Abstract
Fly ashes produced in huge amounts during coal combustion requires proper management. The purpose of this study was to determine the impact of fly ash from burning hard coal used in large doses (250, 500, 1000 and 2000 t ha−1) on soil properties and vegetation of fresh mixed coniferous forest within 43 years from the ash application. The experiment was established in the Podzols in the forest habitat of Czułów, Katowice Forest district, Upper Silesia, Poland. Eight tree species were planted in ridges created by ploughing: Pinus sylvestris, P. nigra, Larix decidua, Betula pendula, Quercus robur, Q., Acer pseudoplatanus and Fagus sylvatica. The changes in soil morphology caused significant transformations in the physical and chemical properties of the soil such as soil texture, pH, macronutrients (P, K and Mg) content and C:N ratio. Increasing of ash doses changed the granulometric composition of the soil levels from loamy sand (250 t/ha−1) to silt loam (2000 t ha−1). Initially, the acidic Podzols were alkalized under the influence of the fly ash and then acidified, possibly due to the impact of accumulated litter layers, and the reaction of organic soil horizons changed from strongly acidic (250–1000 t ha−1) to alkalis (2000 t/ha−1). The macronutrients content increased in proportion to the fly ash dose, but the subsequent acidification resulted in a gradual decrease in the macronutrients share in the soil layers. The value of the C:N ratio grew after the ash application and then it gradually reduced, even by half. The transformations of soil horizons’ properties also increased the capacity of the soil sorption complex (CEC). All these processes led to a change in the trophic status of the habitat expressed by the soil habitat index (SIG) and the initial coniferous forest site can be classified as a mixed forest habitat even with the lowest ash dose used. The composition of plant communities developed forty years after the ash application was similar at the lower ash doses and the most frequent and abundant tree species were L. decidua, P. nigra and P. silvestris. B. pendula was previously co-dominant, but it was eliminated from the tree stands during the experiment. Planted trees characteristic of late stages of succession, such as Q. robur, Q. rubra, F. sylvatica and A. pseudoplatanus either did not survive or remained in very low quantities. The herb and moss layers developed in the process of spontaneous colonization, and together with the trees led to phytostabilisation of the bare substrates. After acidification of the topsoil horizons, the herb layers consisted mostly of coniferous, mixed, and deciduous forest species, and the most frequent or abundant were Lysimachia europea and Pteridium aquilinum. The moss layers were represented by coniferous forest flora. At the ash dose of 2000 t ha−1, Tilia cordata settled in one of the seral stages of spontaneous succession and this species dominated in the community and formed a dense tree stand. After the soil acidification, a shift from calcicole to calcifuge plant strategy took place among species of the herbaceous layer. The transformations of plant communities’ composition occurred in relation to changes in the soil properties.
1. Introduction
Hard coal is still the main material for energy production in many parts of the world [1]. Despite the many applications of fly ash from the coal combustion, for example in agriculture, glass and ceramics manufacturing, zeolites, and mesoporous materials production, geopolymer synthesis and in use as catalysts and catalyst support [2], new ways of its management are still being sought [3]. One of them is the use of the fly ash in forestry [4]. In Europe, many experiments were conducted with the fly ashes mainly in coniferous forest habitats on Podzols [4,5,6,7] and Technosols [8,9] most often in the Norway spruce (Picea abies (L.) H. Karst.) stands on sandy soils [7]. Some tree species such as silver birch (Betula pendula L.), northern red oak (Quercus rubra L.) or common ash (Fraximus excelsior L.) are used in the fly ash management process on ash dumps [9]. In other regions of the world, fly ashes were used in forestry on other soil units as Fluvisols, Alfisols, Ultisols, Inceptosols and Solonietz [10,11,12,13].
The fly ash can get into the soil in different ways. It can spread by industrial emission [14] and fall in a pure raw form on the soil surface [15]. It can also be mixed with the top layer of soil with other materials such as compost [4], sewage sludge [9], biochar, gytja [16] and wastes from paper production [17]. The ash may also be introduced between the existing upper soil levels [6]. This method of application improves the soil water retention in the top layer (0–40 cm) and enables the preservation of the upper horizons, protecting them from over mixing [5]. The fly ash from the coal combustion is often highly reactive in the soil environment and can change physical and chemical properties of the soil profiles, which can lead to the alteration in the trophic status of the habitat [15,18,19]. This may have a positive effect on the plant’s growth and development. Recently, a lot of experiments have been carried out in forest plantations in India, and the influence of fly ashes on the growth and development of trees such as Tecona sp., Acacia sp. or Caesalpinia sp. were investigated. Most of the studied species achieved 20%–30% higher growth compared to those grown without the addition of fly ashes, and the best results were obtained when the soil was mixed with ash in a 1:1 ratio [18]. In Bangladesh, an experiment was carried out with the introduction of mangrove forest vegetation to the surface of setting tanks where fly ashes from hard coal combustion were stored. The species of the mangrove forest showed good growth and development at a dose of 10% ash mixed with soil [19].
In ecosystems, fly ash and vegetation cover act as protection against water and wind erosion by stabilizing the surface [20]. Phytostabilization reduces migration of the ash particles that may have an adverse effect on neighboring habitats due to the harsh properties of fly ashes. (i.e., alkalinity, salinity, ability to crust, and often content of potentially toxic elements and organic pollutants [21]). Establishment of vegetation promotes various processes of soil formation on ash wastes. Vegetation that develops on technogenic substrates has an impact on soil formation. Plant roots that overgrow the cemented ash layers loosen it and contribute to the movement of the ash elements deep into the soil profile [22]. The composition of plants’ communities affects the type of the humus formed gradually over time which determines the soil fertility. Deposition of humus substances may lead to acidification of the alkaline ash layer and change the reaction of the substrata and metal ion bioavailability [22]. However, successful revegetation of fly ash habitats requires appropriate technical reclamation treatments and application of suitable flora species well adapted to the soil texture. The most appropriate approach in the ecological restoration of degraded lands is the reinstatement of native vegetation and this management is the most effective when it involves natural processes of spontaneous plant colonization [23]. Therefore, the knowledge about substrate properties, plant species ecological requirements, soil–plant interactions and course of natural succession is necessary for the formation of sustainable plant communities in the process of revegetation of fly ash habitats.
The aim of the study was to determine the impact of high doses of fly ash from the hard coal combustion on long-term changes of soil properties and vegetation of fresh coniferous forest. This research will try to determine the usefulness of the fly ash as an ameliorating material in the forest environment.
2. Materials and Methods
2.1. Description of the Study Area
The experiment was established in the autumn of 1979 in the fresh mixed coniferous forest habitat, in the 463 Forest Division of Czułów (50°8′46.404″ N, 19°2′9.632″ E), Katowice Forest District, Upper Silesia, Poland on Podzols developed with sand and loamy sand on alluvial deposits. This area belongs to the Silesian Upland macroregion [24], and has an average annual temperature of 7.9 °C, an annual precipitation of 724 mm, and direction of dominant winds of SW, W to NW. Fly ash used in this study came from the IIIrd zone of electrostatic precipitators of the Tychy Power Plant, while hard coal was mined in the Silesia and Wesoła mines (Upper Silesia, Poland). The chemical composition of fly hard coal ash used in the experiment was as follows: pHH2O 10.3, CaCO3 4.05%, C 4.00%, N 0.026%, C/N 154, and CEC 52.58 cmol(+) kg−1 of soil. The ash also showed no elevated levels of radioactivity [7] and the ash was classified as Class F (low lime, <15%), and as the ash with a low content of calcium compounds according to ASTM [25]. The total area of the experiment was 1.5 ha, and it consisted of five plots whose location was determined after cutting the trees and removing trunks and litter. The scheme of the experiment is shown in Figure 1. The fly ash was introduced into the soil in doses 0, 250, 500, 1000 and 2000 t ha−1. The size of the plots ranged from 120 m2 (2000 t ha−1) to 720 m2 (0–1000 t ha−1). In the process of burying the ashes, a heavy forest plough was used, and the ash was mixed with the soil as little as possible. Soil profiles with ash applied are shown in Figure 2. In the soil profile, the ash formed inserts 10–40 cm thick × 60 cm long (250–1000 t ha−1) and 60 cm thick × 60 cm long (2000 t ha−1). The plot with the highest fly ash dose can be treated here as a mini dump. Gaps were left between the ash inserts to provide better conditions for the growth of roots of tree seedlings. One year after the experiment establishment, because of soil compaction, the gaps in the ash layers practically disappeared [4]. Eight tree species were planted in the ridges created by ploughing including: scots pine (Pinus sylvestris L.), black pine (P. nigra Am.), european larch (Larix decidua Mill.), silver birch (Betula pendula Roth.), pedunculate oak (Quercus robur L), red oak (Q. rubra L.), sycamore maple (Acer pseudoplatanus L.) and european beach (Fagus sylvatica L.). These species were evenly mixed and formed groups in which individual specimens were planted at a spacing of 1.5 m × 1.5 m. These species were recommended for the rebuild of forest stands located in the third industrial hazard zone [4]. During the first 14 years after planting the trees, no maintenance work was carried out. The experimental area was little protected from herbivores; only densely planted trees provided some protection from biting branches. After the 20 years of the start experiment, the first thinning was carried out, completely removing B. pendula. The further thinnings were made in subsequent years.
