Abstract
The periderm plays a crucial role in trees, acting as a barrier protecting internal tissues against biotic and abiotic stresses, thus having an impact on tree physiology, ecology, and general performance. It consists of the meristematic phellogen, whose activity gives rise to suberized phellem (cork) cells outwardly and the parenchymatous phelloderm inwardly. Despite the periderm importance, intra-annual and seasonal changes in phellogen activity and phellem and phelloderm differentiation are poorly recognized. Therefore, we aimed to compare periderm development and functioning in successive years in horse chestnut, utilizing standard histological methods. We distinguished six stages of periderm development, including phellogen initiation and the differentiation of its derivatives. In the following years, the phellogen was active for a similar period, but produced fewer derivative cells. Importantly, some phellogen cells lost their meristematic characteristics before the end of the season and differentiated into phellem. To maintain periderm integrity, the remaining phelloderm cells underwent divisions, leading to phellogen re-initiation. Alternatively, when all periderm cells differentiated into the phellem, the new (subsidiary) phellogen originated from the underneath collenchyma. We postulate that phellogen re-initiation could be a mechanism ensuring the functional integrity of the periderm and discuss the role of phelloderm or collenchyma cells in this process.
1. Introduction
In long-lived plants, such as trees, one of the major challenges is to survive in a changing environment, which is especially important during current global climate changes. One of the crucial adaptations is to form the barrier between the environment and internal tissues, protecting trees against variable biotic and abiotic factors. In long-lived plants, during their secondary growth, this function is fulfilled by the periderm [1,2] or by the rhytidome (outer bark), formed later in tree development [3,4].
The periderm is a secondary protective tissue composed of the meristematic phellogen and its derivatives, i.e., the phellem (cork) and the phelloderm. Phellogen cells usually divide periclinally (i.e., parallel to the surface), giving rise to the phelloderm inwardly and the phellem outwardly; thus, phellogen cell activity leads to the formation of regular radial rows of cells [5]. The phelloderm is composed of living parenchymatous cells. Its thickness varies between species, and only rarely is the phelloderm not present [3]. Usually, phelloderm cells are alike, and only in some species, they are thick-walled or even schlerenchymatous, besides thin-walled cells. In addition, phelloderm cells may be filled with different compounds, e.g., tannins or crystals [6,7,8,9]. The phellem cells are dead, suberized, tightly adhering one to another, and without intercellular spaces, forming the main protective layer of the periderm [10]. Phellem layers can be uniform or contain different cell types including thin- or thick-walled cells, phelloids, which are cells devoid of suberin, stone cells, and crystal-bearing cells [5,8,11,12,13]. Various cell types differentiate periodically, making it possible to delimit annual growth rings in some species [10,14].
The first phellogen originates due to two successive periclinal divisions in the epidermis, in the subepidermal and other cells of the primary cortex, or in both tissues [5,12,15]. After the first division, two cells are formed, with the inner one usually differentiating into the phelloderm. The outer cell divides again and gives rise to two subsequent cells, initiating a radial row. Then, the outermost cell undergoes suberization and differentiates into the phellem, whereas the middle one maintains meristematic characteristics of the phellogen [16,17,18]. Alternatively, the formation of phellem cells precedes phelloderm differentiation [12,19,20].
The initiation of the phellogen usually starts in some locations and gradually spreads around the entire stem circumference [12,16,21]. The continuous periderm covers the entire circumference of the stem by the end of the first growing season; rarely, in some species, its formation is delayed for a few years [19,22]. The first established periderm may function for many years, but usually, succeeding periderms develop in deeper layers of the stem, mainly in the secondary phloem, leading to the formation of the rhytidome (outer bark) [5]. The rhytidome contains all earlier functioning periderms intervened with cells of the secondary phloem [3,4].
The significance of the periderm as the protective tissue and the economic importance of the cork account for the increasing interest in this tissue structure and functioning [1,2,10,23]. Current studies focus mostly on processes involved in phellem formation, including programmed cell death, the structure of the cell wall, and suberin biosynthesis [2,3,24]. Attention is also paid to the molecular mechanisms of phellogen maintenance and activity [23,25,26], along with the environmental and intrinsic factors affecting the periderm [14,27,28]. In this context, it is surprising how little is known about the intra-annual changes in the phellogen activity and differentiation of its derivatives during the season [13,29,30]. However, such data are of importance as a starting point for molecular analyses, as well as in light of plant adaptation to the changing environment. It is even more important now, as long-lived organisms, trees, have to cope with global climate changes, and the periderm and rhytidome constitute the interface between environment and internal tissues that are involved in signal perception and response.
Therefore, the main aim of our research was to trace the intra-annual changes in the periderm of Aesculus hippocastanum. We have chosen horse chestnut as it is a common ornamental tree native to the Balkan Peninsula, but is often cultivated in urban ecosystems in Europe due to its esthetic values [31]. It has also been proposed as a model tree to use as a bioindicator of environmental pollution [32,33,34]. In addition, it contains numerous bioactive compounds, such as esculin, escin, esculetin, scopoletin, and flavonoids, which occur in all plant parts including the periderm and are widely analyzed due to their usage in medicine [35,36]. We focused on the first phellogen initiation in Aesculus and phellem and phelloderm cell differentiation. As well, we aimed to compare the phellogen behavior in successive years after initiation, with special attention paid to phellogen maintenance or renewal.
We hypothesize that (1) phellogen cells are transient in their activity and can be re-initiated from phelloderm or collenchyma cells to maintain periderm integrity; (2) phellogen activity changes seasonally and is influenced by the tree age; and (3) phellem and phelloderm cells differentiate according to the intra-annual pattern. We showed that the phellogen initials can be temporary and can be re-initiated from phelloderm or collenchyma cells. The replacement of initial cells will likely be a mechanism that would account for the maintenance of periderm structural and functional continuity. The differentiation of the periderm cells changes intra-annually, with six separate stages distinguished in the first year of the periderm development; after phellogen establishment, in consecutive years, the periderm developmental program is shortened. Our detailed analyses can be a starting point for further deciphering different aspects of periderm functioning, including genetic regulation and periderm physiological and ecological adaptations.
2. Materials and Methods
2.1. Study Site
Analyses were performed in Wrocław (SW Poland). Horse chestnut (Aesculus hippocastanum L.) trees growing in two locations were chosen based on previous observations [37] (51°07′28.1″ N 16°59′44.8″ E, three trees, and 51°06′57.1″ N 17°02′47.5″ E, two trees). All trees grew in urban areas in city parks.
2.2. Sampling, Section Preparation, and Microscopic Observation
To show intra-annual changes in the periderm, one- and two-year-old branches were collected in 2019, 2021, 2022, and 2023, starting from the middle of March to the end of July in weekly intervals, and then in fortnight intervals until the middle of October. Samples from each date were used for further analyses. Analyzed branches were cut into smaller fragments, 0.5 cm high, and fixed in FAA (formalin-acetic acid-50% ethanol). After one week, they were moved to 50% ethanol and stored until further processing. To show the periderm structure upon development, samples were transversally cut (35–40 μm thick sections) on the vibratome (Leica VT 1200S; Leica Instruments GmbH, Wetzlar, Germany) and double-stained with an Alcian Blue–Safranin O mixture [38]; some vibratome sections were left unstained for other histochemical reactions (compare below). Alternatively, to obtain thinner sections, samples were dehydrated in the series of tertiary butyl alcohol (from 50 to 100%), infiltrated in the mixture of butyl alcohol and paraffin, with increasing concentrations of paraffin (20%–100%), and were embedded in paraffin [38]. Then, series of transverse sections, 8–10 mm thick, were cut with the use of the rotary microtome (Leica RM 2135, Leica Instruments GmbH, Wetzlar, Germany). Obtained sections were dewaxed, double-stained with the Alcian Blue–Safranin O mixture, dehydrated in alcohol series (50%–100%), and mounted in Euparal.
