Abstract
It is crucial for northerly Norway spruce to understand how seasonal warming and site conditions influence the intensity of free growth and what the effects of free growth on stem quality and adaptedness are. We studied the intensity of sylleptic and proleptic free growth in 660 6-to-9-year-old Norway spruce trees planted on normally irrigated and temporary overmoistured sites of variable fertility. We focused on the ability of individual trees to retain a type of free growth over three seasons and examined the associations between free growth, stem quality, and phenology traits. The results show that 23% to 50% of trees exhibited free growth, depending on the season. Mild and warm conditions in August and September tended to promote free growth. Among trees aged 6 to 9 years, 82% to 84% of those without free growth maintained this status over the following two seasons. While sylleptic growth decreased with age, proleptic growth increased. Over the seasons, individual trees were more consistent in maintaining proleptic growth than sylleptic growth. Trees on moist site types exhibited significantly more free growth than those in normally irrigated sites across all seasons. Trees with both sylleptic and proleptic free growth were significantly taller than those without free growth; however, sylleptic trees showed a markedly lower frequency of stem defects compared with those with proleptic growth. Free growth intensity was weakly associated with spring phenology and appeared to disrupt the well-established associations between phenology traits within the annual cycle. We conclude that selecting trees for overall height, particularly those with sylleptic free growth, may well exploit the benefits of free growth without significantly increasing the risk of autumn or winter frost damage.
1. Introduction
Over recent decades, the occurrence of free growth in young Norway spruce (Picea abies (L.) Karst.) forests in northern Europe has significantly increased [1,2,3]. Following the forecasts for even warmer autumns [4], the intensity of free growth is expected to further increase in young Norway spruce. Considering the high economic and ecological value of Norway spruce, there is an urgent need for further scrutiny of the environmental and genetic control of free growth in young Norway spruce trees [5]. Specifically, it is crucial for northerly Norway spruce to understand how seasonal warming, in combination with drought and site conditions, influences the incidence and the type of free growth. Additionally, it is important to explore the adaptive significance of free growth—does it lead to reduced vigor and increased susceptibility to injuries, or does it enhance height growth and compatibility [6]?
In Lithuania, Norway spruce naturally grows on 21.1% of the total area of stands, covering an area of 437,300 ha. Compared with 2003, the area of Norway spruce forests decreased by 8000 ha in Lithuania. Norway spruce grows best on loamy and sandy loam soils with fertile to moderately fertile moist soils (the NB, NC, LC site types based on Lithuanian classification). In Lithuania, Norway spruce thrives best on relatively cooler sites of the north-western and north-eastern highlands [7]. In Lithuania, forest sites are indexed to reflect the fertility and moisture content of the soil. Soil fertility is represented by six orders, A (very poor), B, C, D, F (very rich), and soil moisture by these main hydro types: N (normally irrigated), L (temporary overmoistured), U (permanently overmoistured), and P (peatlands). The most widespread forest site indexes in Lithuanian forests are NB, NC, LC, and LD, which account for 61.5% of the forest area [7]. In Lithuania, most newly established Norway spruce forests are planted on fertile mineral soils (mesotrophic mineral soils of normal moisture (NC) or mesotrophic mineral soils of temporary overmoistured (LC) site types). All seeds for Norway spruce plantations are collected in mainly second-generation seed orchards.
Northerly conifers follow a strict timing of phenology stages within an annual cycle: growth onset, active elongation, growth cessation and bud set (apical meristems in endogenic dormancy), development of shoot frost hardiness, transition from endo-to-exogenic dormancy and coming back to budburst in spring [8,9,10]. The timing of transition among the phenology stages is of high adaptive significance for Norway spruce because failure to timely complete a physiological stage before incidence of frost leads to severe damage [11,12]. Within the annual cycle, the phenology traits are strongly correlated in Norway spruce: late growth onset in spring implies late growth cessation, leading to taller trees and lower shoot frost hardiness [13,14]. Dormancy release of the apical meristems is associated with apical dominance control [15]. In Norway spruce, active growth is terminated, and buds are set in response to elongating nights [9]. The critical night length requirement for bud set decreases with increasing latitude of the origin of Norway spruce seedlings [16,17,18,19]. Once apical meristems enter endogenic dormancy, a chilling period is required for transition exogenic dormancy, followed by heat requirements for budburst in spring [17].
However, in young spruce trees (usually up to the age of 10), a phenomenon known as free shoot elongation occurs through the secondary bursting of buds that were set earlier during the same growth period [20,21]. This secondary burst typically happens in late summer or early autumn. Therefore, in young northerly coniferous trees, annual elongation of the leader shoot includes both (a) a predetermined type from buds formed in the previous year [21] and (b) a free type from apical meristems and/or buds formed during the current growth period after the predetermined growth has started [21]. For Norway spruce, based on whether terminal or lateral buds burst and elongate on the leader, two types of free growth have been identified [22,23]: (a) the proleptic type, where there is a secondary burst specifically from the terminal buds on the leader (proleptic = beforehand), and (b) the sylleptic type, where there is no visible secondary burst of the terminal buds on the leader, but there is noticeable elongation from the lateral buds on the leader. In this case, the leader shoot elongates from the apical meristems (bud primordia) that do not form true buds with visible scales. In such a case, the sylleptic growth results in a nearly continuous leader shoot elongation after the predetermined growth ends, without a clear visual transition between the two types of growth on the leader shoot (syllepsis = together with the spring buds). The only clear visual evidence of the sylleptic growth is the elongation of lateral buds on the leader [23]. In contrast to the predetermined growth, the sylleptic free growth immediately expands the stem units continuously set at the tip of the apical meristem. The formation of these new stem units occurs freely as long as the surrounding conditions are favorable, making “free growth” an appropriate term for this type of elongation [8]. Apparently, the sylleptic apical structures do not enter the endogenetic dormancy. For proleptic growth, some authors made a distinction between the secondary burst of the very terminal bud from the remaining apical buds on the leader by calling such shoots lammas shoots [24]. More often, others considered such secondary budburst of all the terminal buds on the leader as lammas shoots and called such growth a synonym to the proleptic type of growth [2,23].
Most of the physiological studies with free growth in Norway spruce were carried out in climate chambers on 1-to-5-year-old seedlings. These studies reported a decrease in free growth and a tendency to switch from a sylleptic to proleptic type of free growth with increasing age in young Norway spruce seedlings [22,23]. Fertile substrate and air moisture promote free growth intensity in young coniferous seedlings [24,25]. Studies in Norway spruce plantations reported less free growth on moist than on normally irrigated sites [2]. As regards the physiological mechanisms of free growth, there is some evidence that apical dominance release is influenced by a shift of auxin/cytokinin ratio, which is affected by such signals as nutrients and water [26]. Ref. [27] found that sylleptic growth requires a high availability of assimilates in young Norway spruce seedlings. However, the main findings on free growth in Norway spruce were obtained from studies on young seedlings tested mainly in climatic chambers. Given that controlled conditions cannot fully replicate the complexity of the natural environment, the novelty of our study lies in gaining a better understanding of free growth in Norway spruce under natural conditions, specifically in larger trees compared with 1–3-year-old seedlings.