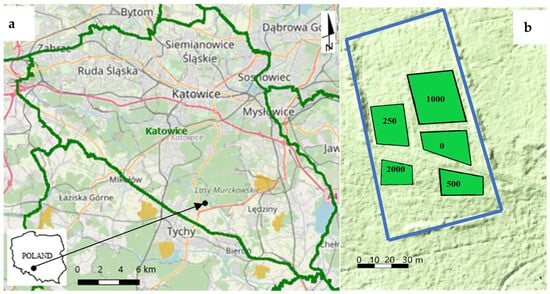
Figure 1.
The place of forest experiment and the lidar map fragment areas with differed fly ashes doses. Geoportal.gov.pl [26], (a)—fragment map with place of experiment, (b)—fragment of lidar map with experiment area,  —borders of entire areas, and
—borders of entire areas, and  —borders of areas with 0, 250, 500, 1000 and 2000 t ha−1 doses.
—borders of areas with 0, 250, 500, 1000 and 2000 t ha−1 doses.
 —borders of entire areas, and
—borders of entire areas, and  —borders of areas with 0, 250, 500, 1000 and 2000 t ha−1 doses.
—borders of areas with 0, 250, 500, 1000 and 2000 t ha−1 doses.

Figure 2.
Soil profiles with doses of fly ash (a) 250, (b) 500, (c) 1000 and (d) 2000 t ha−1.
2.2. Methods of Soil Analyses
In June 2022, fresh soil samples were collected in triplicate from genetic horizons from 0 to 40 cm. In addition, a sample was also taken from a depth of 0–80 cm for a dose of 2000 t ha−1. This was to observe changes in trophies below the fly ash level. The soil sampling sites in the plots were marked with stakes. The samples from horizons were air dried, crushed and sieved through a <1 mm mesh sieve. Laboratory analyses carried out in the fraction (<1 mm) included the following: particle-size distribution by sieving and the hydrometer method after soil dispersion with sodium hexametaphosphate, pH in 1 M KCl (the soil liquid ratio 1:2.5 v/v) potentiometrically, calcium carbonate (CaCO3 equal.) by volumetric Scheibler method, soil organic carbon (SOC) by Walkley-Black method, total nitrogen (N) by high-temperature catalytic combustion by a thermal conductivity detector (Vario MACROCube Elemental Analyse System GmbH, Germany), total acidity (TA) after sample extraction with 1 M sodium chloride, pH 8.2 by potentiometric titration, cation exchange capacity (CEC); the sum of cations (SC) including Ca+2, Mg+2, K+ and Na+ after sample extraction with 1 M (CH3COO)2Ca at pH 8.2 and (TA). The cations were measured in microwave plasma spectrophotometry (MP-AES 4200 Agilent Technologies, Santa Clara, CA, USA). It is a numerical indicator of soil trophies. P and K wereformed by the Egner–Riehm method [27] while Mg was formed by the Schachtschabel method [28]. The properties considered when calculating the (SIG) index are the fraction content < 0.02 mm, the sun of alkaline cations, hydrolytic acidity, and the C:N ratio [29].
2.3. Floristic Investigation
The occurrence of plant species and their abundance were recorded in each plot from an area of 100 m2 in June 2022. The species abundance was estimated using Braun-Blanquet’s method [30]. The names of the species were provided by the checklist of flowering plants and pteridophytes of Poland [31]. The tree canopy cover was estimated and expressed as a percentage. The height of the trees was measured with a laser rangefinder. The stem diameter was measured at breast height at dbh = 1.5 m. The obtained data were compared with the results from the same plots previously published in Bogacz et al. [8] in 1993.
2.4. Statistics
The results obtained were statistically verified using Statistica 13 [32]. Mean values for properties in horizons were statistically compared with each soil profile. Relationships between the selected parameters were expressed as a correlation coefficient r-Persona at a statistically significant level of p < 0.05.
3. Results
3.1. Properties of Soil
After 43 years from the start of the experiment, a layer of forest litter with a thickness of 7 to 9 cm was formed on the soil surface (Table 1). The horizons with fly ashes had a fine-grained structure and differed in soil texture. Horizons with dose’s 250–500 t ha−1 represented loamy sand or sandy loam texture. Higher doses of fly ash 1000–2000 t ha−1 added to the soil represented sandy loam or silt loam texture. The hard coal fly ash used for the experiment was characterized by a dominant silt fraction, with a small clay fraction, and was classified as silt loam [7]. Soil horizons without ashes had generally sand or sometimes loamy sand texture (Figure 3).

Table 1.
Some properties of fly ash and the soil horizons in the experiment.
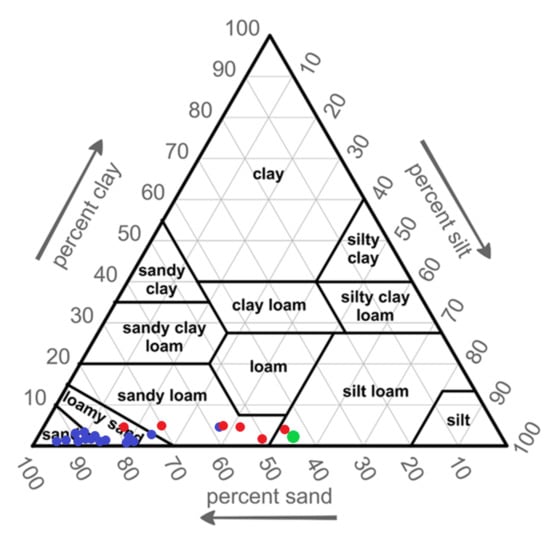
Figure 3.
Soil texture (USDA/FAO), • soil texture horizon without ash, • soil texture horizon in different doses of fly ash, • and texture of fly ash used in experiment.
Fly ash, recognized as a chemical reactive substance, with a minimum layer of 10 cm in the soil. This material according to the classification of anthropogenic material can transform Podzols and other units into Technosols [33,34]. Fly ashes are commonly characterized by a high content of easily leachable salts [31]. In the fly ash used for the experiment, a high content of easily soluble salt was found, corresponding to 5030 mg NaCl L−1, which allows it to be classified into the third salinity group according to FAO [35]. The salinity of the fly ash implicated into the Podzols, determined 11 years after the establishment of the experiment, was low even at the level of their deposition [6]. Soil horizons containing and without fly ash did not present elevated salinity content. For this reason, the measured salinity values are not included in Table 1. Acidification of the soils was observed in comparison to the research carried out in 1990 [7]. The main factor acidifying the soil horizons, over the years, was the development of significant thickness of organic horizons Ol, Of, Oh. The plant material contained in the litter are mainly the remains of coniferous vegetation. The reaction of the litter horizons, developing during the experiment was strongly acidic, and in case of a dose of 2000 t ha−1, the litter was acidic (Table 1). This situation also takes place on a surface with the highest dose of fly ashes applied. The fly ashes levels in 2022 were presently acidic at a dose of 250 t ha−1, slightly acidic at a dose of 500 t ha−1. Horizon, enriched with a dose of fly ash in the amount of 1000 and 2000 t ha−1, still maintained a neutral or alkaline reaction. In the soil through the experimental area, a generally continuous and progressive process of soil acidification was observed (Table 1). The consequence of soil acidification was also the eluviation of CaCO3 from the soil at the doses of 250 and 500 t ha−1. At doses of 1000 and 2000 t ha−1, there was a depletion of calcium carbonate in the soil compared to the research carried out over 30 years ago. The effect of this phenomenon was the alkalization of the levels lying under the fly ashes, especially at doses of 1000 and 2000 t ha−1 (Table 1). The soil horizons enriched with fly ash showed a higher content of organic carbon than other mineral horizons. This issue persists in the described forest soil. It is particularly visible, especially at fly ash doses of 500 t ha−1 or higher.
The fly ash used in the experiment practically did not contain nitrogen (Table 1). In most of the described soil horizons, especially those with ash, the C/N ratio clearly decreased. The exception here is the level of ash at the dose of 250 t ha−1. Compared to the year 1990, the content of the forms of phosphorus, magnesium, and potassium in the mineral horizon of the described soils has clearly decreased by time. The exception are some levels with ashes in which these macronutrients have accumulated. In organic horizons—litters, the contents of available P, K, Mg were the highest in comparison with mineral horizons (Table 1). The highest values among the tested macronutrients were found in organic levels at a fly ash dose of 2000 t ha−1 (Table 1).
