Growth Response of Thai Pine (Pinus latteri) to Climate Drivers in Tak Province of Northwestern Thailand
Abstract
1. Introduction
2. Materials and Methods
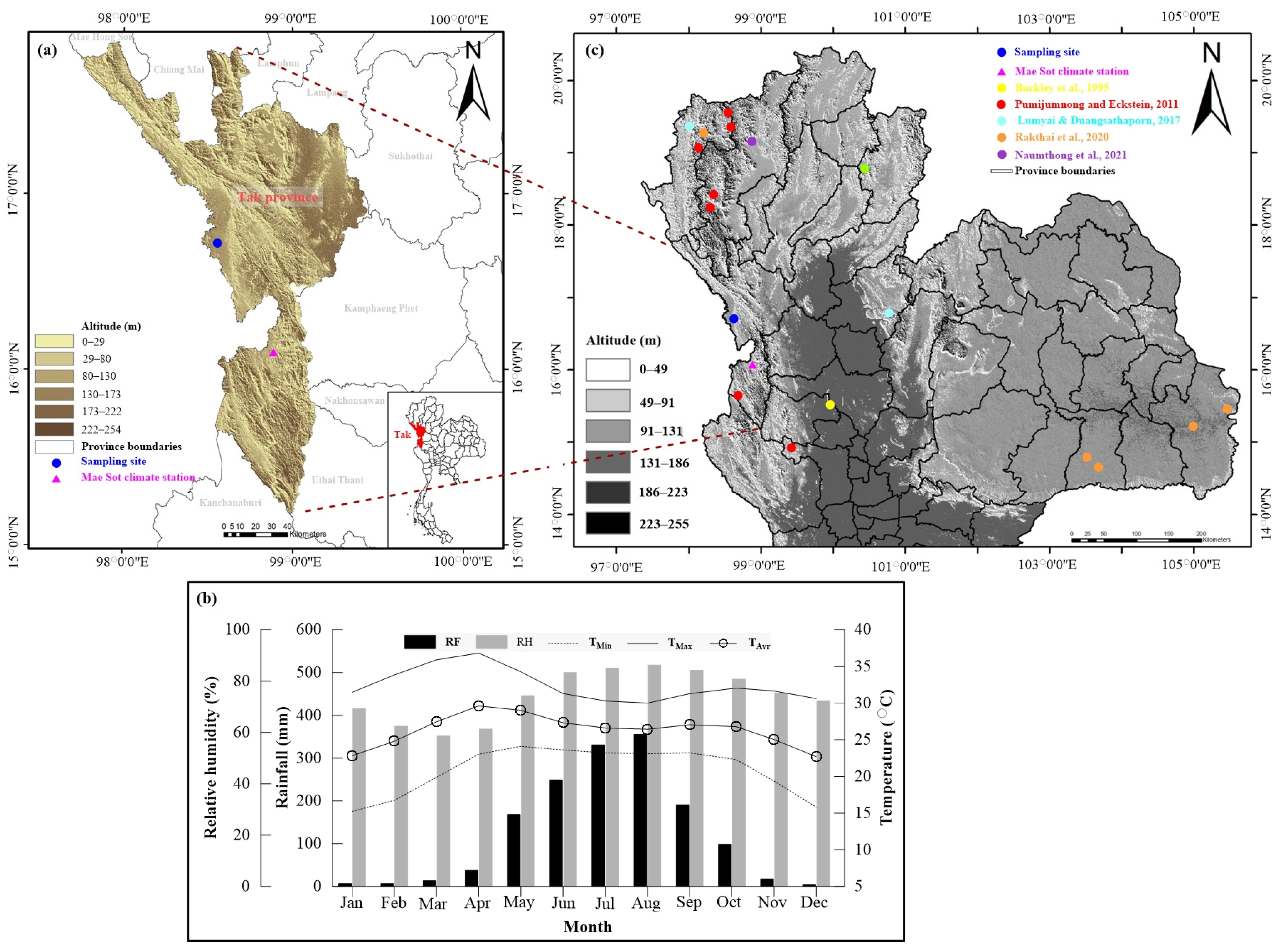
2.1. Study Area and Climate
2.2. Tree Ring Collection, Preparation, and Chronology Development
2.3. Climate Datasets and Data Analysis
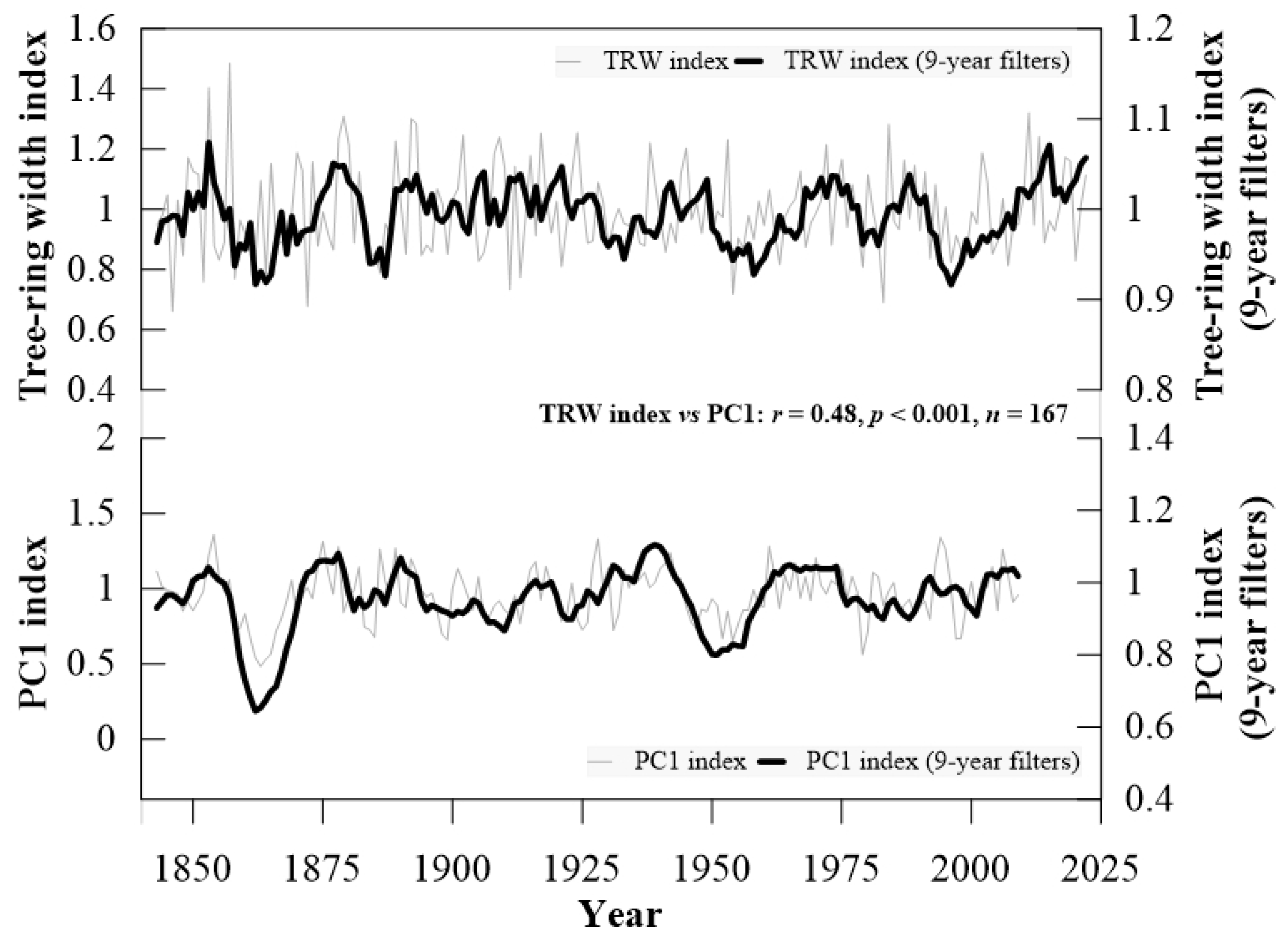
3. Results
3.1. Tree-Ring Chronology Characteristics
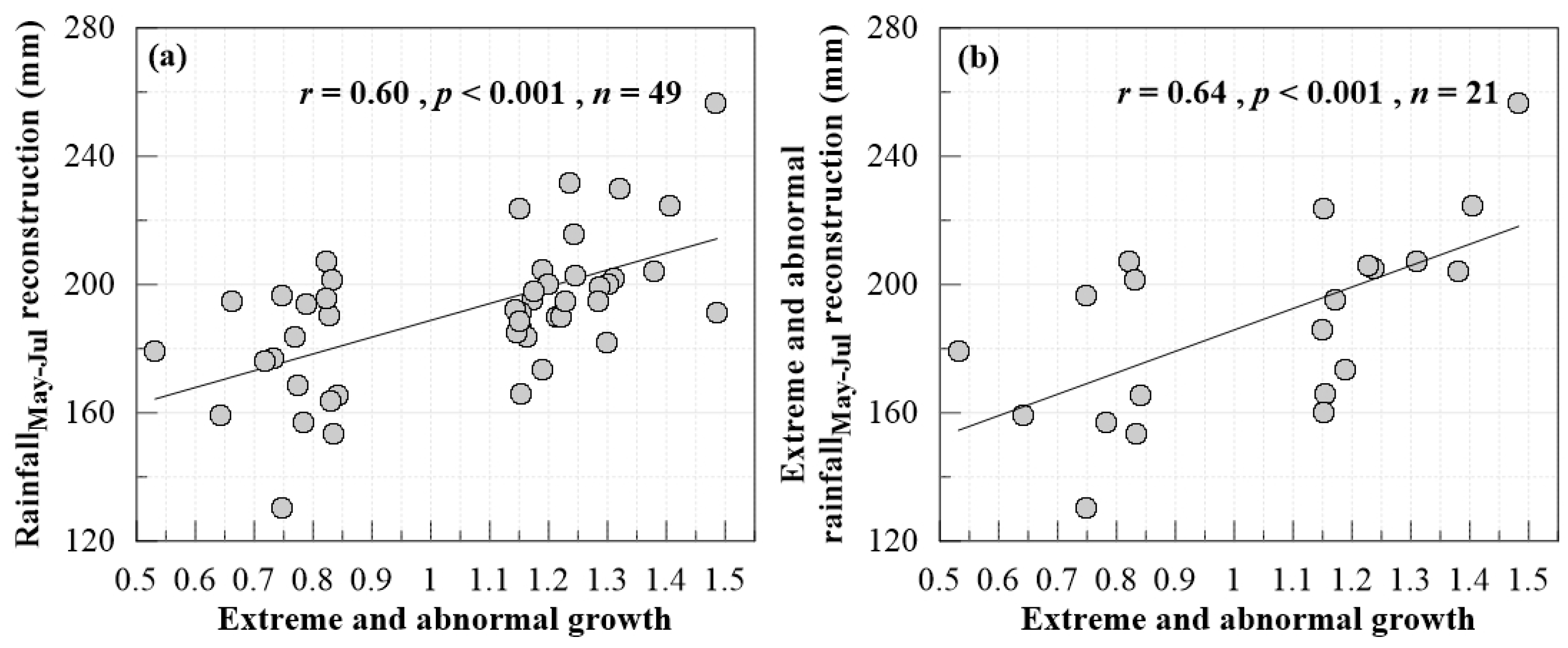
3.2. Growth Anomalies in the Tree Ring Records
3.3. Tree Growth Responses to Climate Drivers
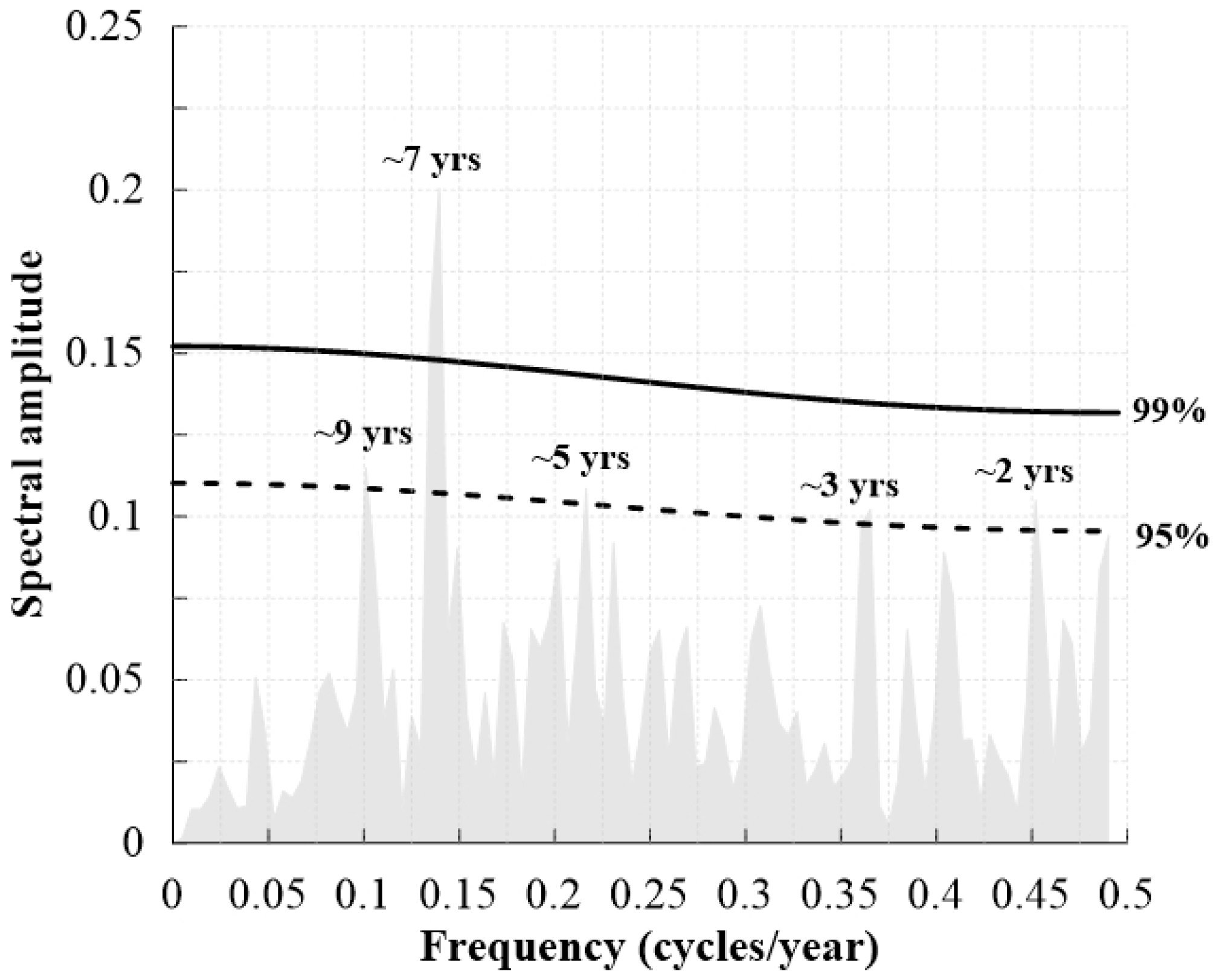
3.4. Periodicity of TRW Chronologies
4. Discussion
4.1. Chronological Characteristics and Comparison with the Pine Tree Ring Index from Northwestern Thailand
4.2. Possible Driving Factors Controlling Tree Growth
4.3. Growth Anomalies and Their Connection with Large-Scale Climate Drivers
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhuiyan, M.A.; Jabeen, M.; Zaman, K.; Khan, A.; Ahmad, J.; Hishan, S.S. The impact of climate change and energy resources on biodiversity loss: Evidence from a panel of selected Asian countries. Renew. Energy 2018, 117, 324–340. [Google Scholar] [CrossRef]
- Weiskopf, S.R.; Rubenstein, M.A.; Crozier, L.G.; Gaichas, S.; Griffis, R.; Halofsky, J.E.; Hyde, K.J.W.; Morelli, T.L.; Morisette, J.T.; Muñoz, R.C.; et al. Climate change effects on biodiversity, ecosystems, ecosystem services, and natural resource management in the United States. Sci. Total Environ. 2020, 733, 137782. [Google Scholar] [CrossRef]
- Chettri, N.; Adhikari, B.; Chaudhary, S.; Wangchuk, K. Changing discourses in the third pole: A systematic review of climate change impact on biodiversity in the Hindu Kush Himalaya. Ecol. Indic. 2023, 155, 111046. [Google Scholar] [CrossRef]
- Rahman, M.; Slam, M.; Bräuning, A. Tree radial growth is projected to decline in South Asian moist forest trees under climate change. Glob. Planet. Chang. 2018, 170, 106–119. [Google Scholar] [CrossRef]
- de Wergifosse, L.; André, F.; Goosse, H.; Boczon, A.; Cecchini, S.; Ciceu, A.; Collalti, A.; Cools, N.; D’Andrea, E.; De Vos, B.; et al. Simulating tree growth response to climate change in structurally diverse oak and beech forests. Sci. Total Environ. 2022, 806, 150422. [Google Scholar] [CrossRef]
- Wu, G.; Xu, G.; Wang, B.; Liu, X.; Chen, T.; Kang, H. Post-drought moisture condition determines tree growth recovery after extreme drought events in the Tianshan Mountains, northwestern China. Ecol. Indic. 2023, 151, 110275. [Google Scholar] [CrossRef]
- Ngo, K.M.; Turner, B.L.; Muller-Landau, H.C.; Davies, S.J.; Larjavaara, M.; Nik Hassan, N.F.; Lum, S. Carbon stocks in primary and secondary tropical forests in Singapore. For. Ecol. Manag. 2013, 296, 81–89. [Google Scholar] [CrossRef]
- Deb, J.C.; Phinn, S.; Butt, N.; McAlpine, C.A. Identifying risks for tropical Asia climate change impacts on tropical forests. J. Trop. For. Sci. 2018, 30, 182–194. [Google Scholar]
- Raj, A.; Jhariya, M.K. Carbon storage, flux and mitigation potential of tropical Sal mixed deciduous forest ecosystem in Chhattisgarh, India. J. Environ. Manag. 2021, 293, 112829. [Google Scholar] [CrossRef] [PubMed]
