Effects of Vegetation Succession on Soil Microbial Communities on Karst Mountain Peaks
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design and Soil Sampling
2.3. Soil Analysis
2.4. DNA Extraction, PCR Amplification, and Sequencing
2.5. Statistical Analyses
3. Results
3.1. Soil Physico-Chemical Properties
3.2. Microbial Biomass and Diversity
3.3. Microbial Community Compositions
3.4. Correlation between Microbial Community Compositions and Soil Environmental Variables
4. Discussion
4.1. Effect of Vegetation Succession on Soil Microbial Biomass and Diversity
4.2. Effect of Vegetation Succession on Microbial Community Compositions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Liu, X.; Yin, Z.; Chen, H.; Cai, X.; Xie, Y.; Wang, S.; Lian, B. Changes in soil microbial communities from exposed rocks to arboreal rhizosphere during vegetation succession in a karst mountainous ecosystem. J. Plant Interact. 2021, 16, 550–563. [Google Scholar] [CrossRef]
- Jiang, S.; Xing, Y.; Liu, G.; Hu, C.; Wang, X.; Yan, G.; Wang, Q. Changes in soil bacterial and fungal community composition and functional groups during the succession of boreal forests. Soil Biol. Biochem. 2021, 161, 108393. [Google Scholar] [CrossRef]
- Li, L.; Wang, D.; Liu, X.; Zhang, B.; Liu, Y.; Xie, T.; Du, Y.; Pan, G. Soil organic carbon fractions and microbial community and functions under changes in vegetation: A case of vegetation succession in karst forest. Environ. Earth Sci. 2014, 71, 3727–3735. [Google Scholar] [CrossRef]
- Amundson, R. The Carbon Budget in Soils. Annu. Rev. Earth Planet. Sci. 2001, 29, 535–562. [Google Scholar] [CrossRef]
- Liang, C.; Amelung, W.; Lehmann, J.; Kästner, M. Quantitative assessment of microbial necromass contribution to soil organic matter. Glob. Change Biol. 2019, 25, 3578–3590. [Google Scholar] [CrossRef] [PubMed]
- Borin, S.; Ventura, S.; Tambone, F.; Mapelli, F.; Schubotz, F.; Brusetti, L.; Scaglia, B.; D’Acqui, L.P.; Solheim, B.; Turicchia, S.; et al. Rock weathering creates oases of life in a High Arctic desert. Environ. Microbiol. 2010, 12, 293–303. [Google Scholar] [CrossRef]
- Tang, Y.; Lian, B. Diversity of endolithic fungal communities in dolomite and limestone rocks from Nanjiang Canyon in Guizhou karst area, China. Can. J. Microbiol. 2012, 58, 685–693. [Google Scholar] [CrossRef]
- Lian, B.; Chen, Y.; Tang, Y. Microbes on carbonate rocks and pedogenesis in karst regions. J. Earth Sci. 2010, 21, 293–296. [Google Scholar] [CrossRef]