In soil horizons with the use of ash, a decrease in (CEC) has been observed in most of the profiles over the last 30 years. The highest values of the described soil sorption indices reached the litter horizons (Table 2). The calculated soil habitat index (SIG) for Podzols without the addition of fly ash classified it as typical for fresh mixed coniferous forest habitats. The value (SIG) here was 21. The addition of fly ash in doses of 250, 500, 1000 and 2000 t ha−1 resulted in a significant increase in the value of this indicator. For various doses of fly ash, the values of this index ranged from 25 to 31. The Technosols with the addition of fly ash and index values ranging from 24 to 33 created potential conditions for the development of mixed forest.

Table 2.
Sorption properties of the soil horizons in experiment.
3.2. Vegetation Characteristic
The composition of plant communities developed in experimental plots after 43 years from the ash application and some plant characteristics were presented in Table 3. The highest total number of 12 species was found at the ash dose of 250 t ha−1 and the lowest of 7 species at the dose of 500 t ha−1 including 5 and 1 tree species, respectively. The tree canopy cover was low for the majority of plots except for those with ash doses of 250 and 2000 t ha−1 where the cover was ≥80%.

Table 3.
Abundance of species and some of their characteristics found on experimental plots treated with different doses of fly ash from burning coal after 43 years from the ash application. The abundance is expressed according to Braun-Blanquet’s cover-abundance scale: +< 5%, few individuals; 1—<5%, numerous individuals; 2–5—25%; 3–25—50%; 4–50—75%; 5–75—100%.
The composition of plant communities differed only slightly at the ash doses of 250–1000 t ha−1 in comparison to the control plot (0 t ha−1) but was significantly different at 2000 t ha−1. Of the eight tree species that were planted at the setting up of the experiment, Betula pendula, Acer pseudoplatanus and Quercus rubra did not persist. B. pendula was intentionally cut down from the experimental area by foresters. On plots with lower ash doses, i.e., from 0 to 1000 t ha−1, the most frequent and abundant tree species were Pinus nigra, P. silvestris and Larix decidua and they showed no signs of stunting. Only at the doses of 2000 t ha−1 small leaved-lime (Tilia cordata) occurred and dominated and this phenomenon set this plot apart from the rest. Individuals of T. cordata had the lowest mean high and dbh value among all the trees from this experiment but they were distinguished by the biggest density of their canopy. The Speciemens of T. cordata were not introduced, so they probably came from self-seeding. Among the planted deciduous tree species, only F. sylvatica and Q. rubra survived at a dose of 250 t ha−1. On all plots, the shrub layer was very poorly developed and consisted of individual specimens of Sorbus aucuparia L. which occurred in the control plot and at the ash dose of 250 t ha−1, as well as of T. cordata which occurred at the dose of 2000 t ha−1. The herb layer was generally sparse, especially at plots with the dense tree canopy cover where elevated shading limited growth of herbaceous plants. The most frequent species in the herb layer was Lysimachia europea (L.) U.Manns and Anderb. which occurred at all plots whereas the most abundant was Pteridium aquilinum (L.) Kuhn. Both of these plants along with frequent Vaccinium myrtillus L. are species characteristic for coniferous and mixed forests (Vaccinio-Picetea class) and they were the most numerous at lower ash doses. Species from the deciduous forest (Querco-Fagetea class) were also present in the herb layer and consisted of Brachypodium sylvaticum (Huds.) Beauv. at ash doses from 500 to 2000 t ha−1 and Stachys sylvatica L. as well as Poa nemoralis L. which were recorded sporadically at different plots. At lower ash doses, Calamagrostis epigejos (L.) Roth was present which is characteristic of clearing communities (Epilobietea angustifolii class) but it was not abundant. Generally, in the herbaceous layer, the species from coniferous, mixed and deciduous forest communities dominated and accounted for 78% of the species composition. The remaining species were from synanthropic (segetal and ruderal) as well as clearing communities with an equal share of 11%. In the moss layer, Pleurozium schreberi (Brid.) Mitt. was dominant and together with Leucobryum glaucum (Hedw.) Angstr. and Polytrichum commune Hedw. they represented the coniferous forest flora.
In comparison to the data obtained after 14 years from the ash application and presented in Bogacz et al. [7], the total number of species decreased by about half in all plots until 2022. The vast majority of the deciduous trees did not persist in communities but with the one exception of T. cordata which significantly increased quantity and occurred only at an ash dose of 2000 t ha−1. At the first date of observation, P. sylvestris, P. nigra, L. decidua and B. pendula dominated in the stands, but the birches were cut down in the following years. Those tree species that did not survive to the second date of observation were planted ones, i.e., Q. robur and A. pseudoplatanus, as well as those that spontaneously colonized experimental plots, i.e., Q. petraea, Populus tremula and Salix cinerea. The tree canopy cover decreased on plots with the ash dose of 500 and 1000 t ha−1 along with the significant loss of deciduous trees as well as the large decline in the cover of coniferous at the ash dose of 1000 t ha−1. Average tree height and dbh values increased at all plots. Species composition of the herbaceous layer accounted for the data from 1993 and represented various vegetation categories, and was as follows: plants from coniferous, mixed, and deciduous forests 37%, synanthropic (segetal and ruderal) vegetation 30%, meadow plants 22%, and species of clearing communities 11%. At the dose, of 2000 t ha−1, calcicole (calciphilous) species were relatively common (38%) in the community composition. Until 2022, all meadow plants and the vast majority of synanthropics as well as clearing community species left from all phytocoenoses. All calcicole species did not survive, and vascular plants with a calcifuge (acidophilous) strategy increased their quantity especially at the lower ash doses. Similarly, the abundance of mosses characteristic of coniferous forests communities increased during succession.
4. Discussion
4.1. Fly Ash and Soil Particles
Some of the fly ash particles are almost completely spherical. Spherical fly ash particles make up 10% of the total coal ash residue. Particle size ranges from 0.005 to 1 mm [36]. The shape of the ash grains is influenced by the temperature of coal processing in the furnace [37] and the controlled oxygen supply [38]. The form of the fly ash particles plays a significant role in their reactivity [39]. The particle size analysis of the fly ash from black coal combustion clearly indicates a high concentration of silt size particles (>60%), followed by sand (>25%) and clay (5%) [40]. The general classification of fly ash in soil texture is silty sand or sandy silt [41]. In the collections of some authors, mineral material mix with fly ash has reached a texture corresponding to sandy loam and silty loam [42]. The application of fly ash into the soil by means of deep ploughing largely limited its mixing with the sandy horizons. After 43 years from the start of the experiment, the development of plants roots may also have contributed to the mixing of fly ash with the soil horizons. In the levels where fly ashes were used, soil texture depends on the applied doses. Based on the increasing amount of silt fraction in the newly created levels in the Czułów experiment, they can be ranked as follows: loamy sand—sandy loam—silt loam (Figure 3).
4.2. Soil pH
The pH of coal fly ash can vary from acidic to alkaline. This situation depends on the Ca/S content of the parent coal. Fly ashe can be classified into acidic ash (pH 1.2 up to 7), mildly alkaline ash (pH 8–9) and strongly alkaline ash (pH 11–13) [43]. In addition, use of fly ash instead of lime can reduce CO2 emission and global worming [44]. Fly ash material can be used for buffering of the soil pH. Alkalic fly ash was used as being chemically equivalent to approximately 20% reagent grade CaCO3 [45]. These alkaline ashes raised the soil pH by 2 and 2.3 units with the addition of 36 and 108 t ha−1, respectively [3]. In the early years of the Czułów experiment, the surface and subsurface levels were alkalized. In an experiment presented in this work in the development of litter, this trend reversed. The ash levels become more acidic and the calcium compounds moving deeper alkalized the deeper levels. The acidification of the soil of an environment clearly progresses with the depth of the examined profiles (r = 0.62, p < 0.05, n = 21) (Table 4). This phenomenon is particularly visible in ash in differed doses (Figure 1). A similar phenomenon, with much smaller doses of fly ash to the Podzols in the province of Ontario, was observed by [46]. In this experiment, most of the soil horizons changed from strongly acidic to slightly acidic or alkaline (Figure 4). The reaction of the litter horizons was strongly acidic or acidic, and showed a tendency to increase the applied dose of fly ashes. The increase in the pH value may also be the result of a different species composition of the remains preserved in a litter. In the boreal forest with Norway spruce and Scots pine experiment, a renewed increase in acidification on surface levels was found. This phenomenon was observed 14 years after the application of ash to the soil [47]. This author points to the passage of time as a factor in the growth of litter acidity.

Table 4.
Values of r Pearson correlation coefficient for soil characteristic.
