The Influence of Forest Litter Characteristics on Bacterial and Fungal Community Diversity in the Picea crassifolia Ecosystem on the Qinghai–Tibet Plateau
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experimental Design
2.3. Litter Properties
2.4. Soil Bacteria and Fungi Analysis
2.5. Data Processing
3. Results
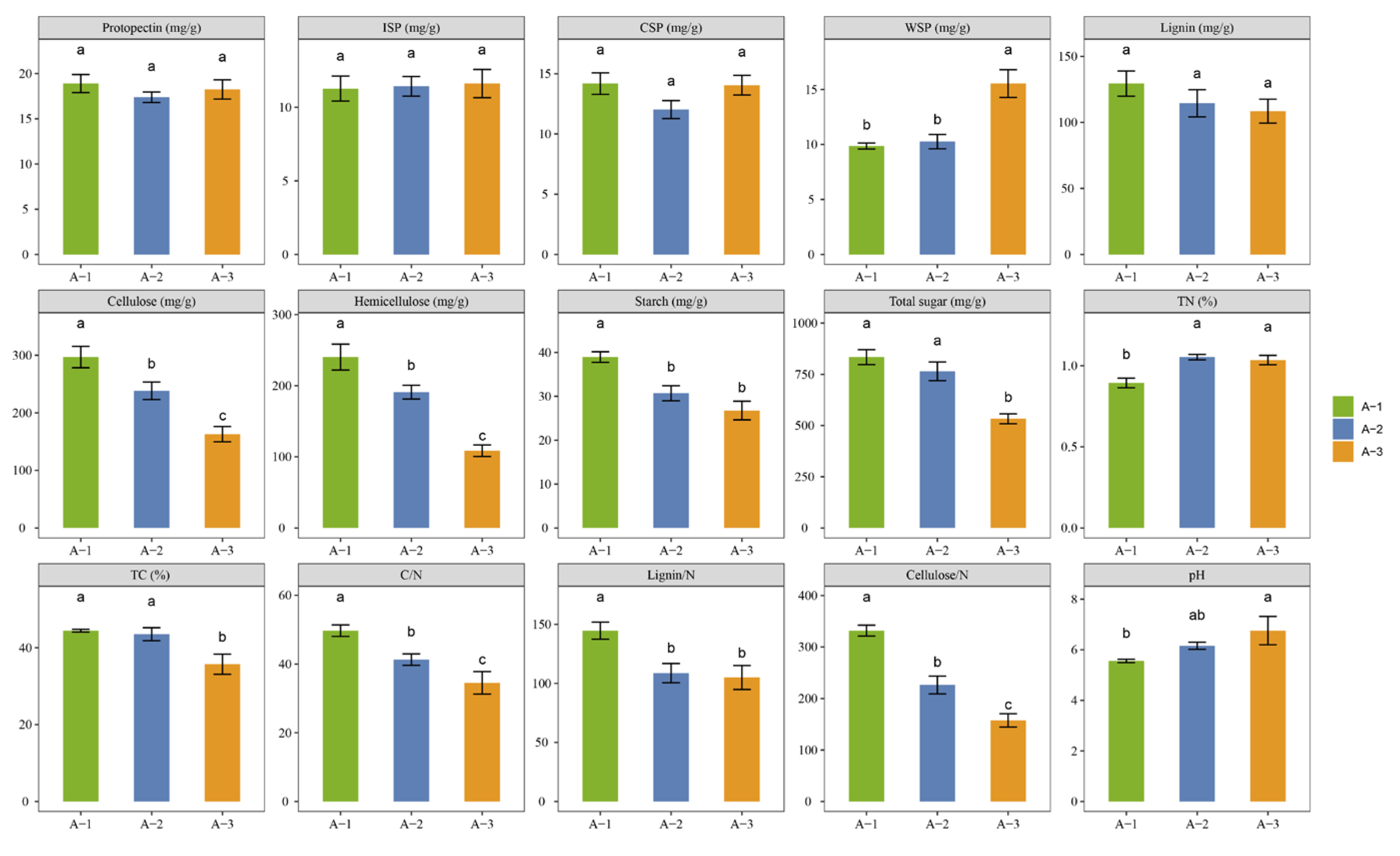
3.1. Chemical and Compositional Components of Different Litter Layers
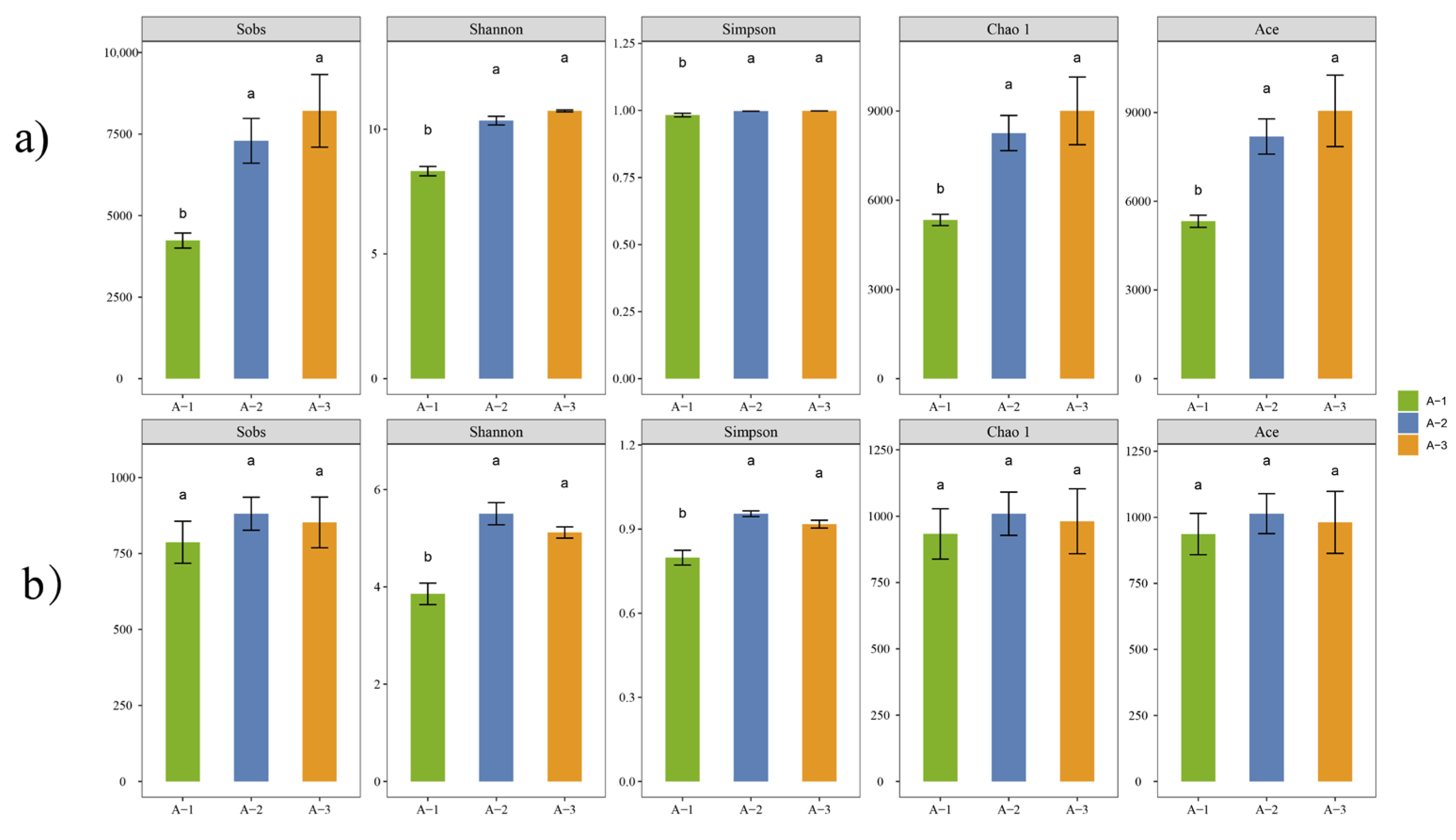
3.2. Analysis of Litter Microbial Community Diversity
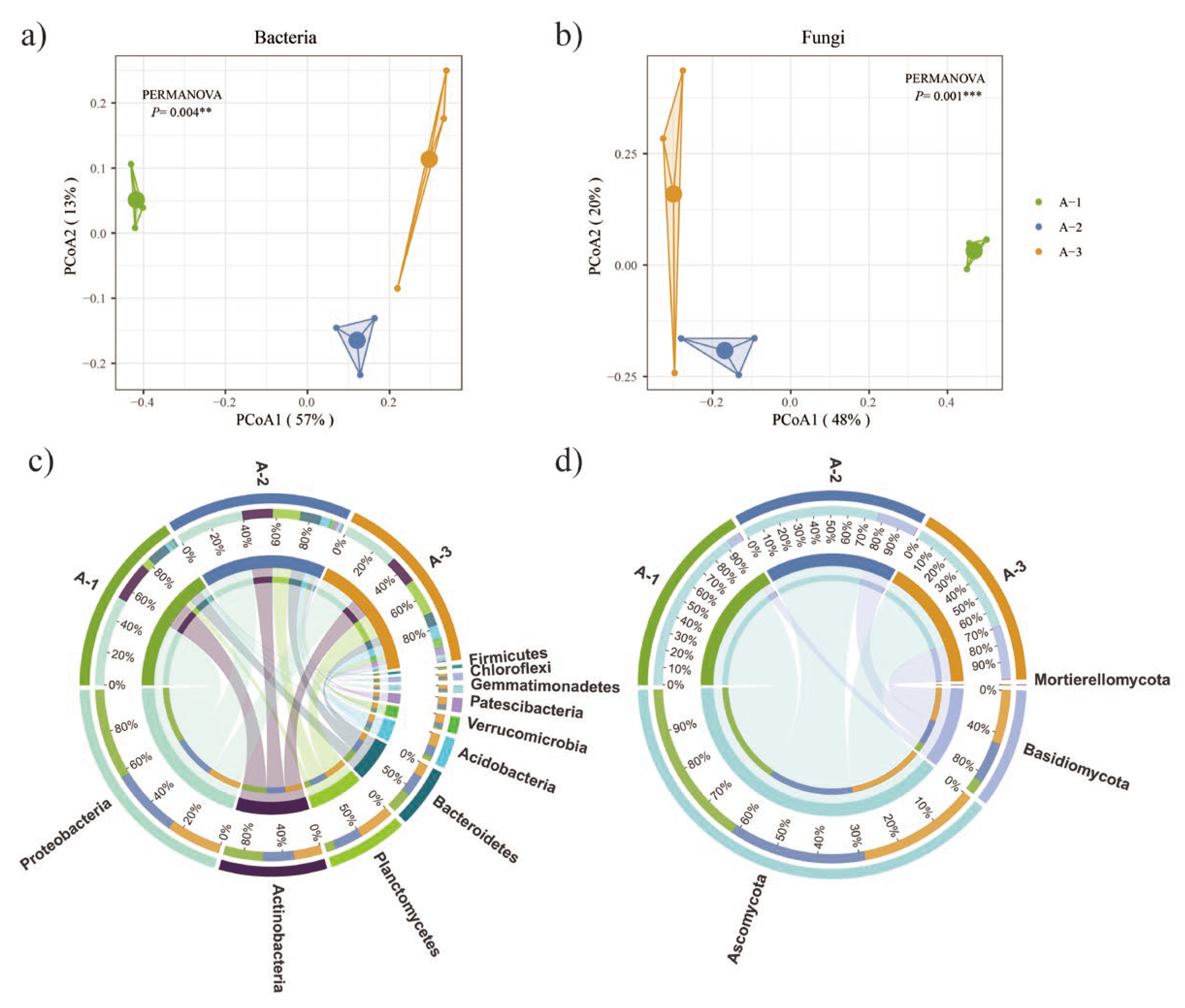
3.3. Litter Microbial Composition
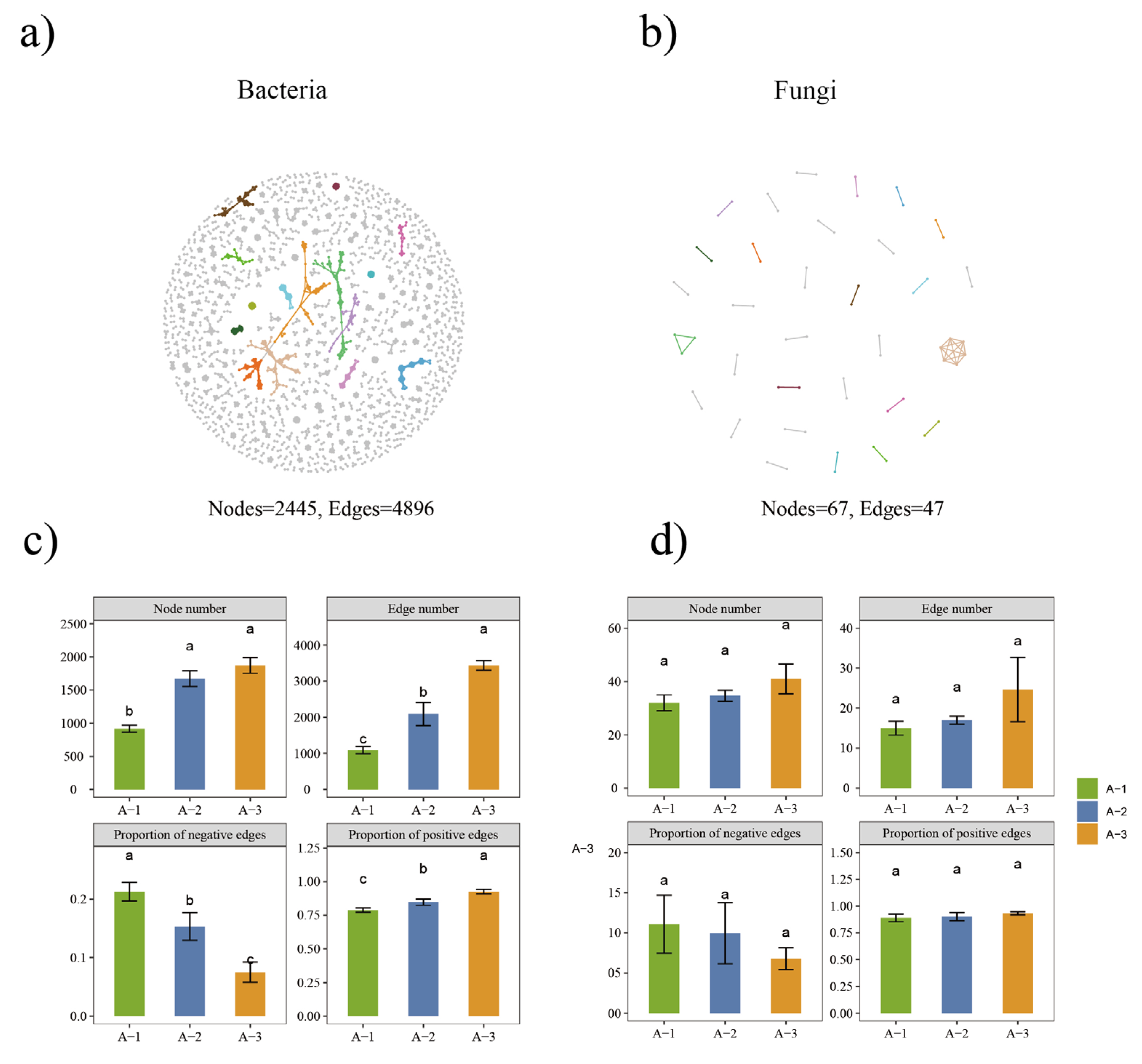
3.4. Bacterial and Fungal Networks in Different Litter Layers
3.5. Factors Influencing Microbial Community Composition in Different Litter Layers
4. Discussion
4.1. Changes in Litter Traits across Different Decomposition Layers
4.2. Changes in Microbial Communities across Different Litter Decomposition Layers
4.3. Changes in Network Complexity across Different Litter Decomposition Layers
4.4. Microbial Community Response to Changes in Litter Traits
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fujii, K.; Uemura, M.; Hayakawa, C.; Funakawa, S.; Kosaki, T. Environmental control of lignin peroxidase, manganese peroxidase, and laccase activities in forest floor layers in humid Asia. Soil Biol. Biochem. 2013, 57, 109–115. [Google Scholar] [CrossRef]
- Wagner, D.; Kobabe, S.; Liebner, S. Bacterial community structure and carbon turnover in permafrost-affected soils of the Lena Delta, northeastern SiberiaThis article is one of a selection of papers in the Special Issue on Polar and Alpine Microbiology. Can. J. Microbiol. 2009, 55, 73–83. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lopez-Mondejar, R.; Zuhlke, D.; Becher, D.; Riedel, K.; Baldrian, P. Cellulose and hemicellulose decomposition by forest soil bacteria proceeds by the action of structurally variable enzymatic systems. Sci. Rep. 2016, 6, 25279. [Google Scholar] [CrossRef] [PubMed]
- Himmel, M.E.; Xu, Q.; Luo, Y.; Ding, S.; Lamed, R.; Bayer, E.A.; National Renewable Energy Laboratory. Microbial enzyme systems for biomass conversion: Emerging paradigms. Biofuels 2010, 1, 323–341. [Google Scholar] [CrossRef]
- Loranger, G.; Ponge, J.; Imbert, D.; Lavelle, P. Leaf decomposition in two semi-evergreen tropical forests: Influence of litter quality. Biol. Fertil. Soils 2002, 35, 247–252. [Google Scholar] [CrossRef]