- Banfield, J.F.; Barker, W.W.; Welch, S.A.; Taunton, A. Biological impact on mineral dissolution: Application of the lichen model to understanding mineral weathering in the rhizosphere. Proc. Natl. Acad. Sci. USA 1999, 96, 3404–3411. [Google Scholar] [CrossRef]
- Peay, K.G.; Kennedy, P.G.; Talbot, J.M. Dimensions of biodiversity in the Earth mycobiome. Nat. Rev. Microbiol. 2016, 14, 434–447. [Google Scholar] [CrossRef]
- Hanif, M.A.; Guo, Z.; Moniruzzaman, M.; He, D.; Yu, Q.; Rao, X.; Liu, S.; Tan, X.; Shen, W. Plant Taxonomic Diversity Better Explains Soil Fungal and Bacterial Diversity than Functional Diversity in Restored Forest Ecosystems. Plants 2019, 8, 479. [Google Scholar] [CrossRef]
- Lladó, S.; López-Mondéjar, R.; Baldrian, P. Drivers of microbial community structure in forest soils. Appl. Microbiol. Biotechnol. 2018, 102, 4331–4338. [Google Scholar] [CrossRef]
- Nakayama, M.; Imamura, S.; Taniguchi, T.; Tateno, R. Does conversion from natural forest to plantation affect fungal and bacterial biodiversity, community structure, and co-occurrence networks in the organic horizon and mineral soil? For. Ecol. Manag. 2019, 446, 238–250. [Google Scholar] [CrossRef]
- Lozano, Y.M.; Hortal, S.; Armas, C.; Pugnaire, F.I. Interactions among soil, plants, and microorganisms drive secondary succession in a dry environment. Soil Biol. Biochem. 2014, 78, 298–306. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, L.; Wang, Z.; Liu, L.-c.; Zhang, P.; Sun, J.; Wang, B.; Song, G.; Li, X. Changes in functional gene structure and metabolic potential of the microbial community in biological soil crusts along a revegetation chronosequence in the Tengger Desert. Soil Biol. Biochem. 2018, 126, 40–48. [Google Scholar] [CrossRef]
- Pugnaire, F.I.; Armas, C.; Maestre, F.T. Positive plant interactions in the Iberian Southeast: Mechanisms, environmental gradients, and ecosystem function. J. Arid Environ. 2011, 75, 1310–1320. [Google Scholar] [CrossRef]
- van der Putten, W.H.; Bardgett, R.D.; Bever, J.D.; Bezemer, T.M.; Casper, B.B.; Fukami, T.; Kardol, P.; Klironomos, J.N.; Kulmatiski, A.; Schweitzer, J.A.; et al. Plant–soil feedbacks: The past, the present and future challenges. J. Ecol. 2013, 101, 265–276. [Google Scholar] [CrossRef]
- Schloter, M.; Dilly, O.; Munch, J.C. Indicators for evaluating soil quality. Agric. Ecosyst. Environ. 2003, 98, 255–262. [Google Scholar] [CrossRef]