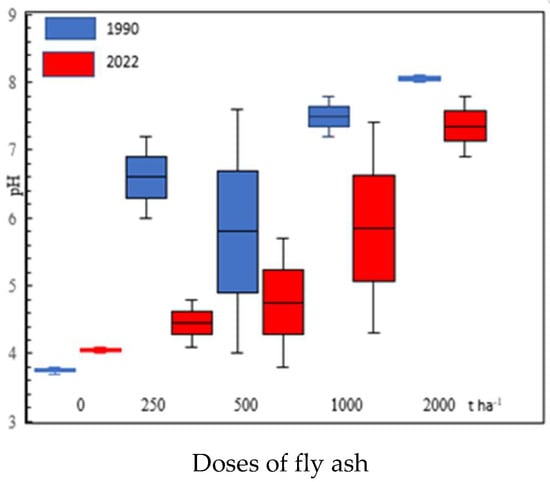
Figure 4.
pH value in surface horizons of soils (0–40 cm) using different doses of fly ash. Explanation: central line—mean values; boxes—mean values; and + standard errors whiskers—mean values.
4.3. C and N
Fly ashes resulting from the combustion of hard coal contain a trace amount of nitrogen [48]. Fly ash is devoiced of humus and N, which is attributed to the oxidation of C and N during coal combustion. The relatively low content of these macronutrients in the fly ash is supplemented by mixing the fly ash with many organic materials [3] or by using additional nitrogen fertilization [49]. Research by Cools et al. [50] showed that the C/N relations in the forests of Northern Europe are strongly associated with the presence of specific tree species. The addition of alkali fly ashes from coal combustion to the soil usually leads to an increase in the value of the C/N ratio [51]. Over time, this relationship changes in the soil, showing a downward trend (Table 1). An increase in the content of both components in the soil clearly reduces the C/N ratio [52]. Some authors using ashes in forestry indicate a stimulating role of fly ash in the development of soil microorganisms [53] and soil mesofauna [54], which decompose organic matter. The CO2 formed in the life processes of the soil is absorbed by the ash. The use of fly ashes in environmentally friendly doses also contributes to the development of nitrification, mineralization, and absorption of NOx forms in the soil [55]. More and more studies on the roots of plants living in a mixture of soil and fly ash, indicate the occurrence of the phenomenon of mycorrhiza [56], leading to soil enrichment with nitrogen. An example of such forest vegetation is Willow sp. [57].
Long-term observations of the effect of introducing fly ashes into the soil, generally indicates an increase in the total carbon content and then decrease—especially in the horizons with ash. Permeability of the soil material, supported by the movement of weak organic acids, formed in the process of transformation of the litter material, contributed to the leaching of carbon into the soil [58]. Such tendencies were probably influenced by the developing, strongly acidic litter level [59]. Soil with very high doses of ash (1000–2000 t ha−1), and an increase in N content in ash horizons was observed over a long period of time. In the remaining cases of the profiles (0, 250, 500 t ha−1), the nitrogen content was very low (Table 1). The exception here are litter levels in which the amount of nitrogen exceeds 1% d.m. of soil. Currently, the content of nitrogen and carbon in the forest soil experiment of the Czułów Forest District shows a downward trend with increasing depth in the profiles (r = −0.53, p < 0.05, n = 21) (r = −0.45, p < 0.05, n = 21) (Table 4).
4.4. P, K, Mg
Phosphorus (P) is a critical nutrition element in the early stages of plant growth. This macronutrient has a relatively high total concentration in fly ash. Many studies have suggested that P in fly ash may not be available to plants [60]. Phosphorus is largely fixed with Fe and Al; this conglomerate is generally unavailable to plants [61]. An increase in pH with high Ca in ashes may cause precipitation of applied P fertilizer and suppress phosphate solubility. In acid soil, applied soluble phosphate fertilizers become fixed through adsorption and precipitation [62]. Soil with a buffering capacity is expected to absorb a lot of phosphorus [63]. When acidic sandy soil is mixed with alkaline fly ashes added from the outside, the solubility of P as a fertilizer changes markedly. The availability of phosphorus for plants probably increases with the neutralization of acidic soils after the application of an appropriate dose of fly ash [64]. P compounds in soil are mainly sorbet clay minerals. After adding fly ash silicate to the soil, they change the availability of phosphorus and reduce its sorption through the soil [65]. Sorption of phosphorus in soil therefore depends on the degree of mixing fly ash with soil [63].
The fly ash used in the experiment strongly enriched the surface horizons with phosphorus. Over the years since its uses, the P content has decreased significantly (Table 1). Enriching the soil with phosphorus by introducing it with big doses of fly ash is associated with the presence of carbon, nitrogen, and magnesium in the soil (Table 1).
Potassium (K) is one of the most abundant elements in the earth’s crust [66]. K is an important yield factor in many field experiments using fly ashes from hard coal combustion [67]. A significant amount of potassium is absorbed in the soil by clay minerals—(illite-smectite). The addition of fly ash to alkaline soils has not yet resulted in an increase in yield [68]. Potassium in fly ash is apparently not as available as fertilizer; there is a possibility that the higher amount of Ca and Mg in the fly ash interfered with K absorption in the plant [69]. The increase in the content of exchangeable K in the soil is generally associated with a decrease in the content of sodium after the use of increasing doses of ash from the combustion of hard coal [70]. The content of available K was moderate in very light and medium soil and low in light soil [71]. In fly ashes, potassium may also occur in the form of chlorides and sulphates [72]. Potassium silicate produced as a fertilizer from burnt coal is not soluble in water but is soluble in weak acids [73].
K introduced into the soil with fly ash increased its content, especially at the dose of 2000 t ha−1. Due to the high mobility of potassium in the soil, its concentration has now decreased (Table 1). High content of K in horizons with introduced fly ash in drainage doses > 250 t ha−1 are strongly correlated to the amount of Mg accumulated in the soil (r = 0.97, p < 0.05, n = 21). This may indicate a similar leaching rate of these microelements from the soil (Table 4).
Magnesium (Mg) is accumulated in significant amounts in fly ashes, often in nodule forms. Generally, Polish soils are poor in magnesium. This is related to the large share of light and very light soils in the total pool of soil in Poland [74]. After fly ash application (5–10%) of soil w/w an insert of magnesium was observed [75]. In the fly ash, magnesium may be present in the form of iron-bound minerals, oxides of Mg, associated with quartz, Mg-Ca complexes [76], or hydrated Mg-carbonate [77]. In the black coal combustion process in some technologies used, dolomite is added, which may remain in the fly ash [78,79].
Mg in the fly ash experiment showed a tendency to accumulate in the surface soil horizons (Table 1). Currently, leaching of the macronutrient from the soil is observed. This phenomenon was caused by the presence of Olfh horizons which, according to Sokołowski et al. [23], participate in the water and weak acids leaching of the component.
4.5. Cation Exchange Capacity (CEC)
CEC value according to particle size distribution and type of clay minerals and presence of organic matter [80]. CEC is used in forest soil as an indicator of fertility. The addition of lime to the soil results in a reactivity stabilization and also the formation of an entryway for soil’s CEC. Grinding of clay increases the CEC because of the increase in the specific surface. Depending on the source of fly ash, such amendments could also provide cation exchange capacity, CEC [81]. Ion Cl− in solutions act as inhibitors of the exchange reaction—“salt effect” [82]. The CEC of fly ashes from Macedonia was 19 to 28 cmol (+) kg−1 of this component. In soil experiments, the CEC decreased with the increase in fly ash and this took place [83] in sandy clay and low plasticity clay. The decrease in CEC values can be explained by the mineralogical changes occurring in the treated soil due to formation of coarser particles with lime treatment which reduce the specific surface area [83]. An important factor reducing the CEC value is also the decrease in the strength of the soil. Similarly, the optimum fly ash content for sand clay and low plasticity clay is 20 and 25%, respectively. The maximum CEC of soil was the dose of 100 g of fly ash on one quadrat meter of soil [80]. The addition of fly ash to light soil from the region of Australia increased the CEC of the sand-ash mixture three times compared to sand alone [83]. In another experiment, an increase in CEC occurred after adding 5% fly ash to sandy soil with additional NPK fertilization [84].
The sorption rates of fly-ash-enriched levels were clearly higher than those of the original Podzols (Table 2). Years after the start of the experiment, this parameter in ash-enriched horizons are still high in doses of 250 and 500 t ha−1 (Table 2). In doses of 1000 and 2000 t ha−1, the CEC parameter decreased. A few described macronutrients (P, K, Mg, C and N) are strongly associated with high CEC values (Table 2).
4.6. Vegetation
The use of fly ash in high doses combined with technical treatments and biological processes resulted in afforestation of the experimental plots. We observed progressive growth and development of some planted tree species as well as the establishment of understory vegetation and T. cordata stand in the process of spontaneous colonization. In plots where the ash doses ranged from 250 to 1000 t ha−1, planted coniferous species L. decidua, P. nigra and P. sylvestris survived and dominated in the communities. According to values of Ellenberg’s ecological indicators [85], L. decidua and P. sylvestris have high light (L) requirements, a wide range of tolerance to soil reaction (R) and they accept low nitrogen (N) levels (L8, Rx, N3; L7, Rx, Nx, respectively). Similarly, P. nigra is a light-demanding plant that prefers neutral or alkaline soils and can live at low nitrogen content (L7, R9, N2). These characteristics suggest that the tolerance of these species to the elevated soil reaction (which became alkalized in the top horizons at the start of our experiment and then alkalized deep into the soil profiles) as well as their resilience to the low nitrogen value made these species able to cope with the fly ash habitat conditions. Moreover, their high light requirements allow them to colonize bare substrates and their investment of energy in fast growth enables them to compete with herbaceous plants [86].
