The Influence of Maturity, Storage, and Embryo Size on Coconut Callus Induction Success
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials Preparations
2.2. Medium Preparations
2.3. Experiment Set-Up
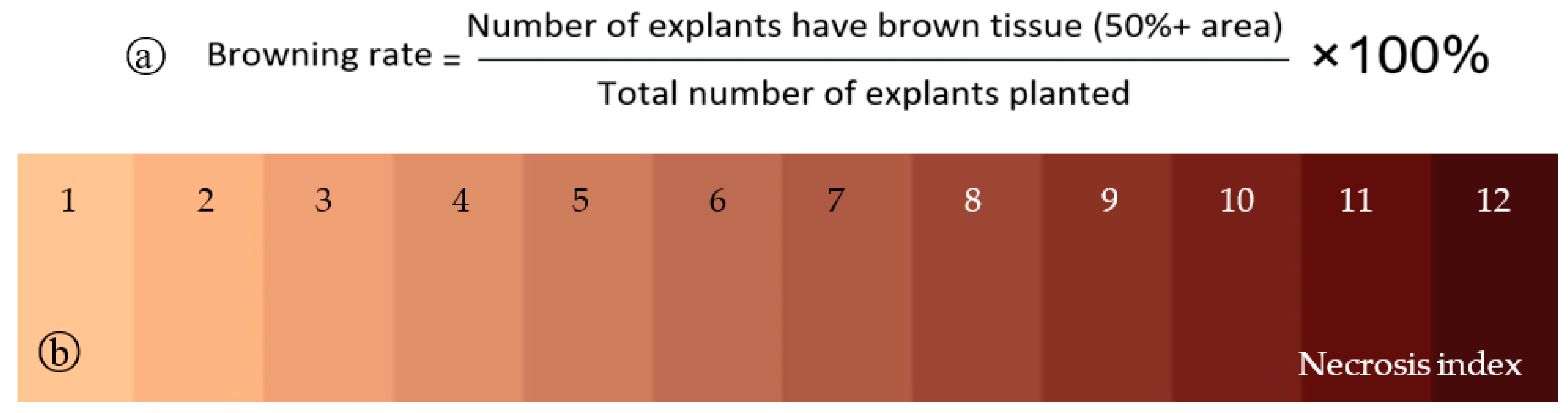
2.4. Assessment and Data Analysis
3. Results
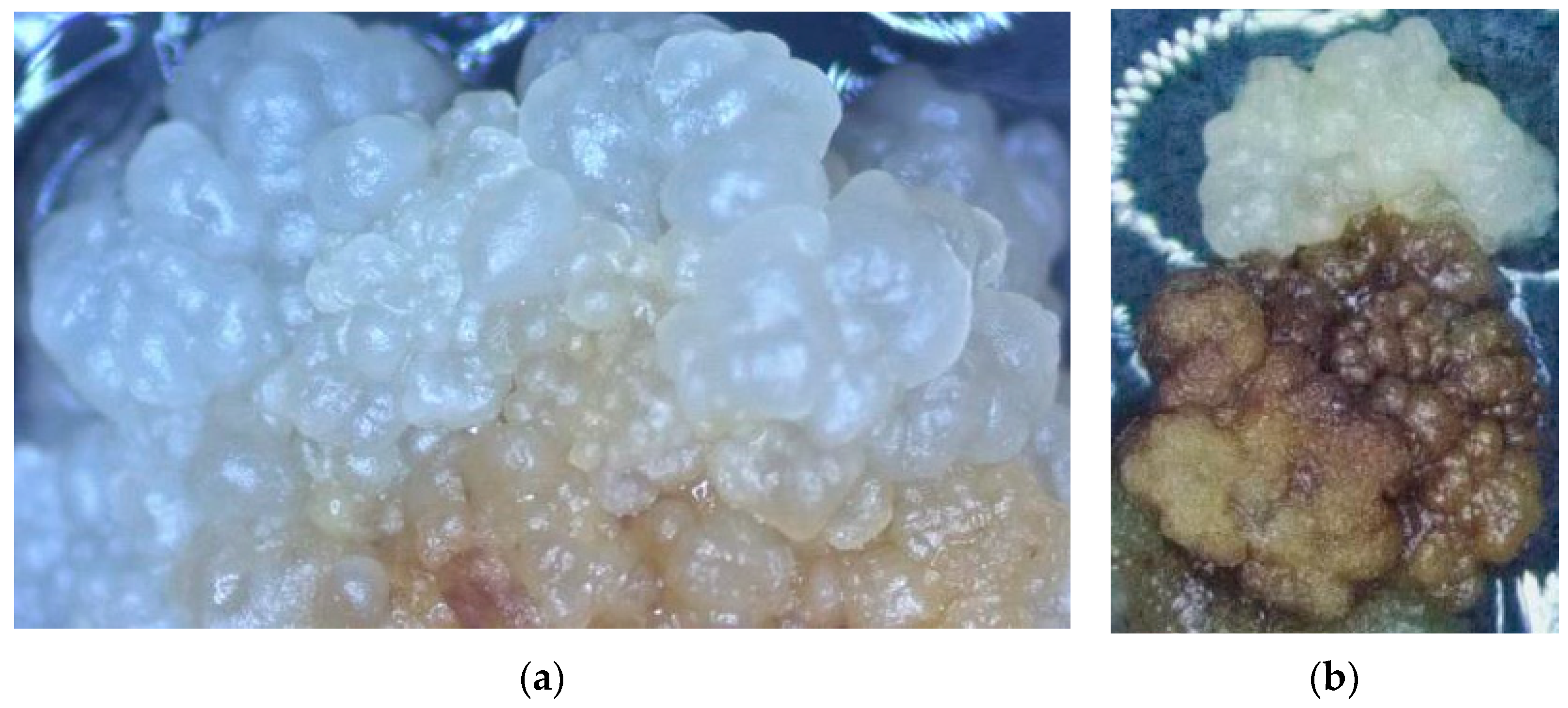
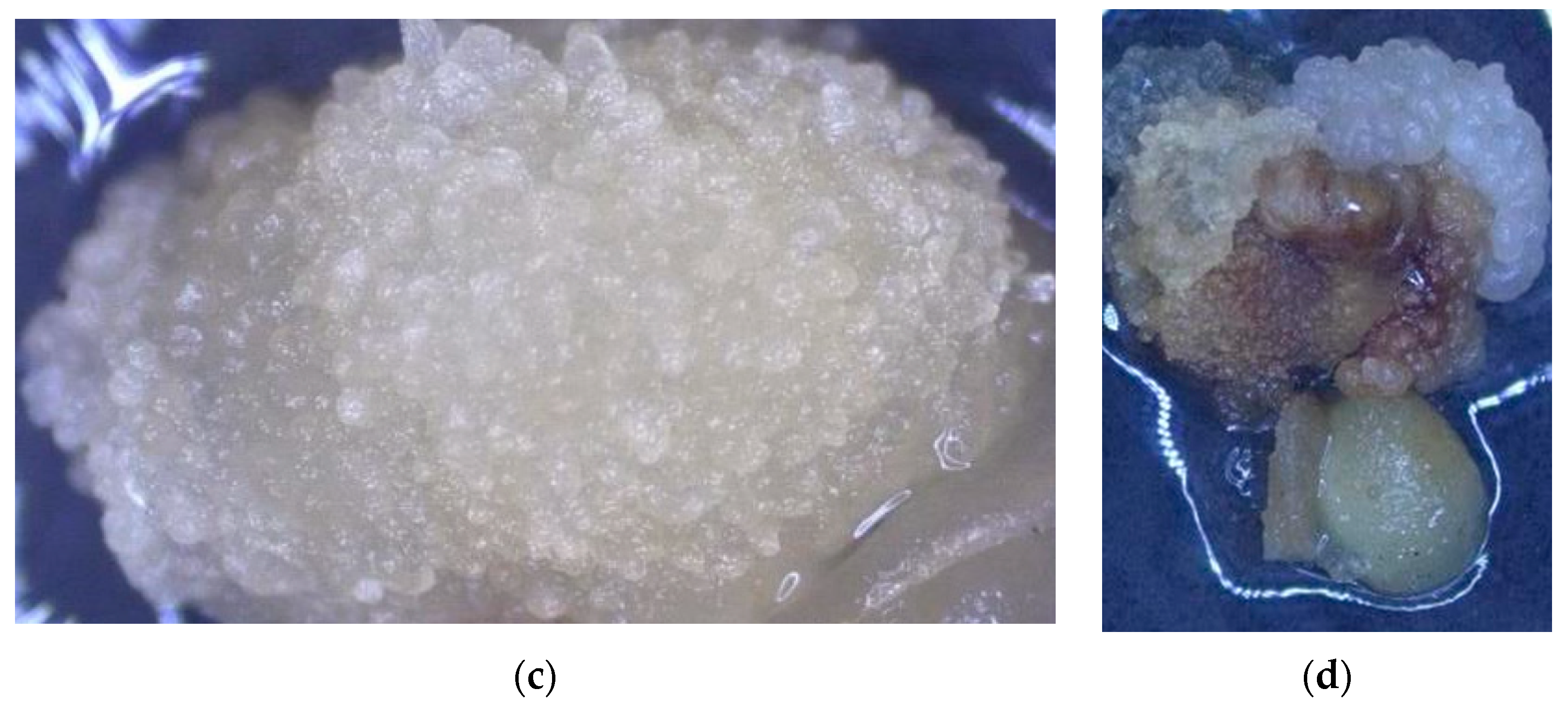
3.1. The Impact of Coconut Fruit Age on the Initiation of Callus
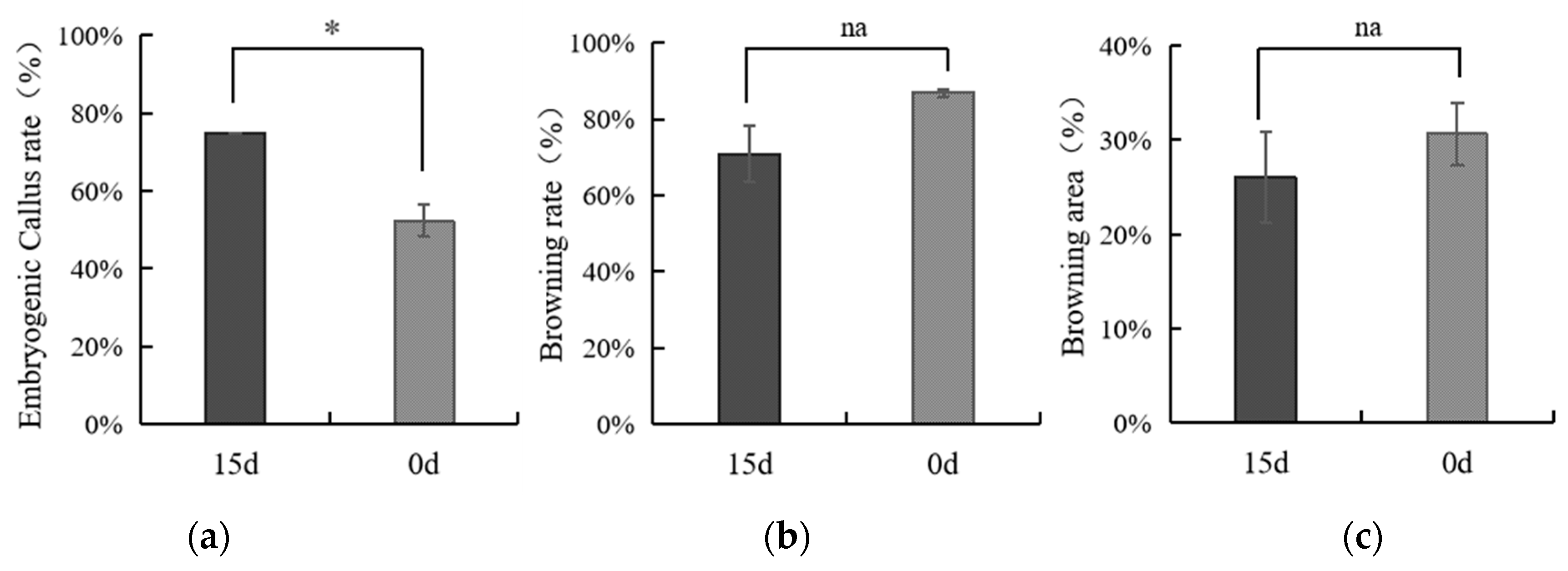
3.2. The Influence of Storage Time of Coconut on the Production of Callus
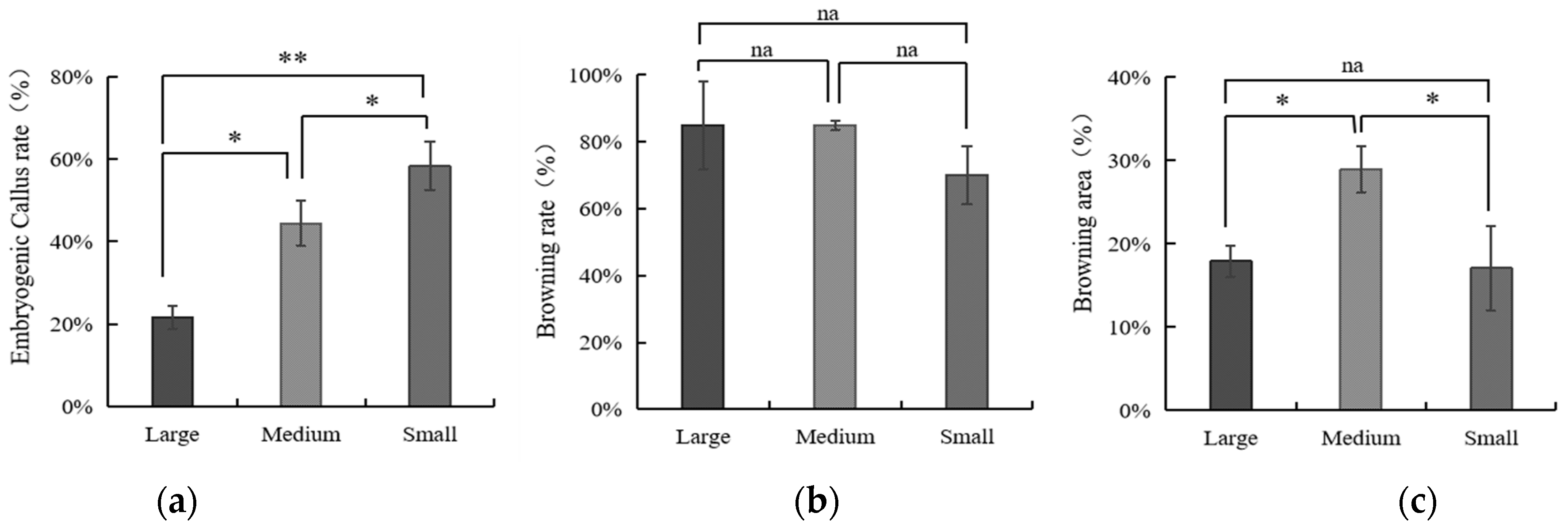
3.3. The Influence of Embryo Size of Coconut Fruits on Callus Initiation
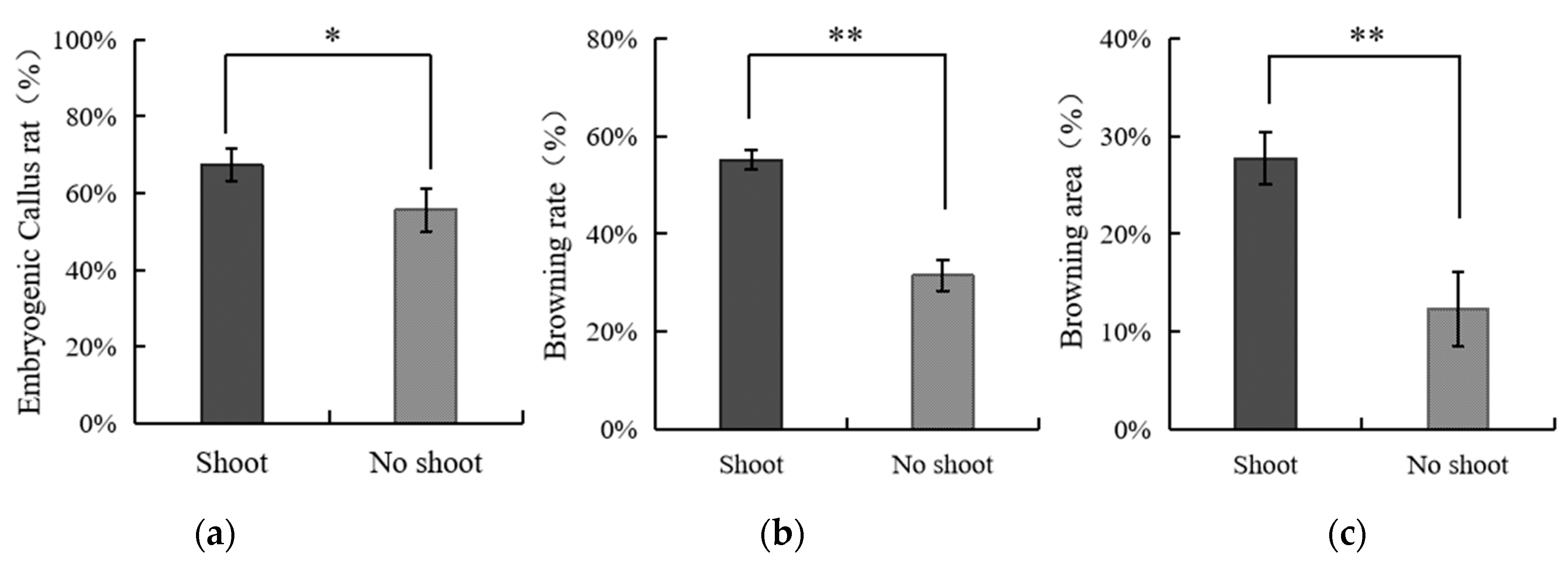
3.4. The Effect of Applying Embryos with Shoot or Non-Shoot as the Explant for Callus Initiation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alshawwa, I.A.; Elsharif, A.A.; Abu-Naser, S.S. An Expert System for Coconut Diseases Diagnosis. Int. J. Acad. Eng. Res. 2019, 3, 8–13. [Google Scholar]
- Mu, Z. Overcoming Bottlenecks in the Pathway of Clonal Propagation of Coconut (Cocos nucifera L.). Ph.D. Thesis, School of Agriculture and Food Sciences, The University of Queensland, Brisbane City, QLD, Australia, 2022. [Google Scholar]
- Deen, A.; Visvanathan, R.; Wickramarachchi, D.; Marikkar, N.; Nammi, S.; Jayawardana, B.C.; Liyanage, R. Chemical composition and health benefits of coconut oil: An overview. J. Sci. Food Agric. 2021, 101, 2182–2193. [Google Scholar] [CrossRef] [PubMed]