- Dong, R.; Wang, X.; Wang, Y.; Ma, Y.; Yang, S.; Zhang, L.; Zhang, M.; Qin, J.; Quzha, R. Differences in soil microbial communities with successional stage depend on vegetation coverage and soil substrates in alpine desert shrublands. Plant Soil 2023, 485, 549–568. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Wu, J.; Shen, Z.; Zhang, Y.; Xu, X.; Fan, Y.; Zhao, Y.; Yan, W. Root biomass distribution in alpine ecosystems of the northern Tibetan Plateau. Environ. Earth Sci. 2011, 64, 1911–1919. [Google Scholar] [CrossRef]
- Smyth, C.E.; Macey, D.; Trofymow, J.A. Long-term litter decay in Canadian forests and the influence of soil microbial community and soil chemistry. Soil Biol. Biochem. 2015, 80, 251–259. [Google Scholar] [CrossRef]
- Cline, L.C.; Zak, D.R. Soil microbial communities are shaped by plant-driven changes in resource availability during secondary succession. Ecology 2015, 96, 3374–3385. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Gao, M.; Zhang, Y.; Long, M.; Wu, Y.; Li, X. Effects of disturbance to moss biocrusts on soil nutrients, enzyme activities, and microbial communities in degraded karst landscapes in southwest China. Soil Biol. Biochem. 2021, 152, 108065. [Google Scholar] [CrossRef]
- Zhao, C.; Long, J.; Liao, H.; Zheng, C.; Li, J.; Liu, L.; Zhang, M. Dynamics of soil microbial communities following vegetation succession in a karst mountain ecosystem, Southwest China. Sci. Rep. 2019, 9, 2160. [Google Scholar] [CrossRef] [PubMed]
- Tedersoo, L.; Bahram, M.; Põlme, S.; Kõljalg, U.; Yorou, N.S.; Wijesundera, R.; Villarreal Ruiz, L.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A.; et al. Fungal biogeography. Global diversity and geography of soil fungi. Science 2014, 346, 1256688. [Google Scholar] [CrossRef]
- Huhe; Chen, X.; Hou, F.; Wu, Y.; Cheng, Y. Bacterial and Fungal Community Structures in Loess Plateau Grasslands with Different Grazing Intensities. Front. Microbiol. 2017, 8, 606. [Google Scholar] [CrossRef]
- Iyyemperumal, K.; Israel, D.W.; Shi, W. Soil microbial biomass, activity and potential nitrogen mineralization in a pasture: Impact of stock camping activity. Soil Biol. Biochem. 2007, 39, 149–157. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Huang, Y.-J.; Tang, S.-L.; Whitman, W.B.; Coleman, D.C.; Chiu, C.-Y. Bacterial Community Diversity in Undisturbed Perhumid Montane Forest Soils in Taiwan. Microb. Ecol. 2010, 59, 369–378. [Google Scholar] [CrossRef]
- Wang, S.J.; Liu, Q.M.; Zhang, D.F. Karst rocky desertification in southwestern China: Geomorphology, landuse, impact and rehabilitation. Land Degrad. Dev. 2004, 15, 115–121. [Google Scholar] [CrossRef]
- Larson, C. An Unsung Carbon Sink. Science 2011, 334, 886–887. [Google Scholar] [CrossRef]