- Gaisberger, H.; Fremout, T.; Kettle, C.J.; Vinceti, B.; Kemalasari, D.; Kanchanarak, T.; Thomas, E.; Serra-Diaz, J.M.; Svenning, J.-C.; Slik, F.; et al. Tropical and subtropical Asia’s valued tree species under threat. Conserv. Biol. 2022, 36, e13873. [Google Scholar] [CrossRef] [PubMed]
- Bordin, K.M.; Esquivel-Muelbert, A.; Bergamin, R.S.; Klipel, J.; Picolotto, R.C.; Frangipani, M.A.; Zanini, K.J.; Cianciaruso, M.V.; Jarenkow, J.A.; Jurinitz, C.F.; et al. Climate and large-sized trees, but not diversity, drive above-ground biomass in subtropical forests. For. Ecol. Manag. 2021, 490, 119126. [Google Scholar] [CrossRef]
- Hauck, M.; Csapek, G.; Dulamsuren, C. The significance of large old trees and tree cavities for forest carbon estimates. For. Ecol. Manag. 2023, 546, 121319. [Google Scholar] [CrossRef]
- Kripalani, R.H.; Singh, S.V.; Panchawagh, N.; Brikshavana, M. Variability of the summer monsoon rainfall over Thailand—Comparison with features over India. Int. J. Climatol. 1995, 15, 657–672. [Google Scholar] [CrossRef]
- Singhrattna, N.; Rajagopalan, B.; Clark, M.; Krishna Kumar, K. Seasonal forecasting of Thailand summer monsoon rainfall. Int. J. Climatol. 2005, 25, 649–664. [Google Scholar] [CrossRef]
- Limsakul, A.; Goes, J.I. Empirical evidence for interannual and longer period variability in Thailand surface air temperatures. Atmos. Res. 2008, 87, 89–102. [Google Scholar] [CrossRef]
- Räsänen, T.A.; Kummu, M. Spatiotemporal influences of ENSO on precipitation and flood pulse in the Mekong River Basin. J. Hydrol. 2013, 476, 154–168. [Google Scholar] [CrossRef]
- Ueangsawat, K. The impacts of ENSO phases on the variation of rainfall and stream flow in the Upper Ping River Basin, northern Thailand. Environ. Nat. Resour. J. 2013, 11, 97–119. [Google Scholar]
- Limsakul, A.; Singhruck, P. Long-term trends and variability of total and extreme precipitation in Thailand. Atmos. Res. 2016, 169, 301–317. [Google Scholar] [CrossRef]
- Limsakul, A.; Singhruck, P.; Wang, L. Climatology and spatio-temporal variability of wintertime total and extreme rainfall in Thailand during 1970–2012. EnvironmentAsia 2017, 10, 162–176. [Google Scholar]
- Trakolkul, C.; Charoenphon, C.; Satirapod, C. Impact of El Niño–Southern Oscillation (ENSO) on the Precipitable Water Vapor in Thailand from Long Term GPS Observation. Int. J. Geoinformatics 2022, 18, 13–20. [Google Scholar]
- Buckley, B.M.; Palakit, K.; Duangsathaporn, K.; Sanguantham, P.; Prasomsin, P. Decadal scale droughts over northwestern Thailand over the past 448 years: Links to the tropical Pacific and Indian Ocean sectors. Clim. Dyn. 2007, 29, 63–71. [Google Scholar] [CrossRef]
- Ummenhofer, C.C.; D’Arrigo, R.D.; Anchukaitis, K.J.; Buckley, B.M.; Cook, E.R. Links between Indo-Pacific climate variability and drought in the Monsoon Asia Drought Atlas. Clim. Dyn. 2013, 40, 1319–1334. [Google Scholar] [CrossRef]
- Muangsong, C.; Cai, B.; Pumijumnong, N.; Hu, C.; Cheng, H. An annually laminated stalagmite record of the changes in Thailand monsoon rainfall over the past 387 years and its relationship to IOD and ENSO. Quat. Int. 2014, 349, 90–97. [Google Scholar] [CrossRef]