Among the deciduous trees planted at the start of the experiment, A. pseudoplatanus and Q. robur did not persist, and F. sylvatica as well as Q. rubra were noted only occasionally at the smallest ash dose. These late-successional broadleaved trees which are members of the stress-tolerant competitor group [87], grow slowly initially and invest a lot of energy in the development of root systems and the protection of tissues against herbivores, but these plants can increase their growth rate in the subsequent life phases and play the role of climax species [86]. The late-successional broadleaved trees have much higher soil requirements than conifers used in our experiment, and they need deep soils with a covering of tree litter rich in nutrients (i.e., Fagus sylvatica N7), and acidic rather than alkaline substrata, especially in the seedling and sapling stage [87]. For these reasons, the fly ash habitat could not provide for them appropriate conditions for growth in the initial phase of our experiment, after removing the previous vegetation, humus layer, and applying the ash. The Swedish research [88] suggests that decreased survival of planted oak seedlings may be a result of interspecific competition for light with seedlings of other trees, like B. pendula which is characterized by fast initial growth in clear-cuts. Birch and pine trees, as pioneer species, have low trophy requirements (B. pendula N4) and may gain a competitive advantage over more demanding species in habitats poor in nutrients. As was shown in our study on the first date of observation [8], the abundance of B. pendula and conifers significantly exceeded the share of late-successional trees, which did not reach any significant quantity even after cutting down birches. Another reason that planted deciduous trees did not persist or reach very small abundance could be their seedlings endangered to browsing and damage to shoots by large herbivores. It was shown [89] that seedlings of Q. robur and other eight broadleaved tree species with F. sylvatica and T. cordata were much more frequently browsing in winter than a control representative of coniferous, i.e., Norway spruce. Among planted trees, Q. rubra is an alien species in Europe which was cultivated for its valuable wood. Currently, red oak is considered an invasive or potentially invasive species in many European countries. Its invasive possibilities depend on local factors [90,91] and has not been revealed in conditions of our experiment.
Along with the development of the tree stands, spontaneous colonization led to the formation of herbaceous and moss layers that enabled stabilization of the substrate with the fly ash deposits and protection against erosion, which is the essential goal of the ash landfills reclamation. The species composition of herbaceous communities was changed during our observations and ruderal, segetal as well as meadow plants did not persist whereas species of deciduous, mixed, and especially coniferous forest significantly increased their numerous, high share of ruderals, segetal and meadow plants in the early stages of natural colonization of fly ash deposits. This was also noted in another experiments in Poland [92,93]. In our study, the transformation of herbaceous vegetation occurred likely in response to the development of the tree stands and the increase in acidification in the horizontal levels of soils. As a result of increased shading in the forest ground layers, the light-demanding species of open environments were replaced by the shade-tolerant forest plants. Moreover, the increase in acidification that was noted in the upper soil levels, i.e., in the herb rooting zones, led to the disappearance of calcicoles and the predominance of calcifuge and neutral species. As was shown [94,95], soil pH affects the solubility and plant availability of essential nutrients, particularly P, Fe, Zn and Mn as well as potentially toxic Al. The relationship between the soil pH, nutrients availabilities and soil physical properties is recognized as a primary driver of plant community composition [96,97].
In our study, an interesting phenomenon is the formation of a high dense stand of T. cordata trees at a fly ash dose of 2000 t ha−1 in the process of natural colonization. The intensive development of lime trees began at the seral succession stage with B. pendula predominance and a well-developed herbaceous layer with calcicole species. At this stage, the humus layer with accumulated nutrients was already formed on the highest fly ash deposit. According to [86,98], lime prefers rich soils (N5) with neutral or alkaline pH (R6) and higher calcium content. This species is also shade tolerant (L5), and more tolerant than Q. petrea and B. pendula. This probably enabled the lime to establish and grow under the canopies of other tree species. T. cordata was classified as a competitor and such plants are able to overgrow and outcompete any other tree species under conditions of high resource availability [87]. Moreover, lime trees are characterized by a high capacity for vegetative reproduction, and the production of sprouts after cutting allows them to gain numerical advantage over other species [99]. The life strategy of T. cordata called ‘waiting’ and ‘persisting’ [100] with a set of other ecological adaptations enabled this species to achieve success in the process afforestation in fly-ash-rich habitat conditions. Formation of T. cordata stand in the process of ecological succession led to the creation of a different habitat in the fresh coniferous forest ecosystem that contributes to the increase in local biodiversity. Such habitats can harbor various plants and animals, and sometimes they become refuges for rare species even without any active restoration techniques [101]. Promoting of ecological processes during fly ash land revegetation would be likely to result in formation of diverse and productive ecosystems that can provide a variety of services.
4.7. Habitat Soil Index (SIG)
The habitat soil index (SIG) is a method of quantitative valorization of soil for the purpose of forest management, including planning the species composition of stands on forest habitats or non-forest land [29]. The SIG values are intended to be resistant to vegetation-modifying habitats. This indicator also reflects the potential fertility of soil. The plant structure modifies the surface layer of the soil the most. In the long term, habitat modification by plants is likely. In a short period of time, a few or several years, the SIG index may change by 1–2 units [102]. Forest habitats were drastically changed by fly ashes added to the soil. In the case of the presented work, the value of the indicator (SIG) increased by more than 10 points. Due to the values of the index (SIG), soils with introduced fly ash already represented abundant forest habitats.
4.8. Soil Definition
The described soils, due to the presence of fly ash, a highly reactive material in a soil environment [103], can be classified as a type of Technogenic soils, subtype of Industrosols or Turbisols in the varieties: carbonate and disturbed. Their soil often has a deep incorporation of fly ashes in the morphological forms of layer or lens. The most frequently written sentences of the described soil are O-A-C(a)-C or O-A(a)-C(a)-C [35]. The presence of fly ash in the soil with a layer depth > 10 cm entitles us to quality the described soil to the Technosols reference group. The main qualifiers that can be used in this case are: Urbic and Isolatic. From the groups of complementary qualifiers, the following were selected: Alcalic, Siltic, Calcaric, Thapto Arenic [36].
5. Conclusions
After the application of doses of fly ash from coal combustion in the forest experiment, the amount of silt fraction in the newly created levels of soil, has increased. The new levels of soils can be classified as loamy sand—sandy loam—silt loam. Alkalization of the soil was observed during the earlier phase of the experiment. Over time, they were found to be acidified in the surface horizons. Deeper horizons continued to be alkaline. Carbon and nitrogen content increase after fly ash application, only to decrease over time. The exception to this is the organic litter horizons. Over time, nutrient (P, K, Mg) abundance decreased except for the 2000 t ha−1 application rate.
The (SIG) index experienced a significant increase, even at the lowest fly ash dose of 250 t ha−1. Sandy forest acidic habitats with no fly ash application were classified as coniferous forests, and others were already as numerous as mixed forest habitats. The presence of fly ash in the soil, classifies it as a Technosols according to the FAO WRB reference group.
The applied doses of fly ash and technical treatments caused differences in reforestation efficiency and led to the development of diverse tree stands. At the lower ash doses (250–1000 t−1) the stands of planted coniferous Pinus nigra, P. silvestris and Larix decidua are established. The saplings of these species are fast-growing, tolerant to the elevated soil reaction and resilient to the low nitrogen levels. The planted late-successional deciduous species did not persist, probably due to interspecific competition in the fly ash habitat conditions in the initial stages of this experiment. At the highest ash dose (2000 t ha−1), a dense Tilia cordata stand developed as a result of natural processes in seral stages of spontaneous succession. Our results demonstrated the importance of application of plant species well-adapted to particular conditions, as well as the role of natural processes of spontaneous succession in the creation of stable vegetation cover in the management of the fly ash in forest habitats.
Author Contributions
Conceptualization, A.B. and D.K.; methodology, A.B. and D.K.; formal analysis, A.B. and D.K.; investigation, A.B., D.K., P.T. and A.D.; resources, D.K., P.T. and A.D.; data curation, A.B. and D.K.; writing—original draft preparation, A.B. and D.K.; writing—review and editing, A.B. and D.K.; visualization, A.B. and D.K.; funding acquisition, A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financed by the statute project: 0-1D010/0040/23 of Wroclaw University of Environmental and Life Science, Institute of Soil Science, Plant Nutrition and Environmental Protection from a subsidy of the Ministry of Education and Science of Poland.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting reported results is available from the corresponding author upon request.
Acknowledgments
The authors highly appreciated the assistance and technical support received from Martyna Uściła, Szymon Jędrzejewski, Jarosław Szadorski, Institute of Soil Science, Plant Nutrition and Environmental Protection, Wroclaw University of Environmental and Life Science.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in a decision to publish the results.
