Abstract
Highlights: Though not highly invasive, bulb and corm ornamental plants can escape cultivation and naturalize in new areas. Studying their naturalization is key to understanding their ecological impact and managing biodiversity. Objectives: This study aimed to document the first naturalization case of Crocus tommasinianus Herb. in Poland and assess the morphological variability of the naturalized population under different environmental conditions. Another objective was to identify diagnostic features in seed testa ornamentation to distinguish C. tommasinianus from related species (C. vernus (L.) Hill. and C. scepusiensis (Rehmann et Wol.) Borbás ex Kulcz.). Methods: The morphometric studies were performed within four subpopulations of C. tommasinianus differing in environmental conditions, determined with Ellenberg indices. Multivariate tests, ANOVA, and post-hoc tests were used to determine the morphometric diversity of specimens and to relate them to environmental factors. Seed micro-ornamentation was examined using a scanning electron microscope. Results: Light and temperature were negatively correlated, while moisture, soil pH, and nitrogen were positively correlated with many morphological traits. Plants spreading into forest ecosystems exhibited better-developed features (larger leaves and flowers) than those in former cultivation sites, indicating higher survival potential. The seed coat is papillate, with distinct differences in the shape, size, and secondary sculpture of the papillae compared to C. vernus and C. scepusiensis. Given that floristic studies often occur during the fruiting period of crocuses, testa ornamentation is crucial for identifying the studied species. Conclusions: The observed naturalization of C. tommasinianus demonstrates the high morphological plasticity of plants, which makes them capable of colonizing new areas, including forest habitats.
1. Introduction
1.1. Problem of Alien and Invasive Plant Species in Europe and in Poland
Any live specimens (or plant parts that can reproduce) of a species, subspecies, or lower taxon introduced outside its natural range as a consequence of human intervention are classified as alien [1]. Species migration and changes in their distribution ranges, resulting from intentional and unintentional human activity, are a global problem that concerns all parts of the world to a greater or lesser extent [2,3,4,5,6,7,8]. It is estimated that the introduction of alien species to Central Europe peaked in the 19th century, and later, it was slightly slower [9]. Currently, Europe ranks second (after North America) in terms of the number of naturalized alien species, estimated at 4140. Most of them are species deriving from other parts of Europe and the temperate zone of Asia but also from Africa, North America, tropical Asia, and South America [4].
Considering exclusively the area of Poland, it can be concluded that the degree of anthropogenic transformation of flora is high, as indicated by the high proportion of alien species to native ones, namely 30% to 70%, respectively [10,11]. Among the alien species introduced since the end of the 15th century, those deriving from Europe prevail (35%), followed by those from North America (30%) and Asia (24%) [10]. About 4% of alien species naturalized in Poland are invasive, i.e., spreading dynamically in the natural environment and threatening biodiversity, as they transform habitats and outcompete native species [12]. It is very challenging to slow down the rate of the spread of invasive species, such as Reynoutria japonica Houtt, R. sachalinensis (F. Schmidt) Nakai, R. ×bohemica Chrtek et Chrtkova, Impatiens parviflora DC, I. glandulifera Royle, Heracleum sosnowskyi Manden., H. mantegazzianum Sommier et Levie, Solidago canadensis L., and S. gigantea Aiton. It requires individualized and complex actions linked with high costs [13,14,15,16]. In the case of non-invasive alien species, which spread slower and less strongly affect the environment, the major threat associated with them is so-called homogenization, i.e., a reduction in the floristic uniqueness of individual regions and the loss of local, specific features within ecosystems [17,18,19,20,21]. That is why one of the key tasks in biodiversity protection, related to the spread of alien species, is currently the early detection of the spread of each alien species and its later monitoring.
1.2. Horticulture and Bulbous Plants in the Context of the Spread of Alien Species
Horticulture—linked with the import of ornamental plants, e-commerce, and plant cultivation—is currently the most important way of introducing new plant species to areas where they did not occur before [22,23]. Most of the introduced and cultivated species remain only in a place of cultivation, but some start to spread, reproduce, and establish in the wild [22]. This phenomenon, called the naturalization of alien species, allows the creation of stable populations in new environments, with or without direct human activity [24]. The number of alien plants escaping from cultivation into native ecosystems is steadily increasing; currently, at least 75% and 93% of the global naturalized alien flora are grown in private and botanical gardens, respectively [25].
An important role in the global ornamental plant trade is played by bulb and corm plants. They belong to over 800 genera, but only a few of them dominate in the trade: Tulipa L., Lilium L., Narcissus L., Gladiolus L., Hyacinthus L., Crocus L., and Iris L. [26], as well as Scilla L., Ornithogalum L., Galanthus L., and Allium L. Bulb and corm geophytes, are not highly invasive plants [27], but many species tend to escape from cultivation and become naturalized in natural and semi-natural ecosystems. All the above-mentioned genera are currently naturalized outside their natural ranges of distribution, and the size of their secondary ranges is usually not larger than their primary ranges [28]. In Poland, only two of them are classified as naturalized—Tulipa sylvestris L. and Crocus vernus (L.) Hill [10]—although the list of naturalized bulb and corm plants will probably be soon extended to include other taxa.
The naturalization success of many ornamental plants results from the preference of a majority of gardeners for plants that are easy to cultivate [29], i.e., have a wide ecological spectrum. In the case of bulb, corm, and tuber plants, their advantage is also the ability to reproduce both vegetatively, by means of new bulbs or tubers, and sexually (with few exceptions) through seed dispersal. The geophytes that escape from cultivation by means of seeds in cemeteries and in former manor parks in Poland include Ornithogallum umbellatum L., O. nutans L., O. boucheanum Asch., Scilla sibirica Haw., Chionodoxa sardensis Barr. et Sudg., Ch. Forbesii Baker, Eranthis hyemalis (L.) Salisb., and Tulipa sylvestris [30,31,32,33,34]. For example, Ornithogallum umbellatum was found on roadsides, meadows, and forest edges, near allotments (small gardens), and even in a national park [35]. Investigations in Lower Saxony [35] showed that due to vegetative reproduction, some populations of Tulipa sylvestris have survived as a cultural relic for at least 250 years in places of their former cultivation (e.g., historical parks and churchyards), forming populations composed of over 10,000 individuals. The spontaneous dispersal of crop plants can be additionally supported by human activity. Ornamental geophytes can escape to the natural environment with discarded plant matter from gardens and composted waste [22]. This applies, for example, to species of the genera Crocus, Galanthus, and Scilla. In contrast, the increasing range and number of localities of the invasive Allium paradoxum are partly due to its intentional dispersal by wild food lovers [36].
1.3. Crocus tommasinianus—A Cultivated, Alien, Naturalized Species
The genus Crocus includes 261 species [37], which are grouped into two subgenera, two sections, and 15 series [38,39,40,41]. Species from this genus are native to the Mediterranean basin (North Africa, Iberian Peninsula), South and Central Europe, and West Asia, reaching as far east as western China, with the largest number of species in the Balkan Peninsula and Asia Minor [42]. As a result of naturalization, the genus Crocus now also occurs in North America [28].
In the past, many Crocus species were introduced to botanic gardens and then distributed [43]. Today, numerous species of this genus are cultivated as ornamental plants [44], but the most popular in cultivation are Crocus chrysanthus (Herb.) Herb., Crocus flavus Weston, Crocus sieberi J. Gay, Crocus tommasinianus Herb. and Crocus vernus, together with hundreds of varieties and hybrids derived from them [45]. Due to their bright colors and multitude of varieties, they are valued for their decorative appeal. They are also relatively easy to grow [43], which makes them popular among both experienced gardeners and amateurs. Crocuses adapt to almost all soils, respond well to organic fertilizers, and tolerate sunny, semi-shady, and shady locations [46].
In Central Europe, most cultivated Crocus species bloom in early spring, after a period of dormancy in winter [44]. One of the smallest [46] and earliest blooming species [47] is Tommasini’s crocus, Crocus tommasinianus. Two synonymous English names are used to refer to this species, which reflect its characteristics well: early crocus [48] and woodland crocus [49]. Sometimes, the less formal name snow crocus is also used [50]. C. tommasinianus can be observed in winter—at the end of February or in early March—and sometimes in spring until early April. It typically begins flowering 2–3 weeks earlier than the very commonly cultivated C. vernus [47], although the cross-pollination of both species and spontaneous introgression are occasionally observed [47,51].