- Sudalaimuthu, S.; Senthilkumar, P.; Sivakumar, B. Coconut industry in a nutshell. Retrieved Novemb. 2012, 23. [Google Scholar]
- Yang, Z.; Liu, Z.; Xu, H.; Li, Y.; Huang, S.; Cao, G.; Shi, M.; Zhu, J.; Zhou, J.; Li, R. ArecaceaeMDB: A comprehensive multi-omics database for Arecaceae breeding and functional genomics studies. Plant Biotechnol. J. 2023, 21, 11. [Google Scholar] [CrossRef] [PubMed]
- De Silva, H.W.S. The Coconut Industry in Sri Lanka: An Analysis of Government Intervention Measures. Master’s Thesis, The Australian National University, Canberra, Australia, 1979. [Google Scholar]
- Nayar, N. Origin and Domestication. In Coconut; Daya Publishing House A Division of Astral International Pvt. Ltd.: New Delhi, India, 2017; pp. 39–72. [Google Scholar]
- Zainol, F.A.; Arumugam, N.; Daud, W.N.W.; Suhaimi, N.A.M.; Ishola, B.D.; Ishak, A.Z.; Afthanorhan, A. Coconut Value Chain Analysis: A Systematic Review. Agriculture 2023, 13, 1379. [Google Scholar] [CrossRef]
- Yousefi, K.; Abdullah, S.N.A.; Hatta, M.A.M.; Ling, K.L. Genomics and Transcriptomics Reveal Genetic Contribution to Population Diversity and Specific Traits in Coconut. Plants 2023, 12, 1913. [Google Scholar] [CrossRef]
- Thadathil, S.T. A comprehensive review of chemical composition and nutritional health benefits of coconut water. Pharma Innov. J. 2023, 12, 343–550. [Google Scholar]