- Räsänen, T.A.; Lindgren, V.; Guillaume, J.H.A.; Buckley, B.M.; Kummu, M. On the spatial and temporal variability of ENSO precipitation and drought teleconnection in mainland Southeast Asia. Clim. Past 2016, 12, 1889–1905. [Google Scholar] [CrossRef]
- Tan, L.; Shen, C.-C.; Löwemark, L.; Chawchai, S.; Edwards, R.L.; Cai, Y.; Breitenbach, S.F.M.; Cheng, H.; Chou, Y.-C.; Duerrast, H.; et al. Rainfall variations in central Indo-Pacific over the past 2700 y. Proc. Natl. Acad. Sci. USA 2019, 116, 17201–17206. [Google Scholar] [CrossRef] [PubMed]
- Chawchai, S.; Liu, G.; Bissen, R.; Scholz, D.; Riechelmann, D.F.C.; Vonhof, H.; Mertz-Kraus, R.; Chiang, H.-W.; Tan, L.; Wang, X. Hydroclimate variability of western Thailand during the last 1400 years. Quat. Sci. Rev. 2020, 241, 106423. [Google Scholar] [CrossRef]
- Hughes, M.K. Dendrochronology in climatology—The state of the art. Dendrochronologia 2002, 20, 95–116. [Google Scholar] [CrossRef]
- Martinelli, N. Climate from dendrochronology: Latest developments and results. Glob. Planet. Change 2004, 40, 129–139. [Google Scholar] [CrossRef]
- Smith, D.; Lewis, D. Dendrochronology. In Encyclopedia of Quaternary Science; Elias, S.A., Ed.; Elsevier: Oxford, UK, 2007; pp. 459–465. [Google Scholar]
- Vlam, M.; Baker, P.J.; Bunyavejchewin, S.; Zuidema, P.A. Temperature and rainfall strongly drive temporal growth variation in Asian tropical forest trees. Oecologia 2014, 174, 1449–1461. [Google Scholar] [CrossRef] [PubMed]
- Thomte, L.; Shah, S.K.; Mehrotra, N.; Saikia, A.; Bhagabati, A.K. Dendrochronology in the tropics using tree-rings of Pinus kesiya. Dendrochronologia 2023, 78, 126070. [Google Scholar] [CrossRef]
- Gebrekirstos, A.; Bräuning, A.; Sass-Klassen, U.; Mbow, C. Opportunities and applications of dendrochronology in Africa. Curr. Opin. Environ. Sustain. 2014, 6, 48–53. [Google Scholar] [CrossRef]
- Allen, K.J.; Freund, M.B.; Palmer, J.G.; Simkin, R.; Williams, L.; Brookhouse, M.; Cook, E.R.; Stewart, S.; Baker, P.J. Hydroclimate extremes in a north Australian drought reconstruction asymmetrically linked with Central Pacific Sea surface temperatures. Glob. Planet. Chang. 2020, 195, 103329. [Google Scholar] [CrossRef]
- Buckley, B.M.; Duangsathaporn, K.; Palakit, K.; Butler, S.; Syhapanya, V.; Xaybouangeun, N. Analyses of growth rings of Pinus merkusii from Lao P.D.R. For. Ecol. Manag. 2007, 253, 120–127. [Google Scholar] [CrossRef]
- Fan, Z.-X.; Bräuning, A.; Tian, Q.-H.; Yang, B.; Cao, K.-F. Tree ring recorded May–August temperature variations since A.D. 1585 in the Gaoligong Mountains, southeastern Tibetan Plateau. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2010, 296, 94–102. [Google Scholar] [CrossRef]
- Ljungqvist, F.C.; Piermattei, A.; Seim, A.; Krusic, P.J.; Büntgen, U.; He, M.; Kirdyanov, A.V.; Luterbacher, J.; Schneider, L.; Seftigen, K.; et al. Ranking of tree-ring based hydroclimate reconstructions of the past millennium. Quat. Sci. Rev. 2020, 230, 106074. [Google Scholar] [CrossRef]