- Dang, C.K.; Chauvet, E.; Gessner, M.O. Magnitude and variability of process rates in fungal diversity-litter decomposition relationships. Ecol. Lett. 2005, 8, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Bååth, E.; Anderson, T.H. Comparison of soil fungal/bacterial ratios in a pH gradient using physiological and PLFA-based techniques. Soil Biol. Biochem. 2003, 35, 955–963. [Google Scholar] [CrossRef]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhu, J.; Zhang, W.; Zhang, Q.; Lu, D.; Zhang, Y.; Zheng, X.; Xu, S.; Wang, G.G. Litter decomposition and nutrient release from monospecific and mixed litters: Comparisons of litter quality, fauna and decomposition site effects. J. Ecol. 2022, 110, 1673–1686. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, M.; Cui, L.; Pan, X.; Liu, W.; Li, W.; Lei, Y. Winter Decomposition of Emergent Macrophytes Affects Water Quality under Ice in a Temperate Shallow Lake. Water 2020, 12, 2640. [Google Scholar] [CrossRef]
- Gessner, M.O.; Swan, C.M.; Dang, C.K.; McKie, B.G.; Bardgett, R.D.; Wall, D.H.; Hättenschwiler, S.; Sveriges, L. Diversity meets decomposition. Trends Ecol. Evol. 2010, 25, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Osono, T. Functional diversity of ligninolytic fungi associated with leaf litter decomposition. Ecol. Res. 2020, 35, 30–43. [Google Scholar] [CrossRef]
- Parton, W.; Silver, W.L.; Burke, I.C.; Grassens, L.; Harmon, M.E.; Currie, W.S.; King, J.Y.; Adair, E.C.; Brandt, L.A.; Hart, S.C.; et al. Global-scale similarities in nitrogen release patterns during long-term decomposition. Science 2007, 315, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.H.; Gallart, M.; Singh, K.; Hannet, G.; Komolong, B.; Yinil, D.; Field, D.J.; Muqaddas, B.; Wallace, H.M. Leaf litter species affects decomposition rate and nutrient release in a cocoa plantation. Agric. Ecosyst. Environ. 2022, 324, 107705. [Google Scholar] [CrossRef]
- Gholz, H.L.; Wedin, D.A.; Smitherman, S.M.; Harmon, M.E.; Parton, W.J. Long-term dynamics of pine and hardwood litter in contrasting environments: Toward a global model of decomposition. Glob. Change Biol. 2000, 6, 751–765. [Google Scholar] [CrossRef]
- Chauvet, E.; Ferreira, V.; Giller, P.S.; McKie, B.G.; Tiegs, S.D.; Woodward, G.; Elosegi, A.; Dobson, M.; Fleituch, T.; Graça, M.A.S.; et al. Chapter Three—Litter Decomposition as an Indicator of Stream Ecosystem Functioning at Local-to-Continental Scales: Insights from the European RivFunction Project. In Advances in Ecological Research; Dumbrell, A.J., Kordas, R.L., Woodward, G., Eds.; Academic Press: Cambridge, MA, USA, 2016; Volume 55, pp. 99–182. [Google Scholar]