References
- Boyanov, V. BP Statistical Review of World Energy. Econ. Policy 2011, 4, 38–55. [Google Scholar]
- Yunusa, I.; Loganathan, P.; Nissanka, S.; Manohaarn, V.; Burchett, M.D.; Skilbeck, C.; Eamus, D. Application of Coal Fly Ash in Agriculture: A Strategic, Perspective. Crit. Revives Environ. Sci. Technol. 2012, 42, 559–600. [Google Scholar] [CrossRef]
- Tsadilas, C.D. Agricultural use of fly ash: Expected benefits and consequences. In Proceedings of the International Workshop on Agricultural Coal Ash Used, WACAU, Bet Dagan, Israel, 24–29 May 2014. [Google Scholar]
- Ram, I.C.; Masto, R.F. An appraisal of the potential use of fly ash for reclaiming coal main spoil. Environ. Manag. 2010, 91, 603–617. [Google Scholar]
- Kuśmierczyk, S. Determination of Physical and Chemical Changes in Soil Properties under the Influence of Melioration Doses of Hard Coal Ash; Report on the First Phase of the Project; Geoportal: Gliwice, Poland, 1980; pp. 5–25. (In Polish) [Google Scholar]
- Giedrojć, B.; Wilczyński, A. Characteristics of physical properties of forest podzolic soil fertilized with high rates of ash from power station. Soil Sci. Ann. 1985, 36, 123–131. (In Polish) [Google Scholar]
- Bogacz, A. Impact of Hard Coal Ashes on Some Properties of Sandy Soil Formed in a Pine Habitat. Ph.D. Thesis, Institute of Soil Science and Agriculture Environmental Protection, Agriculture University of Wrocław, Wrocław, Poland, 1995; pp. 1–95. (In Polish). [Google Scholar]
- Bogacz, A.; Zabawski, J.; Licznar, M. Influence of Amelioration Doses of Ash from Coal on Vegetative Cover of Soil-Fresh Coniferous Sites. Sylwan 1997, 1, 85–92. (In Polish) [Google Scholar]
- Weber, J.; Strączyńska, S.; Kocowicz, A.; Gilewska, M.; Bogacz, A.; Gwiżdż, M.; Dębicka, M. Properties of soil material de-rived from fly ash 11 years after revegetation of post mining excavation. Catena 2017, 148, 35–39. [Google Scholar] [CrossRef]
- Kaczmarek, Z.; Mocek-Płóciniak, A.; Gajewski, P.; Mendyk, Ł.; Bocianowski, J. Physical and soil water properties of technosols developed from lignite fly ash. Arch. Environ. Prot. 2021, 47, 95–102. [Google Scholar]
- Jayaringhe, G.Y.; Tokashiki, Y.; Kiryo, K. Recycling of coal fly ash-based synthetic aggregates as a soil ameliorant for a low productive acid red soil. Water Air Soil Pollut. 2009, 204, 29–41. [Google Scholar] [CrossRef]
- Tsadilas, C.D.; Shaheen, S.M.; Samoras, V.; Gizos, D. Influence of Fly Ash Application on Copper and Zinc Sorption by Acidic Soils Amended with Sewage Sludge. Commun. Soil Sci. Plant Annu. 2009, 40, 273–284. [Google Scholar] [CrossRef]
- Matsumo, S.; Hamanaka, A.; Muracami, K.; Shimada, H.; Sasaoka, T. Securing Topsoil for Rehabilitation Using Fly Ash in Open-cost coal Mines: Effects of fly Ash on Plant Growth. J. Pol. Miner. Eng. Soc. 2019, 43, 13–18. [Google Scholar]
- Klose, S.; Makeschin, F. Chemical properties of forest soil along a fly ash deposition gradient in eastern Germany. Eur. J. For. Res. 2004, 123, 3–11. [Google Scholar] [CrossRef]
- Pandey, V.C.; Bajpai, O.; Singh, N. Plant regeneration potential in fly ash ecosystem. Urban For. Urban Green. 2016, 15, 40–44. [Google Scholar] [CrossRef]
- Vincevica-Gaile, Z.; Stoncevica, K.; Irtiseva, K.; Shiskin, A.; Obuka, V.; Celma, S.; Ozolins, J.; Klavius, M. Granulation of fly ash and biochar with organic lake sediments—A way to sustainable utilization of waste from bioenergy production. Biomass Bioenergy 2019, 125, 23–33. [Google Scholar] [CrossRef]
- Oliveira, V.; Reis, M. Valorisation of pulp and paper industry residues trough composting. In Proceedings of the 12th International Multidisciplinary Scientific Geoconference 2012, SGEM, Albena, Bulgaria, 17–23 June 2012; Volume IV, pp. 813–820. [Google Scholar]
- Kumar, V.; Jha, G.K. Use of fly ash in agriculture: Indian scenario. In Proceedings of the International Workshop and Agricultural Coal Ash Use, Bet Dagan, Israel, 24–29 May 2014. [Google Scholar]
- Mahmood, H.; Saha, C.; Hossain, M.S.; Gahman, M.T. Does coal fly ash influence the growth of mangroves? Environ. Chall. 2022, 8, 100201. [Google Scholar] [CrossRef]
- Pavlović, P.; Mitrović, M.; Djurdjević, L. An ecophysiological study of plants growing on the fly ash deposits from the “Nikola Tesla-A” thermal power station in Serbia. Environ. Manag. 2004, 33, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Necrasova, O.; Radchenko, T.; Filimonova, E.; Lucina, N.; Glazurina, M.; Dergacheva, M.; Uchaev, A.; Betekhtina, A. Natural forestry colonisation and soil formation on ash dump in southern taiga. Folia Forest. Pol. Ser. A—For. 2020, 62, 306–316. [Google Scholar] [CrossRef]
- Yadav, S.; Pandey, V.C.; Kumar, M.; Singh, L. Plant diversity and ecological potential of naturally colonizing vegetation for ecorestoration of fly ash disposal area. Ecol. Eng. 2022, 176, 106533. [Google Scholar] [CrossRef]
- Sokołowski, A.W.; Kliczkowska, A.; Grzyb, M. Determination of phytosociological units falling within the range of forest site types. Pr. Inst. Badaw. Leśnictwa Ser. B 1997, 32, 1–55. (In Polish) [Google Scholar]
- Kondracki, J. Physical Geography of Poland. Physical-Geographical Mesoregions; Wydawnictwo Naukowe PWN: Warszawa, Poland, 1994; p. 339. (In Polish) [Google Scholar]
- ASTM C618; Standard Specification for Coal Fly Ash and Row or Calcined Natural Pozzolan for Use as a Mineral Admixture in Portland Cement Concentrate. Annual Book of ASTM Standards: Philadelphia, PA, USA, 1994; Volume 04.02.
- Geoportal Infrastruktury Informacji Przestrzennej. 2023. Available online: https://www.geoportal.gov.pl (accessed on 1 January 2024). (In Polish)
- Vucans, R.; Lipenite, I.; Livmanis, J. Comparison of methods determination of phosphorus in carbonation soil. Litvian J. Agron. 2008, 11, 229–304. [Google Scholar]
- Stangaitis, G.; Rutkansciene, R. Comparison of magnesium determination methods as influenced by soil properties. Zemdirbyste 2010, 97, 105–116. [Google Scholar]
- Brożek, S. Soil quality numerical valorisation—A tool in forest site diagnoses. Sylwan 2007, CLI, 35–42. (In Polish) [Google Scholar]
- Braun-Blanquet, J. Planzensoziologie; Springer: Vienna, Austria, 1951. [Google Scholar]
- Mirek, Z.; Piękoś-Mirkowa, H.; Zając, M. Flowering Plants and Pteridophytes of Poland. A Checklist, Biodiversity of Poland; Polish Academy of Sciences: Kraków, Poland, 2002. [Google Scholar]
- STATISTICA (Data Analysis Software System), version 13.1; Stat Soft Inc.: Tulsa, OK, USA, 2023.