Additionally, the micro-cores, which were sampled in earlier studies (in 2014, 2015) for the secondary xylem differentiation [37], were utilized to analyze the periderm of the tree trunks. The micro-cores were taken from the main stem at a height of approx. 1.20–1.60 m above the ground [39], using the Trephor’s tool, in weekly intervals from the beginning of April to the middle of May, and then in fortnight intervals until the middle of October, in 2014 and 2015. From each micro-core, transverse sections were prepared using the same method as described above.
To analyze the chemical composition of phellem cell walls, unstained vibratome sections of one- and two-year-old branches were used (described above). They were viewed in UV light (360–370 nm) to detect the autofluorescence of suberin [12,40,41,42]; the suberin presence was further confirmed by a specific staining with 0.01% Fluorol Yellow 088 (Santa Cruz Biotechnology, TX, USA) in lactic acid, and visualized in blue light (488 nm) [43]. In addition, sections were stained with phloroglucinol and 50% HCl for lignin detection [38] or were observed in polarized light to show the crystalline structure of the secondary cell wall.
To visualize the ultrastructure of the mature periderm after the first year of functioning, the material was collected during the dormancy period in January 2021. The outer fragments of the stem were trimmed to smaller pieces (ca. 2 × 2 × 3 mm) and fixed in a mixture of 4% paraformaldehyde (PFA; Thermo Fisher Scientific, Waltham, MA, USA) and 5% glutaraldehyde (Sigma-Aldrich), with the addition of cacodylic acid (solved 1:1 in miliQ; protocol optimized for trees after [44]). Samples were left for 2 h at room temperature under low vacuum conditions and then placed in 4 °C overnight. Subsequently, the material was treated with a mixture of 1% osmium tetroxide and 0.8% potassium ferrocyanide at 4 °C for 24 h [45]. After that, samples were thoroughly rinsed, dehydrated, infused with resin using the acetone series (10%–100%) and acetone–resin series (4:1, 3:1, 2:1, 1:1), and then embedded in Epon 812 (Serva, Heidelberg, Germany). Ultrathin sections were cut using an ultramicrotome (Reichert Ultracut E, Munich, Germany) and contrasted with uranyl acetate and lead citrate [46].
Microscopic sections were observed using the epifluorescent microscope Olympus BX50 in bright field, UV, or blue emission light or using a polarizing adapter. Images were taken with an Olympus DP71 camera and Cell B software (Olympus Optical Co., Warsaw, Poland). Ultrathin sections were analyzed using transmission electron microscopy (TEM, Zeiss 900 EM). Figures were prepared using CorelDraw 2017 (Corel Co., Ottawa, ON, Canada). The contrast and brightness of the entire images were evenly increased, if necessary.
2.3. Estimating the Phellogen Activity
For each tree and each sampling date, at least three transverse sections were used to analyze the divisional activity of the phellogen. The date of the phellogen initiation, subsequent divisions, and differentiation of derivatives, as well as the date of cessation of divisions, were established. The number of cells in a radial row was counted in at least 10 rows per 1 transverse section. In addition, the number of rows with a detectable phellogen cell was estimated at the end of the year. The extremes, average values, and standard deviations were calculated using Microsoft Excel (Windows 365 for University of Wrocław).
3. Results
3.1. Initiation of the Phellogen and Its Activity in the First Year
In horse chestnut, the growth of new shoots is pre-determined, meaning that all leaves developing in the current year are initiated in a previous growing season and are already present in the bud. In analyzed trees, buds swelled usually in the first ten days of April, and the entire one-year-old branches developed before the end of April, when the first flowers appeared. New stems were covered by the epidermis, and collenchyma cells were located beneath the covering tissue (Figure 1, stage 1). In some location in the stem circumference, lenticels were visible already before the appearance of the first continuous periderm. However, as the continuous phellogen formation was not related to lenticels, their development was not further analyzed.
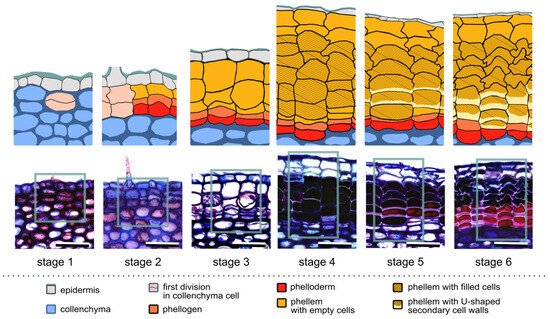
Figure 1.
Successive stages of the first periderm formation in Aesculus hippocastanum. The lower panel presents representative cross sections of one-year-old branches during successive developmental stages: stage 1—the first periclinal division in the collenchyma cell; stage 2—the second periclinal division, establishing the phellogen; stage 3—formation of the first type of phellem cells, empty inside; stage 4—formation of the second type of phellem cells, filled inside; stage 5—formation of the U-shaped secondary cell wall; stage 6—accomplishment of periderm differentiation. Interpretations of the cell identities in the section part, framed by rectangles, are shown in the corresponding diagrams in the upper panel. For details, see the text. Scale bars = 50 µm.
Based on structural changes during the development of the first periderm, six succeeding stages were determined (Figure 1). Regardless of the fluctuations in the following years, all stages occurred in a comparable period during a year (Figure 2). Even in 2021, when periderm formation started 2–3 weeks later comparing to the remaining years, the duration of successive stages and the number of produced cells were comparable.
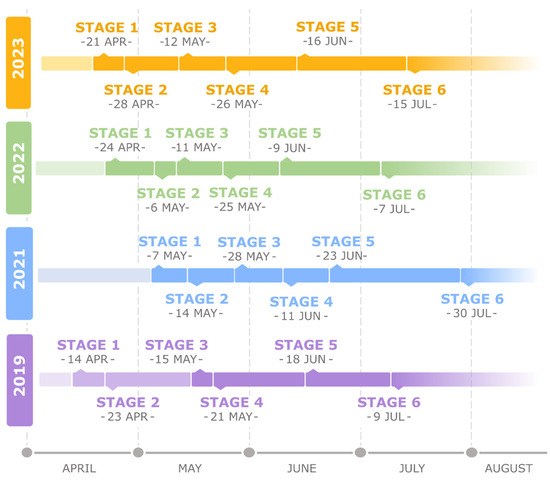
Figure 2.
Comparison of the beginning and duration of successive stages during the first periderm development in Aesculus hippocastanum in four analyzed years. Developmental stages were determined based on observed structural changes: stage 1—the first periclinal division in the collenchyma cell; stage 2—the second periclinal division, establishing the phellogen; stage 3—formation of the first type of phellem cells, empty inside; stage 4—formation of the second type of phellem cells, filled inside; stage 5—formation of the U-shaped secondary cell wall; stage 6—accomplishment of periderm differentiation.