- Hu, P.; Xiao, J.; Zhang, W.; Xiao, L.; Yang, R.; Xiao, D.; Zhao, J.; Wang, K. Response of soil microbial communities to natural and managed vegetation restoration in a subtropical karst region. Catena 2020, 195, 104849. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, W.; Xiao, L.; Yang, R.; Xiao, D.; Zhao, J.; Wang, W.; Chen, H.; Wang, K. Moss-dominated biological soil crusts modulate soil nitrogen following vegetation restoration in a subtropical karst region. Geoderma 2019, 352, 70–79. [Google Scholar] [CrossRef]
- Zhao, J.; He, X.; Nie, Y.; Zhang, W.; Fu, Z.; Wang, K. Unusual soil nematode communities on karst mountain peaks in southwest China. Soil Biol. Biochem. 2015, 88, 414–419. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, W.; Kuzyakov, Y.; Xiao, L.; Xiao, D.; Xu, L.; Chen, H.; Zhao, J.; Wang, K. Linking bacterial life strategies with soil organic matter accrual by karst vegetation restoration. Soil Biol. Biochem. 2023, 177, 108925. [Google Scholar] [CrossRef]
- Tang, T.; Hu, P.; Zhang, W.; Xiao, D.; Tang, L.; Xiao, J.; Zhao, J.; Wang, K. The Role of Bedrock Geochemistry and Climate in Soil Organic Matter Stability in Subtropical Karst Forests of Southwest China. Forests 2023, 14, 1467. [Google Scholar] [CrossRef]
- Bossio, D.A.; Scow, K.M. Impact of carbon and flooding on the metabolic diversity of microbial communities in soils. Appl. Environ. Microbiol. 1995, 61, 4043–4050. [Google Scholar] [CrossRef]
- Zhao, J.; Zeng, Z.; He, X.; Chen, H.; Wang, K. Effects of monoculture and mixed culture of grass and legume forage species on soil microbial community structure under different levels of nitrogen fertilization. Eur. J. Soil Biol. 2015, 68, 61–68. [Google Scholar] [CrossRef]
- Ruess, L.; Chamberlain, P.M. The fat that matters: Soil food web analysis using fatty acids and their carbon stable isotope signature. Soil Biol. Biochem. 2010, 42, 1898–1910. [Google Scholar] [CrossRef]
- Frostegård, Å.; Tunlid, A.; Bååth, E. Use and misuse of PLFA measurements in soils. Soil Biol. Biochem. 2011, 43, 1621–1625. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.; Liu, S.; Huang, W.; He, L.; Zhou, J. Enhanced hydrolysis-acidification of high-solids and low-organic-content sludge by biological thermal-alkaline synergism. Bioresour. Technol. 2019, 294, 122234. [Google Scholar] [CrossRef]