- Makkonen, M.; Berg, M.P.; Handa, I.T.; Hattenschwiler, S.; van Ruijven, J.; van Bodegom, P.M.; Aerts, R. Highly consistent effects of plant litter identity and functional traits on decomposition across a latitudinal gradient. Ecol. Lett. 2012, 15, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Liu, X.; Gong, L.; Chen, X.; Zhao, J.; Chen, W. Response of litter decomposition and the soil environment to one-year nitrogen addition in a Schrenk spruce forest in the Tianshan Mountains, China. Sci. Rep. 2022, 12, 648. [Google Scholar] [CrossRef] [PubMed]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Change Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zeng, D.; Yu, Z.; Fan, Z.; Yang, D.; Liu, Y. Impact of litter quality and soil nutrient availability on leaf decomposition rate in a semi-arid grassland of Northeast China. J. Arid Environ. 2011, 75, 787–792. [Google Scholar] [CrossRef]
- Zhang, J.; Li, H.; Zhang, H.; Zhang, H.; Tang, Z. Responses of Litter Decomposition and Nutrient Dynamics to Nitrogen Addition in Temperate Shrublands of North China. Front. Plant Sci. 2021, 11, 618675. [Google Scholar] [CrossRef]
- Liu, P.; Sun, O.J.; Huang, J.; Li, L.; Han, X. Nonadditive effects of litter mixtures on decomposition and correlation with initial litter N and P concentrations in grassland plant species of northern China. Biol. Fertil. Soils 2007, 44, 211–216. [Google Scholar] [CrossRef]
- Berg, B.; Berg, M.P.; Bottner, P.; Box, E.; Breymeyer, A.; De Anta, R.C.; Couteaux, M.; Escudero, A.; Gallardo, A.; Kratz, W.; et al. Litter Mass Loss Rates in Pine Forests of Europe and Eastern United States: Some Relationships with Climate and Litter Quality. Biogeochemistry 1993, 20, 127–159. [Google Scholar] [CrossRef]
- Ibáñez, I.; Schupp, E.W. Effects of litter, soil surface conditions, and microhabitat on Cercocarpus ledifolius Nutt. Seedling emergence and establishment. J. Arid Environ. 2002, 52, 209–221. [Google Scholar] [CrossRef]
- Gege, Y.; Peng, Z.; Yinghui, W.; Aftab, B.; Penghui, D.; Qiang, Z.; Guoping, C.; Mengke, W.; Biwei, Y.; Senhao, W.; et al. Photochemical transformation of terrestrial dissolved organic matter derived from multiple sources in tropical plantations. Geochim. Cosmochim. Acta 2023, 358, 162–173. [Google Scholar] [CrossRef]
- Austin, A.T.; Ballare, C.L. Dual role of lignin in plant litter decomposition in terrestrial ecosystems. Proc. Natl. Acad. Sci. USA 2010, 107, 4618–4622. [Google Scholar] [CrossRef]