Stage 1—the first periclinal division: The first stage was defined by the first periclinal division of a collenchyma cell, resulting in the appearance of two daughter cells, being the first symptom of phellogen initiation. It occurred prevalently in the second half of April, about three weeks after bud swelling, and lasted about one week (Figure 1, stage 1; Figure 2).
Stage 2—phellogen initiation: The second periclinal division indicated the beginning of stage 2 and lead to the formation of three cells arranged in a radial row. From these three radially aligned cells, the middle one became the phellogen cell, whereas the innermost cell differentiated into the parenchymatic phelloderm. Concomitantly, the outermost cell in the row suberized, as was confirmed by suberin autofluorescence and a specific staining with Fluorol Yellow, and differentiated into the phellem cell (Figure 1, stage 2, and Figure S1). This stage lasted 1–2 weeks.
Stage 3—formation of empty phellem cells: During stage 3, which lasted for next 2–3 weeks, divisions of the phellogen gave rise to 1–2 phellem cells that were empty inside and had suberized cell walls (Figure 1, stage 3; Figure S1).
Stage 4—formation of filled phellem cells: Intensive divisions of the phellogen resulted in the formation of distinct rows of phellem cells. However, in this stage, phellem cells differed morphologically from those initiated in the previous stage (1–2 outermost cells in the row) and were filled inside (Figure 1, stage 4; Figure S1). Stage 4 lasted 2–3 weeks.
Stage 5—formation of phellem cells with the U-shaped cell wall: The following stage of periderm development was the longest, lasting at least for a month. During this stage, the phellogen accomplished its divisions and the thick U-shaped secondary cell walls were deposited on the periclinal innermost side of the phellem cells (Figure 1, stage 5; Figure S1). Such secondary cell walls were formed in two or three of the youngest phellem cells adjacent to the phellogen.
Stage 6—accomplishment of differentiation: In the final stage, no phellogen divisions were observed and all derivative cells were completely differentiated (Figure 1, stage 6; Figure S1). This stage was already achieved in July, meaning that the process of periderm initiation and differentiation was rapid. Stage 6 lasted until the consecutive vegetative season, when the phellogen resumed its activity.
Importantly, phellogen, from the time of its origin at the end of April, was active until the middle of June. Ultimately, at the end of the first year of phellogen activity, on average, seven phellem cells (from 4 to 11; ±1.67 SD), one phellogen, and one phelloderm cell were present per each radial row. However, it is important to stress here that in 32% of radial rows (in 69 rows out of 216 analyzed in stage 6), only one living cell was present at this stage, and, based on the position in the radial row and morphological features, it was identified as the phelloderm cell. This means that, in such rows, the phellogen lost its meristematic identity and differentiated into the phellem cell (Figure 1, stage 6).
3.2. Initiation of the Subsidiary Phellogen and Its Activity
In some locations at the stem circumference, the development of the periderm did not proceed according to the sequence described above (Figure 3). Although two subsequent periclinal divisions that resulted in the formation of three aligned cells in a row occurred normally (stages 1 and 2), then all the cells differentiated into the phellem, which was confirmed by the presence of suberin in their cell walls. This means that in such radial rows, the phellogen lost its meristematic characteristics and a normal structure of the periderm was not present at that moment as all the cells maturated like the phellem. Thus, to maintain the continuity of the secondary protective tissue and enable the formation of new layers of the periderm, the initiation of the subsidiary phellogen occurred. The process proceeded in the same mode as described above for the first phellogen, but occurred in deeper collenchyma cells (Figure 3a, stage re1) adjacent to the earlier differentiated phellem cells (Figure 3a, stage re1). After two subsequent periclinal divisions of the collenchyma cell (Figure 3a, stage re2), the new subsidiary phellogen was established, laying below previously differentiated phellem cells. Due to such a positioning of the new phellogen cell, the radial rows of the first and subsidiary periderm were shifted and lost their continuity (Figure 3a, stage re2). Subsequent periclinal divisions of the newly initiated subsidiary phellogen produced derivatives, which differentiated into the phellem and phelloderm (Figure 3a, stage re3). It is worth noting that the derivatives, which differentiated into the phellem, omitted developmental stage 3 and went directly to stages 4 and 5, resulting in the circumferential continuity of the cell types of both periderms (Figure 3b). Such a subsidiary periderm could develop randomly at the stem circumference without any specific pattern, and no specific reasons or factors triggering its initiation could be observed or related to the process (Figure S1).
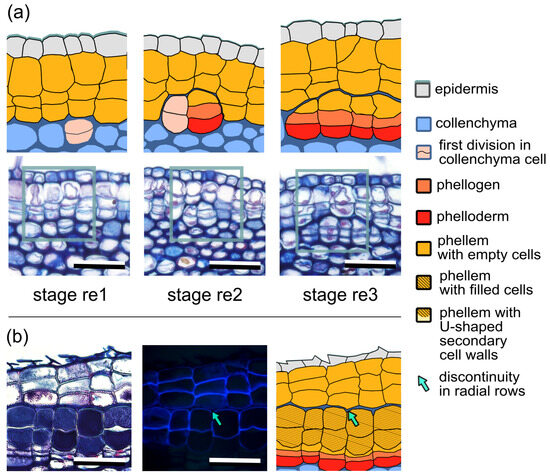
Figure 3.
Development and functioning of the subsidiary phellogen in Aesculus hippocastanum. (a) Initiation of the subsidiary phellogen. Successive developmental stages (re 1–3) are shown in the lower panel and their graphic interpretations in the upper panel. Rectangles frame the interpreted fragments of the sections. Stage re1—the first periclinal division in the collenchyma cell underneath the differentiated phellem cells of the first periderm; stage re2—the second periclinal division, establishing the subsidiary phellogen; stage re3—formation of the subsidiary periderm. (b) Functioning of the subsidiary periderm. Differentiation of the subsidiary phellogen derivatives leads to the formation of the periderm with filled phellem cells (shown in bright field; left panel), whose identity are confirmed by suberin autofluorescence (seen in UV light; middle panel) and the graphic interpretation of the subsidiary periderm structure (right panel). Arrows indicate the border between the first (at the top) and the subsidiary periderm (at the bottom); note the circumferential shift in the radial rows. Scale bars = 50 µm.
3.3. Phellogen Activity and Periderm Development in 2- and 3-Year-Old Stems
Seasonal changes in phellogen activity and the differentiation of phellem and phelloderm cells were further analyzed in 2- and 3-year-old stems. At the beginning of a new growing season, at the end of April, 2-year-old stems were covered by the periderm formed in the previous year; thus, the cell arrangement corresponded to stage 6 described above (Figure 1, stage 6). This means that in each radial row, one phellogen cell was preserved and could resume its activity in a new growing season, or that, as mentioned earlier, only one living cell, identified as the phelloderm, was present at the bottom of the row (Figure 4a). In such rows, with only one living cell, the phelloderm cell underwent a periclinal division to re-initiate the phellogen. Accordingly, two living cells were present below the phellem during the first 10 days of May, from which the inner cell differentiated into the phelloderm, whereas the outer one became the phellogen. The process of phellogen re-initiation lasted through all of May (Figure 4b).