- Nguyen, Q.T.; Bandupriya, H.D.; López-Villalobos, A.; Sisunandar, S.; Foale, M.; Adkins, S.W. Tissue culture and associated biotechnological interventions for the improvement of coconut (Cocos nucifera L.): A review. Planta 2015, 242, 1059–1076. [Google Scholar] [CrossRef]
- Wilms, H.; Swennen, R.; Panis, B. Developing New In Vitro Micropropagation and Cryopreservation Techniques in Coconut; KU Leuven: Leuven, Belgium, 2023. [Google Scholar]
- Guo, Q.; Wang, Y.; Zou, J.; Jing, H.; Li, D. Efficient isolation and transformation of protoplasts in coconut endosperm and leaves for gene function studies. Trop. Plants 2023, 2, 16. [Google Scholar] [CrossRef]
- Kong, E.Y.; Biddle, J.; Kalaipandian, S.; Adkins, S.W. Coconut Callus Initiation for Cell Suspension Culture. Plants 2023, 12, 968. [Google Scholar] [CrossRef]
- Mu, Z.; Tran, B.-M.; Xu, H.; Yang, Z.; Qamar, U.Z.; Wang, X.; Xiao, Y.; Luo, J. Exploring the potential application of coconut water in healthcare and biotechnology: A review. Beverage Plant Res. 2024, 1–9. [Google Scholar] [CrossRef]
- Nwite, P.A.; Eke, C.; Osemwegie, Q.; Ikhajiagbe, B. Assessment of media modification and induction of calli on coconut (Cocos nucifera L.) palm explants. Res. J. Agric. Sci. 2023, 2320, 6063. [Google Scholar]
- Antonova, I. Somatic Embryogenesis for Micropropagation of Coconut (Cocos nucifera L.). Ph.D. Thesis, School of Land, Crop and Food Sciences, The University of Queensland, Brisbane City, QLD, Australia, 2009. [Google Scholar]