- Strickland, M.S.; Osburn, E.; Lauber, C.; Fierer, N.; Bradford, M.A. Litter quality is in the eye of the beholder: Initial decomposition rates as a function of inoculum characteristics. Funct. Ecol. 2009, 23, 627–636. [Google Scholar] [CrossRef]
- Liu, P.; Huang, J.; Sun, O.J.; Han, X. Litter decomposition and nutrient release as affected by soil nitrogen availability and litter quality in a semiarid grassland ecosystem. Oecologia 2010, 162, 771–780. [Google Scholar] [CrossRef]
- Sarmiento, R.; Aguinsatan, R.; Gorgonio, C. Variations in the microclimatic conditions and litter decomposition rates between mixed forest and grassland areas. Soc. Sci. Res. Netw. 2021, 8, 10–14. [Google Scholar] [CrossRef]
- Harris, R.B. Rangeland degradation on the Qinghai-Tibetan plateau: A review of the evidence of its magnitude and causes. J. Arid. Environ. 2010, 74, 1–12. [Google Scholar] [CrossRef]
- Shen, M.; Piao, S.; Jeong, S.J.; Zhou, L.; Zeng, Z.; Ciais, P.; Chen, D.; Huang, M.; Jin, C.S.; Li, L.Z.; et al. Evaporative cooling over the Tibetan Plateau induced by vegetation growth. Proc. Natl. Acad. Sci. USA 2015, 112, 9299–9304. [Google Scholar] [CrossRef]
- Yao, T.; Wu, F.; Ding, L.; Sun, J.; Zhu, L.; Piao, S.; Deng, T.; Ni, X.; Zheng, H.; Ouyang, H. Multispherical interactions and their effects on the Tibetan Plateau’s earth system: A review of the recent researches. Natl. Sci. Rev. 2015, 2, 468–488. [Google Scholar] [CrossRef]
- Huirong, H.; Rongta, B.; Yanxia, W. Forest Soil Science; China Forestry Publishing House: Beijing, China, 2019. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Lamlom, S.H.; Savidge, R.A. A reassessment of carbon content in wood: Variation within and between 41 North American species. Biomass Bioenerg. 2003, 25, 381–388. [Google Scholar] [CrossRef]
- Lotse, E.G. Chemical Analysis of Ecological Materials; Blackwell Scientific Publications: London, UK, 1976; Volume 121, p. 373. [Google Scholar]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Magoc, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Abarenkov, K.; Tedersoo, L.; Nilsson, R.H.; Vellak, K.; Saar, I.; Veldre, V.; Parmasto, E.; Prous, M.; Aan, A.; Ots, M.; et al. PlutoF—A Web Based Workbench for Ecological and Taxonomic Research, with an Online Implementation for Fungal ITS Sequences. Evol. Bioinform. 2010, 6, EBO.S6271. [Google Scholar] [CrossRef]
- Liu, W.S.; Wei, Y.X.; Deng, P.P.; Oladele, O.P.; N’Dri, B.Y.; Dang, Y.P.; Zhao, X.; Zhang, H.L. Conservation tillage increases surface soil organic carbon stock by altering fungal communities and enzyme activity. Environ. Sci. Pollut. Res. 2023, 30, 80901–80915. [Google Scholar] [CrossRef]