- Lopez-Saez, J.; Corona, C.; von Arx, G.; Fonti, P.; Slamova, L.; Stoffel, M. Tree-ring anatomy of Pinus cembra trees opens new avenues for climate reconstructions in the European Alps. Sci. Total Environ. 2023, 855, 158605. [Google Scholar] [CrossRef] [PubMed]
- Bolles, K.C.; Williams, A.P.; Cook, E.R.; Cook, B.I.; Bishop, D.A. Tree-Ring Reconstruction of the Atmospheric Ridging Feature That Causes Flash Drought in the Central United States Since 1500. Geophys. Res. Lett. 2021, 48, e2020GL091271. [Google Scholar] [CrossRef]
- Zheng, Z.; Jin, L.; Li, J.; Chen, J.; Zhang, X.; Wang, Z. Moisture variation inferred from tree rings in north central China and its links with the remote oceans. Sci. Rep. 2021, 11, 16463. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Y.; Nakatsuka, T.; Song, H.; McCarroll, D.; Yang, Y.; Qi, J. The 225-year precipitation variability inferred from tree-ring records in Shanxi Province, the North China, and its teleconnection with Indian summer monsoon. Glob. Planet. Chang. 2015, 132, 11–19. [Google Scholar] [CrossRef]
- Harada, M.; Watanabe, Y.; Nakatsuka, T.; Tazuru-Mizuno, S.; Horikawa, Y.; Subiyanto, B.; Sugiyama, J.; Tsuda, T.; Tagami, T. Assessment of sungkai tree-ring δ18O proxy for paleoclimate reconstruction in western Java, Indonesia. Quat. Int. 2017, 432, 33–38. [Google Scholar] [CrossRef]
- Sano, M.; Li, Z.; Murakami, Y.; Jinno, M.; Ura, Y.; Kaneda, A.; Nakatsuka, T. Tree ring oxygen isotope dating of wood recovered from a canal in the ancient capital of Japan. J. Archaeol. Sci. Rep. 2022, 45, 103626. [Google Scholar] [CrossRef]
- Tang, W.; Fang, K.; Mei, Z.; Zhou, F.; Wu, H.; Seftigen, K.; Cao, X.; Dong, Z.; Bai, M.; Chen, Y. Drought variations in the southeastern Tibetan Plateau region since 1704 and their link to the Atlantic Multidecadal Oscillation. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2023, 630, 111813. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, C.; Liu, Y.; An, W.; Guo, Z. Irrawaddy River experienced more frequent hydrological drought events with global warming: Evidence from a 400-year tree ring oxygen isotope record. Catena 2023, 232, 107455. [Google Scholar] [CrossRef]
- Liu, S.; Xu, C.; Fontana, C.; An, W.; Tomazello-Filho, M.; Zhao, Q.; Zhao, Y.; Liu, Y.; Guo, Z. Monthly Precipitation Reconstruction in Subtropical South America Using Seasonal Tree-Ring Oxygen Isotopes. J. Geophys. Res. Biogeosciences 2023, 128, e2023JG007490. [Google Scholar] [CrossRef]
- Xu, C.; Huang, R.; An, W.; Zhao, Q.; Zhao, Y.; Ren, J.; Liu, Y.; Guo, Z. Tree ring oxygen isotope in Asia. Glob. Planet. Chang. 2024, 232, 104348. [Google Scholar] [CrossRef]
- Pumijumnong, N.; Muangsong, C.; Buajan, S.; Songtrirat, P.; Chatwatthana, R.; Chareonwong, U. Factors Affecting Cambial Growth Periodicity and Wood Formation in Tropical Forest Trees: A Review. Forests 2023, 14, 1025. [Google Scholar] [CrossRef]
- Palakit, K.; Duangsathaporn, K.; Siripatanadilok, S. Climatic fluctuations trigger false ring occurrence and radial-growth variation in teak (Tectona grandis L.f.). iForest 2015, 9, 286–293. [Google Scholar] [CrossRef]
- Sharma, B.; Fan, Z.-X.; Panthi, S.; Gaire, N.P.; Fu, P.-L.; Zaw, Z. Warming induced tree-growth decline of Toona ciliata in (sub-) tropical southwestern China. Dendrochronologia 2022, 73, 125954. [Google Scholar] [CrossRef]