C. tommasinianus forms corms with a distinctly reticulate tunic from which three to five linear leaves grow 4–8 mm wide. The flowers are large and slender, 2–4 cm long and 4–5 cm in diameter, with a perianth tube diameter of about 2–3 mm. Their color ranges from deep violet or purple to snow white, but the perianth tube is always clear white. The style is shortly divided into three branches, each of them fimbriate at the apex; the stigma does not exceed the stamens. The plant height is 7–10 cm [38,42,52,53].
This species naturally occurs only on the Balkan Peninsula: from southern Hungary and northwestern Bulgaria to the West Balkans, namely Croatia, Bosnia-Herzegovina, Montenegro, and Serbia [28,38,54], where it is found in forests and other shaded areas [55,56]. It is a particularly useful winter or early spring flowering species in designed landscapes [57], often grown in gardens and parks. Under favorable conditions, it multiplies quickly by tubers and by self-seeding, so it can escape from cultivation [58,59]. Its naturalization has been recorded in Europe (Belgium, Germany, Great Britain, The Netherlands) and in the eastern part of North America (Delaware, New York, Virginia) [28]. The process of escaping from cultivation was interestingly described by [47], based on observations in London, where colonies of fair size spread especially along shady, lightly wooded parkland verges, where the soil is not heavily compacted.
Since the naturalization of this species was not recorded earlier in Poland [28,60], the main goal of our study was to document the first naturalized case of C. tommasinianus and to analyze the causes and possible consequences of this process.
Another goal was to determine the morphological variability of the local population depending on environmental conditions, in the context of species expansion from the place of introduction to a forest community.
The basis of every species monitoring effort is species identification. In the case of crocuses (especially from the Verni series, to which C. tommasinianus belongs), identification is difficult due to the close relationship of other species, related to polyploidization [38,40,61,62,63]. This results in high interspecific morphological similarity and the high intraspecific morphological variability of macroscopic features. In such cases, searching for microscopic features is very justified. In the case of the Verni series, the features analyzed so far concerned the anatomical structure of the leaves [64,65,66]. There are some studies on seeds of C. tommasinianus and C. vernus, but so far, they have only considered the primary sculpture of the testa without characterizing the secondary sculpture [67,68]. Seed micro-ornamentation patterns are constant and not very susceptible to environmental influences, making them more reliable for systematic purposes than other macro- and micromorphological features [69]. For these reasons, the third aim of the study was to search for new diagnostic features of C. tommasinianus based on the primary and secondary testa sculpture and comparison with seeds of two closely related species from the Verni series occurring in Poland: C. vernus and C. scepusiensis (Rehmann and Woł.) Borbás (not yet studied for testa micro-ornamentation).
2. Materials and Methods
2.1. Description of the New Location
Field research was conducted in 2023 in the Wielkopolska National Park (WNP, western Poland; 52°16′09.5″ N 16°47′82.0″ E; Figure 1). WNP climate is temperate, transitional between oceanic and continental, with mean annual temperature of 8.4 °C and mean annual precipitation of 521 mm, for the years 1951—[70].
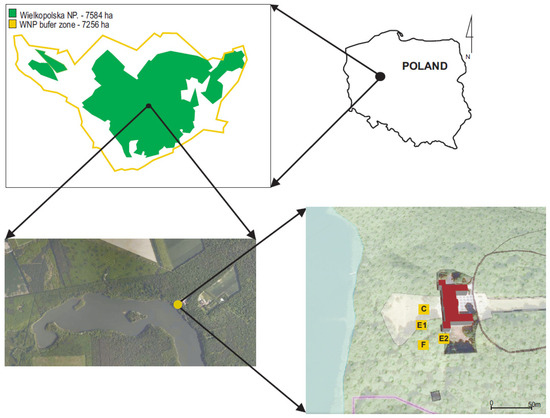
Figure 1.
Location map of the study area. Abbreviations: WNP—Wielkopolska National Park, C—clearing, F—oak forest (Potentillo albae-Quercetum), E1—ecotone1, E2—ecotone2.
Crocus tommasinianus was found here to be spreading from a former cultivation site (in a clearing near a palace building) to a nearby forest. The local population was first observed at the described site in 1994, but it was not identified as a species at that time (A. Czarna—unpublished data). Recently, the species has been determined at the flowering stage, based on the key and descriptions included in the works of Mathew et al. [38] and Dostál [53]—Figure 2.

Figure 2.
Crocus tommasinianus in the Wielkopolska National Park: (a) plant habit; (b) group of specimens (photo: A. Czarna, 9 March 2024).
In the study area, the species covers about 500 m2. Currently, four groups (i.e., subpopulations) can be distinguished: (1) in a clearing (place of former cultivation), (2) in a nearby oak forest, (3) at the edge of the oak forest and the clearing (hereinafter referred to as ecotone1) and far away from buildings, and (4) at the edge of the oak forest and the clearing, but in a site partly shaded by the palace building (hereinafter referred to as ecotone2)—Figure 1.
In a representative part of each subpopulation (i.e., representing its typical density and distribution of specimens), a plan of the distribution of specimens was made in a 1 m × 1 m plot. In these places, in areas of 10 m2, phytosociological relevés characterizing the local vegetation were also taken. The species lists were prepared separately for three layers of vegetation: trees (A), shrubs (B), and herbaceous (C). The names of species were given after Mirek [60]. The Braun-Blanquet cover-abundance scale was used to show the shares of the recorded species [71].
Phytosociological data are often used to infer environmental characteristics [72]. Therefore, based on the prepared relevés and Ellenberg’s indices [73], average values of temperature, light, soil moisture, pH, and soil nutrients were calculated for the areas occupied by individual subpopulations.
2.2. Morphometric Measurements of Individuals
To determine whether habitat conditions affect the diversity of C. tommasinianus, the morphological features of 30 individuals from each subpopulation were measured. The number of leaves emerging from the corm was counted; the length of the longest leaf, the length of the flower (i.e., the sum of the total length of the perianth tube and the longest tepal), the length of the outer tepal, the width of the outer tepal, the length of the anther and filament, as well as the length of the stigma and style were measured. The measurements were taken using a 10 cm VIN caliper with an accuracy of 0.05 mm.
2.3. Carpological Examination
To determine whether C. tommasinianus is carpologically distinguishable from two closely related species from the Verni series (C. vernus and C. scepusiensis), their seeds were analyzed. Carpological material for C. tommasinianus and C. scepusiensis came from the Botanical Garden of Adam Mickiewicz University in Poznan (Poland), and that for C. vernus came from the Index Seminum of the University of Ljubljana Botanical Garden (Slovenia). Three features (seed length, width, and elaiosome length) of 30 fully developed seeds of each species were measured under a stereoscopic microscope PZO MST 132 with an eyepiece micrometer scale (Polskie Zakłady Optyczne PZO, Warsaw, Poland). Seed-coat sculpture was studied using scanning electron microscope (SEM) micrographs prepared with a Zeis EVO 10 microscope (Carl Zeiss Microscopy GmbH, Jena, Germany).
2.4. Statistical Analyses
Principal component analysis (PCA) on the correlation matrix was used to examine the combined effects of environmental factors (i.e., average values of Ellenberg indicators) on morphological characters of Crocus tommasinianus. The analysis included morphometric measurements of all the studied specimens without prior division into subpopulations. The scree test was used to select the main components that significantly explained the variability of the set [74].
Discriminant function analysis (DFA) was used to determine which morphological characters most effectively differentiate the studied subpopulations. Before analysis, the quantitative characters were standardized to compare characters that were previously recorded in different units. The data were not transformed because they were normally or near-normally distributed (checked with a normality plot combined with the Kolmogorov–Smirnov test). The models were built using a backward stepwise procedure. The contribution of particular characters to the discrimination between groups was interpreted based on standardized coefficients.
Differences in morphometric and carpological characters between subpopulations were tested using ANOVA and Tukey’s HSD test.