- Zhu, Z.; Fang, Y.; Liang, Y.; Li, Y.; Liu, S.; Li, Y.; Li, B.; Gao, W.; Yuan, H.; Kuzyakov, Y.; et al. Stoichiometric regulation of priming effects and soil carbon balance by microbial life strategies. Soil Biol. Biochem. 2022, 169, 108669. [Google Scholar] [CrossRef]
- Schreiner, K.M.; Blair, N.E.; Levinson, W.; Egerton-Warburton, L.M. Contribution of Fungal Macromolecules to Soil Carbon Sequestration; Springer International Publishing: Cham, Switzerland, 2014; pp. 155–161. [Google Scholar]
- Xu, Y.; Gao, X.; Liu, Y.; Li, S.; Liang, C.; Lal, R.; Wang, J. Differential accumulation patterns of microbial necromass induced by maize root vs. shoot residue addition in agricultural Alfisols. Soil Biol. Biochem. 2022, 164, 108474. [Google Scholar] [CrossRef]
- Zhang, G.; Yu, X.; Xu, J.; Duan, H.; Rafay, L.; Zhang, Q.; Li, Y.; Liu, Y.; Xia, S. Effects of environmental variation on stable isotope abundances during typical seasonal floodplain dry season litter decomposition. Sci. Total Environ. 2018, 630, 1205–1215. [Google Scholar] [CrossRef] [PubMed]
- Penalva, M.A.; Tilburn, J.; Bignell, E.; Arst, H.J. Ambient pH gene regulation in fungi: Making connections. Trends Microbiol. 2008, 16, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Keuskamp, J.A.; Hefting, M.M.; Dingemans, B.J.; Verhoeven, J.T.; Feller, I.C. Effects of nutrient enrichment on mangrove leaf litter decomposition. Sci. Total Environ. 2015, 508, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Steffens, C.; Helfrich, M.; Joergensen, R.G.; Eissfeller, V.; Flessa, H. Translocation of 13C-labeled leaf or root litter carbon of beech (Fagus sylvatica L.) and ash (Fraxinus excelsior L.) during decomposition—A laboratory incubation experiment. Soil Biol. Biochem. 2015, 83, 125–137. [Google Scholar] [CrossRef]
- Aerts, R. Climate, Leaf Litter Chemistry and Leaf Litter Decomposition in Terrestrial Ecosystems: A Triangular Relationship. Oikos 1997, 79, 439–449. [Google Scholar] [CrossRef]
- Davis, S.E.; Corronado-Molina, C.; Childers, D.L.; Day, J.W. Temporally dependent C, N, and P dynamics associated with the decay of Rhizophora mangle L. leaf litter in oligotrophic mangrove wetlands of the Southern Everglades. Aquat. Bot. 2003, 75, 199–215. [Google Scholar] [CrossRef]
- van der Wal, A.; Geydan, T.D.; Kuyper, T.W.; de Boer, W. A thready affair: Linking fungal diversity and community dynamics to terrestrial decomposition processes. FEMS Microbiol. Rev. 2013, 37, 477–494. [Google Scholar] [CrossRef] [PubMed]