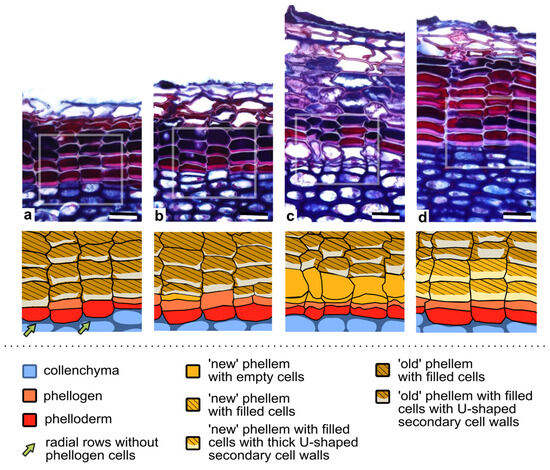
Figure 4.
Phellogen activity and the differentiation of its derivatives in 2- and 3-year old branches of Aesculs hippocastanum. (a–d) The upper panels present inter-annual changes in the periderm functioning, from (a) the phellogen dormancy, (b) the re-activation of the phellogen divisions, and (c) the formation of a few derivative cells to (d) the accomplishment of the differentiation of U-shaped phellem cells at the end of a successive year of phellogen activity. Framed fragments of the sections are graphically presented in corresponding lower panels. Note that in some radial rows, after phellogen dormancy, only one living cell (the phelloderm) can be present (in rows pointed by arrows). The term ‘old’ refers to the phellem cells differentiated in the previous season; ‘new’ phellem cells are these formed in that season. The number of ‘new’ phellem cells (formed in that year) is reduced compared to those differentiated in the previous season (‘old’ phellem cells). For details, see the text. Scale bars = 25 µm.
The phellogen, either preserved in a radial row over the dormancy period or re-initiated from the phelloderm, resumed its divisions, producing new periderm layers and continuing its activity until the middle of July. Importantly, in 2- and 3-year-old stems, the first divisions of the phellogen occurred about one month later than in the first year of its activity. During the entire growing season, in 2- and 3-year-old stems, the youngest phellem cells adjacent to the phellogen already had well-developed, thick, U-shaped secondary cell walls (Figure 4b), and only rarely was phellem cell differentiation observable (Figure 4c), indicating the rapidness of the process. At the end of the growing season, in the radial row, on average, 11 (7–14, ±1.51 SD) and 13 (10–15, ±1.32 SD) phellem cells were present in 2- and 3-year-old stems, respectively, indicating that approximately only 2–3 new phellem cells developed in each year, and in addition, only one phelloderm cell was added inwardly. This number of periderm cells further suggests that the phellogen in 2- and 3- year-old stems had lower divisional activity than in the first year. The annual increments were delineated due to the differently stained content of the phellem cells (Figure 4d), although they were not always clearly visible. In addition, thick U-shaped secondary cell walls of the phellem cells differed between successive seasons. Those deposited in the current season adhered tightly to the primary cell wall, while those formed in a previous year slightly detached from the anticlinal primary walls (Figure 4d), probably due to the significant circumferential increase in the intensively growing stem.
3.4. Periderm Development in Old Trunks
In horse chestnut trees, the first periderm may function even in 50-year-old trees. However, as our analyses showed, even in trees of such age, the intra-annual changes in phellogen activity and periderm formation were comparable to those in 2- and 3-year-old stems. In old trees, the phellogen was active from the middle of May to the middle of July, with sporadic divisions occurring, thus producing the scarce amount of derivative cells (Figure 5a). In all analyzed sections, phellem cells with thick secondary cell walls were adjacent to the phellogen; differentiating phellem cells were not observed, probably due to only a few differentiating cells being present in a growing season and the rapidness of the process (Figure 5a).
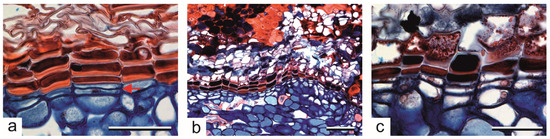
Figure 5.
Periderm of old trees of Aesculus hippocastanum. (a) The first divisions of the phellogen (pointed by an arrow); phellem cells with the thick U-shaped secondary cell wall, differentiated in the previous season, are adjacent to the phellogen. (b,c) The second periderm formed below the older one; three types of phellem cells are differentiated, i.e., thin-walled empty cells, dark-filled cells and cells with the thick U-shaped secondary cell wall, indicating the reiteration of the developmental program of phellem differentiation as in one-year-old branches. Scale bars: (a,c) = 50 µm, (b) = 100 µm.
In old trees, the second periderm developed beneath the first, old periderm (Figure 5b,c). This was visible in several tree cores, although rather occasionally, and the new periderm consisted of nine phellem cells on average (7–11, ±1.16 SD), the phellogen and 1–2 phelloderm cells in each radial row. The phellogen for the second periderm originated from the parenchymatic living cells of the secondary phloem. During the expansion of this new periderm, the same developmental stages as in one-year-old stems occurred. First, the derivative cells were produced outwardly due to periclinal divisions of the phellogen, which differentiated into large suberized and thin-walled phellem cells (stage 3); then, the next cells in a row had dark-stained contents (stage 4); and ultimately, the cells with the thick U-shaped secondary cell walls were formed close to the phellogen cell (stage 5) (Figure 5b,c). Presumably, the formation of the new periderm was a rapid process, as in the one-year-old stems because, in old trees, only the mature multi-layered periderm was noticeable. The new periderm was formed only in limited locations, not across the entire trunk circumference, as it was not visible on sections taken from the same tree in later dates of sampling.
3.5. Structure of the Thick U-Shaped Cell Wall of the Phellem Cells
The nature of the thick U-shaped secondary cell wall, which was formed in the inner periclinal side of the phellem cell, was further analyzed using different microscopic methods and staining reactions (Figure 6). The distinct shining of such walls in polarized light indicated the crystalline structure of cellulose fibrils, typical of the secondary cell wall (Figure 6a). The lignification of these walls was further shown by staining with Safranin O (Figure 6b). In addition, the U-shaped layer of such secondary cell walls was not suberized, as shown by the lack of autofluorescence in UV light and it not being a detectable signal after using Flourol Yellow (Figure 6c,c′ and Figure 6d,d′, respectively; lack of the signal in the thick U-shaped cell wall; visible autofluorescence comes from the remaining cell walls). The ultrastructural analyses in TEM showed that the U-shaped cell walls adhered tightly to the primary cell wall on the inner side of the cell and had a multilayered structure (Figure 6e).
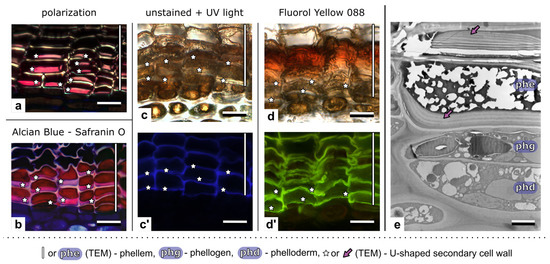
Figure 6.