- Kabała, C.; Charzyński, P.; Chodoroski, J.; Drewnik, M.; Glina, B.; Greinert, A.; Hulisz, P.; Jankowski, M.; Jonczak, J.; Łabaz, B.; et al. Polish Soil Classfication—Principles, classification scheme and correlations. Soil Sci. Ann. 2019, 70, 71–97. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources. In International Soil Classification System for Naming Soil and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Science (IUSS): Vienna, Austria, 2022. [Google Scholar]
- Kim, H.; Purev, O.; Choi, N.; Lee, J.; Yoon, S. Removal of Inorganic Salts in Municipal Solid Waste Incineration Fly Ash Using a Washing Ejector, and Its Application for CO2 Capture. Int. J. Environ. Res. Public Health 2022, 19, 2306. [Google Scholar] [CrossRef] [PubMed]
- FAO. Geographic Information Systems in Sustainable Development; FAO: Rome, Italy, 1998. [Google Scholar]
- Jagadeeson, D.; Manssor, U.; Mandel, P.; Sudaresan, A.; Eswar Moorthy, M. Hollow Spheres to Nanocaps: Tuning the Morphology and Magnetic Properties of Single Crystalline Fe2O3 Nanostructures. Angewante Chem. Int. Ed. 2008, 47, 7564–7771. [Google Scholar]
- Mualem, Y.; Dogan, G. A dependent model of capillary hysteresis. Water Res. Res. 1975, 11, 452–460. [Google Scholar] [CrossRef]
- Bieniek, J.; Ściubidło, A.; Majchrzak-Kocięba, J. Properties of fly ash derived from coal combustion in air and in oxygen enriched atmosphere in a pilot plant installation Oxy-Fuel CFB0.1 MW2. Energetica 2013, 11, 821–826. [Google Scholar]
- Fernandez-Himenez, A.; Palonio, A. Characterization of fly ashes. Potential reactivity as alkaline cement. Fuel 2003, 18, 2259–2265. [Google Scholar] [CrossRef]
- Mishra, L.C.; Shukla, K.N. Effects of fly ash deposition on growth, metabolism and dry matter production of maize and soybean. Environ. Pol. Ser. A Ecol. Biol. 1986, 42, 1–13. [Google Scholar] [CrossRef]
- Ram, A.K.; Mohanry, S. State of the art review on physicochemical and engineering characteristics of fly ash and its applications. Int. J. Coal Sci. A Technol. 2022, 9, 9. [Google Scholar] [CrossRef]
- Kolbe, J.; Lee, L.S.; Jafvert, C.T.; Maruka, I.P. Use of Alkaline Coal Ash for Reclamation of a Former Strip Mine. In Proceedings of the World of Coal Ash (WOCA) Conference, Denver, CO, USA, 9–12 May 2011. [Google Scholar]
- Jafri, M.M.; Kumar, P. A feasibility study in low volume road embankment constructions using fly ash. Int. J. Electr. Electron. Commun. Eng. 2013, 3, 12. [Google Scholar]
- Singh, R.; Singh, D.P.; Kumar, N.; Bhargava, S.K.; Barman, S.C. Accumulation, and translocation of heavy metals in soil and plants from fly ash contaminated area. J. Environ. Biol. 2010, 31, 421–430. [Google Scholar]
- Moliner, A.M.; Street, J.J. Effect of fly ash and lime on growth and composition of corn (Zea mays L.) on acid sandy soil. Proc. Soil Crop Sci. Soc. Fla. 1982, 41, 217–220. [Google Scholar]
- Deighton, H.D.; Watmough, S.A. Effects of Non-Industrial Wood Ash (NIWA) Application on Soil Chemistry and Sugar Maple (Acer saccharum, Marsh.) Seeding Growth in an Acidic Sugar Bush in Central Ontario. Forestry 2020, 11, 693. [Google Scholar]
- Saarsalmi, A.; Malconen, E.; Pirainem, S. Effect of Wood Ash Fertilization on Forest Soil Chemical Properties. Silva Fenn. 2001, 35, 355–368. [Google Scholar] [CrossRef]
- Brożek, S.; Lasota, J.; Zwydak, M.; Wanic, T.; Gruba, P.; Błońska, E. Methodological approach to research on the relations between plants. Soil Sci. Ann. 2011, 62, 13–38. [Google Scholar]
- Kumar, U.; Mathur, M.; Preeti, K.S. Fly ash management: Vision for the new millennium. In Proceedings of the 2nd International Conference on Fly Ash Disposal and Utilization, FAM&CBIP, New Delhi, India, 2–4 February 2000; Volume 1, pp. 1–9. [Google Scholar]
- Cools, N.; Vesterdal, L.; De Vos, B.; Vanguelova, E.; Hausen, K. Tree species in the major factor explaining C/N ratios in European forest soils. For. Ecol. Manag. 2014, 311, 3–16. [Google Scholar] [CrossRef]
- Gaind, S.; Gaur, A.C. Quality assessment of compost prepared from fly ash and crop residue. Biores. Technol. 2003, 87, 125–127. [Google Scholar] [CrossRef] [PubMed]
- Mupambwa, H.A.; Dube, E.; Mnkeni, P.N.S. Fly ash composting to improve fertiliser value—A review. S. Afr. Journey Sci. 2015, 111, 1–6. [Google Scholar] [CrossRef][Green Version]
- Schutler, S.M.E.; Jeffry, J.; Fuhrmann, J.J. Microbial responses to coal fly ash under field conditions. J. Environ. Qual. 1999, 28, 648–652. [Google Scholar] [CrossRef]
- Yunusa, I.A.M.; Braun, M.; Lawrie, R. Amount of soil coal ash modified the burrowing habitats of two earthworm species. Appl. Soil Ecol. 2009, 42, 63–68. [Google Scholar] [CrossRef]
- Odlare, M.; Peel, M. Effect of Wood fly ash and compost on nitrification and denitrification in agricultural soil. Appl. Energy 2009, 86, 74–80. [Google Scholar] [CrossRef]
- Ultra, V.U.J.; Manyiwa, T. Influence of mycorrhiza and fly ash on the survival, growth and heavy metal accumulation in tree Acacia species grown in Cu-Ni mine soil. Environ. Geochem. Health 2021, 43, 1337–1353. [Google Scholar] [CrossRef] [PubMed]
- Hrynkiewicz, K.; Baum, C.; Niedojadło, J.; Dahm, M. Promotion of mycorrhiza formation and growth of willows by the bacterial stain Sphingomonas sp. 23L on fly ash. Biol. Fert Soil 2009, 45, 385–394. [Google Scholar] [CrossRef]
- Rubinio, M.; Dungai, J.A.J.; Evershed, R.P.; Bertolini, T.; De Angelis, P.; D’Onofio, A.; Lagomarsino, A.; Lubritto, C.; Merola, A.; Terrasi, F.; et al. Carbon input belowground in the major C flux contributing to leaf litter mases loss: Evidence from a 13C labelled-leaf litter experiment. Soil Biol. Biochem. 2010, 42, 1009–1016. [Google Scholar] [CrossRef]
- Abelenda, A.M.; Sample, K.T.; Lag-Brotons, A.J.; Herbert, B.M.J.; Aggidis, G.; Aionache, F. Alkaline Wood Ash, Turbulence, and Traps with Excess of Sulfuric Acid do Not Strip Completely the Ammonia off an Agrowaste Digestate. Edelweiss Chem. Sci. J. 2021, 4, 19–24. [Google Scholar] [CrossRef]
- O’Reilly, S.E.; Sims, J.T. Phosphorus adsorption and desorption in a sand soil amended with light rates of coal fly ash. Comm. Soil Sci. Plant Anal. 1995, 26, 2983–2993. [Google Scholar] [CrossRef]
- Masto, R.; Mahato, M.K.; Ram, L. The Effect of Fly Ash Application on Phosphorus Availability in an Acid soil. Energy Sources Part A Recovery Util. Environ. Eff. 2013, 35, 1556–7230. [Google Scholar] [CrossRef]
- Bera, R.; Seal, A.; Bhattacharyya, P.; Mukhopadhyay, K.; Giri, R. Phosphate sorption desorption characteristics of some farraginous soil in tropical region in Eastern India. Environ. Geol. 2006, 51, 399–407. [Google Scholar] [CrossRef]
- Li, X.; Rubaek, G.H.; Sorensen, P. High plant availability of phosphorus and low availability of cadmium in four biomass combustion ashes. Sci. Total Environ. 2016, 557–558, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Masto, R.E.; Anasari, M.A.; George, J.; Selvi, V.A.; Ram, L.C. Co application of biochar and lignite fly ash on soil nutrients and biological parameters and different crop growth stage of Zea mays. Ecol. Eng. 2013, 58, 314–322. [Google Scholar] [CrossRef]
- Grubb, D.G.; Guimares, M.S.; Valencia, R. Phosphate immobilization using an acidic type F fly ash. J. Hazard. Mater. 2000, 76, 217–236. [Google Scholar] [CrossRef] [PubMed]
- Wedepohl, K.H. The composition of the continental crust. Geochim. Cosnochim. Acta 1995, 57, 1217–1232. [Google Scholar] [CrossRef]
- Ashfanque, M.; Hecralal, M.; Reddy, P.H.P. A Study on Strength Behaviour of Alkali—Contaminated Soil Treated with Fly Ash. Rec. Waste Mater. 2019, 32, 137–143. [Google Scholar]