- Nguyen, T.Q. Clonal Propagation of Coconut (Cocos nucifera L.) for Elite Seedling Production and Germplasm Exchange. Ph.D. Thesis, School of Agriculture and Food Sciences, The University of Queensland, Brisbane City, QLD, Australia, 2018. [Google Scholar]
- Bazrafshan, A. Ex situ conservation of coconut (Cocos nucifera L.) germplasm using cryopreservation. Cryobiology 2022, 97, 297. [Google Scholar]
- Mu, Z. Optimization of Coconut Micropropagation via Somatic Embryogenesis. Multidiscip. Digit. Publ. Inst. Proc. 2020, 36, 55. [Google Scholar]
- Eeuwens, C. Mineral requirements for growth and callus initiation of tissue explants excised from mature coconut palms (Cocos nucifera) and cultured in vitro. Physiol. Plant. 1976, 36, 23–28. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- EL-Gioushy, S.; Liu, R.; Fan, H. A complete protocol to reduce browning during coconut (Cocos nucifera L.) tissue culture through shoot tips and inflorescence explants. Plant Arch. 2020, 20, 2196–2204. [Google Scholar]
- Bhojwani, S.S.; Dantu, P.K. Plant Tissue Culture: An Introductory Text; Springer: Berlin/Heidelberg, Germany, 2013; Volume 318. [Google Scholar]
- Adkins, S.W.; Samosir, Y.M.; Nikmatullah, A.; Ogle, H. Coconut (Cocos nucifera) in vitro ecology: Modifications of headspace and medium additives can optimize somatic embryogenesis. In Proceedings of the II International Symposium on Biotechnology of Tropical and Subtropical Species, Taipei, Taiwan, 5–9 November 2001; pp. 21–32. [Google Scholar]