- McGuire, K.L.; Bent, E.; Borneman, J.; Majumder, A.; Allison, S.D.; Treseder, K.K.; Rillig, M.C. Functional diversity in resource use by fungi. Ecology 2010, 91, 2324–2332. [Google Scholar] [CrossRef] [PubMed]
- Fukami, T.; Dickie, I.A.; Paula, W.J.; Paulus, B.C.; Park, D.; Roberts, A.; Buchanan, P.K.; Allen, R.B. Assembly history dictates ecosystem functioning: Evidence from wood decomposer communities. Ecol. Lett. 2010, 13, 675–684. [Google Scholar] [CrossRef]
- Strickland, M.S.; Rousk, J. Considering fungal:bacterial dominance in soils—Methods, controls, and ecosystem implications. Soil Biol. Biochem. 2010, 42, 1385–1395. [Google Scholar] [CrossRef]
- Kaiser, C.; Franklin, O.; Dieckmann, U.; Richter, A. Microbial community dynamics alleviate stoichiometric constraints during litter decay. Ecol. Lett. 2014, 17, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Eichorst, S.A.; Kuske, C.R. Identification of cellulose-responsive bacterial and fungal communities in geographically and edaphically different soils by using stable isotope probing. Appl. Environ. Microbiol. 2012, 78, 2316–2327. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Dai, Z.; Wang, H.; Dsouza, M.; Liu, X.; He, Y.; Wu, J.; Rodrigues, J.L.; Gilbert, J.A.; Brookes, P.C.; et al. Distinct Biogeographic Patterns for Archaea, Bacteria, and Fungi along the Vegetation Gradient at the Continental Scale in Eastern China. mSystems 2017, 2, e00174-16. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, W.S.; Tripathi, B.M.; Adams, J. Distinct bacterial communities dominate tropical and temperate zone leaf litter. Microb. Ecol. 2014, 67, 837–848. [Google Scholar] [CrossRef]
- Schneider, T.; Keiblinger, K.M.; Schmid, E.; Sterflinger-Gleixner, K.; Ellersdorfer, G.; Roschitzki, B.; Richter, A.; Eberl, L.; Zechmeister-Boltenstern, S.; Riedel, K. Who is who in litter decomposition? Metaproteomics reveals major microbial players and their biogeochemical functions. ISME J. 2012, 6, 1749–1762. [Google Scholar] [CrossRef] [PubMed]
- Baldrian, P.; Kolarik, M.; Stursova, M.; Kopecky, J.; Valaskova, V.; Vetrovsky, T.; Zifcakova, L.; Snajdr, J.; Ridl, J.; Vlcek, C.; et al. Active and total microbial communities in forest soil are largely different and highly stratified during decomposition. ISME J. 2012, 6, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Barns, S.M.; Cain, E.C.; Sommerville, L.; Kuske, C.R. Acidobacteria phylum sequences in uranium-contaminated subsurface sediments greatly expand the known diversity within the phylum. Appl. Environ. Microbiol. 2007, 73, 3113–3116. [Google Scholar] [CrossRef]