Structure of the thick U-shaped cell wall of the phellem cells in the periderm of Aesculus hippocastanum, visualized by different microscopic methods. (a) The crystalline structure of cellulose fibrils visualized in polarized light shows the secondary nature of the cell wall. (b) Lignification of U-shaped secondary cell walls indicated by staining with an Alcian Blue and Safranin O mixture. (c,c’) Detection of suberin by autofluorescence in UV light: (c) The unstained section in bright field, and (c’) The same section in UV light, suberin is visible as a thin layer in the phellem cell wall, but the signal in thick U-shaped secondary cell walls is not detectable; autoflorescence comes from the remaining cell wall layers; (d,d’) Detection of suberin by staining with a suberin-specific dye, Fluorol Yellow; (d) The analyzed section in bright field, and (d’) The same section in blue light, fluorescent signals of suberin detectable in phellem cell walls, with exception of the U-shaped cell wall. (e) The ultrastructure of the periderm in TEM; the U-shaped secondary cell wall of phellem cells is thick, multilayered, and adheres tightly to the primary cell wall. The U-shaped secondary cell wall marked by asterisks (a–d) or by arrows (e). Scale bars: (a–d) = 20 µm; (e) = 5 µm.
4. Discussion
In times of global warming and high levels of environmental pollution, long-lived perennial plants, such as trees, are continuously exposed to harmful environmental factors, which affect plant development. Crucial for their survival in a changing environment is the establishment of the secondary protective tissue, the periderm, and then the rhytidome (outer bark) [1,3]. Regardless of the importance of these tissues for tree performance, their development and functioning are rarely analyzed in contrast to the intensively studied cambium, responsible for wood (secondary xylem) formation, which is commonly utilized in the industry [47,48,49,50,51]. In this light, periderm initiation and differentiation in A. hippocastanum, as well as mechanisms related to the maintenance of the secondary protective tissue, presented here in detail, fill the gaps in the knowledge on the periderm development and performance. In particular, we suggest that the repetitive re-initiation of the phellogen in different developmental stages, which we described in Aesculus, is a vital mechanism maintaining periderm integrity.
The initiation of the first phellogen in one-year-old branches of horse chestnut trees occurs in the subepidermal cells of the collenchyma, similarly as in other tree species [5,15,16,52]. This happens relatively early, i.e., about three weeks after bud bursting, and the established phellogen maintains its divisional activity for about two months. Interestingly, in older branches, the activity of the phellogen is restored about a month later compared to the initiation of the first phellogen, but also lasts for about two months. In old horse chestnut stems, due to only occasional divisions of the phellogen, the detection of meristem activity was not so obvious, as noted, e.g., in Quercus petraea [13]. On the contrary, the activity of the other secondary meristem, cambium, in the same trees is maintained for more than four months [37]. Based on the available data, the phellogen activity in other tree species can be similarly short, such as from May to June/July, as, e.g., in Betula maximowicziana [29], whereas, in Abies alba, the period of divisions can last from May to September, although in older branches, only during September [52]. The longest activity of the phellogen was observed in Quercus suber from April to October [10,14,28]. The scarcity of data in the literature points to the necessity of the wide-scale analysis of phellogen functioning in other trees that would allow for a conclusion on the factors involved in and regulating the process [53].
The differentiation of the derivatives during the first year of phellogen functioning in A. hippocastanum is rapid and is accomplished in mid-July. Based on the intra-annual changes in cell morphology, six developmental stages can be distinguished, starting from two consecutive periclinal divisions establishing the first phellogen to the maturation of three different types of the phellem cells. In general, the differentiation of phellem cells is a multistep process, including cell expansion, suberization, the deposition of waxes, and programmed cell death. Suberization increases cell wall rigidity, reduces its permeability and prevents the penetration of pathogens, enhancing the protective and defensive function of the phellem [2,24,54,55]. In addition, the diversification of the phellem cell types is a common feature of many tree species, which leads to the distinctiveness of annual growth rings if the sequences of different cell types are regularly repeated every year [7,11,14,27,29]. However, the causes and adaptive significance of phellem cell diversification remain elusive. The characteristic feature of the phellem cells is the deposition of suberin, which forms a multilayered lamellate structure on the primary cell wall [3,56]. In Aesculus, we observed a specific additional U-shaped secondary cell wall in phellem cells. The presence of such an extra secondary cell wall, which is rich in cellulose and lignin, is known in some species (e.g., Viburnum opulus, Ceratonia siliqua, or Berberis pruinosa) [5,17,21], but its function is not known. The presence of thick multi-layered secondary cell walls in Aesculus seems to ensure the mechanical properties of the periderm, especially regarding the fact that during a season, only few phellem cells are produced. It could be suggested that, as in other cell types with thick cellulose–lignin cell walls, such as wood or phloem fibers, this structure can account for the mechanical properties of the cells [57]. In addition, the U-shaped cell wall tightly adheres to anticlinal walls of the phellem cells only during the first year after differentiation and, in the next season, is then flattened and detached from anticlinal walls. This morphological change probably indicates that this thick cell wall counteracts the circumferential tension during the girth increase.
Interestingly, the entire developmental program for phellogen initiation and the differentiation of its derivatives with three different phenotypes of the phellem cells is repeated in older horse chestnut trees, where the phellogen is formed de novo from the secondary phloem, showing the reiteration of the entire developmental program for the periderm formation.
Our detailed analyses revealed that the meristematic identity of the first phellogen can be lost. At the end of the vegetative season, about one-third of phellogen cells differentiate into the phellem, and in a radial row, only one cell, the phelloderm, remains alive. Subsequently, in the next season, the remaining phelloderm cell becomes the source for the restoration of the phellogen due to resumed periclinal divisions. Similarly, in Acer negundo [19], at the end of the vegetative season, in a radial row, two or one living cells were observed, determined as the phellogen and phelloderm or only the phelloderm cell, respectively. If only the phelloderm cell was present, in a new vegetative season, this divided periclinally, re-initiating the phellogen [19], similarly to what we observed in Aesculus. Interestingly, Acer negundo belongs to the same family (Sapindaceae) as Aesculus [58]. Although it seems that this phenomenon can occur in different species, its frequency and significance have not been widely discussed. In addition, the reasons for the loss of meristematic identity are not fully recognized, not only in the case of the phellogen but in general, for initial cells in other plant meristems [59,60,61]. However, it could be assumed that the process of phellogen re-initiation from the phelloderm cells could be the mechanism protecting the undamaged genetic information for the meristematic phellogen cells. As the earlier studies on meristems showed, due to the repetitive divisions of the initial cells, the probability of errors/somatic mutations increases; the replacement of the initial cell by such that divides only occasionally and potentially has less errors/mutations, may ensure the functioning of the initial cell that has the original (unchanged) genetic information [61]. The phelloderm is a direct derivative of the collenchyma cell, the mother cell for the entire periderm tissue (row); this means that it was also the source of the first phellogen. The phelloderm cell did not undergo the divisions; thus, it can be a reservoir of the potentially unchanged genetic information for the newly established initials of the phellogen.