3. Results
3.1. Description of the New Location
The terrain where Crocus tommasinianus occurs is undulating, with a slope of 10%–30%. Rusty brown soils were formed here on clayey sands [75]. The plants grew in a clearing in the adjacent forest ecosystem. In the clearing with a meadow ecosystem, the cover of the herbaceous layer is about 60%. The dominant species are Festuca pratensis Huds. and F. heterophyllaI Lam., with an admixture of dicotyledonous plants, among which the most abundant are Hieracium pilosella L. and Trifiolium repens L. (Appendix A). The forest ecosystem represents the habitat type of moderately moist deciduous forest, phytosociologically closest to an oak forest (Ass. Potentillo albae-Quercetum Libb. 1933, All. Potentillo albae-Quercion petraeae; O. Quercetalia pubescenti-petraeae). The forest stand is formed by approximately 100-year-old sessile oaks (Quercus petraea (Matt.) Liebl.) with an admixture of Carpinus betulus L., Acer platanoides L., Fraxinus excelsior L., Ulmus laevis Pall., and Pinus sylvestris L. The average density of the tree layer is about 50%. The shrub layer is poorly developed, covering up to 5%. The herbaceous layer coverage is about 60%, with no dominant species, although Poa nemoralis L. and Quercus petraea are abundant in some places. In the forest edge zones (referred to here as ecotones), a separate plant community is not formed. ecotone1 and ecotone2 differ in tree density (40% and 15%, respectively), the number of species in the herbaceous layer (36 and 11, respectively), and their coverage (50% and 65%, respectively). In the ecotones, certain ornamental bulbous species occur with a low degree of cover: Chionodoxa sardensis, Galanthus nivalis L., and Scilla sibirica.
The mean Ellenberg indices, calculated separately for each site of C. tommasinianus, were as follows: light (L) 5.00–7.61; temperature (T) 5.00–5.79; moisture (F) 4.07–5.40; soil pH (R) 5.22–7.00; nitrogen content (N) 3.80–5.83. The habitat conditions were quite similar in the forest and ecotones (especially ecotone1) and markedly different in the clearing, where the L value was significantly higher in the clearing, while the nitrogen content (N) was significantly lower (Figure 3).
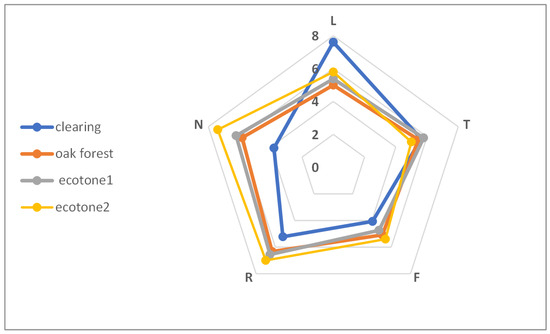
Figure 3.
Mean Ellenberg indicator values for light (L), temperature (T), moisture (F), soil pH (R), and nitrogen (N) calculated for sites of Crocus tommasinianus occurrence.
In 1994, the population of Crocus tommasinianus was larger, but it was limited to the clearing where it was cultivated, covering about 200 m2. The species did not show any tendency to spread at that time. Since then, the population has spread to the adjacent forested areas, now occupying about 500 m2, with only about 30 m2 remaining at the original cultivation site. In 2023, the density of this species (Figure 4) was the highest at the edges of the forest (about 100–140 individuals/m2) and the lowest in the clearing (fewer than 20 individuals/m2). The share of flowering individuals was very high—at least 95%—regardless of the place of occurrence.
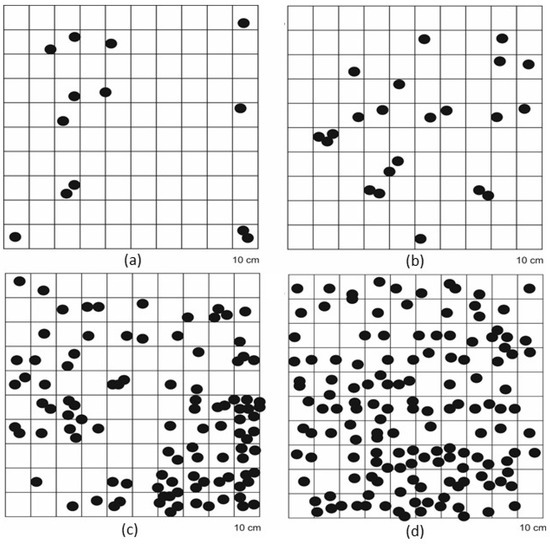
Figure 4.
Distribution of Crocus tommasinianus specimens on 1 m × 1 m plots: (a) clearing, (b) oak forest, (c) ecotone1, (d) ecotone2.
3.2. Morphometric Measurements of Individuals
The examined specimens markedly varied morphologically. Individual specimens had three to five leaves, with a mean of 3.90. At the time of flowering, the mean length of the longest leaf was 5.94 cm, with a range from 2 cm to 14.5 cm. Flower length varied from 4.5 cm to 16.5 cm (mean 8.49 cm), while the outer tepal length varied from 2.2 cm to 4.5 cm (mean 3.10 cm). Among the sexual parts of the flowers, the filament length was the most variable, ranging from 4 mm to 14 mm, with a mean of 8.35 mm (Table 1).

Table 1.
Descriptive statistics of the analyzed features of Crocus tommasinianus.
Principal component analysis (PCA) was used to examine the relationships between Ellenberg indicators and morphological characters. The first two principal components explained almost 60% of the total variance (Figure 5). Light (L) was negatively correlated with all the analyzed characters, most strongly with the length of the longest leaf (−0.54) and flower length (−0.47). Temperature (T) was negatively correlated with all characters except style length; the strongest correlations were with the length of the longest leaf (−0.61) and number of leaves (−0.56). Moisture (F), soil pH (R), and nitrogen content (N) were very strongly (r > 0.90) positively correlated with each other. Among the last three environmental traits, moisture had the strongest influence on the morphological characters, particularly on the length of the longest leaf (0.65), flower length (0.51), and outer tepal length (0.49).
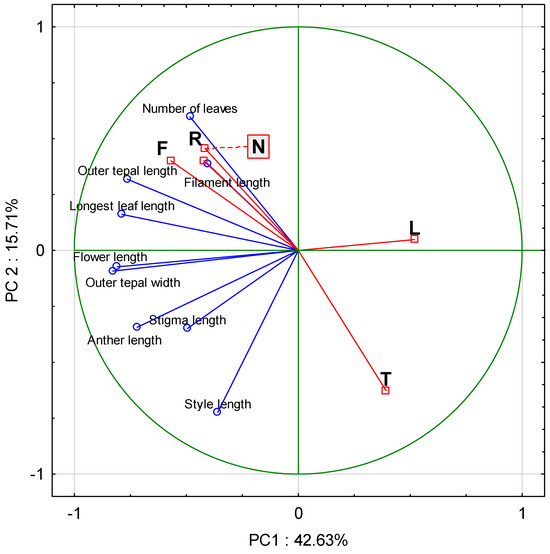
Figure 5.
Principal component analysis (PCA) biplot of morphological characters of Crocus tommasinianus and mean Ellenberg indicator values for light (L), temperature (T), moisture (F), soil pH (R), and nitrogen (N).
Based on the stepwise discriminant function analysis (DFA), seven out of nine morphological features significantly differentiated the studied subpopulations (Wilks’ lambda = 0.05038; F(27,316) = 20.859, p < 0.001). The first two discriminant functions explained 96% of the total variability in the tested material and had a significant impact on the discrimination (chi-square tests; p < 0.001). Almost 60% of the total discriminatory power was contributed by the first function, which primarily separated the forest subpopulation from ecotone1 and the clearing subpopulations (Figure 6).
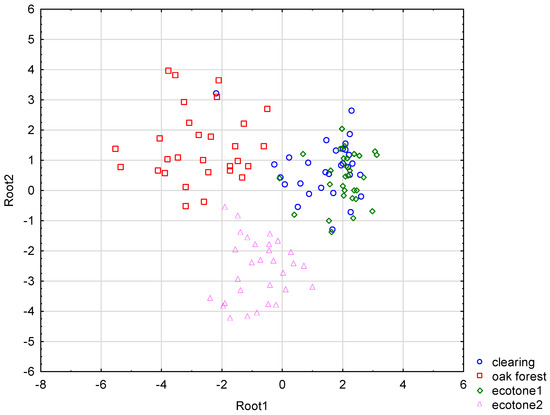
Figure 6.
Results of discriminant function analysis (DFA) based on 9 morphological features of specimens from the studied subpopulations of Crocus tommasinianus.