Clearly, from an adaptive perspective, free growth may have a competitive advantage in warm, southerly environments [28], whereas in the north, with the abrupt autumn frost spells, such a strategy of maintaining frost-sensitive apical meristems towards the winter is clearly unfavorable. Often, in northerly regions, such stem defects as spike knots or forking may be attributed to the high intensity of free growth in Norway spruce [2]. This hypothesis was supported by a higher free growth intensity observed for southern provinces in common garden tests with Norway spruce [16,22,29,30]. Furthermore, repeatedly appearing free proleptic growth leads to the formation of several whorls during one season, in such a way, stem branch frequency increases, leading to low sawn wood quality [10]. Therefore, the high incidence of free growth is highly significant for the quality of wood and the adaptability of Norway spruce forests.
The objectives of our study were to assess the year and site effect on the intensity of sylleptic and proleptic types of free growth in 6–9-year-old commercial Norway spruce plantations in Lithuania. We also analyzed relationships between the intensity of either type of free growth and phenology, stem quality, and height growth traits.
2. Materials and Methods
2.1. The Study Sites
We studied the free growth intensity, height growth, and phenology of 674 young Norway spruce trees in commercial forest plantations for three consecutive years: 2020, 2021, and 2022. These Norway spruce plantations were established with 2-year-old seedlings in 2016. The seedlings originate from a 2nd-generation seed orchard with 50–60 clones of local origin. Considering this, we assume a common genetic background for the plantations established in different years.
We chose Norway spruce plantations that are located within a 20 km radius of the four major forest site types for planting Norway spruce (Table 1): normally irrigated rich (indexed as NC, 225 trees studied), temporarily overmoistured poor (LB, 125 trees), rich (LC, 175 trees), and very rich (LD, 149 trees). On each site, the same individual trees were measured repeatedly in the years 2020, 2021, and 2022. For each site type, we established 4–5 study sites to be treated as replications. The size of a study site was 0.5 to 1 ha, depending on the plantation size and suitability of the planting site for our study (Table 1). The suitability criteria for the study sites were as follows: no shadow from the ancient forest edge, no browsing or other types of damage, and no evident effects of competition with naturally regenerating groups of other forest tree species. Within each study site, we randomly selected 25 to 100 trees satisfying the above-given criteria and located at least 10 m apart from each other. Finally, we used a nested ANOVA to check for the replication effects on the intensity of free growth by testing the ANOVA model with the site type effect and the effect of replication nested within the site type. The results reveal no significant effect of replication on the free growth intensity and, therefore, the replication was not included as a classification variable in the subsequent ANOVAs.

Table 1.
List and geographic coordinates (WGS) of the study sites and number of studied trees.
The normally irrigated rich site type (coded as NC) dominates in Norway spruce forests of Lithuania. The soils of the Nc site type are irrigated by atmospheric precipitation, usually are calcar–hyperstagnic luvisols, sandy, 2–3 cm forest litter, the micro-relief is not pronounced, the water table is deeper than 2 m, and ground vegetation is usually dominated by wood sorrel (Oxalis acetosella L.) [31,32]. Temporarily overmoistured (L) site types are common on landscape depressions in lower elevation plains, with a water table at a depth of 0.5–1.5 m; the soils show signs of shrinkage, glutinosity, or stagnation [31,32]. Based on soil fertility, we chose several L site types on poor to rich soils indexed as b, c, d. The soils of site type Lb (poorest in our study) are orthi-endohypogleyic podzols, 4–9 cm forest litter, with dominant ground vegetation of European blueberry (Vaccinium myrtillus L.), Norway spruce grows as an admixture of Scots pine (Pinus sylvestris L.) and silver birch (Betula pendula Roth) [31,32]. The Lc site type is on richer orthi-endohypogleyic podzols with heavy clay horizon 70 to 100 cm deep, 12–14 cm forest litter, with European blueberry and wood sorrel in the ground vegetation. Norway spruce dominates with an admixture of Scots pine, silver birch and black alder (Alnus glutinosa (L.) Gaertn.). The Ld site is the richest with calcar–hypostagnic luvisoils, mainly sub-clay, 1–2 cm forest litter, wood sorrel with other broadleaved grass species, and Norway spruce as admixture to silver birch, aspen (Populus tremula L.), and English oak (Quercus robur L.) forests [31,32].
2.2. The Assessments
The height (cm) and root collar diameter (mm) of the Norway spruce trees were measured. The leader height (cm) was measured at the end of each growing season, and the number of buds on the leader shoot was counted. To assess free growth occurrence and intensity (Figure 1), we assessed (a) proleptic growth secondary budburst of the apical buds on the leader shoot and (b) secondary budburst of the lateral bud on the leader with no secondary budburst of the apical buds on the same leaders. We also measured the length of the longest lateral shoot on the leader after the secondary budburst. As a secondary budburst, we considered a budburst and its elongation for more than 0.5 cm. We scored stem defects as forking and spike knots. We also followed the timing of onset of active growth in spring at 10-day intervals of each of the three growth periods (the same dates every year from 20 April to 20 June, Table 2).
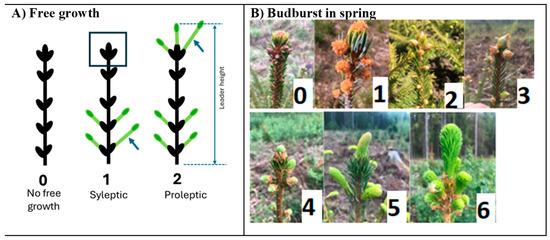
Figure 1.
(A) Types of free growth of the leader shoot in fall of three years, 2020, 2021, 2022, in the Norway spruce plantations established in 2016: 0—no free growth. 1—sylleptic type of free growth, where no visible bud scale burst is observed on the apical surrounding terminal buds at the top of the leader shoot (the boxed top). In fall, all buds counted on the leader, including the terminals (11 buds in the figure with type 1), along with the buds that broke the scales and elongated for at least a few millimeters (4 such buds in the figure with type 1); length of the longest lateral shoot that formed after the second flush was measured (in type 1, marked by an arrow, the lower right); 2—proleptic type of free growth where budburst of the terminal buds on the leader shoot was observed. The buds were counted in the same way as for sylleptic growth (11 buds in total of these 7 with budbust). Length of the longest elongating terminal shot was measured (marked by arrow, upper right in type 2). (B) Spring budburst scores from 0 to 6.