- Yang, R.-Q.; Fu, P.-L.; Fan, Z.-X.; Panthi, S.; Gao, J.; Niu, Y.; Li, Z.-S.; Bräuning, A. Growth-climate sensitivity of two pine species shows species-specific changes along temperature and moisture gradients in southwest China. Agric. For. Meteorol. 2022, 318, 108907. [Google Scholar] [CrossRef]
- Zhou, B.; Sterck, F.; Kruijt, B.; Fan, Z.-X.; Zuidema, P.A. Diel and seasonal stem growth responses to climatic variation are consistent across species in a subtropical tree community. New Phytol. 2023, 240, 2253–2264. [Google Scholar] [CrossRef]
- Quesada-Román, A.; Ballesteros-Cánovas, J.A.; St. George, S.; Stoffel, M. Tropical and subtropical dendrochronology: Approaches, applications, and prospects. Ecol. Indic. 2022, 144, 109506. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, A.; Duan, A.; Wu, H.; Zhang, J. Responses to subtropical climate in radial growth and wood density of Chinese fir provenances, southern China. For. Ecol. Manag. 2022, 521, 120428. [Google Scholar] [CrossRef]
- Zhang, R.; Hu, Z.; Cherubini, P.; Cooper, D.J.; Zhu, L.; Lei, P. Tree-ring data reveal trees are suffering from severe drought stress in the humid subtropical forest. For. Ecol. Manag. 2023, 546, 121330. [Google Scholar] [CrossRef]
- Lumyai, P.; Duangsathaporn, K. Climate reconstruction on the growth of teak in umphang wildlife sanctuary, Thailand. Environ. Nat. Resour. J. 2018, 16, 21–30. [Google Scholar]
- Pumijumnong, N. Teak Tree Ring Widths: Ecology and Climatology Research in Northwest Thailand. Sci. Technol. Dev. 2012, 31, 165–174. [Google Scholar]
- Lumyai, P.; Palakit, K.; Duangsathaporn, K.; Wanthongchai, K. A 324-years temperature reconstruction from Pinus latteri mason at highland in Chiang Mai Province, Thailand. Biodiversitas 2020, 21, 3938–3945. [Google Scholar] [CrossRef]
- Nguyen, H.T.T.; Galelli, S.; Xu, C.; Buckley, B.M. Droughts, Pluvials, and Wet Season Timing Across the Chao Phraya River Basin: A 254-Year Monthly Reconstruction from Tree Ring Widths and δ18O. Geophys. Res. Lett. 2022, 49, e2022GL100442. [Google Scholar] [CrossRef]
- Pumijumnong, N.; Wanyaphet, T. Seasonal cambial activity and tree-ring formation of Pinus merkusii and Pinus kesiya in Northern Thailand in dependence on climate. For. Ecol. Manag. 2006, 226, 279–289. [Google Scholar] [CrossRef]
- Lumyai, P.L.; Duangsathaporn, K. Pine growth variation and climate change: Opportunities for dendroclimatology in central Thailand. J. Trop. For. Res. 2017, 1, 23–35. [Google Scholar]
- Pumimnong, N.; Eckstein, D. Reconstruction of pre-monsoon weather conditions in northwestern Thailand from the tree-ring widths of Pinus merkusii and Pinus kesiya. Trees 2011, 25, 125–132. [Google Scholar] [CrossRef]
- Xu, C.; Pumijumnong, N.; Nakatsuka, T.; Sano, M.; Li, Z. A tree-ring cellulose δ18O-based July–October precipitation reconstruction since AD 1828, northwest Thailand. J. Hydrol. 2015, 529, 433–441. [Google Scholar] [CrossRef]
- Duangsathaporn, K.; Palakit, K. Climatic Signals Derived from the Growth Variation and Cycles of Pinus merkusii in Easternmost Thailand. Thai J. For. 2022, 32, 9–23. [Google Scholar]