- Janusz, G.; Pawlik, A.; Sulej, J.; Swiderska-Burek, U.; Jarosz-Wilkolazka, A.; Paszczynski, A. Lignin degradation: Microorganisms, enzymes involved, genomes analysis and evolution. FEMS Microbiol. Rev. 2017, 41, 941–962. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Yang, T.; Bao, Y.; He, P.; Yang, K.; Mei, X.; Wei, Z.; Xu, Y.; Shen, Q.; Banerjee, S. Network analysis and subsequent culturing reveal keystone taxa involved in microbial litter decomposition dynamics. Soil Biol. Biochem. 2021, 157, 108230. [Google Scholar] [CrossRef]
- Snajdr, J.; Cajthaml, T.; Valaskova, V.; Merhautova, V.; Petrankova, M.; Spetz, P.; Leppanen, K.; Baldrian, P. Transformation of Quercus petraea litter: Successive changes in litter chemistry are reflected in differential enzyme activity and changes in the microbial community composition. FEMS Microbiol. Ecol. 2011, 75, 291–303. [Google Scholar] [CrossRef]
- Boer, W.D.; Folman, L.B.; Summerbell, R.C.; Boddy, L. Living in a fungal world: Impact of fungi on soil bacterial niche development. FEMS Microbiol. Rev. 2005, 29, 795–811. [Google Scholar] [CrossRef]
- Meidute, S.; Demoling, F.; Bååth, E. Antagonistic and synergistic effects of fungal and bacterial growth in soil after adding different carbon and nitrogen sources. Soil Biol. Biochem. 2008, 40, 2334–2343. [Google Scholar] [CrossRef]
- Op, D.B.M.; Troein, C.; Siregar, S.; Gentile, L.; Abbondanza, G.; Peterson, C.; Persson, P.; Tunlid, A. Regulation of fungal decomposition at single-cell level. ISME J. 2020, 14, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Osono, T. Ecology of ligninolytic fungi associated with leaf litter decomposition. Ecol. Res. 2007, 22, 955–974. [Google Scholar] [CrossRef]
- Torres, P.A.; Abril, A.B.; Bucher, E.H. Microbial succession in litter decomposition in the semi-arid Chaco woodland. Soil Biol. Biochem. 2005, 37, 49–54. [Google Scholar] [CrossRef]
- Ramirez, K.S.; Geisen, S.; Morrien, E.; Snoek, B.L.; van der Putten, W.H. Network Analyses Can Advance Above-Belowground Ecology. Trends Plant Sci. 2018, 23, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Ni, H.; Jiao, S.; Lu, Y.; Zhou, J.; Sun, B.; Liang, Y. Coexistence patterns of soil methanogens are closely tied to methane generation and community assembly in rice paddies. Microbiome 2021, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Xu, C.; Wu, F. Huhe Microbial co-occurrence networks driven by low-abundance microbial taxa during composting dominate lignocellulose degradation. Sci. Total Environ. 2022, 845, 157197. [Google Scholar] [CrossRef] [PubMed]