Table 2.
Abbreviations of the variables explained. The same individual trees were assessed each year.
The following variables were constructed for the data analyses:
FREEBUD—number of buds on the leader shoot (all buds, lateral and apical on the leader) that had visible budburst and elongation at the end of November. This variable serves as the major indicator of free growth intensity.
FREECLASS—a categorical variable constructed from FREEBUD to compare the properties of trees with variable intensity of free growth: 0 = no budburst, 1 = 1 to 30%, 2 = 31 to 60%, 3 ≥ 61%.
FREETYPE—type of free growth of the leader: 0—no free growth (no secondary budburst on the leader in autumn); 1—sylleptic type, where no secondary budburst of the terminal buds of the leader was observed and at the same time elongation of at least a single lateral bud on the leader for more than 5 mm was observed; and 2—proleptic type, where secondary budburst of the at least single terminal bud on the leader was observed (Figure 1).
FENO—budburst stage of the terminal apical bud on the leader was recorded every 10 days from 20 April to 20 June (2020, 2021, 2022): 0—dormant bud; 1—swollen but still brown; 2—bud swollen, whitish to green, bud scales still closed; 3—burst of bud scales, tips of needles emerging; 4—needles elongated to about 1 cm; 5—first spread of needles, buds now have the appearance of a painter’s brush, shoots length between 1 to 5 cm; 6—basal needles spread, shoot length over 5 cm based on [33].
To assess the influence of temperature and humidity on Norway spruce tree growth, temperature and humidity were recorded. Mean daily temperature and mean daily air humidity (%) were obtained from temperature loggers installed 0.5 m above ground in the shade.
2.3. Statistical Analysis
The significance of differences between the mean values of the traits was tested by the Tukey LSD test, available in PROC GLM (SAS statistical package ver. 9.4), using the following model in the ANOVAs:
where Yij is an individual observation, Mj is the fixed effect of a classification variable (e.g., site type, free growth type, etc.), and eij is a random error. The Tukey LCD test was preferred over other post-hoc tests as being particularly powerful for multiple comparisons among group means following an ANOVA especially when comparing quantitative variables with no marked deviation from a quantitative normal distribution (such as in our study).
Yij = Mj + eij,
Pearson product–moment correlation coefficients and their significance were calculated by PROC CORR (SAS statistical package ver. 9.4) on individual tree-level code.
3. Results
3.1. General Growth Features by Growth Period
Before presenting the free growth effects, we provide key growth characteristics to better represent the status of the Norway spruce plantations on the most common site types for Norway spruce.
Game browsing was found on only 0.01% of the 1996 Norway spruce trees studied and was ignored in the subsequent analysis. At the end of the 5–6 growth period (GP) after planting (fall 2020), the Norway spruce trees reached an average height of 106, 119, 138, and 109 cm on the LB, LC, LD, and Nc site types (annual sample size per site type of 125 to 225 trees, Figure S1, Table S1). The mean height of the leader shoot steadily increased from 36.9 to 49.9 and 58.2 cm after the GPs in 2020, 2021, and 2022, respectively (average annual sample size of 660 trees, Table S1). The maximum elongation of the leader shoot was 95, 125, and 115 cm for the GPs, as above. The number of lateral buds on the leader shoots was 21.1 (max. 50), 24.6 (max. 59), and 26.4 (max. 68) for the GPs, as above. Only 8 and 20 trees out of ca. 650 trees had annual leader increments exceeding 100 cm in 2021 and 2022 (no such trees found in 2020). Nearly half of these trees with over one-meter annual growth capacity had free growth with over 50% fall budburst intensity, and 80% had the proleptic type of free growth, while tree numbers with dwarfing leaders (<10 cm) were 8, 6, 5 for the years 2020, 2021, and 2022, and all of them had no free growth at all.
Site type had a significant effect on height growth of the Norway spruce trees in the plantations established 2015–2016: (a) the tallest trees were growing on a temporary overmastered very rich (LD) site type and the shortest on normally irrigated rich (NC) and temporary overmastered poor (LB) type (Figure S2); (b) leader shoot elongation was significantly lower in the N than in the L site types for all three GPs of 2020, 2021, and 2022 (Figure S2). Spring budburst on L site types tended to occur later than on N site type, though the difference was not strong (Figure S2). Spring budburst occurred earlier in 2021 than in the other two seasons (Figure S3). There was a slightly higher number of spike knots on the L site types than on the N site type. However, the overall number of Norway spruce trees with spike knots was as low as 147, which is 7% of all trees studied. Similarly, there were merely 74 forked trees, making up 4% of all trees studied.
Finally, we suggest reviewing the key features of the tallest 20% trees at the final stage of the experiment at age 8 (year 2022). Such trees would be highly preferable commercially. The intriguing question is whether these 20% best trees are superior because of free growth or other factors, such as longer growth in the season. The largest superiority of the 20% best trees over the mean was 157% of the mean in the richest LD site type, and it varied at about 135% in the remaining sites (Figure 2). At all site types, the superior trees had markedly more free growth than the overall mean; however, the significant difference between the superior trees and the overall mean was found in the LD site only (Figure 2). In all site types except LD, the best trees started active growth in spring a few days later; however, again, the difference was not significant (Figure 2). Spike knot frequency was slightly but not significantly higher for the 20% superior trees on most of the site types (Figure 2).
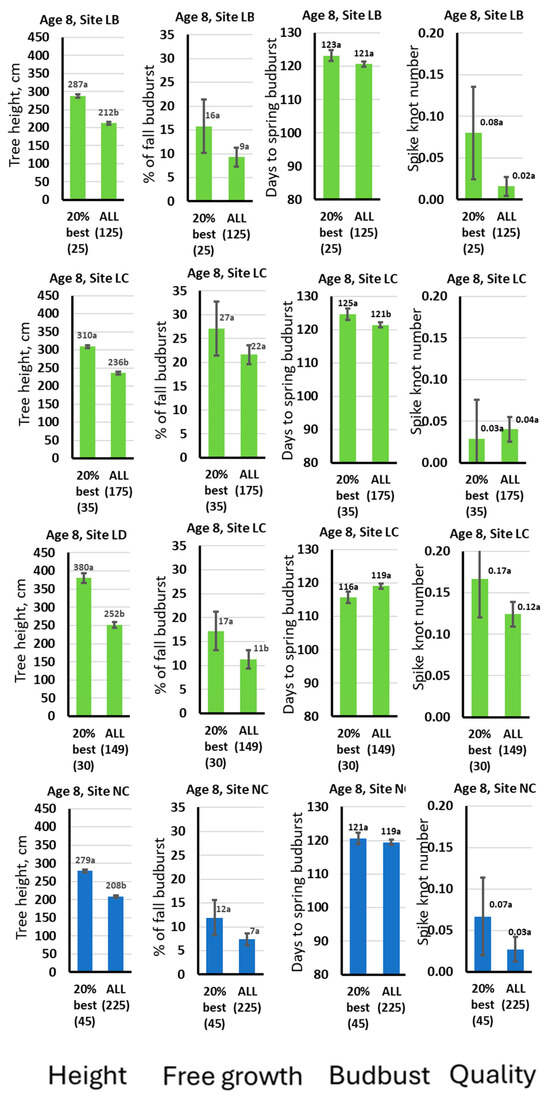
Figure 2.