- Pumijumnong, N.; Muangsong, C.; Panthi, S.; Buajan, S.; Cai, B.; Kulsuwan, P.; Kongsombat, P. A 225-year pine (Pinus latteri) tree-ring record of pre-monsoon relative humidity variation in Nan province of northern Thailand and the linkage with large-scale ocean-atmospheric circulations. Glob. Planet. Chang. 2023, 230, 104277. [Google Scholar] [CrossRef]
- Thai Meteorological Department. Average Rainfall, Temperature, and Relative Humidity Data. 2023. Available online: http://www.tmd.go.th/ (accessed on 1 January 2023).
- Buckley, B.M.; Barbetti, M.; Watanasak, M.; Arrigo, R.D.; Boonchirdchoo, S.; Sarutanon, S.J.I.J. Dendrochronological Investigations in Thailand. Iawa J. 1995, 16, 393–409. [Google Scholar] [CrossRef]
- Rakthai, S.; Fu, P.-L.; Fan, Z.-X.; Gaire, N.P.; Pumijumnong, N.; Eiadthong, W.; Tangmitcharoen, S. Increased Drought Sensitivity Results in a Declining Tree Growth of Pinus latteri in Northeastern Thailand. Forests 2020, 11, 361. [Google Scholar] [CrossRef]
- Naumthong, M.; Palakit, K.; Duangsathaporn, K.; Prasomsin, P.; Lumyai, P. Climate effects on the growth of Pinus latteri and Pinus kesiya at the intakin silvicultural research station, Chiang mai province, Thailand. Biodiversitas 2021, 22, 2512–2519. [Google Scholar] [CrossRef]
- Rinn, F. TSAP-WinTM: Time Series Analysis and Presentation for Dendrochronology and Related Applications; Version 4.64 for Microsoft Windows User Reference; Rinntech: Heidelberg, Germany, 2011. [Google Scholar]
- Ng’andwe, P.; Chungu, D.; Tailoka, F.; Bwembya, M. Tree-ring chronology data of non-native Pinus kesiya (Royle ex Gordon) in Zambia. Data Brief 2021, 38, 107447. [Google Scholar] [CrossRef] [PubMed]
- Rohner, B.; Weber, P.; Thürig, E. Bridging tree rings and forest inventories: How climate effects on spruce and beech growth aggregate over time. For. Ecol. Manag. 2016, 360, 159–169. [Google Scholar] [CrossRef]
- Altieri, S.; Niccoli, F.; Kabala, J.P.; Liyaqat, I.; Battipaglia, G. Influence of drought and minimum temperature on tree growth and water use efficiency of Mediterranean species. Dendrochronologia 2024, 83, 126162. [Google Scholar] [CrossRef]
- Holmes, R.L. Dendrochronology Program Library, Installation and Program Manual; Lab. of Tree-Ring Research, University of Arizona: Tucson, AZ, USA, 1992. [Google Scholar]
- Grissino-Mayer, H.D. Evaluating crossdating accuracy: A manual and tutorial for the computer program COFECHA. Tree-Ring Res. 2001, 57, 205–221. [Google Scholar]
- Diao, H.; Wang, A.; Gharun, M.; Saurer, M.; Yuan, F.; Guan, D.; Dai, G.; Wu, J. Tree-ring δ13C of Pinus koraiensis is a better tracer of gross primary productivity than tree-ring width index in an old-growth temperate forest. Ecol. Indic. 2023, 153, 110418. [Google Scholar] [CrossRef]
- Cook, E.; Krusic, P. Program ARSTAN: A Tree-Ring Standardization Program Based on Detrending and Autoregressive Time Series Modeling, with Interactive Graphics; Lamont-Doherty Earth Observatory, Columbia University: New York, NY, USA, 2005. [Google Scholar]
- Cook, E.R.; Peters, K. The smoothing spline: A new approach to standardizing forest interior Tree-Ring widths series for dendroclimatic studies. Tree-Ring Bull. 1981, 41, 45–53. [Google Scholar]