- Adriano, D.C.; Page, A.L.; Elseewi, A.A.; Chang, A.C.; Straughan, I. Utilization and Disposal of Fly Ash and Other Coal Residues in Terrestrial Ecosystems: A review. J. Environ. Qual. 1980, 9, 333–344. [Google Scholar] [CrossRef]
- Sikka, R.; Kansal, B.D. Effect of fly ash application on yield and nutrient composition of rice, wheat on pH and available nutrients status of soils. Biores. Technol. 1995, 51, 199–203. [Google Scholar] [CrossRef]
- Lai, K.; Chnobra, R.; Mogia, A.D.; Meena, R.L. Release and Uptake of Potassium and Sodium with Fly Ash Application in Rise on Reclaimed Alkali Soil. J. Indian Soc. Soil Sci. 2012, 60, 1–6. [Google Scholar]
- Grewald, K.S.; Metha, S.C.; Oswald, M.C.; Yadov, P.S. Effect of Fly Ash on Release Behaviour of Potassium in Soil of Arid Region. J. Indian Soc. Soil Sci. 1998, 46, 203–206. [Google Scholar]
- Wierzbowska, J.; Sienkiewicz, S.; Żarczyński, P.; Knebietke, S. Environmental Application of Ash from Incinerated Biomass. Agronomy 2020, 10, 482. [Google Scholar] [CrossRef]
- Goto, S.; Aoki, M.; Lang, C.D.; Takada, C.; Hayashi, H.; Chino, M. Potassium silicate fertilizer using Chinese fly ash and fertilizer response test. Japanese J. Soil Sci. Plant Nutr. 2000, 71, 378–384. [Google Scholar]
- Igras, S.; Lipiński, W. Evolution of selected elements of soil fertility and quality of shallow ground water on the background of crop production intensity in the region of frame. Pamiętnik Puławski 2006, 142, 147–161. (In Polish) [Google Scholar]
- Lai, K.M.; Ye, D.Y.; Wong, J.W.C. Enzyme activities in a sandy soil amended with sewage sludge and coal fly ash. Water Air Soil Pol. 1999, 113, 261–272. [Google Scholar] [CrossRef]
- Valentim, B.; Białecka, B.; Goncalves, P.A.; Guedes, A.; Guimaraes, R.; Cruceru, M.; Całus-Moszko, J.; Popescu, L.G.; Predeanu, G.; Santos, A.C. Undifferentiated Inorganics in Coal Fly Ash, and Bottom Ash: Calcispheres, Magnesia Calcispheres and Magnesia Spheres. Minerals 2008, 8, 140. [Google Scholar] [CrossRef]
- Cho, H.K.; Lee, H.S.; Wang, X.Y.; Ismal, M.; Park, W.J. Evaluation of CO2 emission-absorption of fly-ash-blended concentrate structures using cement-hydration-based carbonation model. Mater. Struct. 2015, 48, 3949–3963. [Google Scholar] [CrossRef]
- Pietrzykowski, M.; Woś, B.; Haus, N. Scotch pine needles macronutrient (N, P, K, Ca, Mg and S) supply at different reclaimed mine soil substrates as an indicator of the stability of developed forest ecosystems. Environ. Monit. Assess. 2013, 185, 7445–7457. [Google Scholar] [CrossRef] [PubMed]
- Adari, M.P.; Prasad, A.D. Cation exchange capacity (CEC) and unconfined compressive strength (UCS) of soil under the in-fluence of lime and fly ashes. In Proceedings of the National Conference on Technological Innovations in Civil Engineering—NCTICE 2017, Vadlamudi, India, 17–20 May 2017; Volume 3, pp. 2455–2462. [Google Scholar]
- Akbulut, S.; Seracettin, A. The Variations of Cation Exchange Capacity, pH, and Zeta Potential in Expansive soil Treated by Additives. Int. J. Civ. Struct. Eng. 2010, 1, 139–154. [Google Scholar]
- Rajput, V.D.; Minkina, T.; Sushkova, S.; Mandzhieva, S.; Gorovtsov, A.; Nievidomskyaya, D.; Gromacova, N. Effect of nanoparticles on crops and soil microbe communities. J. Soil Sediments 2018, 18, 2179–2187. [Google Scholar] [CrossRef]
- Pathan, S.M.; Aylmore LA, G.; Aylmore, G.; Colmer, T. Fly Ash Amendment of Sandy Soil to Improve Water and Nutrient Use Efficiency in Turf Culture. Int. Turfgrass Soc. Res. J. 2001, 9, 33–39. [Google Scholar]
- Kovacik, P.; Macak, M.; Ducsay, L.; Helcinova, M.; Jancich, M. Effect of fly-ash mixture application on soil fertility. J. Elem. 2011, 16, 215–225. [Google Scholar]
- Ellenberg, H.; Düll, R.; Wirth, V.; Werner, W.; Paulißen, D. Zeigerwerte von Pflanzen in Mitteleuropa. In Scripta Geobotanica; 18. 2 Auflage; Erich Goltze KG: Göttingen, Germany, 1992; p. 248. [Google Scholar]
- Brzeziecki, B.; Kienast, F. Classifying the life-history strategies of trees based on the Grimian model. For. Ecol. Manag. 1992, 69, 167–187. [Google Scholar] [CrossRef]
- Grime, J.P.; Hodgson, J.G.; Hunt, R. Comparative Plant Ecology: A Functional Approach to Common British Species; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Löf, M.; Bergquist, J.; Brunet, J.; Karlsson, M.; Welander, N.T. Conversion of Norway spruce stands ds to broadleaved woodland—Regeneration systems, fencing and performance of planted seedlings. Ecol. Bull. 2010, 53, 165–173. [Google Scholar]
- Kullberg, Y.; Bergström, R. Winter browsing by large herbivores on planted deciduous seedlings in southern Sweden. Scand. J. For. Res. 2001, 16, 371–378. [Google Scholar] [CrossRef]
- Dyderski, M.K.; Jagodziński, A.M. Context-dependence of urban forest vegetation invasion level and alien species’ ecological success. Forests 2019, 10, 26. [Google Scholar] [CrossRef]
- Chmura, D. The spread and role of the invasive alien tree Quercus rubra (L.) in novel forest ecosystems in Central Europe. Forests 2020, 11, 586. [Google Scholar] [CrossRef]
- Jasionkowski, R.; Wojciechowska, A.; Kamiński, D.; Piernik, A. Meadow species in early stages of succession on the ash settler of power plant EDF Toruń SA in Toruń, Poland. Ecol. Quest. 2016, 23, 79–86. [Google Scholar] [CrossRef]
- Żołnierz, L.; Weber, J.; Gilewska, M.; Strączyńska, S.; Pruchniewicz, D. The spontaneous development of understory vegetation on reclaimed and afforested post-mine excavation filled with fly ash. Catena 2016, 136, 84–90. [Google Scholar] [CrossRef]
- Lambers, H.; Oliveira, R.S. Plant Physiological Ecology, 3rd ed.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Singh, S.; Tripathi, D.K.; Singh, S.; Sharma, S.; Dubey, N.K.; Chauhan, D.K.; Vaculík, M. Toxicity of aluminium on various levels of plant cells and organism: A review. Environ. Exp. Bot. 2017, 137, 177–193. [Google Scholar] [CrossRef]
- Gough, L.; Shaver, G.R.; Carroll, J.; Royer, D.L.; Laundre, J.A. Vascular plant species richness in Alaskan arctic tundra: The importance of soil pH. J. Ecol. 2000, 88, 54–66. [Google Scholar] [CrossRef]
- van der Welle, M.E.W.; Vermeulen, P.J.; Berendse, F.; Shaver, G.R. Factors determining plant species richness in Alaskan arctic tundra. J. Veg. Sci. 2003, 14, 711–720. [Google Scholar] [CrossRef]
- Jaworski, A. Silviculture Characteristic of Forest Trees; Gutenberg: Kraków, Poland, 1995. [Google Scholar]
- Pigott, C.D. Biological flora of the British Isles Tilia cordata (Miller) (T. europaea, L. pro parte, T. parvifolia Ehrh. Ex Hoffm., T. sylvestris Desf., T. foemina folio minure Bauhin). J. Ecol. 1991, 79, 1147–1207. [Google Scholar] [CrossRef]
- Radoglu, K.; Dobrowolska, D.; Spyroglu, G.; Nikolescu, V.N. A Review on the Ecology and Silviculture of Limes (Tilia cordata Mill, Tilia platyphyllos Scop. and Tilia tomentosa Moench) in Europe. 2008. Available online: http://www.valbro.uni-freiburg.de/pdf/paper_tilia.pdf (accessed on 1 January 2020).
- Pandey, V.C.; Singh, K.; Singh, K.P.; Singh, B. Naturally growing Saccharum munja L.on the fly ash lagoons: A potential ecologicalengineer for the revegetation and stabilization. Ecol. Eng. 2012, 40, 95–99. [Google Scholar] [CrossRef]
- Brożek, S.; Zwydak, M.; Lasota, J. Numerical index of trophy varieties and rusty soil. Soil Sci. Ann. 2008, 59, 7–17. [Google Scholar]
- Yao, Z.T.; Ji, X.S.; Sarker, P.K.; Tang, J.H.; Ge, L.Q.; Xie, M.S.; Xi, Y.Q. A comprehensive review on the applications of coal fly ash. Earth-Sci. Rev. 2015, 141, 105–121. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).