The main characteristics of 20% superior by height trees at age 8 (that was the oldest age of the assessments) compared with the overall mean and standard error for the same year 2022 (age 8). Budburst is the budburst in spring (growth onset). Columns of the bar plots are abbreviated at the bottom of the figure to indicate what features the corresponding variable represents. Numbers in the brackets below the X-axis labels show the sample sizes. At the bar top, the mean trait values are shown along with the results for Tukey LSD test from the ANOVA, where the same letter at the top of each bar indicates no significant difference at the 0.05 significance level. The error bars are the standard errors. “% of fall bud burst” is the % of the burst buds on the leader shoot in fall (the main indicator of free growth intensity).
3.2. Variation in Free Growth Intensity and Type Between the Years
Of the total 674–666 trees assessed annually, 50%, 31%, and 23% had free growth in 2020, 2021, and 2022, respectively (Figure 3). Out of 335 trees with free growth in 2020, 78% had an above medium intensity of free growth (based on % autumn budburst on the leader). As regards the type of free growth in autumn 2020, 2021, and 2022, 41%/9%, 14%/16%, and 7%/17% of all trees had sylleptic/proleptic free growth, respectively. The mean length of the longest sylleptic shoot that formed out of autumn budburst of the lateral buds on the leader of each tree sampled was 2.5 cm, 3.7 cm, and 3.8 cm in 2020, 2021, and 2022, respectively (the max. values, respectively were 29 cm, 26 cm, and 21 cm; Table S1), whereas the mean length of the longest proleptic shoot that formed out of the budburst of the apical buds on the leader was 10.1 cm, 7.6 cm, and 5.1 cm for 2020, 2021, and 2022, respectively (the max. values, respectively, were 38 cm, 11 cm, and 8 cm; Table S1).
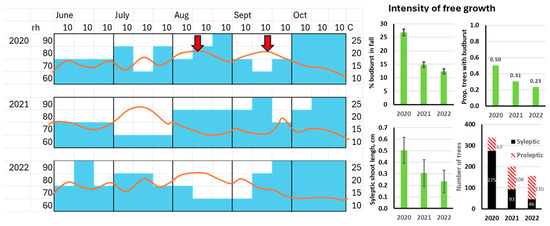
Figure 3.
Effect of autumn air temperature (T) and air humidity (rh) on intensity of free growth during the three seasons in 2020, 2021, 2022 (age 6 to 9 years). Simplified diagram of mean daily values of T and rh is given (see actual climatic data in Figure S4). The blue shadings show rh by decade to be accounted for in the rh scores to the left (60%–90%). The red line drawn over the humidity bars shows temperature to be accounted for by the scale on the right side of the box. The three bar plots show free growth intensity: % of budburst of all buds on the leader in fall (upper left), proportion of trees with more than one budburst on the leader in fall (upper right), and length of the longest lateral (sylleptic) shoot on the leader (lower left). Number of trees by type of free growth (lower right). Moist and warm August together with warm September, such as in 2020, is likely to promote free growth (shown by arrows).
The intensity of free growth decreased significantly with increasing age over the three years of observation (Figure 3). The most intensive free growth was recorded in autumn 2020, which was distinguished from the remaining two seasons by a moist and warm August together with a very warm September (Figure 3). For instance, for August 2020, 2021, and 2022, the mean monthly temperatures (standard error of 0.5 °C) were 18.0; 15.2, and 19.9, respectively (monthly means for maximum daily temperatures (°C) were 25.5, 21.7, and 28.9 for years 2020, 2021, and 2022, respectively). Seasonal variation in actual climatic data is shown in Figure S4.
The type of free growth also shifted markedly with age—from obvious dominance of sylleptic over proleptic free growth in 2020 to vice versa in 2022 (tree numbers with proleptic growth were 62, 107, and 110 in 2020, 2021, and 2022; Figure 3). A warm and moist August, together with the very warm September of 2020, may have promoted the sylleptic type of free growth (Figure 4). The heat spells in July of 2021 and August of 2022 seem to not have promoted sylleptic growth during the following autumn (Figure 3). The moist but cool August–September 2021 also did not raise sylleptic growth intensity to the levels of 2020. However, relatively higher moisture in September 2021 and 2022 may have led to a higher intensity of proleptic growth (Figure 3).
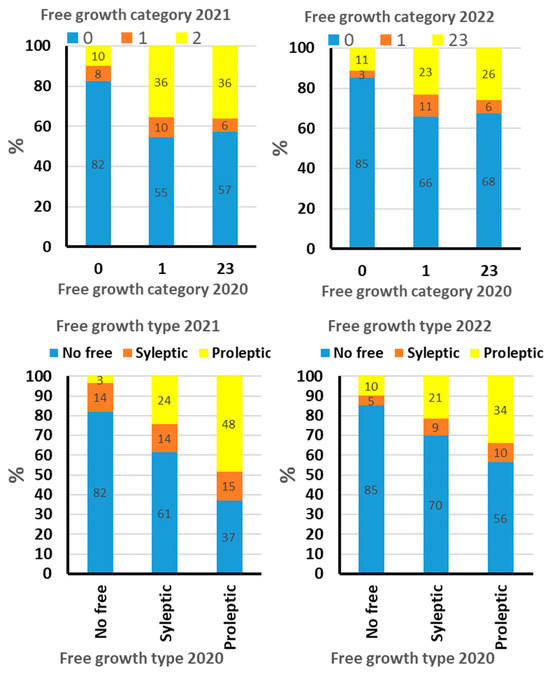
Figure 4.
The two upper plots: associations between intensity of free growth between 2020 and the two following seasons by taking the % of the autumn budburst on the leader as the indicator variable. Free growth category shows the shares of trees that fall into the following three autumn budbust intensity classes: 0 = no budbust (no free growth), 1 = up to 30% of the buds on the leader busted in autumn and 23 over 30% autumn budbust ton the leader (classes 2 and 3 pooled). The lower two plots show ability to retain specific type of free growth during the three seasons.
Of the 315 trees without free growth in 2020, only 18%–15% switched to free growth in 2021 and 2022. Of all the trees with intensive free growth in 2020 (over 30% autumn budburst on the leader), nearly half (37%) retained the same free growth intensity in 2021 and one-third (37%) in 2022 (Figure 4).