- Yao, Q.; Liu, J.; Yu, Z.; Li, Y.; Jin, J.; Liu, X.; Wang, G. Three years of biochar amendment alters soil physiochemical properties and fungal community composition in a black soil of northeast China. Soil Biol. Biochem. 2017, 110, 56–67. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson-Palme, J.; Ryberg, M.; Hartmann, M.; Branco, S.; Wang, Z.; Godhe, A.; Wit, P.D.; Sánchez-García, M.; Ebersberger, I.; Sousa, F.; et al. Improved software detection and extraction of ITS1 and ITS2 from ribosomal ITS sequences of fungi and other eukaryotes for analysis of environmental sequencing data. Methods Ecol. Evol. 2013, 4, 914–919. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Huang, X.; Shen, J.; Xu, F.; Su, J.J.G. Effects of plant diversity and soil properties on soil fungal community structure with secondary succession in the Pinus yunnanensis forest. Geoderma 2020, 379, 114646. [Google Scholar] [CrossRef]
- Maron, P.A.; Sarr, A.; Kaisermann, A.; Lévêque, J.; Mathieu, O.; Guigue, J.; Karimi, B.; Bernard, L.; Dequiedt, S.; Terrat, S.; et al. High Microbial Diversity Promotes Soil Ecosystem Functioning. Appl. Environ. Microbiol. 2018, 84, e02738-17. [Google Scholar] [CrossRef]
- Qiang, W.; He, L.; Zhang, Y.; Liu, B.; Liu, Y.; Liu, Q.; Pang, X. Aboveground vegetation and soil physicochemical properties jointly drive the shift of soil microbial community during subalpine secondary succession in southwest China. Catena 2021, 202, 105251. [Google Scholar] [CrossRef]
- Liu, J.; Jia, X.; Yan, W.; Zhong, Y.; Shangguan, Z. Changes in soil microbial community structure during long-term secondary succession. Land Degrad. Dev. 2020, 31, 1151–1166. [Google Scholar] [CrossRef]
- Zhang, X.; Pei, G.; Sun, J.; Huang, Y.; Huang, Q.; Xie, H.; Mo, J.; Zhao, M.; Hu, B. Responses of soil nitrogen cycling to changes in aboveground plant litter inputs: A meta-analysis. Geoderma 2023, 439, 116678. [Google Scholar] [CrossRef]
- Cornwell, W.K.; Cornelissen, J.H.; Amatangelo, K.; Dorrepaal, E.; Eviner, V.T.; Godoy, O.; Hobbie, S.E.; Hoorens, B.; Kurokawa, H.; Pérez-Harguindeguy, N.; et al. Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol. Lett. 2008, 11, 1065–1071. [Google Scholar] [CrossRef]
- Berg, G.; Smalla, K. Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiol. Ecol. 2009, 68, 1–13. [Google Scholar] [CrossRef]
- Wang, S.; Zuo, X.; Zhao, X.; Awada, T.; Luo, Y.; Li, Y.; Qu, H. Dominant plant species shape soil bacterial community in semiarid sandy land of northern China. Ecol. Evol. 2018, 8, 1693–1704. [Google Scholar] [CrossRef]
- Maestre, F.T.; Delgado-Baquerizo, M.; Jeffries, T.C.; Eldridge, D.J.; Ochoa, V.; Gozalo, B.; Quero, J.L.; García-Gómez, M.; Gallardo, A.; Ulrich, W.; et al. Increasing aridity reduces soil microbial diversity and abundance in global drylands. Proc. Natl. Acad. Sci. USA 2015, 112, 15684–15689. [Google Scholar] [CrossRef]