- Davis, C.J.; Hanna, E.G. Temperature and rainfall trends in northern Australia 1911−2013mimplications for human activity and regional development. Clim. Res. 2016, 71, 1–16. [Google Scholar] [CrossRef]
- Zaveri, E.; Russ, J.; Damania, R. Rainfall anomalies are a significant driver of cropland expansion. Proc. Natl. Acad. Sci. USA 2020, 117, 10225–10233. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Bao, A.; Xu, W.; Yu, R.; Zhang, Q.; Jiang, L.; Nzabarinda, V. Tree-ring-based assessments of drought variability during the past 400 years in the Tianshan mountains, arid Central Asia. Ecol. Indic. 2021, 126, 107702. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Menking, K.M. Decadal to millennial-scale solar forcing of Last Glacial Maximum climate in the Estancia Basin of central New Mexico. Quat. Res. 2015, 83, 545–554. [Google Scholar] [CrossRef]
- Azharuddin, S.; Govil, P.; Singh, A.D.; Mishra, R.; Shekhar, M. Solar insolation driven periodicities in southwest monsoon and its impact on NE Arabian Sea paleoceanography. Geosci. Front. 2019, 10, 2251–2263. [Google Scholar] [CrossRef]
- Hackett, W.P. Juvenility and Maturity. In Cell and Tissue Culture in Forestry: General Principles and Biotechnology; Bonga, J.M., Durzan, D.J., Eds.; Springer: Dordrecht, The Netherlands, 1987; pp. 216–231. [Google Scholar]
- Pretzsch, H. The course of tree growth. Theory and reality. For. Ecol. Manag. 2020, 478, 118508. [Google Scholar] [CrossRef]
- Tonelli, E.; Vitali, A.; Piermattei, A.; Urbinati, C. Are young trees suitable for climate-growth analysis? A trial with Pinus nigra in the central Apennines treeline. Dendrochronologia 2020, 62, 125720. [Google Scholar] [CrossRef]
- Esper, J.; Niederer, R.; Bebi, P.; Frank, D. Climate signal age effects—Evidence from young and old trees in the Swiss Engadin. For. Ecol. Manag. 2008, 255, 3783–3789. [Google Scholar] [CrossRef]
- Schulz, M.; Mudelsee, M. REDFIT: Estimating red-noise spectra directly from unevenly spaced paleoclimatic time series. Comput. Geosci. 2002, 28, 421–426. [Google Scholar] [CrossRef]
- Wigley, T.M.L.; Briffa, K.R.; Jones, P.D. On the Average Value of Correlated Time Series, with Applications in Dendroclimatology and Hydrometeorology. J. Appl. Meteorol. Climatol. 1984, 23, 201–213. [Google Scholar] [CrossRef]
- Briffa, K.R.; Jones, P.D. Basic chronology statistics and assessment. In Methods of Dendrochronology: Applications in the Environmental Sciences; Cook, E.R., Kairiukstis, L.A., Eds.; Kluwer Academic Publishers: Norwell, MA, USA, 1990; pp. 137–152. Available online: https://www.springer.com/gb/book/9780792305866 (accessed on 30 August 2023).
- Pumijumnong, N.; Palakit, K. Effects of Climate Variability on the Annual and Intra-annual Ring Formation of Pinus merkusii growing in Central Thailand. Environ. Nat. Resour. J. 2020, 18, 234–248. [Google Scholar] [CrossRef]
- Panthi, S.; Fan, Z.-X.; van der Sleen, P.; Zuidema, P.A. Long-term physiological and growth responses of Himalayan fir to environmental change are mediated by mean climate. Glob. Change Biol. 2020, 26, 1778–1794. [Google Scholar] [CrossRef] [PubMed]