As regards the ability to retain the type of free growth over the seasons, (a) only 38% to 30% of the 272 trees with sylleptic growth in 2020 retained the sylleptic type of free growth over the two subsequent seasons by mainly losing free growth (only 14 to 9% switching to proleptic), and 61 to 70% of the sylleptic trees lost free grow in 2021 and 2022, (b) while the trees with proleptic free growth in 2020 (61 of such trees out of 660) better retained the proleptic type of free growth—48 and 34% of the proleptic trees remain as such in 2021 and 2020; only 37 and 56% of the proleptic trees lost their free growth the two subsequent seasons (Figure 4).
The age–age correlations estimated on individual tree levels between the free growth variables were medium-high but highly significant. An exception was the elongation length of the proleptic top shoots, which had low age–age correlations. The corresponding age–age correlations between spring budburst variables were high and significant (Table 3).

Table 3.
Age–age correlation coefficient and their significance between free growth and spring phenology variables (n = 649 trees individual level).
3.3. Site Effect on Free Growth
Site type had a highly significant effect on free growth intensity: The highest intensity was on the medium fertility temporarily overmoistured site types of LB and LC (Figure 4, within-year ANOVAs on-site effects return 0.0001 significance of p-values). In 2022, when the free growth was least intensive, the site effect was less pronounced (but significant on 0.05 p level). If considering the L site types, the free growth intensity decreased with increasing fertility of the site (Figure 4). Most of the trees with a proleptic type of free growth were observed on poor to medium rich L sites (especially the LC type, Figure 5).
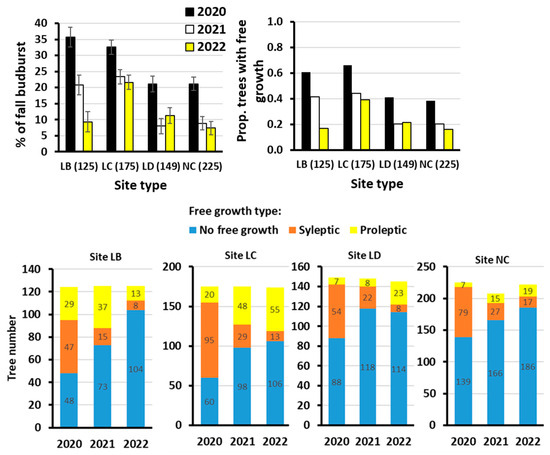
Figure 5.
Site effect on intensity free growth (based on % autumn budburst on the leader (upper left); the error bars are standard errors and tree frequency with autumn budburst on the leader (upper right)). The lower line of bar plots shows the effects of site type on shifting between the free growth types over the three years (data values at the bars are the tree numbers). Over the years, the proleptic type increased mainly in moist fertile sites (LC).
Another interesting question is what the site type effect is on retaining the intensity and type of free growth. On the LC site type, free growth intensity dropped the least over time (Figure 5). Also, on the LC type, the number of trees with proleptic free growth increased markedly over time (Figure 5).
3.4. Is Free Growth Related to the Size or Phenology of the Trees?
At the end of the experiment in 2022, the trees with free growth were significantly taller than the trees with no free growth (Figure 6). When compared within each site type in 2022, the proleptic trees were ca. 20 cm taller than sylleptic trees on most of the sites and 20–70 cm taller than the trees with no free growth on all sites. For the leader length, the differences were even more pronounced (Figure 7). In 2020, the proleptic trees had twice as long leaders as the trees with no free growth on all site types, followed by a lower superiority of leaders of trees with free growth in the two later seasons (LC and NC shown in Figure 7). The intensity of stem defects (spike knots and forking) was relatively higher for the trees with free growth, in particular for the proleptic type of free growth (Figure 6). The timing of spring budburst varied little among the trees, with different free growth intensities in the autumn of the same year (Figure 6). Trees with proleptic growth had stronger free growth intensity, as judged by the number of autumn budburst intensities (Figure 6).
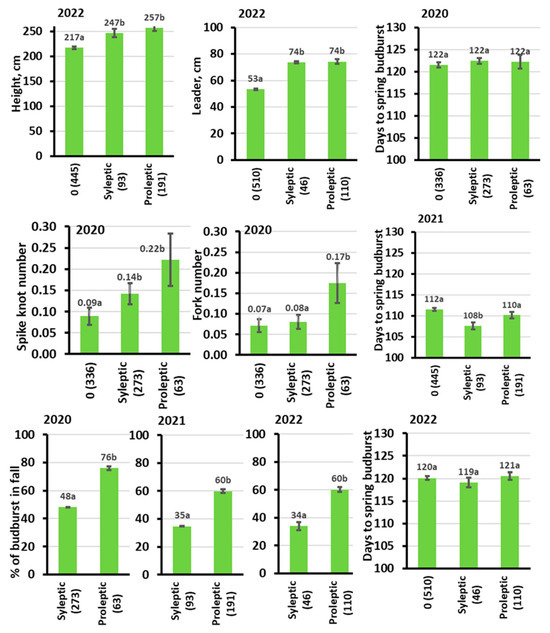
Figure 6.
Associations between the type of free growth and growth, sprig phenology, stem quality, and free growth intensity variables. The numbers at the X-axis labels are the sample sizes for trees with no free growth (0), sylleptic, and proleptic types of free growth. The letters at the data labels show the Tukey LSD test results, where the same letter indicates no significant difference at 0.05 p level. The error bars are standard errors.
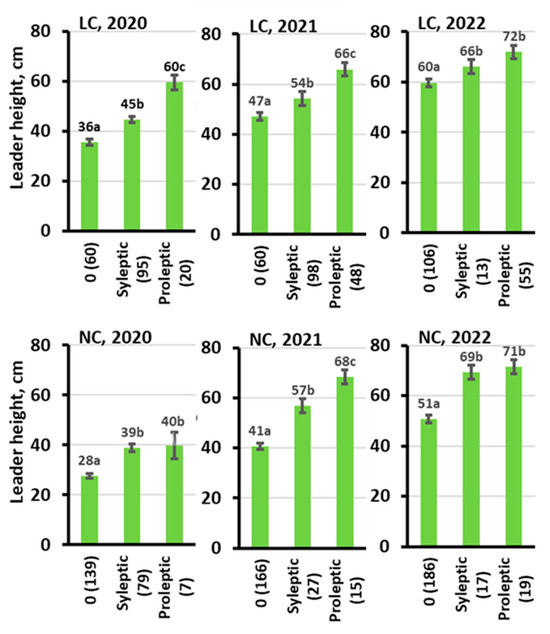
Figure 7.
Leader height for the trees with no free growth (0): sylleptic and proleptic types are given by site type and year. LC—temporarily overmoistured rich, NC—normally irrigated rich. The letters at the data labels show the Tukey LSD test results, where the same letter indicates no significant difference at 0.05 p level. The error bars are standard errors.