A similar mechanism, selecting new meristematic cells of the phellogen, which has not been described so far in other plants, occurs during the formation of the subsidiary phellogen in A. hippocastanum. During the initiation of the first phellogen, in some radial rows, the phellogen cell loses its meristematic activity and the entire row differentiates into phellem cells. To maintain the continuity of the periderm, the collenchyma cell laying underneath such a row gains meristematic potential and undergoes two successive periclinal divisions, producing a new subsidiary phellogen. Importantly, both mechanisms of the re-initiation of the phellogen—from the phelloderm or collenchyma—could ensure the recruitment of the meristematic cells with potentially undamaged genetic information, pointing to the adaptive plasticity of a plant and its meristem (phellogen). To some extent, the process of the replacement of the initial cells resembles the functioning of stochastic shoot apical meristems (SAMs), in which the stem cells are impermanent and can be exchanged from the pool of meristematic cells to counteract potential mutations [59,60,61,62]. It is worth stressing that, as observed in Aesculus, mechanisms that ensure the proper development of the periderm undoubtedly are related to the effective functioning of the protective barrier against the external environmental factors.
Although we showed the re-initiation of the phellogen only in one-year-old branches, it cannot be ruled out that a similar process takes place also in older stems. It is worth stressing that in A. hippocastanum, usually only one phelloderm cell is formed during the vegetative season. This further suggests that phelloderm could be a reservoir of potential initial cells due to their restricted divisions. Seemingly, the occurrence of so-called thin phelloderm in other species, such as, e.g., Picea abies, Abies balsamea, and Berberis pruinosa [5,11], may also point to the phelloderm as a reservoir for phellogen re-initiation, suggesting that the presented phenomenon is more common. However, the development of the periderm and the role of phelloderm cells in phellogen re-initiation were not studied in detail in other plant species.
The ability of the phelloderm and collenchyma to be a source for the re-initiated phellogen, revealed here, could be a specific adaptation of trees to cope with the abiotic harmful stress-inducing factors, e.g., environmental pollution. For example, heavy metals can be accumulated in the rhytidome and periderm [63,64,65] and can affect the proper cell divisions in the phellogen of roots [66]. Therefore, the mechanism restoring the phellogen initial cells may possibly play a role in the defense against environmental loads. This could be of great importance, especially in trees grown in urban environments, such as Aesculus, where they have to deal with high pollution [67]. It has also been shown that the response of Quercus illex to cell damage caused by environmental stress-inducing factors included the upregulation of the bark genes [23]. Analyzing the periderm development under different stress factors, e.g., water-stress or low/high temperatures, during acclimation would broaden our knowledge about tree performance in urban ecosystems.
It is worth stressing that the subsidiary phellogen, supplementing circumferentially the periderm formed earlier and ensuring its continuity, adjusts its developmental program. It does not go through all the developmental stages, but continues the pattern started by the previous periderm. This, in turn, suggests that the periderm developmental program is under the control of the stationary epigenetic pattern (morphogenetic map) that orchestrates its development. Such a morphogenetic map, known from research on the cambium [68,69], constitutes information about the spatial distribution of the cells in the tissue and governs developmental events in the cells. The exemplary morphogenetic map could be the storied or double-storied pattern of the initial cells at the tangential surface of the cambium, which could be maintained even in the presence of some events (e.g., directional divisions, intrusive growth) which disturb the proper cell arrangement [70,71,72,73]. A specific example of the epigenetic map could be the initiation of the new phellogen in the secondary phloem in old Aesculus trees. In such cases, newly formed phellogen repeats the entire program of periderm cell differentiation as in one-year-old branches, with three distinct types of phellem cells.
In summary, a plant’s ability to select new initial cells and the presence of the epigenetic map determining the pattern of cell distribution are generally accepted as the mechanisms governing meristem functioning, but so far have not been recognized in the phellogen. In addition, both phenomena suggest the plasticity and developmental potential of this tissue to adapt to changing environments. This assumption is further confirmed by the ability of the phellogen to form a pool of meristematic cells after injury [3]; however, the ultimate response of the phellogen and the mechanisms involved require further studies.
5. Conclusions
Our results showed the following:
- Meristematic phellogen cells are not permanent and can be replaced by new ones, re-initiated from the phelloderm or collenchyma cells. Together with the subsidiary phellogen initiation, both mechanisms play a crucial role in the maintenance of the periderm integrity.
- Only 1–2 phelloderm cells are formed per year, suggesting their role as a source for meristematic cell re-initiation.
- The phellogen is active for a short time during the vegetative season; its activity changes seasonally, with the intensive divisions in the first year and the decrease in activity in consecutive years.
- The differentiation of the periderm cells changes intra-annually, with six developmental stages distinguished in the first year of periderm development and upon the re-initiation of the phellogen from living cells of the secondary phloem. However, in consecutive years, the developmental program is shortened, and only one type of phellem cell, with the U-shaped secondary cell wall, is formed.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/f16010176/s1, Figure S1: Successive stages of the first periderm formation in Aesculus hippocastanum; Figure S2: Development of the subsidiary periderm in Aesculus hippocastanum.
Author Contributions
Study concept and design, E.M. and E.M.G.; data collection and analysis, A.B. and E.M.; supervision, E.M.; writing—original draft preparation E.M. and E.M.G.; figure preparation, A.B. and E.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the University of Wrocław research subvention 501/73/10110/MPK 2599150000/2024.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Acknowledgments
We would like to thank to Sylwia Nowak (Laboratory of Microscopic Techniques, University of Wrocław) for ultrathin section preparation.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lev-Yadun, S. Bark. eLS 2022, 3, 1–7. [Google Scholar] [CrossRef]