- Fernando, S.; Santha, E.; Hewarathna, D. Activated coconut shell charcoal as a component of tissue culture media of Cocos nucifera L. J. Natl. Sci. Found. Sri Lanka 2010, 38. [Google Scholar] [CrossRef]
- Pérez-Núñez, M.; Chan, J.; Saenz, L.; González, T.; Verdeil, J.-L.; Oropeza, C. Improved somatic embryogenesis from Cocos nucifera (L.) plumule explants. Vitr. Cell. Dev. Biol. Plant 2006, 42, 37–43. [Google Scholar] [CrossRef]
- Sáenz, L.; Chan, J.L.; Narvaez, M.; Oropeza, C. Protocol for the micropropagation of coconut from plumule explants. In Plant Cell Culture Protocols; Humana Press: New York, NY, USA, 2018; Volume 1815, pp. 161–170. [Google Scholar]
- Beveridge, F.C.; Kalaipandian, S.; Yang, C.; Adkins, S.W. Fruit Biology of Coconut (Cocos nucifera L.). Plants 2022, 11, 3293. [Google Scholar] [CrossRef]
- Lopez Villalobos, A. Roles of Lipids in Coconut (Cocos nucifera L.) Embryogenesis. Ph.D. Thesis, University of London, London, UK, 2002. [Google Scholar]
- Fernando, S.; Weerakoon, L.; Gunathilake, T. Micropropagation of coconut through plumule culture. Print. Sri Lanka 2004, 16, 1–10. [Google Scholar] [CrossRef][Green Version]
- Chan, J.; Saenz, L.; Talavera, C.; Hornung, R.; Robert, M.; Oropeza, C. Regeneration of coconut (Cocos nucifera L.) from plumule explants through somatic embryogenesis. Plant Cell Rep. 1998, 17, 515–521. [Google Scholar] [CrossRef]