The strong and positive association between the intensity of free growth and tree height was confirmed by significant individual tree-level correlation coefficients (Table 4). The trees with more free growth tended to have more spike knots and forked trees. Surprisingly, the timing of spring budburst was uncorrelated with height growth during all years (Table 3 and Table S2). The correlation patterns among the traits were similar between the years (Table S2). However, when running the correlation analysis separately for seedlings without free growth, the correlation coefficients among tree height and days to budbust sage 3 in spring turned to significant (individual level for years 2020, 2021, 2022 r = 0.12 *, r = 0.11 *, r = 0.12 **, where the stars show significances at * = 0.01–0.05, and ** = 0.001–0.01, n = 336, n = 455, and n = 510 for the corresponding years). Meanwhile, for the trees with both types of free growth, the correlations between height and budburst in spring were highly insignificant and similar between the two styles of free growth (p for the r = from 0.4 to 0.8).

Table 4.
Pearson product–moment correlation coefficients and their significance (below the coefficient) among the growth, free growth, stem quality, and spring budburst traits for the year 2022 on individual tree level (n = 674). For the correlations in the remaining years, see Table S2. The variables are explained in Table 2.
4. Discussion
Our observations on 23%–50% intensity of free growth (9%–17% intensity of proleptic type) were higher than those for similar age, site type, and climate plantations of Norway spruce in Latvia (second flush/lammas shoots in 6.5% of all studied trees by Katrevics et al. [2] and 8% to 27% by Neimane et al. [34]). This result may support the theoretical forecasts for high intensity of free growth in Norway spruce plantations in the Baltic region and suggests initiating a regional monitoring initiative on free growth.
Year effects. In agreement with earlier studies, we observed a drastic decrease in sylleptic free growth in the same individual trees from age 6 to age 9. By age 9, our assessments approached the typical age limit for free growth in Norway spruce [20,21]. Consequently, in our study, it is challenging to determine what had the decisive effect on the intensity of free growth—the age of trees or favorable climatic conditions. For instance, in a Norway spruce field test in Sweden, free growth intensity dropped from 38% to 22% between ages 6 and 5 [6]. In 2021, August–September was moist but cool, while in 2022, August and the first half of September were warm but dry (Figure 3). Only in 2020 was August–September simultaneously moist and warm, providing the conditions promoting free growth in spruce [8,24]. In contrast to [2], based on observations of 2021, our study shows that in the absence of moist weather, high temperatures in July do not promote free growth in Norway spruce. It rather seems to lead to a drought and exhaust the resources of trees.
How stable free growth is retained by individual trees over the seasons. For ages 6 to 9, as much as 82%–84% of the trees without free growth tended to remain such over the following two seasons (Figure 4). This indicates a strong genetic component in sustaining young Norway spruce from switching to free growth. However, for the trees that already had free growth in 2020, as much as 55 to 68% lost free growth in the following seasons (Figure 4). Such a result may indicate that for Norway spruce trees with strong free growth competence, the environmental conditions affect free growth intensity more strongly than for those with free growth avoidance.
As regards retaining the specific type of free growth, clearly, the proleptic type was more stable over the years than the sylleptic type (on average, 45% of proleptic trees remained as such over the two seasons, versus 12% for the sylleptic trees, Figure 4). Consequently, the sylleptic type is under stronger environmental control than the proleptic type of free growth. Similar findings were reported by Neimane et al. [34], who found that 32.3% of the Norway spruce trees repeatedly had proleptic growth during three seasons in Latvia. Physiologically, the proleptic type may require a more discrete endogenic control mechanism, as the status of apical meristem follows discrete stages: setting of visible buds in July, followed by a pause in active growth, and visible bud burst and elongation towards the autumn. It seems that in comparison with the predetermined buds, the proleptic buds do not enter the endogenic dormancy immediately after the budset and remain longer in the state of exogenic dormancy; therefore, they do not require chilling for secondary budbreak during the coming fall. Our study supports the hypothesis that such a proleptic type of free growth is under a stronger genetic control than the sylleptic type, where no true buds are set, and the initiation of a stem unit is immediately followed by its expansion.
Site type effects. In agreement with numerous studies [2,25,34], we also found a significant site effect on the intensity of free growth. The moist site types (L type) had more free growth during all seasons than the normally irrigated site (N), and this result is based on large sample sizes. A possible explanation of this finding is that as a nutrition resource, moisture promotes free growth [25]. However, high soil fertility had an effect opposite to that of moisture, reducing free growth and increasing soil fertility (LB (poor) to LD (rich) site types in Figure 5). Consistently over all three seasons, most of the proleptic type of free growth occurred on the temporarily overmoistured poor to medium rich sites (9%–29% of all trees in LB, LC versus 3%–15% in the remaining sites). Our finding is not easy to compare with other studies reporting positive effects of site fertility on the free growth of Norway spruce because these studies were carried out in plantations on normally irrigated sites [2,25,34].
Shall we select for or against free growth (trait associations)? The first scrutiny could be on what we obtain if, at age 8, we select the 20% tallest trees by ignoring free growth and other traits (Figure 2). The highest height growth improvement we would obtain is on the richest LD site type. This result can be explained by a higher differentiation in tree height in Norway spruce plantations on rich sites, such as LD (380 cm for the 20% tallest vs. 252 cm mean of the remaining trees on the LD site, Figure 2). This is likely due to relatively stronger microsite variation in such sites as LD. Next, on all the site types, we would (a) markedly elevate the intensity of free growth, (b) not markedly change the timing of growth onset in spring, and (c) markedly increase the incidence of spike knot defects (Figure 2). Thus, certainly, free growth should be included in selection indexes for Norway spruce trees tested in experimental plantations for commercial purposes to differentiate between the candidates that are superior because of free growth or because of other reasons, such as late growth cessation or growth vigor. The absence of significant associations between free growth and spring phenology has a positive aspect because selecting for free growth will not significantly alter the phenology rhythm of the selected material out of adaptively required limits.
The significant individual-level correlation coefficients observed during the three growth periods indicate that free growth significantly increases tree height. However, this comes with a significant drawback—an increased number of trees with stem defects, such as spike knots and stem forking (as observed for Norway spruce by, e.g., Søgaard et al. [6]). In this way, a higher intensity of free growth may reduce the adaptedness of Norway spruce trees on frost-prone sites. We explain these results by the low frost hardiness of the trees with free growth to withstand frost spells in late autumn and winter [6,35]. At a mature age, such trees will likely have a lower wood quality because of spike knots, forks, and double whorls.