- Lima-Mendez, G.; Faust, K.; Henry, N.; Decelle, J.; Colin, S.; Carcillo, F.; Chaffron, S.; Ignacio-Espinosa, J.C.; Roux, S.; Vincent, F.; et al. Ocean plankton. Determinants of community structure in the global plankton interactome. Science 2015, 348, 1262073. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, U.N.; Ayres, E.; Wall, D.H.; Bardgett, R.D. Soil biodiversity and carbon cycling: A review and synthesis of studies examining diversity-function relationships. Eur. J. Soil Sci. 2011, 62, 105–116. [Google Scholar] [CrossRef]
- Zhao, X.; Tian, P.; Liu, S.; Yin, P.; Sun, Z.; Wang, Q. Mean annual temperature and carbon availability respectively controlled the contributions of bacterial and fungal residues to organic carbon accumulation in topsoil across China’s forests. Glob. Ecol. Biogeogr. 2023, 32, 120–131. [Google Scholar] [CrossRef]
- Bahram, M.; Hildebrand, F.; Forslund, S.K.; Anderson, J.L.; Soudzilovskaia, N.A.; Bodegom, P.M.; Bengtsson-Palme, J.; Anslan, S.; Coelho, L.P.; Harend, H.; et al. Structure and function of the global topsoil microbiome. Nature 2018, 560, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Huang, Y.; An, S.; Zeng, Q.; Wang, B.; Bai, X.; Huang, Q. Decay stages and meteorological factors affect microbial community during leaf litter in situ decomposition. Soil Ecol. Lett. 2023, 5, 220160. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Li, H.; Zhang, S.; Zhang, M.; Pan, H.; Zhou, F.; Wang, L. The Influence of Forest Litter Characteristics on Bacterial and Fungal Community Diversity in the Picea crassifolia Ecosystem on the Qinghai–Tibet Plateau. Forests 2024, 15, 797. https://doi.org/10.3390/f15050797
Chen Y, Li H, Zhang S, Zhang M, Pan H, Zhou F, Wang L. The Influence of Forest Litter Characteristics on Bacterial and Fungal Community Diversity in the Picea crassifolia Ecosystem on the Qinghai–Tibet Plateau. Forests. 2024; 15(5):797. https://doi.org/10.3390/f15050797
Chicago/Turabian StyleChen, Yahui, Haijia Li, Shiyang Zhang, Min Zhang, Hui Pan, Fangwei Zhou, and Lei Wang. 2024. "The Influence of Forest Litter Characteristics on Bacterial and Fungal Community Diversity in the Picea crassifolia Ecosystem on the Qinghai–Tibet Plateau" Forests 15, no. 5: 797. https://doi.org/10.3390/f15050797
APA StyleChen, Y., Li, H., Zhang, S., Zhang, M., Pan, H., Zhou, F., & Wang, L. (2024). The Influence of Forest Litter Characteristics on Bacterial and Fungal Community Diversity in the Picea crassifolia Ecosystem on the Qinghai–Tibet Plateau. Forests, 15(5), 797. https://doi.org/10.3390/f15050797