The first function was most heavily affected by the length of the longest leaf as well as the flower length (Table 2). The second function explained nearly 40% of the total variability and primarily separated the subpopulation in ecotone2 from the remaining subpopulations, with this distinction being driven primarily by various flower characters (flower length, outer tepal length, style length, and filament length), as well as the number of leaves.

Table 2.
Results of discriminant function analysis (DFA) based on morphological characters of Crocus tommasinianus. The highest values of standardized coefficients are bolded.
The results of the analysis of variance (ANOVA, Table 2) and the post-hoc tests (Figure 7) confirmed that the characters related to the size and robustness of the plants (number of leaves, longest leaf length, flower length) were crucial for distinguishing the described subpopulations.
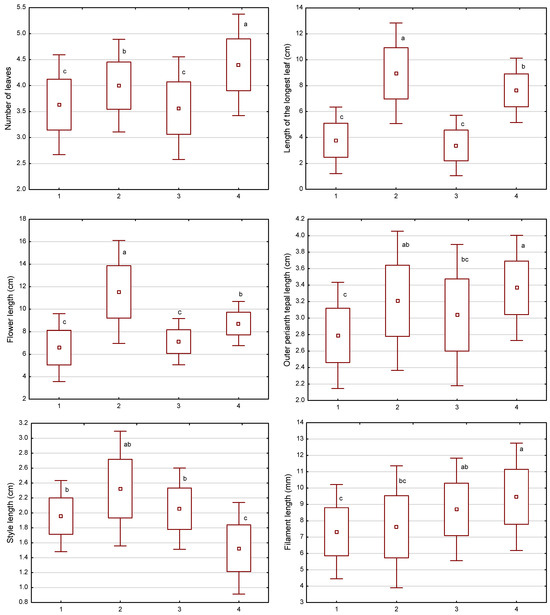
Figure 7.
The mean (point), mean ± SD (standard deviation, box), and mean ± 1.96*SD (whisker) for the morphological characters of Crocus tommasinianus identified by discriminant function analysis. The horizontal axis shows the groups (subpopulations) from different habitats: 1—clearing; 2—oak forest; 3—ecotone1; 4—ecotone2. Different lowercase letters indicate subpopulations that differ in a given character (Tukey’s HSD test, p < 0.05).
3.3. Carpological Examinaton
Crocus tommasinianus has brown seeds that are rounded, 2.43–3.00 mm long, and 1.63–2.24 mm wide, with an elaiosome length of 0.34–0.61 mm. The seed coat micro-ornamentation is papillate, with long papillae that taper towards the top, are flattened on both sides, and are slightly bent at the top. All papillae are more or less the same size, about 40 µm long and 10 µm wide at the base with wrinkled secondary sculpture (Figure 8, Table 3).
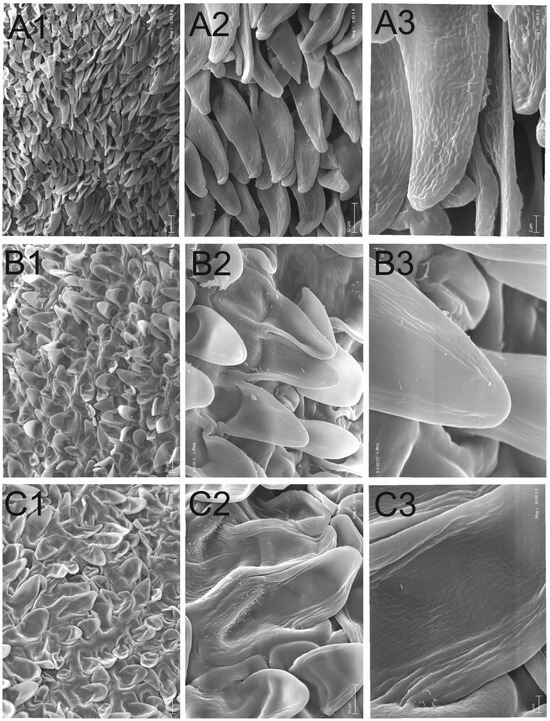
Figure 8.
Seed sculpture of examined taxa (SEM): Crocus tommasinianus (A), Crocus vernus (B) and Crocus scepusiensis (C). Magnifications: ×1000 (1), ×3500 (2), and ×10,000 (3).

Table 3.
Seed size in three species of the genus Crocus occurring in Poland. Different lowercase letters indicate species that differ in a given characteristic (Tukey’s HSD test, p < 0.05).
Crocus vernus has brown seeds that are rounded, 2.13–3.15 mm long, and 1.71–2.43 mm wide, with an elaiosome length of 0.30–0.53 mm. The seed coat micro-ornamentation is papillate; the papillae are equally long (about 25 µm), slightly tapering towards the top. Their width at the base is about 20 µm. Their dorsal side is convex, and the ventral side is concave. The base of the papillae is often visibly sunken. As for the secondary sculpture, the cuticle surface is slightly longitudinally wrinkled at the edges of the papillae, as well as on their concave side.
Crocus scepusiensis has brown seeds that are rounded, 2.77–3.69 mm long, and 1.90–2.28 mm wide, with an elaiosome length of 0.30–0.65 mm. The micro-ornamentation seed coat is papillate; the papillae are equally long, with an extended base and a slightly tapering top. They are about 30 µm long and 20 µm wide at the base. Their dorsal side is convex, and the ventral side is concave. As for the secondary sculpture, the cuticle surface is irregularly, longitudinally, and deeply wrinkled at the edges of the papillae and on the concave side, with wax platelets near the base.
A statistical analysis of seed size showed significant differences only in seed length: C. tommasinianus seeds are significantly shorter than C. scepusiensis seeds but do not differ significantly in length from C. vernus seeds.
4. Discussion
So far, only two species from the genus Crocus have been recorded in the wild in Poland: C. scepusiensis Rehmann et Wol. and C. vernus Hill [60], both of the Verni series. The first species is native and occurs in the southern part of Poland, particularly in the mountain ranges of the Western Carpathians [76]. In contrast, C. vernus is an alien species that often escapes from cultivation and becomes naturalized [10]. Our research indicates that a third species, C. tommasinianus, can now be included in the flora of Poland. According to the geographical-historical classification of floras [77], this species should be considered as an alien (anthropophyte) introduced and naturalized after the 15th century (metaphyte, kenophyte).
Although there have been no previous reports of C. tommasinianus escaping from cultivation in Poland, the described site is not unique within the country. Searching for other locations of C. tommasinianus in Poland, we examined the iNaturalist database [78]. Of the 36 locations identified by observers as C. tommasinianus, 10 are classified as reliable, and 3 are regarded as probable. Some of the confirmed locations were marked by observers as wild-growing individuals, although this is not always corroborated by the visible surroundings in the photographs. The vast majority of probably wild specimens are found in urban or rural areas near cultivation sites. However, at least one site seems to be situated in a forest, as indicated by the abundant presence of Ficaria verna Huds.
4.1. Description of the New Location
Crocus tommasinianus has been cultivated at the study site for at least 30 years. During that period, the distribution of individuals has changed markedly, reflecting the transformation from a cultivated species into a naturalized one. At the original introduction site (i.e., the clearing), the number of individuals is currently very small. The population has dispersed into nearby forest areas. We assume that the small number of individuals in the clearing is at least partly due to the unsuitable location for cultivation of this species, as C. tommasinianus is moderately shade-tolerant [38,53]. In the study area, it is more abundant under the canopy of trees, and all of the studied morphological characters are more or less negatively correlated with light indicator values. Due to its similarity to the commonly cultivated and sold C. vernus, it may have been mistakenly planted as C. vernus, which is known to prefer sunlight.