Further analysis of the trait associations based on the type of free growth reveals that in comparison with sylleptic growth, proleptic growth produces significantly longer leader shoots but only slightly taller trees with noticeably lower stem quality and more intense free growth (as indicated by increased lateral budburst on the leader) (Figure 6 and Figure 7). Therefore, in Norway spruce breeding, trees with proleptic growth are generally less desirable than those with sylleptic growth. Selecting for sylleptic growth could lead to a 14% height increase by age 9, with stem defect scores remaining comparable to those of trees without free growth (Figure 6). As regards proleptic growth, our findings agree well with conclusions from other studies on markedly higher incidences of tree forking for the trees with lammas shooting in young Norway spruce plantations [2,6,25,34]. However, for sylleptic growth, we would rather support the suggestions of Mboyi and Lee [36] for Sitka spruce that selecting trees for total tree height may exploit the potential benefits of free growth, without markedly increasing the risk of autumn or winter frost damage.
Our finding of low and insignificant correlations between tree height (of all trees) and growth onset in spring contradicts most of the earlier studies in Norway spruce [18]. Therefore, after investigating this association in greater detail, we found that for the trees without free growth, the correlations between tree height and the day number to budburst in spring turns out to be significantly positive, while there was no improvement for the trees with free growth. Such results are consistent for all three seasons with large sample sizes. These correlations show that for the trees without free growth, the taller trees had later growth onset in spring, which agrees well with earlier studies [18]. One way to explain such weak associations between the height and spring phenology of the trees with free growth is the effect of random environmental variation on the length of free growth shoots. Apparently, in the case of free growth, the trees with late and early spring growth onset may approach similar height if, after the second budburst, the earlier elongates less than the latter because of microsite or other random variation. For instance, von Wuehlisch and Muhs [37] reported that free growth in young Norway spruce is influenced strongly by the environmental conditions and seems not to be influenced by the amount of preceding predetermined growth, which holds strong phenology cycle associations. Here, the type of free growth may also play a role, where the trees of sylleptic type tend to have an earlier spring growth onset than the trees of proleptic type (Figure 6). Regarding other studies, Neimane et al. [34] and Skrøppa and Steffenrem [25] reported that the relatively taller and earlier flushing young Norway spruce trees had a significantly higher frequency of free growth in field plantations in Latvia and Norway.
5. Conclusions
In conclusion, our study of 6-to-9-year-old Norway spruce plantations on common site types for Norway spruce in Lithuania showed that (a) mild and warm August to early September promote free growth, while hot and dry summer does the opposite; (b) over the seasons, the proleptic growth is maintained by individual trees stronger than the sylleptic type of free growth; (c) free growth intensity is weakly associated with spring phenology and seems to disrupt the well-known associations between phenology traits within the annual cycle; and (d) free growth leads to taller trees but high stem defects; however, when selecting for breeding purpose, sylleptic is preferable over proleptic growth owing to markedly lower numbers of forked trees.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f15111965/s1, Table S1. Mean data for the traits by year (n is the number of trees). CV coefficient of variation. Table S2. Pearson product moment correlation coefficients and their significance (below the coefficient) among the growth, free growth, stem quality and spring budbust trats for year 2022. The variables are explained in Table 1. Figure S1. Effect of site type on mean height and leader shoot elongation in the Norway spruce plantations established in 2015–2016. The error bars show standard errors, the number at the X-axis labels are the sample sizes. L–temporarily overmoistured, N-normally irrigated. B, C, D in poor, medium and rich soils. The plantations are located within a 20 km radius. Leader height on the N site type was shorter than on the L types. Figure S2. Overall mean phenology scores and the standard errors for the years 2020, 2020, 2022 in the Norway spruce plantations studied. The diagram bars of assessment occasions with the largest overall variation at the budburst stages 3–4 are highlighted in red. Figure S3. Comparison of mean budbust timing in spring among the site types. Norway spruce on high moisture sites (L) tends to start active growth earlier than normally irrigated sites. Budburst on the L types occurs later than on N type. Figure S4. Seasonal variation in mean daily air temperature (°C, left) and relative humidity (%, right) in the study area for years 2020, 2021 and 2022) shown as the line series in the plots. The X axis labels are the starting date of a decade (e.g., 9.21 means the 21st of September).
Author Contributions
Formal analysis, D.D.; Data curation, S.Š. and G.Š. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kvaalen, H.; Søgaard, G.; Steffenrem, A. Environmental and genetic effects on lammas growth of Norway spruce. In Proceedings of the Abstracts of International Scientific Conference Adaptation of Trees and Stands to Forest Disturbances: Management Considerations, Riga, Latvia, 18–21 October 2010; p. 13. [Google Scholar]
- Katrevics, J.; Neimane, U.; Dzerina, B.; Kitenberga, M.; Jansons, J.; Jansons, A. Environmental Factors Affecting Formation of Lammas Shoots in Young Stands of Norway Spruce (Picea abies Karst.) in Latvia. iForest-Biogeosci. For. 2018, 11, 809–815. [Google Scholar] [CrossRef]
- Häggström, B.; Lutter, R.; Lundmark, T.; Sjödin, F.; Nordin, A. Effect of arginine-phosphate addition on early survival and growth of Scots pine, Norway spruce and silver birch. Silva Fenn. 2023, 57, 22013. [Google Scholar] [CrossRef]
- Stöckli, R.; Vidale, P.L. European Plant Phenology and Climate as Seen in a 20-Year AVHRR Land-Surface Parameter Dataset. Int. J. Remote Sens. 2004, 25, 3303–3330. [Google Scholar] [CrossRef]
- Hänninen, H. Effects of climatic change on trees from cool and temperate regions: An ecophysiological approach to modelling of bud burst phenology. Can. J. Bot. 1995, 73, 183–199. [Google Scholar] [CrossRef]
- Søgaard, G.; Fløistad, I.; Granhus, A.; Hanssen, K.H.; Kvaalen, H.; Skrøppa, T.; Steffenrem, A. Lammas shoots in spruce-occurrence, genetics, and climate. In Proceedings of the Forest Management and Silviculture in the North-Balancing Future Needs, Book of Abstracts for the Conference, Stjørdal, Norway, 6–8 September 2011. [Google Scholar]
- Forestry Statistics. Available online: https://amvmt.lrv.lt/lt/atviri-duomenys-1/misku-statistikos-leidiniai/misku-ukio-statistika (accessed on 1 October 2021).
