, B.; Brodribb, T.J.; Brodersen, C.R.; Duursma, R.A.; Lopez, R.; Medlyn, B.E. Triggers of tree mortality under drought. Nature 2018, 558, 531–539. [Google Scholar] [CrossRef]
- Oliveira-Filho, A.T.; Mello, J.M. Effects of past disturbance and edges on tree community structure and dynamics within a fragment of tropical semideciduous forest in south-eastern Brazil over a five-year period (1987–1992). Plant Ecol. 1997, 131, 45–66. [Google Scholar] [CrossRef]
- Oliveira-Filho, A.T.; Fontes, M.A.L. Patterns of floristic differentiation among Atlantic Forests in Southeastern Brazil and the influence of climate. Biotropica 2000, 32, 793–810. [Google Scholar] [CrossRef]
- INMET-Instituto Nacional de Meteorologia. Normais Climatológicas do Brasil 1991–2020. 2020. Available online: https://portal.inmet.gov.br/normais (accessed on 24 July 2024).
- Ratuchne, L.C.; Koehler, H.S.; Watzlawick, L.F.; Sanquetta, C.R.; Schamne, P.A. Estado da arte na quantificação de biomassa em raízes de formações florestais. FLORAM 2016, 23, 450–462. [Google Scholar] [CrossRef]
- Ramos, H.M.N.; Vasconcelos, S.S.; Kato, O.R.; Castellani, D.C. Above-and belowground carbon stocks of two organic, agroforestry-based oil palm production systems in eastern Amazonia. Agrofor. Syst. 2018, 92, 221–237. [Google Scholar] [CrossRef]
- Ribeiro, J.A.H.; de Almeida, L.H.D.; de Souza, T.G.; Lima, P.L.S.; de Sousa Cardoso, S.; de Souza, P.H.P.; Lacerda, F.D.C.B. Importância ecossistêmica das raízes: Uma revisão de literatura. Res. Soc. Dev. 2024, 13, e0313345177. [Google Scholar] [CrossRef]
- Chave, J.; Réjou-Méchain, M.; Búrquez, A.; Chidumayo, E.; Colgan, M.S.; Delitti, W.B.; Vieilledent, G. Improved allometric models to estimate the aboveground biomass of tropical trees. Glob. Change Biol. 2014, 20, 3177–3190. [Google Scholar] [CrossRef]
- Reynolds, W.D.; Elrick, D.E.; Youngs, E.G.; Booltink, H.W.G.; Bouma, J. Laboratory Methods. In Methods of Soil Analysis, Part 1, 2nd ed.; Klute, A., Ed.; SSSA Book Series; SSSA Publications: Madison, WI, USA, 1986; pp. 802–816. [Google Scholar]
- Basílio, J.J.N.; Campoe, O.C.; Queiroz, T.B.; de Souza, C.R.; Carneiro, R.L.; Alvares, C.A.; Figura, M.A. Fine Root Density Dynamics and Carbon Stock of Eucalyptus spp.: Interplay of Age, Genotype, and Edaphoclimatic Conditions. Plants 2024, 13, 1503. [Google Scholar] [CrossRef]
- Leonova, A.; Heger, A.; Vásconez Navas, L.K.; Jensen, K.; Reisdorff, C. Fine root mortality under severe drought reflects different root distribution of Quercus robur and Ulmus laevis trees in hardwood floodplain forests. Trees 2022, 36, 1105–1115. [Google Scholar] [CrossRef]
- Kulmann, M.S.D.S.; Dick, G.; Eufrade Junior, H.J.; Guerra, S.P.S.; Schumacher, M.V. Soil physical-chemical aspects influence the fine roots parameters of Pinus elliottii Engelm. stands in southern Brazil. Sci. For. 2022, 50, e3839. [Google Scholar] [CrossRef]
- Morais, V.A.; Santos, C.A.; Mello, J.M.; Dadid, H.C.; Araújo, E.J.G.; Scolforo, J.R.S. Spatial and vertical distribution of litter and belowground carbon in a Brazilian Cerrado vegetation. Cerne 2017, 23, 43–52. [Google Scholar] [CrossRef][Green Version]
- King, J.R.; Köry, J.; Ptashnyk, M. Multiscale analysis of nutrient uptake by plant roots with sparse distribution of root hairs: Nonstandard scaling. SIAM J. Appl. Math. 2021, 81, 1361–1388. [Google Scholar] [CrossRef]
- Demir, G.; Guswa, A.J.; Filipzik, J.; Metzger, J.C.; Römermann, C.; Hildebrandt, A. Root water uptake patterns are controlled by tree species interactions and soil water variability. Hydrol. Earth Syst. Sci. 2024, 28, 1441–1461. [Google Scholar] [CrossRef]
- Lian, X.; Zhao, W.; Gentine, P. Recent global decline in rainfall interception loss due to altered rainfall regimes. Nat. Commun. 2022, 13, 7642. [Google Scholar] [CrossRef]
- Mello, C.R.; Guo, L.; Yuan, C.; Rodrigues, A.F.; Lima, R.R.; Terra, M.C. Deciphering global patterns of forest canopy rainfall interception (FCRI): A synthesis of geographical, forest species, and methodological influences. J. Environ. Manag. 2024, 358, 120879. [Google Scholar] [CrossRef]
- Oliveira, S.; Cunha, J.; Nóbrega, R.L.; Gash, J.H.; Valente, F. Enhancing global rainfall interception loss estimation through vegetation structure modeling. J. Hydrol. 2024, 631, 130672. [Google Scholar] [CrossRef]
- Terra, M.D.C.N.; Mello, C.R.; de Mello, J.M.; de Oliveira, V.A.; Nunes, M.H.; Silva, V.O.; Alves, G.J. Stemflow in a neotropical forest remnant: Vegetative determinants, spatial distribution and correlation with soil moisture. Trees 2018, 32, 323–335. [Google Scholar] [CrossRef]
- Oliveira-Filho, A.T.; Scolforo, J.R.S.; Mello, J.M. Composição florística e estrutura comunitária de um remanescente de floresta semidecídua montana em Lavras, MG. Braz. J. Bot. 1994, 17, 167–182. [Google Scholar]
- Agee, E.; He, L.; Bisht, G.; Couvreur, V.; Shahbaz, F.M.; Gough, C.M.; Matheny, A.M.; Bohrer, G.; Ivanov, V. Root lateral interactions drive water uptake patterns under water limitation. Adv. Water Resour. 2021, 151, 103896. [Google Scholar] [CrossRef]
- Pedroni, F.; Sanchez, M.; Santos, F.A. Fenologia da copaíba (Copaifera langsdorffii Desf. Leguminosae, Caesalpinioideae) em uma floresta semidecídua no sudeste do Brasil. Braz. J. Bot. 2002, 25, 183–194. [Google Scholar] [CrossRef]
- INPE Painel El Niño 2023-2024 BOLETIM MENSAL, No. 05 2024. Available online: https://dataserver.cptec.inpe.br/dataserver_diptc/web/Painel-Elnino/2023-2024/painel_el_nino_boletim_mensal_no_05.pdf (accessed on 10 July 2024).