Another factor that affects the subpopulation of C. tommasinianus in the clearing is mowing. For all crocus species cultivated on lawns, it is recommended to delay mowing until the end of May to avoid damaging the leaves during photosynthesis, allowing the corms to accumulate the necessary nutrients for flowering in the following year [79,80]. Since C. tommasinianus is one of the smallest cultivated crocus species [46], mowing might not significantly damage its leaves. An experiment [24] on the growth and development of C. tommasinianus ‘Ruby Giant’ on a lawn composed of highly competitive warm-season turfgrasses showed that strong competition, herbicides, and lawn mowing did not affect the cultivar’s bulb survival and flowering, suggesting that typical lawn management practices are not detrimental to the bulbs. However, the cultivar ‘Ruby Giant’ used in the experiment is sterile [81]. The population observed in our study sets fruits (capsules) that ripen in summer. During ripening, the pedicel elongates significantly, raising the capsules above the ground surface. Mowing can damage or destroy unripe fruit, thus reducing the number of viable seeds, which likely affects dispersal within the clearing. This effect does not occur in the ecotone or the forest. As indicated by [82,83], generative reproduction significantly contributes to the increase in the population size of C. tommasinianus. This species disperses its seeds primarily through autochory, with further dissemination aided by ants (myrmecochory) attracted by the elaiosomes on the seeds. This two-phase dispersal (diplochory) is exceptionally effective, allowing relatively long-range seed dispersal and reducing seed mortality [84]. The adaptation of seed traits for ant-mediated dispersal is widespread among plants inhabiting temperate deciduous and mixed forests [85].
In the case of bulb and corm plants, we must take into account their vegetative propagation by the formation of daughter bulbs and corms. Although in the study area, we did not conduct detailed research on the proportions of sexual and vegetative reproduction in this population and subpopulations, such observations will be initiated in the coming years as an element of monitoring of this species. In 2024, traces of rooting by wild boars were found in ecotone2, i.e., in the place where C. tommasinianus was the most abundant. Wild boars could search for tubers of Ficaria verna (which grows there) but also for corms of C. tommasinianus. Crocuses can be part of the food base of wild boars, as reported earlier for C. heufelianus Herb. [55] and C. ilvensis Peruzzi and Carta [86]. Published studies [87,88] indicate that rooting by wild boars causes the fragmentation of underground organs of forest geophytes, such as Ficaria verna or Dentaria bulbifera L., thereby promoting the dispersion of these organs and intensifying their multiplication. As a result of rooting by wild boars, the density of geophytes increases. It seems that the activity of wild boars can also have a positive influence on asexual reproduction and population density in this crocus species. If wild boars root there repeatedly, as is often the case [89], then further expansion of the alien species can be expected.
When assessing the influence of this species on native flora and plant communities, we concluded that according to the classification proposed by Faliński [90,91], it currently enriches the species composition, i.e., the new species does not outcompete the coexisting species and does not replace other species found earlier in the plant community. It seems, however, that if its density increases in the future, then its competitiveness may also increase. The geophytes that develop in spring in forest ecosystems may slow down the development of other plants in the herb layer, mostly annuals, as reported, e.g., in the case of the influence of Ficaria verna on Impatiens parviflora DC. [92]. A specific form of competition may be also allelopathy, which is understood in this context as inhibition of the development of other plants by means of chemical substances (secondary metabolites) leached from plants by rain, exuded from roots, or volatilized or released during decomposition of dead plant matter [93]. Although there is no available publication on the possible allelopathic properties of C. tommasinianus, such research was conducted on the closely related C. sativus L. Due to the high phylogenetic conservation of the composition of secondary metabolites [94], such a comparison may be justified. Extracts from the leaves, corms, or flowers of C. sativus have allelopathic properties, hampering seed germination and leading to a decrease in the biomass and leaf area of various plant species (both field weeds and cultivated plants) [95,96,97,98]. Thus, similar effects can be expected in the case of C. tommasinianus. A meta-analysis of 16,810 allelopathic effects obtained from 384 studies showed that native plants suffer more from leachates of naturalized alien plants than from leachates of other native plants [99], so allelopathy may aid alien plant species in colonizing new plant communities.
4.2. Morphometric Measurements of Individuals
Crocuses undeniably exhibit substantial morphological variation, even within a single population [100]. Discriminant function analysis revealed that individual subpopulations differed in features contributing to the larger size of individuals, such as the number of leaves, the length of the longest leaf, as well as flower length and the length of the outer tepal; individuals growing in moderate shade were clearly larger. This is likely because plants can fully exploit their growth potential in better conditions, e.g., in partial shade for C. tommasinianus. Seed dimensions, however, tend to be more conservative and less variable in response to environmental conditions [101], as confirmed by the data in Table 3. Ecological studies conducted on various species show that the conditions under which a plant matures affect the number of seeds produced and their germination capacity rather than seed size [101].
4.3. Carpological Examination
Seed sculpture is also a very conservative feature [69]. Our study shows that C. tommasinianus clearly differs from C. vernus and C. scepusiensis in terms of both ornamentation and secondary testa sculpture. Since many floristic studies are conducted during the fruiting period of crocuses rather than during flowering, the diagnostic usefulness of testa ornamentation for species identification is crucial. This is particularly important in the case of the pair C. tommasinianus and C. vernus. Both species are widely cultivated throughout Poland, and both may escape from cultivation, so their ranges overlap. These species are morphologically very similar, so outside the flowering period, they are extremely difficult to distinguish.
5. Conclusions
The naturalization of alien plant species, their further expansion, and even invasion are most often the result of a combination of several factors: their inherent potential, supported by human activity, and certain random events. For these reasons, the future of alien species is often difficult to predict. The documentation of the first cases of alien species naturalization is very important, as it provides an opportunity to implement preventive and protective strategies to minimize the risks associated with their introduction.
For the first time, we observed the naturalization of the ornamental Crocus tommasinianus in Poland. This shade-tolerant species, initially cultivated in a location situated near forest ecosystems, has spread and successfully reproduced in a nearby oak forest. This may lead to an increase in population size and further expansion of the species in the future. In this study, we examined the morphological plasticity of this species in response to environmental conditions outside its cultivated area. We identified the probable causes of its spread in the forest ecosystem and the reduction in the number of individuals at its former cultivation site. We also analyzed the current and potential future impact of this species on the forest ecosystem and other coexisting organisms.
Due to the lack of data on the ecology of Crocus tommasinianus in naturalized sites in Central Europe, this area will be used for monitoring studies. Long-term monitoring will track the species’ dispersal dynamics, the proportion of vegetative and sexual reproduction in different habitat conditions, morphological variability, and potential interactions with other species. This approach will capture the effects of seasonal and annual changes on the naturalization of Crocus tommasinianus, providing a deeper understanding of the species’ long-term dynamics. A limitation of this study is that it covers only an area of about 500 m2. Considering the climatic and soil variability in Poland, future sampling from a larger number of locations would provide a better understanding of C. tommasinianus adaptation to different ecological conditions.
Author Contributions
Conceptualization, A.C. and R.N.; methodology, R.N. and A.C.; formal analysis, R.N.; investigation, A.C. and R.N.; writing—original draft preparation, R.N. and A.C.; writing—review and editing, R.N. and A.C.; visualization R.N. and A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This publication was financed by the Polish Minister of Science and Higher Education as part of the Strategy of the Poznan University of Life Sciences for 2024–2026 within the field of improving scientific research and development work in priority research areas.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We would like to express our gratitude to Maria Morozowska for her assistance with the study of seed coat micro-ornamentation. We also sincerely thank the editor and the anonymous reviewers for their valuable comments and suggestions, which have significantly improved the quality of this paper.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
Plant Communities with Crocus tommasinianus subpopulation studied. Abbreviations: 1—clearing; 2—oak forest (Potentillo albae-Quercetum); 3—ecotone1; 4—ecotone2. For individual species, the values of the Ellenberg indices are provided for light (L), temperature (T), moisture (F), soil pH (R), and nitrogen content (N); x—no data.
Table A1.