- Li, J.; Zhao, J.; Liao, X.; Yi, Q.; Zhang, W.; Lin, H.; Liu, K.; Peng, P.; Wang, K. Long-term returning agricultural residues increases soil microbe-nematode network complexity and ecosystem multifunctionality. Geoderma 2023, 430, 116340. [Google Scholar] [CrossRef]
- Li, H.; Yang, S.; Semenov, M.V.; Yao, F.; Ye, J.; Bu, R.; Ma, R.; Lin, J.; Kurganova, I.; Wang, X.; et al. Temperature sensitivity of SOM decomposition is linked with a K-selected microbial community. Glob. Change Biol. 2021, 27, 2763–2779. [Google Scholar] [CrossRef]
- Yu, Y.; Zheng, L.; Zhou, Y.; Sang, W.; Zhao, J.; Liu, L.; Li, C.; Xiao, C. Changes in soil microbial community structure and function following degradation in a temperate grassland. J. Plant Ecol. 2021, 14, 384–397. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, Y.; Tang, Z.; Shangguan, Z.; Chang, F.; Jia, F.a.; Chen, Y.; He, X.; Shi, W.; Deng, L. Effects of grassland afforestation on structure and function of soil bacterial and fungal communities. Sci. Total Environ. 2019, 676, 396–406. [Google Scholar] [CrossRef]
- Spain, A.M.; Krumholz, L.R.; Elshahed, M.S. Abundance, composition, diversity and novelty of soil Proteobacteria. ISME J. 2009, 3, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Hodkinson, B.P.; Lutzoni, F. A microbiotic survey of lichen-associated bacteria reveals a new lineage from the Rhizobiales. Symbiosis 2009, 49, 163–180. [Google Scholar] [CrossRef]
- Bai, Y.; She, W.; Miao, L.; Qin, S.; Zhang, Y. Soil microbial interactions modulate the effect of Artemisia ordosica on herbaceous species in a desert ecosystem, northern China. Soil Biol. Biochem. 2020, 150, 108013. [Google Scholar] [CrossRef]
- Chen, W.; Wang, J.; Meng, Z.; Xu, R.; Chen, J.; Zhang, Y.; Hu, T. Fertility-related interplay between fungal guilds underlies plant richness-productivity relationships in natural grasslands. New Phytol. 2020, 226, 1129–1143. [Google Scholar] [CrossRef] [PubMed]
- Hendershot, J.N.; Read, Q.D.; Henning, J.A.; Sanders, N.J.; Classen, A.T. Consistently inconsistent drivers of microbial diversity and abundance at macroecological scales. Ecology 2017, 98, 1757–1763. [Google Scholar] [CrossRef]