- Sano, M.; Buckley, B.M.; Sweda, T. Tree-ring based hydroclimate reconstruction over northern Vietnam from Fokienia hodginsii: Eighteenth century mega-drought and tropical Pacific influence. Clim. Dyn. 2009, 33, 331–340. [Google Scholar] [CrossRef]
- Gergis, J.L.; Fowler, A.M. Classification of synchronous oceanic and atmospheric El Niño-Southern Oscillation (ENSO) events for palaeoclimate reconstruction. Int. J. Climatol. 2005, 25, 1541–1565. [Google Scholar] [CrossRef]
- Gergis, J.L.; Fowler, A.M. A history of ENSO events since A.D. 1525: Implications for future climate change. Clim. Chang. 2009, 92, 343–387. [Google Scholar] [CrossRef]
- D’Arrigo, R.; Cook, E.R.; Wilson, R.J.; Allan, R.; Mann, M.E. On the variability of ENSO over the past six centuries. Geophys. Res. Lett. 2005, 32, L03711. [Google Scholar] [CrossRef]
- Li, J.; Xie, S.-P.; Cook, E.R.; Huang, G.; D’Arrigo, R.; Liu, F.; Ma, J.; Zheng, X.-T. Interdecadal modulation of El Niño amplitude during the past millennium. Nat. Clim. Chang. 2011, 1, 114–118. [Google Scholar] [CrossRef]
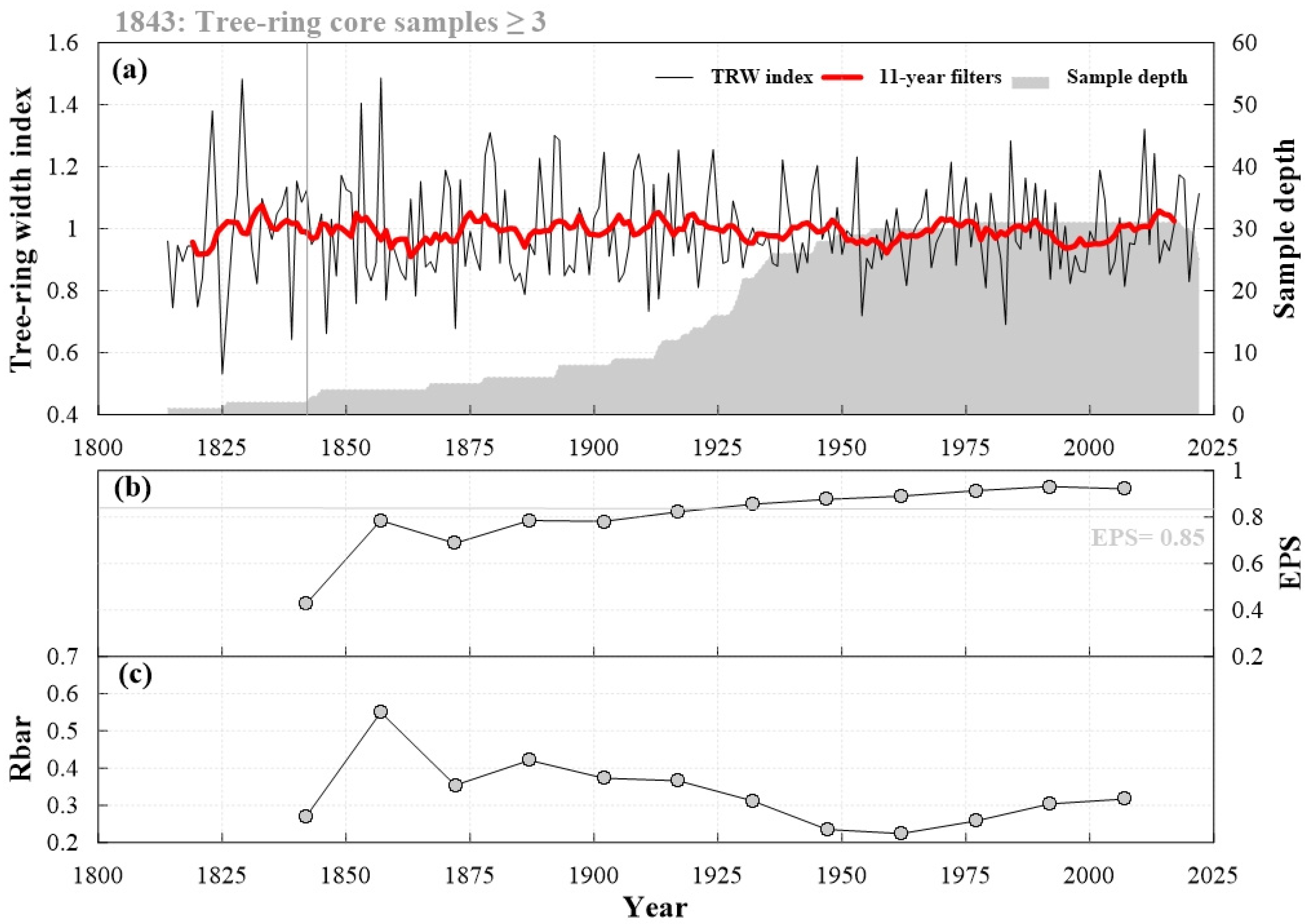


| Statistical Items | Statistical Values |
|---|---|
| Cores/Trees (n) | 31/16 |
| Time span (AD) | 1843–2022 |
| Number of years | 180 |
| Mean length of series (year) | 111 |
| Mean sensitivity (MS) | 0.25 |
| Mean interseries correlation (Rbar) | 0.34 |
| Expressed population signal (EPS) | 0.84 |
| First year in which EPS value ≥0.85 (number of trees) | 1918 (13) |
| Classified | Years |
|---|---|
| Abnormally positive | 1870, 1873,1878, 1880, 1889, 1892, 1893, 1902, 1908, 1910, 1915, 1917, 1924, 1938, 1945, 1953, 1972, 1984, 1987, 2013, 2018, 2019 |
| Extremely positive | 1853, 1857, 1879, 2011 |
| Abnormally negative | 1852, 1855, 1858, 1862, 1864, 1884, 1885, 1905, 1911, 1913, 1921, 1954, 1983, 1992, 1996 |
| Extremely negative | 1846, 1872 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inthawong, S.; Pumijumnong, N.; Muangsong, C.; Buajan, S.; Cai, B.; Chatwatthana, R.; Chareonwong, U.; Phewphan, U. Growth Response of Thai Pine (Pinus latteri) to Climate Drivers in Tak Province of Northwestern Thailand. Forests 2024, 15, 345. https://doi.org/10.3390/f15020345
Inthawong S, Pumijumnong N, Muangsong C, Buajan S, Cai B, Chatwatthana R, Chareonwong U, Phewphan U. Growth Response of Thai Pine (Pinus latteri) to Climate Drivers in Tak Province of Northwestern Thailand. Forests. 2024; 15(2):345. https://doi.org/10.3390/f15020345
Chicago/Turabian StyleInthawong, Sasiwimol, Nathsuda Pumijumnong, Chotika Muangsong, Supaporn Buajan, Binggui Cai, Rattanakorn Chatwatthana, Uthai Chareonwong, and Uthaiwan Phewphan. 2024. "Growth Response of Thai Pine (Pinus latteri) to Climate Drivers in Tak Province of Northwestern Thailand" Forests 15, no. 2: 345. https://doi.org/10.3390/f15020345
APA StyleInthawong, S., Pumijumnong, N., Muangsong, C., Buajan, S., Cai, B., Chatwatthana, R., Chareonwong, U., & Phewphan, U. (2024). Growth Response of Thai Pine (Pinus latteri) to Climate Drivers in Tak Province of Northwestern Thailand. Forests, 15(2), 345. https://doi.org/10.3390/f15020345