Plant Communities with Crocus tommasinianus subpopulation studied. Abbreviations: 1—clearing; 2—oak forest (Potentillo albae-Quercetum); 3—ecotone1; 4—ecotone2. For individual species, the values of the Ellenberg indices are provided for light (L), temperature (T), moisture (F), soil pH (R), and nitrogen content (N); x—no data.
| Position of Locality | 1 | 2 | 3 | 4 | Ellenberg Indicators | ||||
|---|---|---|---|---|---|---|---|---|---|
| L | T | F | R | N | |||||
| Date | 26.03.2021 | 26.03.2021 | 26.03.2021 | 26.03.2021 | |||||
| Coordinates | 52°16′11.0″ N 16°47′81.0″ E | 52°16′08.4″ N 16°47′81.2″ E | 52°16′11.6″ N 16°47′82.2″ E | 52°16′09.5″ N 16°47′84.5″ E | |||||
| Sample collection area [m2] | 10 | 10 | 10 | 8 | |||||
| Cover of tree layer a [%] | 0 | 35 | 40 | 15 | |||||
| Cover of shrub layer b [%] | 0 | 5 | 2 | 0 | |||||
| Cover of herbaceous layer c [%] | 65 | 60 | 50 | 65 | |||||
| Number of species | 22 | 21 | 38 | 11 | |||||
| Crocus tommasinianus Herb. | 1.2 | 1.1 | 2.2 | 3.4 | |||||
| ChO. Arrhenatheretalia Pawł. 1928 | |||||||||
| Achillea millefolium L. | r | . | . | . | 8 | x | 4 | x | 5 |
| Dactylis glomerata L. | . | . | . | r | 7 | x | 5 | x | 6 |
| Taraxacum officinale Web. | + | . | r | 8 | x | 5 | x | 7 | |
| ChCl. Molinio-Arrhenatheretea T.Tx. 1937 | |||||||||
| Festuca pratensis Huds. | 2.2 | . | . | . | 8 | x | 6 | x | 6 |
| Plantago lanceolata L. | + | . | . | . | 6 | x | x | x | x |
| Rumex acetosa L. | r | . | . | . | 8 | x | x | x | 5 |
| ChCl. Querco-Fagetea Br.-Bl. et Vlieg. 1937 | |||||||||
| Acer platanoides L. (a) | . | 1.1 | 1.1 | 1.1 | (5) | 6 | x | x | x |
| Acer platanoides L. (b) | r | + | + | . | |||||
| Aegopodium podagraria L. | . | 1.1 | . | + | 5 | x | 6 | 7 | 8 |
| Anemone nemorosa L. | . | . | + | . | x | x | x | 5 | x |
| Fraxinus excelsior L. (a) | . | . | 1.1 | . | (4) | 5 | x | 7 | 7 |
| Fraxinus excelsior L. (c) | r | . | . | ||||||
| Lonicera xylosteum L. | . | + | . | . | 5 | 5 | 5 | 7 | x |
| Poa nemoralis L. | . | 2.2 | 1.1 | . | 5 | x | 5 | 5 | 3 |
| ChCl. Nardo-Callunetea Prsg 1949 | |||||||||
| Luzula campestris (L.) DC. | . | r | . | . | 7 | x | 4 | 3 | 2 |
| Pilosella officinarum Vaill. | 1.1 | . | . | . | 7 | x | 4 | x | 2 |
| ChCl. Koelerio-Corynephoretea Klika in Klika et Novak, 1941 | |||||||||
| Helichrysum arenarium (L.) Moench | + | . | . | . | 7 | 6 | 3 | 5 | 1 |
| Sedum sexangulare L. | + | . | . | . | 7 | 5 | 7 | 8 | 1 |
| ChAll. Vicio lathyroides-Potentillion Brzeg in Brzeg et M. Woj. 1996 | |||||||||
| Hypochoeris radicata L. | r | . | . | . | 8 | 5 | 5 | 4 | 3 |
| Potentilla argentea L. | r | . | . | . | 9 | x | 2 | 3 | 1 |
| Vicia lathyroides L. | + | . | . | . | 8 | 7 | 2 | 3 | 2 |
| ChAll. Carpinion Issl. 1931 em. Oeberd. 1953 | |||||||||
| Carpinus betulus L. (a) | . | 1.1 | . | 1.1 | (4) | 6 | x | x | x |
| ChAll. Fagion R.Tx. et Diem. 1936 | |||||||||
| Fagus sylvatica L. (c) | . | . | r | . | (3) | 5 | 5 | x | x |
| ChO. Fagetalia Pawł. in Pawł., Sokoł. et Wall. 1928 | . | ||||||||
| Corydalis intermedia (L.) Mérat | . | r | + | . | 3 | 5 | 5 | 7 | 7 |
| Ficaria verna Huds. | 1.1 | + | 1.1 | 4 | 5 | 6 | 7 | 7 | |
| ChAss. Lolio-Cynosuretum R.Tx. 1947 | |||||||||
| Trifiolium repens L. | 1.1 | . | . | . | 8 | x | x | x | 7 |
| ChAll. Alliarion Oberd. (1957) 1962 | . | ||||||||
| Geranium robertianum L. | . | + | + | . | 4 | x | x | x | 7 |
| ChAss. Alliario-Chaerophylletum temuli (Kreh 1935) Lohm. 1949 | |||||||||
| Chaerophyllum temulum L. | . | . | + | . | 5 | 6 | 5 | x | 8 |
| Hedera helix L. | . | + | + | . | (4) | 5 | 5 | x | x |
| ChO. Glechometalia R.Tx. in R.Tx. et Brun.-Hool 1975 | . | ||||||||
| Geum urbanum L. | . | + | . | . | 4 | 5 | 5 | x | 7 |
| ChCl. Festuco-Brometea Br.Bl. et R.Tx. 1943 | . | ||||||||
| Ajuga genevensis L. | . | r | + | . | 8 | x | 4 | 7 | 2 |
| Euphorbia cyparissias L. | . | . | r | . | 8 | x | 3 | x | 3 |
| ChAll. Alliarion Oberd. (1957) 1962 | |||||||||
| Impatiens parviflora DC. | . | + | . | 4 | 6 | 5 | x | 6 | |
| ChO. Glechometalia R.Tx. in R.Tx. et Braun-Hool 1975 | |||||||||
| Alliaria petiolata (M. Bieb.) Cavara and Grande | . | . | + | . | 5 | 6 | 5 | 7 | 9 |
| Glechoma hedaracea L. | + | + | . | 6 | 5 | 6 | x | 7 | |
| ChAss. Hordeo-Brometeum (Allorge 1922) Lohm. 1950 | . | ||||||||
| Bromus sterilis L. | . | + | . | 7 | 7 | 4 | x | 5 | |
| ChAll. Ulmenion minoris Oberd. 1953 | |||||||||
| Ulmus laevis Pall. (a) | . | . | 1.1 | (4) | 6 | 8 | 7 | 7 | |
| ChCl. Stellarietea mediae R.Tx., Lohm. et Prsg. 1950 | . | ||||||||
| Stellaria media (L.) Vill. | . | r | + | 6 | x | 4 | 7 | 8 | |
| Others | |||||||||
| Aesculus hippocastanum L. (a) | . | . | 1.1 | . | . | . | . | . | . |
| Allium vineale L. | r | . | + | . | 5 | 7 | 4 | x | 7 |
| Arenaria serpyllifolia L. | . | . | + | . | 9 | 5 | 4 | x | x |
| Carex spicata Huds. | . | . | r | . | 7 | 5 | 5 | x | 6 |
| Chionodoxa sardensis Barr. et Sudg. | r | . | r | . | |||||
| Conyza canadensis (L.) Cronquist | . | . | + | . | 8 | x | 4 | x | 4 |
| Euonymus alatus (Thunb.) Siebold (b) | . | + | . | . | . | . | . | . | . |
| Festuca trachyphylla Sibth. | 2.2 | . | . | . | . | . | . | . | . |
| Gagea pratensis (Pers.) Dumort. | . | . | + | . | . | . | . | . | . |
| Galanthus nivalis L. | r | . | + | . | 5 | 7 | x | 7 | 7 |
| Geranium molle L. ‘Alba’ | + | . | . | . | 7 | 6 | 3 | 5 | 4 |
| Medicago lupulina L. | r | . | . | . | 7 | 5 | 4 | 8 | x |
| Myosotis stricta Link ex Roem. and Schult. | . | . | + | . | 8 | x | 3 | 4 | 2 |
| Ornithogalum nutans L. | . | . | + | . | . | . | . | . | . |
| Poa annua L. | . | . | . | r | 7 | x | 6 | x | 8 |
| Prunus domestica L. subsp. syriaca (Borkh.) Janch. (b) | . | . | + | . | . | . | . | . | . |
| Pyrus pyraster (L.) Burgsd. (c) | . | r | . | . | . | . | . | . | . |
| Quercus petraea (Matt.) Liebl. (a) | r | 2.2 | 2.2 | . | (6) | 6 | 5 | x | x |
| Scilla sibirica Haw. | . | . | r | 1.1 | . | . | . | . | . |
| Scilla sibirica Haw. ‘Alba’ | . | . | + | + | . | . | . | . | . |
| Veronica chamaedrys L. | + | . | r | . | 6 | x | 4 | x | x |
| Veronica sublobata M. A. Fisch. | . | 1.1 | 1.1 | 6 | 6 | 5 | 7 | 7 | |
| Vinca minor L. | . | 1.1 | + | . | 4 | 6 | 5 | x | 6 |
| Viola suavis M. Bieb. | . | . | r | . | . | . | . | . | . |
References
- Regulation (EU) No 1143/2014 of the European Parliament and of the Council of 22 October 2014 on the Prevention and Management of the Introduction and Spread of Invasive Alien Species. Available online: https://eur-lex.europa.eu/eli/reg/2014/1143/oj (accessed on 25 August 2024).