- Scola, V.; Ramond, J.-B.; Frossard, A.; Zablocki, O.; Adriaenssens, E.M.; Johnson, R.M.; Seely, M.; Cowan, D.A. Namib Desert Soil Microbial Community Diversity, Assembly, and Function Along a Natural Xeric Gradient. Microb. Ecol. 2018, 75, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Siles, J.A.; Margesin, R. Abundance and Diversity of Bacterial, Archaeal, and Fungal Communities Along an Altitudinal Gradient in Alpine Forest Soils: What Are the Driving Factors? Microb. Ecol. 2016, 72, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Chen, R.; Wang, L.; Jiang, L.; Yang, F.; Zheng, S.; Wang, G.; Lin, X. Soil pH, total phosphorus, climate and distance are the major factors influencing microbial activity at a regional spatial scale. Sci. Rep. 2016, 6, 25815. [Google Scholar] [CrossRef]
- Ochoa-Hueso, R.; Collins, S.L.; Delgado-Baquerizo, M.; Hamonts, K.; Pockman, W.T.; Sinsabaugh, R.L.; Smith, M.D.; Knapp, A.K.; Power, S.A. Drought consistently alters the composition of soil fungal and bacterial communities in grasslands from two continents. Glob. Change Biol. 2018, 24, 2818–2827. [Google Scholar] [CrossRef]
- Zuo, X.; Cheng, H.; Zhao, S.; Yue, P.; Liu, X.; Shaokun, W.; Liu, L.; Xu, C.; Luo, W.; Knops, J.M.H.; et al. Observational and experimental evidence for the effect of altered precipitation on desert and steppe communities. Glob. Ecol. Conserv. 2019, 21, e00864. [Google Scholar] [CrossRef]
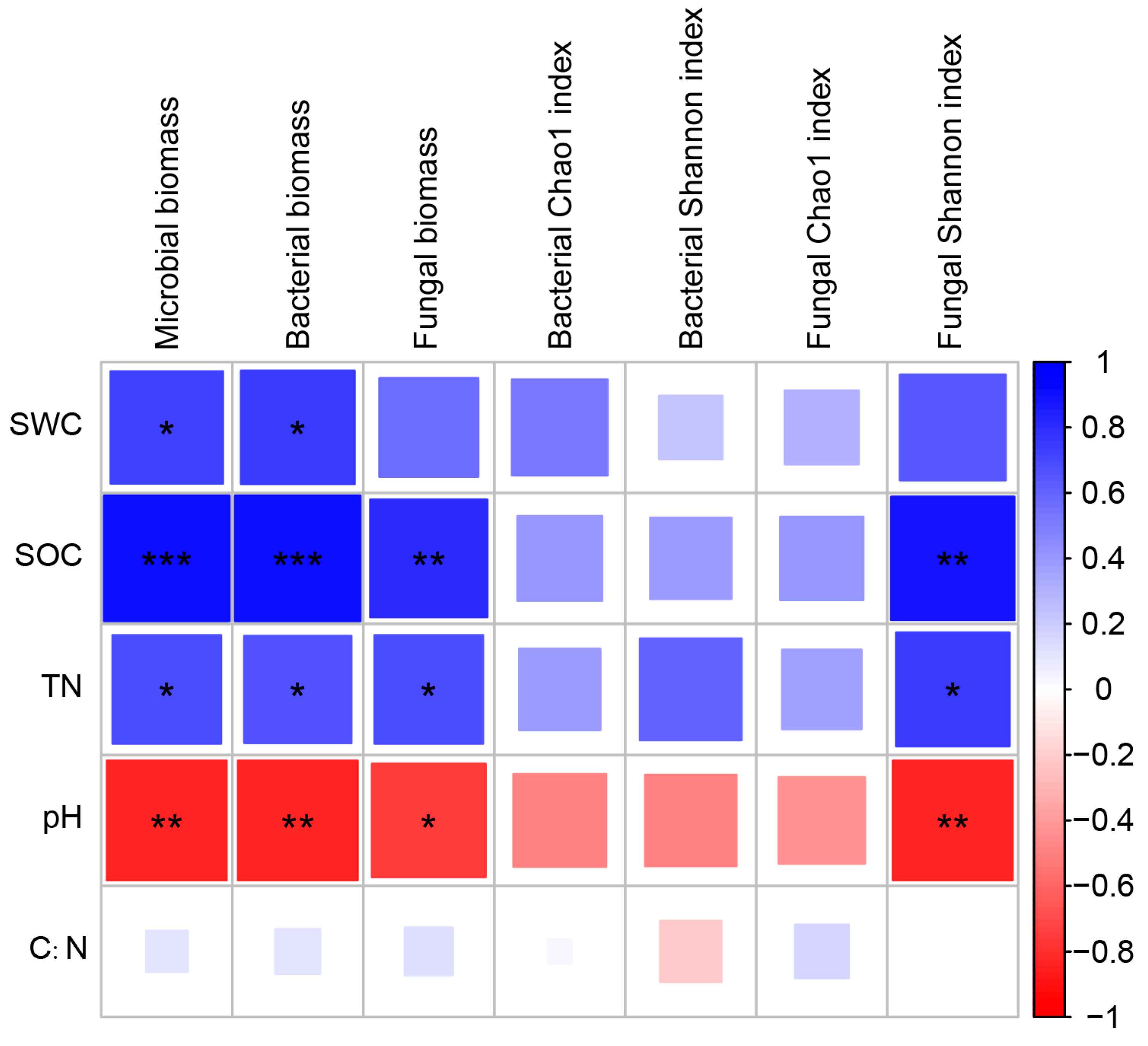
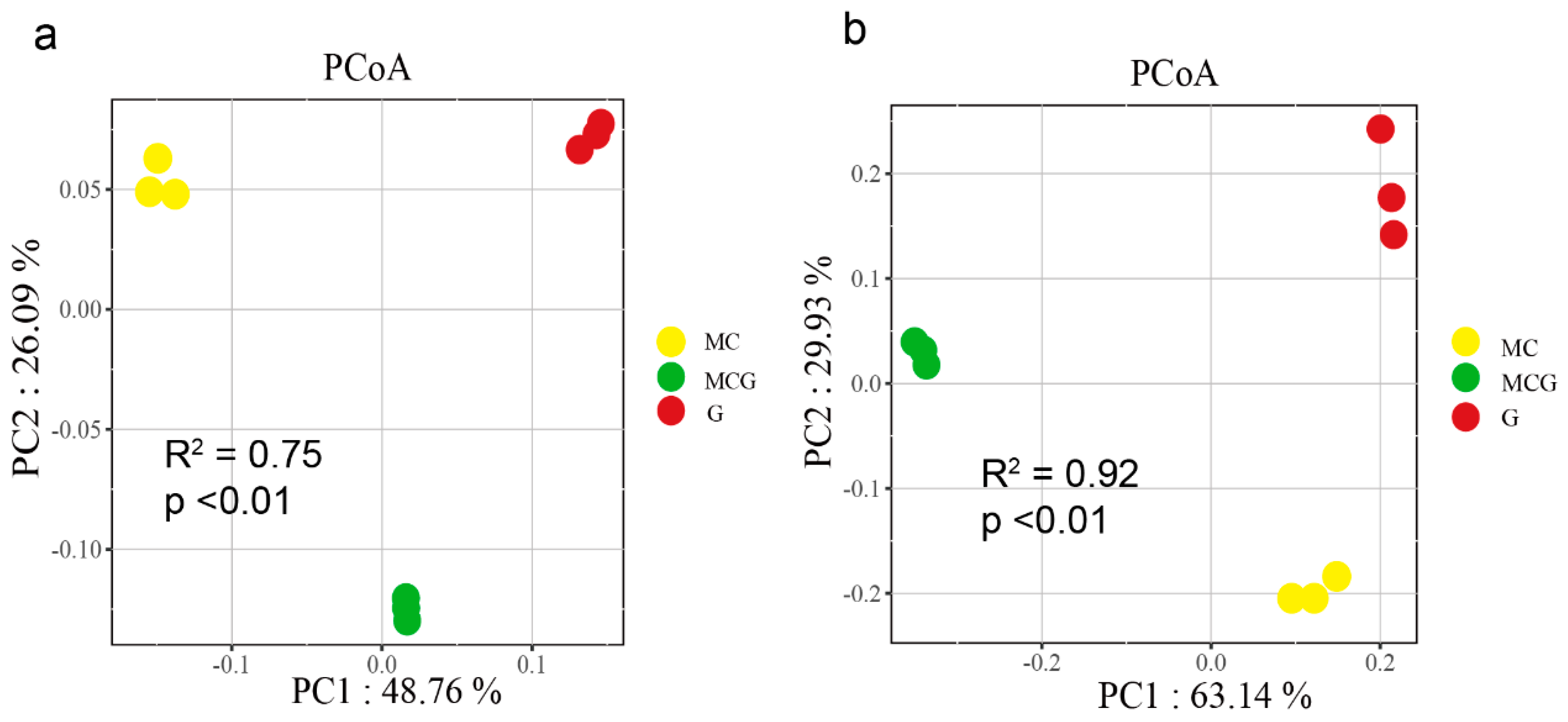

| Property | Vegetation Succession | ||
|---|---|---|---|
| MC | MCG | G | |
| SWC (%) | 8.98 ± 2.13 | 15.02 ± 4.02 | 13.28 ± 2.85 |
| SOC (g·kg−1) | 31.99 ± 4.32 b | 47.81 ± 5.71 a | 52.89 ± 6.03 a |
| TN (g·kg−1) | 2.62 ± 0.41 b | 4.07 ± 0.35 ab | 4.32 ± 0.67 a |
| pH | 8.44 ± 0.05 | 8.22 ± 0.10 | 8.19 ± 0.08 |
| C:N | 12.34 ± 0.35 | 11.68 ± 0.45 | 12.51 ± 1.10 |
| Variable | Vegetation Successional Stages | ||
|---|---|---|---|
| MC | MCG | G | |
| Microbial biomass | 18.87 ± 0.49 b | 24.98 ± 2.37 a | 25.93 ± 2.68 a |
| Bacterial biomass | 15.12 ± 0.47 b | 19.92 ± 1.78 a | 20.54 ± 1.72 a |
| Fungal biomass | 3.75 ± 0.31 b | 5.26 ± 0.49 a | 5.39 ± 1.24 a |
| Bacterial Chao1 index | 2054 ± 32 | 2083 ± 5 | 2100 ± 17 |
| Bacterial Shannon index | 9.39 ± 0.04 b | 9.47 ± 0.01 a | 9.48 ± 0.02 a |
| Fungal Chao1 index | 302 ± 12 b | 300 ± 5 b | 419 ± 20 a |
| Fungal Shannon index | 3.99 ± 0.09 b | 4.38 ± 0.02 b | 5.06 ± 0.21 a |
| Phyla | Class | Vegetation Successional Stages | ANOVA | |||
|---|---|---|---|---|---|---|
| MC | MCG | G | F | p | ||
| Prot | 29.48 ± 0.57 c | 32.01 ± 0.09 b | 35.02 ± 0.41 a | 46.32 | <0.001 | |
| Alph | 18.38 ± 0.33 a | 17.13 ± 0.07 b | 19.15 ± 0.37 a | 12.74 | <0.01 | |
| Beta | 3.83 ± 0.22 c | 6.63 ± 0.11 a | 5.84 ± 0.10 b | 88.08 | <0.001 | |