- Feher, A.; Pasternak, T.P.; Dudits, D. Transition of somatic plant cells to an embryogenic state. Plant Cell Tissue Organ Cult. 2003, 74, 201–228. [Google Scholar] [CrossRef]
- Solís-Ramos, L.Y.; Andrade-Torres, A.; Sáenz-Carbonell, L.; Oropeza-Salín, C.; Castaño de la Serna, E. Somatic embryogenesis in recalcitrant plants. In Embryogenesis; Intech Open: London, UK, 2012; pp. 597–618. [Google Scholar]
- Verdeil, J.-L.; Huet, C.; Grosdemange, F.; Buffard-Morel, J. Plant regeneration from cultured immature inflorescences of coconut (Cocos nucifera L.): Evidence for somatic embryogenesis. Plant Cell Rep. 1994, 13, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Buchsbaum, R.M. Size of explant and volume of medium in tissue cultures. J. Exp. Zool. 1932, 63, 483–508. [Google Scholar] [CrossRef]
- Dal Vesco, L.L.; de Almeida Pinto, A.; Zaffari, G.R.; Nodari, R.O.; Dos Reis, M.S.; Guerra, M.P. Improving pineapple micropropagation protocol through explant size and medium composition manipulation. Fruits 2001, 56, 143–154. [Google Scholar] [CrossRef]
- Singh, B.; Sood, N. Significance of explant preparation and sizing in Aloe vera L.—A highly efficient method for in vitro multiple shoot induction. Sci. Hortic. 2009, 122, 146–151. [Google Scholar] [CrossRef]
- Nhut, D.T.; An, T.T.T.; Huong, N.T.D.; Don, N.T.; Hai, N.T.; Thien, N.Q.; Vu, N.H. Effect of genotype, explant size, position, and culture medium on shoot generation of Gerbera jamesonii by receptacle transverse thin cell layer culture. Sci. Hortic. 2007, 111, 146–151. [Google Scholar] [CrossRef]