- Pyšek, P.; Richardson, D.M. The biogeography of naturalization in alien plants. J. Biogeogr. 2006, 33, 2040–2050. [Google Scholar] [CrossRef]
- Aguin-Pombo, D. Biological Invasions and Global Trade: An Urgent Issue. In Natural Resources, Sustainability and Humanity: A Comprehensive View; Aguin-Pombo, D., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 83–99. [Google Scholar]
- Van Kleunen, M.; Dawson, W.; Essl, F.; Pergl, J.; Winter, M.; Weber, E.; Pyšek, P. Global exchange and accumulation of non-native plants. Nature 2015, 525, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Early, R.; Bradley, B.A.; Dukes, J.S.; Lawler, J.J.; Olden, J.D.; Blumenthal, D.M.; Gonzalez, P.; Grosholz, E.D.; Ibáñez, I.; Miller, L.P.; et al. Global threats from invasive alien species in the twenty-first century and national response capacities. Nat. Commun. 2016, 7, 12485. [Google Scholar] [CrossRef] [PubMed]
- Zenni, R.D.; Essl, F.; García-Berthou, E.; McDermott, S.M. The economic costs of biological invasions around the world. NeoBiota 2021, 67, 1–9. [Google Scholar] [CrossRef]
- Seebens, H.; Essl, F.; Hulme, P.E.; van Kleunen, M. Development of pathways of global plant invasions in space and time. In Global Plant Invasions; Clements, D.R., Upadhyaya, M.K., Joshi, S., Shrestha, A., Eds.; Springer: Cham, Switzerland, 2022; pp. 35–50. [Google Scholar] [CrossRef]
- Lenzner, B.; Latombe, G.; Schertler, A.; Seebens, H.; Yang, Q.; Winter, M.; Weigelt, P.; van Kleunen, M.; Pyšek, P.; Pergl, J.; et al. Naturalized alien floras still carry the legacy of European colonialism. Nat. Ecol. Evol. 2022, 6, 1723–1732. [Google Scholar] [CrossRef]
- Sudnik-Wójcikowska, B. Rośliny Synantropijne; Multico, Oficyna Wydawnicza: Warsaw, Poland, 2011. [Google Scholar]
- Tokarska-Guzik, B. The Establishment and Spread of Alien Plant Species (Kenophytes) in the Flora of Poland; Wydawnictwo Uniwersytetu Śląskiego: Katowice, Poland, 2005. [Google Scholar]
- Jackowiak, B. Man-made changes in the flora and vegetation of Poland: Current review. Diversity 2023, 15, 618. [Google Scholar] [CrossRef]
- Tokarska-Guzik, B.; Dajdok, Z.; Zając, M.; Zając, A.; Urbisz, A.; Danielewicz, W.; Hołdyński, C. Rośliny Obcego Pochodzenia w Polsce; Generalna Dyrekcja Ochrony Środowiska: Warsaw, Poland, 2012. [Google Scholar]
- Obidziński, A.; Kołaczkowska, E.; Otręba, A. Metody Zwalczania Obcych Gatunków Roślin Występujących na Terenie Puszczy Kampinoskiej; Kampinoski Park Narodowy: Izabelin, Poland; Wydawnictwo BioDar: Kraków, Poland, 2016. [Google Scholar]
- Słowiński, K.; Grygierzec, B.; Synowiec, A.; Tabor, S.; Araniti, F. Preliminary study of control and biochemical characteristics of giant hogweed (Heracleum sosnowskyi Manden.) treated with microwaves. Agronomy 2022, 12, 1335. [Google Scholar] [CrossRef]
- Švec, P.; Perglová, I.; Fröhlich, V.; Laštovička, J.; Seidl, J.; Růžičková, K.; Horáková, I.; Lukavský, J.; Ferko, M.; Štych, P.; et al. Perseverance of management is needed–Efficient long-term strategy of Reynoutria management. NeoBiota 2024, 94, 261–288. [Google Scholar] [CrossRef]
- Szymura, M.; Szymura, T.H.; Wolski, K. Invasive Solidago species: How large area do they occupy and what would be the cost of their removal? Pol. J. Ecol. 2016, 64, 25–34. [Google Scholar] [CrossRef]
- Winter, M.; Schweiger, O.; Klotz, S.; Nentwig, W.; Andriopoulos, P.; Arianoutsou, M.; Basnou, C.; Delipetrou, P.; Didžiulis, V.; Hejda, M.; et al. Plant extinctions and introductions lead to phylogenetic and taxonomic homogenization of the European flora. Proc. Natl. Acad. Sci. USA 2009, 106, 21721–21725. [Google Scholar] [CrossRef]
- Yang, Q.; Weigelt, P.; Fristoe, T.S.; Zhang, Z.; Kreft, H.; Stein, A.; Seebens, H.; Dawson, W.; Essl, F.; König, C.; et al. The global loss of floristic uniqueness. Nat. Commun. 2021, 12, 7290. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Guo, Q. Linking biotic homogenization to habitat type, invasiveness, and growth form of naturalized alien plants in North America. Divers. Distrib. 2010, 16, 119–125. [Google Scholar] [CrossRef]
- Daru, B.H.; Davies, T.J.; Willis, C.G.; Meineke, E.K.; Ronk, A.; Zobel, M.; Pärtel, M.; Antonelli, A.; Davis, C.C. Widespread homogenization of plant communities in the Anthropocene. Nat. Commun. 2021, 12, 6983. [Google Scholar] [CrossRef] [PubMed]
- Šibíková, M.; Jarolímek, I.; Hegedüšová, K.; Májeková, J.; Mikulová, K.; Slabejová, D.; Škodová, I.; Zaliberová, M.; Medvecká, J. Effect of planting alien Robinia pseudoacacia trees on homogenization of Central European forest vegetation. Sci. Total Environ. 2019, 687, 1164–1175. [Google Scholar] [CrossRef]
- Heywood, V.H.; Brunel, S. Code of Conduct on Horticulture and Invasive Alien Plants; Council of Europe Publ: Strasbourg, France, 2008. [Google Scholar]
- Fry, C. The Plant Hunters: The Adventures of the World’s Greatest Botanical Explorers; Univ. Chicago Press: Chicago, IL, USA, 2013. [Google Scholar]
- Richardson, M.D.; McCalla, J.; Buxton, T.; Lulli, F. Incorporating early spring bulbs into dormant warm-season turfgrasses. HortTechnology 2015, 25, 228–232. [Google Scholar] [CrossRef]
- van Kleunen, M.; Essl, F.; Pergl, J.; Brundu, G.; Carboni, M.; Dullinger, S.; Early, R.; González-Moreno, P.; Groom, Q.J.; Hulme, P.E.; et al. The changing role of ornamental horticulture in alien plant invasions. Biol. Rev. 2018, 93, 1421–1437. [Google Scholar] [CrossRef]
- Alam, A.; Iqbal, M.; Vats, S. Cultivation of Some Overlooked Bulbous Ornamentals—A Review on its Commercial Viability. Rep. Opin. 2013, 5, 9–34. [Google Scholar]
- Invasive Species Specialist Group ISSG. The Global Invasive Species Database. Version 2015.1. Available online: https://www.iucngisd.org/gisd/ (accessed on 24 September 2024).
- Plants of the World Online (POWO). Available online: https://powo.science.kew.org (accessed on 25 February 2024).