- Grünhofer, P.; Schreiber, L. Humboldt Review: Cutinized and suberized barriers in leaves and roots: Similarities and differences. J. Plant Physiol. 2023, 282, 153921. [Google Scholar] [CrossRef] [PubMed]
- Serra, O.; Mähönen, A.P.; Hetherington, A.J.; Ragni, L. The making of plant armor: The periderm. Ann. Rev. Plant Biol. 2022, 20, 405–432. [Google Scholar] [CrossRef]
- Ohse, M.; Irohara, R.; Iizuka, E.; Arakawa, I.; Kitin, P.; Funada, R.; Nakaba, S. Sequent periderm formation and changes in the cellular contents of phloem parenchyma during rhytidome development in Cryptomeria japonica. J. Wood Sci. 2022, 68, 19. [Google Scholar] [CrossRef]
- Angyalossy, V.; Pace, M.R.; Evert, R.F.; Marcati, C.R.; Oskolski, A.A.; Terrazas, T.; Kotina, E.; Lens, F.; Mazzoni-Viveiros, S.C.; Angeles, G.; et al. IAWA list of microscopic bark features. IAWA J. 2016, 37, 517–615. [Google Scholar] [CrossRef]
- Arzee, T.; Waisel, Y.; Liphschitz, N. Periderm development and phellogen activity in the shoots of Acacia raddiana Savi. New Phytol. 1970, 69, 395–398. [Google Scholar] [CrossRef]
- Patel, R.N. Bark anatomy of radiata pine, corsican pine, and douglas fir grown in New Zealand. N. Z. J. Bot. 1975, 13, 149–167. [Google Scholar] [CrossRef]
- Chang, Y.P. Bark Structure of North American Conifers; US Department of Agriculture: Washington, DC, USA, 1954. [Google Scholar]
- Godkin, S.E.; Grozdits, G.A.; Keith, C.T. The periderms of three north American conifers: Part 2: Fine structure. Wood Sci. Technol. 1983, 17, 13–30. [Google Scholar] [CrossRef]
- Teixeira, R.T. Cork development: What lies within. Plants 2022, 11, 2671. [Google Scholar] [CrossRef] [PubMed]
- Grozdits, G.A.; Godkin, S.E.; Keith, C.T. The periderms of three North American conifers: Part 1: Anatomy. Wood Sci. Technol. 1982, 16, 305–316. [Google Scholar] [CrossRef]
- Myśkow, E. Occurrence of atypical phellem in representatives of Cornus. Int. J. Plant Sci. 2014, 175, 328–335. [Google Scholar] [CrossRef]
- Gričar, J.; Jagodic, Š.; Prislan, P. Structure and subsequent seasonal changes in the bark of sessile oak (Quercus petraea). Trees 2015, 29, 747–757. [Google Scholar] [CrossRef]
- Caritat, A.; Gutiérrez, E.; Molinas, M. Influence of weather on cork-ring width. Tree Physiol. 2000, 20, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Trockenbrodt, M. Survey and discussion of the terminology used in bark anatomy. IAWA J. 1990, 11, 141–166. [Google Scholar] [CrossRef]
- Arzee, T.; Liphschitz, N.; Waisel, Y. The origin and development of the phellogen in Robinia pseudoaccacia L. New Phytol. 1968, 67, 87–93. [Google Scholar] [CrossRef]
- Wacowska, M.; Tarkowska, J.A. Ontogenesis and structure of phelloid in Viburnum opulus L. Acta Soc. Bot. Pol. 1983, 52, 107–114. [Google Scholar] [CrossRef]
- Graça, J.; Pereira, H. The periderm development in Quercus suber. IAWA J 2004, 25, 325–335. [Google Scholar] [CrossRef]
- Wacowska, M. Ontogenesis and structure of periderm in Acer negundo L. and × Fatshedera lizei Guillaum. Acta Soc. Bot. Pol. 1985, 54, 17–27. [Google Scholar] [CrossRef]
- Słupianek, A.; Wojtuń, B.; Myśkow, E. Origin, activity and environmental acclimation of stem secondary tissues of the polar willow (Salix polaris) in high-Arctic Spitsbergen. Polar Biol. 2019, 42, 759–770. [Google Scholar] [CrossRef]
- Arzee, T.; Arbel, E.; Cohen, L. Ontogeny of periderm and phellogen activity in Ceratonia siliqua L. Bot. Gaz. 1977, 138, 329–333. [Google Scholar] [CrossRef]
- Arbel, E.; Arzee, T. Development of peripheral periderm from cork strips in Ceratonia siliqua. Can. J. For. Res. 1976, 6, 425–428. [Google Scholar] [CrossRef]
- Boher, P.; Soler, M.; Sánchez, A.; Hoede, C.; Noirot, C.; Paiva, J.A.P.; Serra, O.; Figueras, M. A comparative transcriptomic approach to understanding the formation of cork. Plant Mol. Biol. 2018, 96, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Woolfson, K.N.; Esfandiari, M.; Bernards, M.A. Suberin biosynthesis, assembly, and regulation. Plants 2022, 11, 555. [Google Scholar] [CrossRef]
- Campilho, A.; Nieminen, K.; Ragni, L. The development of the periderm: The final frontier between a plant and its environment. Curr. Opin. Plant Biol. 2020, 53, 10–14. [Google Scholar] [CrossRef]
- Wunderling, A.; Ripper, D.; Barra-Jimenez, A.; Mahn, S.; Sajak, K.; Targem, M.B.; Ragni, L. A molecular framework to study periderm formation in Arabidopsis. New Phytol. 2018, 219, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Caritat, A.; Molinas, M.; Gutierrez, E. Annual cork-ring width variability of Quercus suber L. in relation to temperature and precipitation (Extremadura, southwestern Spain). For. Ecol. Managem. 1996, 86, 113–120. [Google Scholar] [CrossRef]
- Costa, A.; Graça, J.; Barbosa, I.; Spiecker, H. Effect of climate on cork-ring width and density of Quercus suber L. in Southern Portugal. Trees 2022, 36, 1711–1720. [Google Scholar] [CrossRef]
- Shibui, H.; Sano, Y. Structure and formation of phellem of Betula maximowicziana. IAWA J. 2018, 39, 18–36. [Google Scholar] [CrossRef]
- Waisel, Y.; Liphschitz, N.; Arzee, T. Phellogen activity in Robinia pseudacacia L. New Phytol. 1995, 66, 331–335. [Google Scholar] [CrossRef]
- Lack, H.W. The discovery and rediscovery of the horse chestnut. Arnoldia 2002, 61, 15–19. [Google Scholar] [CrossRef]
- Petrova, S.; Yurukova, L.; Velcheva, I. Horse chestnut (Aesculus hippocastanum L.) as a biomonitor of air pollution in the town of Plovdiv (Bulgaria). J. BioSci. Biotech. 2012, 1, 241–247. [Google Scholar]
- Chonova, P.; Gecheva, G.M.; Gribacheva, P. Air pollution biomonitoring in urban ecosystems with Aesculus hippocastanum. Ecol. Balk. 2019, 11, 85–92. [Google Scholar]
- Konarska, A.; Grochowska, M.; Haratym, W.; Tietze, M.; Weryszko-Chmielewska, E.; Lechowski, L. Changes in Aesculus hippocastanum leaves during development of Cameraria ohridella. Urban For. Urban Green. 2020, 56, 126793. [Google Scholar] [CrossRef]
- Idris, S.; Mishra, A.; Khustar, M. Phytochemical, ethanomedicinal and pharmacological applications of escin from Aesculus hippocastanum L. towards future medicine. J. Basic Clin. Physiol. Pharmacol. 2020, 31, 20190115. [Google Scholar] [CrossRef] [PubMed]
- Owczarek, A.; Kolodziejczyk-Czepas, J.; Woźniak-Serwata, J.; Magiera, A.; Kobiela, N.; Wąsowicz, K.; Olszewska, M.A. Potential activity mechanisms of Aesculus hippocastanum bark: Antioxidant effects in chemical and biological in vitro models. Antioxidants 2021, 10, 995. [Google Scholar] [CrossRef]
- Myśkow, E.; Sokołowska, K.; Słupianek, A.; Gryc, V. Description of intra-annual changes in cambial activity and differentiation of secondary conductive tissues of Aesculus hippocastanum trees affected by the leaf miner Cameraria ohridella. Forests 2021, 12, 1537. [Google Scholar] [CrossRef]
- Ruzin, S.E. Plant Microtechnique and Microscopy; Oxford University Press Inc.: New York, NY, USA, 1999. [Google Scholar]
- Rossi, S.; Anfodillo, T.; Menardi, R. Trephor: A new tool for sampling microcores from tree stems. IAWA J. 2006, 27, 89–97. [Google Scholar] [CrossRef]