- Malaurie, B.; Tregear, J.; N’Nan, O.; Bandupriya, H.; Borges, M.; Verdeil, J.-L. Cryopreservation as a tool for the management of coconut germplasm. In Proceedings of the I International Symposium on Cryopreservation in Horticultural Species, Leuven, Belgium, 5–8 April 2009; pp. 461–466. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mu, Z.; Yang, S.; Xu, H.; Yang, Z.; Haque, M.M.; Tran, B.-M.; Chen, J.; Wang, X.; Peng, H.; Luo, J. The Influence of Maturity, Storage, and Embryo Size on Coconut Callus Induction Success. Forests 2024, 15, 764. https://doi.org/10.3390/f15050764
Mu Z, Yang S, Xu H, Yang Z, Haque MM, Tran B-M, Chen J, Wang X, Peng H, Luo J. The Influence of Maturity, Storage, and Embryo Size on Coconut Callus Induction Success. Forests. 2024; 15(5):764. https://doi.org/10.3390/f15050764
Chicago/Turabian StyleMu, Zhihua, Shuya Yang, Hang Xu, Zhuang Yang, Mirza Mobashwerul Haque, Binh-Minh Tran, Jiepeng Chen, Xingwei Wang, Hui Peng, and Jie Luo. 2024. "The Influence of Maturity, Storage, and Embryo Size on Coconut Callus Induction Success" Forests 15, no. 5: 764. https://doi.org/10.3390/f15050764
APA StyleMu, Z., Yang, S., Xu, H., Yang, Z., Haque, M. M., Tran, B.-M., Chen, J., Wang, X., Peng, H., & Luo, J. (2024). The Influence of Maturity, Storage, and Embryo Size on Coconut Callus Induction Success. Forests, 15(5), 764. https://doi.org/10.3390/f15050764