| Delt | 5.31 ± 0.10 b | 6.10 ± 0.03 a | 6.09 ± 0.12 a | 23.91 | <0.01 | |
| Gamm | 1.96 ± 0.05 b | 2.15 ± 0.12 b | 3.93 ± 0.08 a | 166.81 | <0.001 | |
| Acid | 22.61 ± 0.91 ab | 24.23 ± 0.11 a | 21.1 ± 0.29 b | 8.00 | <0.05 | |
| Acid | 3.04 ± 0.16 | 3.05 ± 0.11 | 2.88 ± 0.14 | 0.45 | 0.65 | |
| Blas | 12.42 ± 0.77 a | 11.57 ± 0.04 a | 8.77 ± 0.04 b | 18.46 | <0.01 | |
| Subg | 12.42 ± 0.77 a | 6.54 ± 0.12 b | 7.30 ± 0.21 a | 47.26 | <0.001 | |
| Acti | 24.27 ± 0.75 a | 21.32 ± 0.1 b | 20.64 ± 0.87 b | 8.34 | <0.05 | |
| Actin | 8.87 ± 0.38 a | 5.97 ± 0.07 b | 6.23 ± 0.21 b | 39.35 | <0.001 | |
| Chlo | 6.27 ± 0.10 a | 5.61 ± 0.04 b | 5.43 ± 0.18 b | 13.52 | <0.01 | |
| Ther | 11.07 ± 0.59 | 10.18 ± 0.31 | 10.32 ± 0.47 | 1.03 | 0.41 | |
| Gemm | 5.84 ± 0.12 b | 6.32 ± 0.13 a | 5.46 ± 0.15 b | 10.40 | <0.05 | |
| Gemm | 4.22 ± 0.10 b | 4.53 ± 0.07 a | 3.90 ± 0.08 c | 14.35 | <0.01 | |
| Bact | 5.92 ± 0.98 | 5.08 ± 0.08 | 5.56 ± 0.35 | 0.48 | 0.64 | |
| Plan | 1.08 ± 0.05 | 1.18 ± 0.05 | 1.15 ± 0.04 | 1.26 | 0.35 | |
| Phyla | Class | Vegetation Successional Stages | ANOVA | |||
|---|---|---|---|---|---|---|
| MC | MCG | G | F | p | ||
| Basi | 72.31 ± 0.83 a | 73.24 ± 0.32 a | 64.39 ± 2.59 b | 9.47 | <0.05 | |
| Agar | 72.09 ± 0.83 a | 73.07 ± 0.31 a | 64.28 ± 2.63 b | 9.05 | <0.05 | |
| Asco | 12.93 ± 0.08 b | 20.05 ± 0.56 a | 24.12 ± 2.40 a | 15.82 | <0.01 | |
| Sord | 3.68 ± 0.11 b | 4.87 ± 0.38 b | 7.10 ± 0.71 a | 13.63 | <0.01 | |
| Doth | 1.94 ± 0.06 c | 2.76 ± 0.18 b | 4.52 ± 0.13 a | 97.80 | <0.001 | |
| Euro | 1.65 ± 0.11 | 2.35 ± 0.20 | 3.71 ± 1.46 | 1.51 | 0.29 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Peng, P.; Li, J.; Liao, X.; Zhang, W.; Wang, K.; Zhao, J. Effects of Vegetation Succession on Soil Microbial Communities on Karst Mountain Peaks. Forests 2024, 15, 586. https://doi.org/10.3390/f15040586
Wang W, Peng P, Li J, Liao X, Zhang W, Wang K, Zhao J. Effects of Vegetation Succession on Soil Microbial Communities on Karst Mountain Peaks. Forests. 2024; 15(4):586. https://doi.org/10.3390/f15040586
Chicago/Turabian StyleWang, Wenyu, Peiqin Peng, Jiangnan Li, Xionghui Liao, Wei Zhang, Kelin Wang, and Jie Zhao. 2024. "Effects of Vegetation Succession on Soil Microbial Communities on Karst Mountain Peaks" Forests 15, no. 4: 586. https://doi.org/10.3390/f15040586
APA StyleWang, W., Peng, P., Li, J., Liao, X., Zhang, W., Wang, K., & Zhao, J. (2024). Effects of Vegetation Succession on Soil Microbial Communities on Karst Mountain Peaks. Forests, 15(4), 586. https://doi.org/10.3390/f15040586