- Barbosa, R.A.; de Faria, R.S.; da Silveira, L.J.; Dias, H.C.T.; Pimenta, L.V.A.; de Souza, C.M.; Ferreira, A.C.S. Variação Temporal Da Umidade Do Solo Sob Diferentes Coberturas Vegetais. Rev. Ifes Ci. 2019, 5, 11–23. [Google Scholar]
- Xiao, L.; Xue, S.; Liu, G.B.; Zhang, C. Soil moisture variability under different land uses in the Zhifanggou catchment of the Loess Plateau, China. Arid Land Res. Manag. 2014, 28, 274–290. [Google Scholar] [CrossRef]
- Carranza, C.; Nolet, C.; Pezij, M.; van der Ploeg, M. Root zone soil moisture estimation with Random Forest. J. Hydrol. 2021, 593, 125840. [Google Scholar] [CrossRef]
- Liu, D.; She, D. The effect of fracture properties on preferential flow in carbonate-derived laterite from karst moun tainous agroforestry lands. Soil Till Res. 2020, 203, 104670. [Google Scholar] [CrossRef]
- Luo, Z.; Niu, J.; Xie, B.; Zhang, L.; Chen, X.; Berndtsson, R.; Du, J.; Ao, J.; Yang, L.; Zhu, S. Influence of root distribution on preferential flow in deciduous and coniferous Forest soils. Forests 2019, 10, 986. [Google Scholar] [CrossRef]
- Lin, H.; Zhou, X. Evidence of subsurface preferential flow using soil hydrologic monitoring in the Shale Hills catchment. Eur. J. Soil Sci. 2008, 59, 34–49. [Google Scholar] [CrossRef]




| Scientific Name | Popular Name in Brazil | Height-H (m) | DBH (cm) |
|---|---|---|---|
| Blepharocalyx salicifolius (Kunth) | Murta | 12.0 | 19.50 |
| Copaifera langsdorffii (Desf.) | Copaíba | 16.4 | 32.10 |
| Miconia willdenowii (Klotzsch ex Naudin) | Miconia prateada | 12.5 | 23.10 |
| Xylopia brasiliensis (Spreng.) | Pindaíba | 15.4 | 35.00 |
| Species | AGB (kg) | CI (mm) | ETwb (mm) |
|---|---|---|---|
| Blepharocalyx salicifolius (Kunth) | 14.31 | 44.98 | 284.27 |
| Copaifera langsdorffii (Desf.) | 48.96 | 58.84 | 316.04 |
| Miconia willdenowii (Klotzsch ex Naudin) | 20.41 | 58.84 | 331.43 |
| Xylopia brasiliensis (Spreng.) | 54.52 | 88.35 | 278.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cau, A.C.; Junqueira Junior, J.A.; Vega, A.B.; Macôo, S.J.; Rodrigues, A.F.; Terra, M.C.N.S.; Guo, L.; Mello, C.R. Water Balance in an Atlantic Forest Remnant: Focus on Representative Tree Species. Forests 2025, 16, 812. https://doi.org/10.3390/f16050812
Cau AC, Junqueira Junior JA, Vega AB, Macôo SJ, Rodrigues AF, Terra MCNS, Guo L, Mello CR. Water Balance in an Atlantic Forest Remnant: Focus on Representative Tree Species. Forests. 2025; 16(5):812. https://doi.org/10.3390/f16050812
Chicago/Turabian StyleCau, Adérito C., José A. Junqueira Junior, Alejandra B. Vega, Severino J. Macôo, André F. Rodrigues, Marcela C. N. S. Terra, Li Guo, and Carlos R. Mello. 2025. "Water Balance in an Atlantic Forest Remnant: Focus on Representative Tree Species" Forests 16, no. 5: 812. https://doi.org/10.3390/f16050812
APA StyleCau, A. C., Junqueira Junior, J. A., Vega, A. B., Macôo, S. J., Rodrigues, A. F., Terra, M. C. N. S., Guo, L., & Mello, C. R. (2025). Water Balance in an Atlantic Forest Remnant: Focus on Representative Tree Species. Forests, 16(5), 812. https://doi.org/10.3390/f16050812