- Schreiber, L.; Franke, R.; Hartmann, K. Wax and suberin development of native and wound periderm of potato (Solanum tuberosum L.) and its relation to peridermal transpiration. Planta 2005, 220, 520–530. [Google Scholar] [CrossRef]
- Surový, P.; Olbrich, A.; Polle, A.; Ribeiro, N.A.; Sloboda, B.; Langenfeld−Heyser, R. A new method for measurement of annual growth rings in cork by means of autofluorescence. Trees 2009, 23, 1237–1246. [Google Scholar] [CrossRef]
- Meyer, C.J.; Peterson, C.A. Casparian bands occur in the periderm of Pelargonium hortorum stem and root. Ann. Bot. 2011, 107, 591–598. [Google Scholar] [CrossRef]
- Rains, M.K.; Gardiyehewa de Silva, N.D.; Molina, I. Reconstructing the suberin pathway in poplar by chemical and transcriptomic analysis of bark tissues. Tree Physiol. 2018, 38, 340–361. [Google Scholar] [CrossRef] [PubMed]
- Słupianek, A.; Kasprowicz-Maluśki, A.; Myśkow, E.; Turzańska, M.; Sokołowska, K. Endocytosis acts as transport pathway in wood. New Phytol. 2019, 222, 1846–1861. [Google Scholar] [CrossRef] [PubMed]
- McDonald, K. . Osmium ferricyanide fixation improves microfilament preservation and membrane visualization in a variety of animal cell types. J. Ultrastruct. Res. 1984, 86, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, E.S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 1963, 17, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Forner, N.; Vieira, J.; Nabais, C.; Carvalho, A.; Martínez-Vilalta, J.; Campelo, F. Climatic and physiological regulation of the bimodal xylem formation pattern in Pinus pinaster saplings. Tree Physiol. 2019, 39, 2008–2018. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Rossi, S.; Deslauriers, A.; Liu, J. Contrasting strategies of xylem formation between black spruce and balsam fir in Quebec, Canada. Tree Physiol. 2019, 39, 747–754. [Google Scholar] [CrossRef]
- Yu, B.; Rossi, S.; Su, H.; Zhao, P.; Zhang, S.; Hu, B.; Li, X.; Chen, L.; Liang, H.; Huang, J.-G. Mismatch between primary and secondary growth and its consequences on wood formation in Qinghai spruce. Tree Physiol. 2023, 43, 1886–1902. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S. Climatic influence on tree wood anatomy: A review. J. Wood Sci. 2021, 67, 24. [Google Scholar] [CrossRef]
- Groover, A. The vascular cambium revisited. IAWA J. 2023, 44, 531–538. [Google Scholar] [CrossRef]
- Golinowski, W.O. The anatomical structure of the common fir (Abies alba Mill.) bark. I. Development of bark tissues. Acta Soc. Bot. Pol. 1971, 40, 149–181. [Google Scholar] [CrossRef]
- Rosner, S.; Morris, H. Breathing life into trees: The physiological and biomechanical functions of lenticels. IAWA J. 2022, 43, 234–262. [Google Scholar] [CrossRef]
- Faustino, A.; Pires, R.C.; Marum, L. Periderm differentiation: A cellular and molecular approach to cork oak. Trees 2023, 37, 627–639. [Google Scholar] [CrossRef]
- Graça, J. Suberin: The biopolyester at the frontier of plants. Front. Chem. 2015, 3, 62. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, R.T.; Pereira, H. Suberized cell walls of cork from cork oak differ from other species. Microsc. Microanal. 2010, 16, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Evert, R.F. Esau’s Plant Anatomy: Meristems, Cells, and Tissues of the Plant Body—Their Structure, Function, and Development, 3rd ed.; John Wiley & Sons. Inc.: Hoboken, NJ, USA, 2005.
- POWO Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. 2025, Published on the Internet. Available online: https://powo.science.kew.org/ (accessed on 11 January 2025).
- Zagórska-Marek, B.; Turzańska, M. Clonal analysis provides evidence for transient initial cells in shoot apical meristems of seed plants. J. Plant Growth Regul. 2000, 19, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Gola, E.M.; Jernstedt, J.A. Impermanency of initial cells in Huperzia lucidula (Huperziaceae) shoot apices. Int. J. Plant Sci. 2011, 172, 847–855. [Google Scholar] [CrossRef]
- Klekowski, E.J. Plant clonality, mutation, diplontic selection and mutational meltdown. Biol. J. Linn. Soc. 2003, 79, 61–67. [Google Scholar] [CrossRef]
- Burian, A. Does shoot apical meristem function as the germline in safeguarding against excess of mutations? Front. Plant Sci. 2021, 12, 707740. [Google Scholar] [CrossRef]
- Chrabąszcz, M.; Mróz, L. Tree bark, a valuable source of information on air quality. Pol. J. Environ. Stud. 2017, 26, 453–466. [Google Scholar] [CrossRef]
- Klink, A.; Polechońska, L.; Dambiec, M.; Białas, K. A comparative study on macro- and microelement bioaccumulation properties of leaves and bark of Quercus petraea and Pinus sylvestris. Arch. Environ. Contam. Toxicol. 2018, 74, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Caldana, C.R.G.; Hanai-Yoshida, V.M.; Paulino, T.H.; Baldo, D.A.; Freitas, N.P.; Aranha, N.; Vila, M.D.C.; Balcão, V.M.; Oliveira, J.M. Evaluation of urban tree barks as bioindicators of environmental pollution using the X-ray fluorescence technique. Chemosphere 2023, 312, 137257. [Google Scholar] [CrossRef] [PubMed]
- Krzesłowska, M.; Goliński, P.; Szostek, M.; Mocek-Płóciniak, A.; Drzewiecka, A.; Piechalak, A.; Ilek, A.; Neumann, U.; Timmers, A.C.J.; Budzyńska, S.; et al. Morphology and physiology of plants growing on highly polluted mining wastes. In Phytoremediation for Environmental Sustainability; Prasad, R., Ed.; Springer: Singapore, 2021; pp. 151–200. [Google Scholar] [CrossRef]
- Araminienė, V.; Sicard, P.; Černiauskas, V.; Coulibaly, F.; Varnagirytė-Kabašinskienė, I. Estimation of air pollution removal capacity by urban vegetation from very high-resolution satellite images in Lithuania. Urban Clim. 2023, 51, 101594. [Google Scholar] [CrossRef]
- Romberger, J.A.; Hejnowicz, Z.; Hill, J.F. Plant structure: Function and development. In A Treatise on Anatomy and Vegetative Development, with Special Reference to Woody Plants; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1993. [Google Scholar]
- Zagórska-Marek, B. Plant meristems and their patterns. In Pattern Formation in Biology, Vision and Dynamics; Carbone, A., Gromov, M., Prusinkiewicz, P., Eds.; World Scientific Publishing: Hackensack, NJ, USA, 2000; pp. 217–239. [Google Scholar] [CrossRef]
- Zagórska-Marek, B. Pseudotransverse divisions and intrusive elongation of fusiform initials in storeyed cambium of Tilia. Can. J. Bot. 1984, 62, 20–27. [Google Scholar] [CrossRef]
- Kojs, P.; Rusin, A.; Iqbal, M.; Włoch, W.; Jura, J. Readjustments of cambial initials in Wisteria floribunda (Willd.) DC. for development of storeyed structure. New Phytol. 2004, 163, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Myśkow, E.; Zagórska-Marek, B. Ontogenetic development of storied ray pattern in cambium of Hippophaë rhamnoides L. Acta Soc. Bot. Pol. 2004, 73, 93–101. [Google Scholar] [CrossRef][Green Version]
- Myśkow, E.; Zagórska-Marek, B. Vertical migration of rays leads to the development of a double- storied phenotype in the cambium of Aesculus turbinata. Botany 2008, 86, 36–44. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).