- Dormling, I.; Gustafsson, A.; Von Wettstein, D. The Experimental Control of the Life Cycle in Picea abies (L.) Karst. Silvae Genet. 1968, 17, 44–64. [Google Scholar]
- Hänninen, H. The annual phenological cycle. In Boreal and Temperate Trees in a Changing Climate: Modelling the Ecophysiology of Seasonality; Springer: Berlin/Heidelberg, Germany, 2016; pp. 35–138. [Google Scholar]
- Jansson, G.; Danusevičius, D.; Grotehusman, H.; Kowalczyk, J.; Krajmerova, D.; Skrøppa, T.; Wolf, H. Norway Spruce (Picea abies (L.) H.Karst.); Springer: Dordrecht, The Netherlands; Heidelberg, Germany; New York, NY, USA; London, UK, 2013; pp. 123–176. [Google Scholar]
- Langvall, O. Impact of climate change, seedling type and provenance on the risk of damage to Norway spruce (Picea abies (L.) Karst.) seedlings in Sweden due to early summer frosts. Scand. J. For. Res. 2011, 26 (Suppl. S11), 56–63. [Google Scholar] [CrossRef]
- Beuker, E. Adaptation to climatic changes of the timing of bud burst in populations of Pinus sylvestris L. and Picea abies (L.) Karst. Tree Physiol. 1994, 14, 961–970. [Google Scholar] [CrossRef]
- Hannerz, M.; Sonesson, J.; Ekberg, I. Genetic correlations between growth and growth rhythm observed in a short-term test and performance in long-term field trials of Norway spruce. Can. J. For. Res. 1999, 29, 768–778. [Google Scholar] [CrossRef]
- Skrøppa, T. Withinpopulation variation in autumn frost hardiness and its relationship to budset and height growth in Picea abies. Scand. J. For. Res. 1991, 6, 353–363. [Google Scholar] [CrossRef]
- Partanen, J.; Häkkinen, R.; Sirkka, S.; Viherä-Aarnio, A.; Zhang, R.; Hänninen, H. Endodormancy release in Norway spruce grafts representing trees of different ages. Tree Physiol. 2021, 41, 631–643. [Google Scholar] [CrossRef]
- Danusevičius, D.; Persson, B. Phenology of natural Swedish population of Picea abies as compared with introduced seed sources. For. Genet. 1998, 5, 211–220. [Google Scholar]
- Hannerz, M. Genetic and Seasonal Variation in Hardiness and Growth Rhythm in Boreal and Temperate Conifers; No. 2. Report; Skogforsk: Boca Raton, FL, USA, 1998. [Google Scholar]
- Danusevičius, D.; Garbrilavičius, R. Variation in juvenile growth rhythm among Picea abies provenances from the Baltic states and adjacent regions. Scand. J. For. Res. 2001, 16, 305–317. [Google Scholar] [CrossRef]
- Ekberg, I.; Eriksson, G.; Namkoong, G.; Nilsson, C.; Norell, L. Genetic correlations for growth rhythm and growth capacity at ages 3–8 years in provenance hybrids of Picea abies. Scand. J. For. Res. 1994, 9, 25–33. [Google Scholar] [CrossRef]
- Jablanczy, A. Changes due to age in apical development in spruce and fir. Bi-Mon. Res. Notes Can. For. Serv. 1971, 27, 10. [Google Scholar]
- Pollard, D.F.W.; Logan, K.T. The Role of Free Growth in the Differentiation of Provenances of Black Spruce Pice amariana (Mill.) B.S.P. Can. J. For. Res. 1974, 4, 308–311. [Google Scholar] [CrossRef]
- Ununger, J.; Ekberg, I.; Kang, H. Genetic control and age-related changes of juvenile growth characteristics in Picea abies. Scand. J. For. Res. 1988, 3, 55–56. [Google Scholar] [CrossRef]
- Wühlisch, G.; Muhs, H.J. Influence of Age on Sylleptic and Proleptic Free Growth of Norway Spruce Seedlings. 1986. Available online: https://literatur.thuenen.de/digbib_extern/dn051699.pdf (accessed on 20 August 2024).
- Aldén, T. Influence of CO2, Moisture and Nutrients on the Formation of Lammas Growth and Prolepsis in Seedlings of Pinus silvestris L. 1971. Available online: https://pub.epsilon.slu.se/5772/ (accessed on 20 August 2024).
- Skrøppa, T.; Steffenrem, A. Selection in a provenance trial of Norway spruce (Picea abies L. Karst) produced a land race with desirable properties. Scand. J. For. Res. 2016, 31, 439–449. [Google Scholar] [CrossRef]
- Cline, M.G.; Constance, A.H. Apical Dominance and Apical Control in Multiple Flushing of Temperate Woody Species. Can. J. For. Res. 2007, 37, 74–83. [Google Scholar] [CrossRef]
- Lundströmer, J. Adaptation of Norway Spruce (Picea abies (L.) Karst.) to Current and Future Climatic Conditions. Ph.D. Thesis, Acta Universitatis Agriculturae Sueciae, Original Trukeri, Umea, Sweden, 2021. Volume 2, 86p, ISSN 1652-688. [Google Scholar]
- Schmidt-Vogt, H. Genetics of Picea abies (L.) Karst. Ann. For. 1978, 7/5, 147–186. [Google Scholar]
- Eriksson, G. Picea abies: Recent Genetic Research; Genetic Center, Department of Plant Biology and Forest Genetics, Swedish University of Agricultural Sciences: Uppsala, Sweden, 2010; p. 197. [Google Scholar]
- Kohmann, K.; Johnsen, Ø. Effects of early long-night treatment on diameter and height growth, second flush and frost tolerance in two-year-old Picea abies container seedlings. Scand. J. For. Res. 2007, 22, 375–383. [Google Scholar] [CrossRef]
- Karazija, S. Types of Lithuanian Forests. Monograph; Mokslas: Vilnius, Lithuania, 1988; 210p. [Google Scholar]
- Vaičys, M. Types of Forest Vegetation; Lututė: Kaunas, Lithuania, 2006; 96p. [Google Scholar]
- Krutzsch, P. An Investigation on Bud Set in Norway Spruce (Picea abies); Report no. 6; Department of Forest Genetics and Plant Physiology, Swedish University of Agriculture Sciences: Umea, Sweden, 1986; pp. 21–32. [Google Scholar]
- Neimane, U.; Zadina, M.; Sisenis, L.; Dzerina, B.; Pobiarzens, A. Influence of Lammas Shoots on Productivity of Norway Spruce in Latvia. Agron. Res. 2015, 13, 354–360. [Google Scholar]
- Hannerz, M.; Westin, J. Autumn Frost Hardiness in Norway Spruce plus Tree Progeny and Trees of the Local and Transferred Provenances in Central Sweden. Tree Physiol. 2005, 25, 1181–1186. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mboyi, W.M.; Lee, S.J. Incidence of autumn frost damage and lammas growth in a 4-year-old clonal trial of Sitka spruce (Picea sitchensis) in Britain. For. Int. J. For. Res. 1999, 72, 135–146. [Google Scholar] [CrossRef][Green Version]
- Wuehlisch, G.; Muhs, H. Environmental influences on juvenile shoot growth in Picea abies. Scand. J. For. Res. 1991, 6, 479–498. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).