- Hoste, I. Ornamentals and Invasive Plants: If You Choose One You Get the Other as Well. In Mini Symposium of Aliens and Invasive Species; National Botanic Garden of Belgium, Bouchout Castle: Meise, Belgium, 2011. [Google Scholar]
- Czarna, A.; Klimko, M.; Janyszek, S. Vascular Flora and Vegetation of the Former Manor Park in Radojewo (Wielkopolska Region, Poland). Rocz. AR Pozn. 2009, 388, 37–47. [Google Scholar]
- Czarna, A. Vascular Flora in the Park at Jeziory in the National Park of Wielkopolska. Rocz. AR Pozn. 2010, 389, 39–44. [Google Scholar]
- Czarna, A. Vascular Plant Flora in the Cytadela Cemeteries in Poznań (Poland). Acta Agrobot. 2016, 69, 1–17. [Google Scholar] [CrossRef]
- Czarna, A. Vascular Plants in the Cemetery of the Meritorious (Cmentarz Zasłużonych) in Poznań (Poland). Ann. UMCS 2016, 71, 59–73. [Google Scholar] [CrossRef]
- Nowińska, R.; Czarna, A.; Czekalski, M.; Morozowska, M. Vascular Flora of Selected Palace Parks in the Wielkopolska Region. Steciana 2016, 20, 137–157. [Google Scholar] [CrossRef]
- Żukowski, W.; Latowski, K.; Jackowiak, B.; Chmiel, J. Rośliny Naczyniowe Wielkopolskiego Parku Narodowego. In Prace Zakładu Taksonomii Roślin Uniwersytetu im. Adama Mickiewicza w Poznaniu; Bogucki Wydawnictwo Naukowe: Poznań, Poland, 1995. [Google Scholar]
- Drobnik, J. Allium paradoxum from Asia to Europe: Ornamental, Invasive, Edible, and Medicinal. Hum. Ecol. 2023, 51, 559–567. [Google Scholar] [CrossRef]
- Rukšāns, J. The World of Crocuses, the First Supplement; The Latvian Academy of Sciences: Riga, Latvia, 2023. [Google Scholar]
- Mathew, B.F. Crocus L. In Flora Europaea, Vol. 5: Alismataceae to Orchidaceae (Monocotyledones); Tutin, T.G., Heywood, V.H., Burges, N.A., Moore, D.M., Valentine, D.H., Walters, S.M., Webb, D.A., Eds.; Cambridge University Press: Cambridge, UK, 2010; pp. 92–97. [Google Scholar]
- Petersen, G.; Seberg, O.; Thorsøe, S.; Jørgensen, T.; Mathew, B. A phylogeny of the genus Crocus (Iridaceae) based on sequence data from five plastid regions. Taxon 2008, 57, 487–499. [Google Scholar] [CrossRef]
- Surányi, G.; Máthé, C.; Mosolygó, Á.; Borbély, G.; Vasas, G. Analysis of genetic diversity in crocuses with Carpathian Basin origin using AFLP-markers. Acta Biol. Hung. 2010, 61, 149–155. [Google Scholar] [CrossRef]
- Alsayied, N.F.; Fernández, J.A.; Schwarzacher, T.; Heslop-Harrison, J.S. Diversity and relationships of Crocus sativus and its relatives analysed by inter-retroelement amplified polymorphism (IRAP). Ann. Bot. 2015, 116, 359–368. [Google Scholar] [CrossRef]
- Rukšans, J. The World of Crocuses; Latvian Academy of Sciences: Riga, Latvia, 2017. [Google Scholar]
- Rand, E.S. Popular Flowers, and How to Cultivate Them; Hurd and Houghton: New York, NY, USA, 1876. [Google Scholar]
- Snowarski, M. Flora Polski, Atlas Roślin.pl. Available online: https://atlas-roslin.pl/index.html (accessed on 25 February 2024).
- Harris, S. Crocus species (Iridaceae). Oxford University Plants 400. Department of Plant Sciences; Oxford University. Available online: https://herbaria.plants.ox.ac.uk/bol/plants400/Profiles/cd/crocus (accessed on 18 October 2024).
- Szilagyi, C.; Băla, M.; Toța, C. The behaviour of some crocus species upon different fertilizers and substrates. J. Name 2018, 22, 98–101. [Google Scholar]
- Wurzell, B. Spring flowering crocuses. BSBI News 1992, 60, 36–38. [Google Scholar]
- The Complete List of Taxon Names from the BSBI’s Database. Available online: https://bsbi.org/taxon-lists (accessed on 14 October 2024).
- McAwoy, M. Non-Native Plants of Delaware; Species Conservation and Research Program, Delaware Department of Natural Resources and Environmental Control, Division of Fish and Wildlife: Dover, DE, USA, 2024. [Google Scholar]
- Tuttle, J. Plant spring blooming bulbs now. In Ask a Master Gardener; University of Minnesota Extension: St Paul, MN, USA, 2023; pp. 1–6. [Google Scholar]
- Brighton, C.A. Cytological problems in the genus Crocus (Iridaceae): I. Crocus vernus aggregate. Kew Bull. 1976, 31, 33–46. [Google Scholar] [CrossRef]
- Fedorov, A.A. Flora Partis Europaeae URSS. 1979; Volume 4. [Google Scholar]
- Dostál, J. Nová Květena ČSSR; Academia: Prague, Czech Republic, 1989; 1548p. [Google Scholar]
- Prisžter, S.Z. Uj säfränyfaj (Crocus tommasinianus HERB.) Magyarorszägon. Bot. Közlemények 1964, 51, 183–186. [Google Scholar]
- Mihaly, A.; Kricsfalusy, V. Population biology and ecology of Crocus heuffelianus Herb. (Iridaceae) in Ukraine. Linz. Biol. Beiträge 1997, 29, 641–681. [Google Scholar]
- Ževrnja, N.; Vladović, D. The genus Crocus L. in the flora of Svilaja Mountain. Nat. Croat. Period. Musei Hist. Nat. Croat. 2005, 14, 363–368. [Google Scholar]
- Hitchmough, J.; Fieldhouse, K. Plant User Handbook: A Guide to Effective Specifying; Blackwell Science: Oxford, UK, 2004. [Google Scholar] [CrossRef]
- Ogle, C.C.; La Cock, G.D. Additional records and observations of monocotyledons naturalised or casual in Manawatu Ecological Region, New Zealand. Perspect. Biosecurity 2019, 4, 7–31. [Google Scholar]
- Hill, M.O.; Preston, C.D.; Shanklin, J.D. Geographical patterns in the flora of Cambridgeshire (vc 29). Br. Ir. Bot. 2020, 2, 285–308. [Google Scholar] [CrossRef]
- Mirek, Z.; Piękoś-Mirkowa, H.; Zając, A.; Zając, M. Vascular Plants of Poland: An Annotated Checklist; W. Szafer Institute of Botany, Polish Academy of Sciences: Kraków, Poland, 2020. [Google Scholar]
- Harpke, D.; Carta, A.; Tomović, G.; Ranđelović, V.; Ranđelović, N.; Blattner, F.R.; Peruzzi, L. Phylogeny, karyotype evolution and taxonomy of Crocus series Verni (Iridaceae). Plant Syst. Evol. 2015, 301, 309–325. [Google Scholar] [CrossRef]
- Mosolygó, Á.; Sramkó, G.; Barabás, S.; Czeglédi, L.; Jávor, A.; Molnár, A.V.; Surányi, G. Molecular genetic evidence for allotetraploid hybrid speciation in the genus Crocus L. (Iridaceae). Phytotaxa 2016, 258, 121–136. [Google Scholar] [CrossRef]
- Raca, I.; Blattner, F.R.; Waminal, N.E.; Kerndorff, H.; Ranđelović, V.; Harpke, D. Disentangling Crocus series Verni and its polyploids. Biology 2023, 12, 303. [Google Scholar] [CrossRef]
- Kandemir, N. Comparative leaf anatomy of some endemic Crocus L. taxa from Turkey. Bangladesh J. Bot. 2011, 40, 155–162. [Google Scholar] [CrossRef]
- Raca, I.; Ljubisavljević, I.; Jušković, M.; Ranđelović, N.; Ranđelović, V. Comparative anatomical study of the taxa from series Verni Mathew (Crocus L.) in Serbia. Biol. Nyssana 2017, 8, 15–22. [Google Scholar] [CrossRef]
- Raca, I.; Jovanovic, M.; Ljubisavljevic, I.; Juskovic, M.; Randelovic, V. Morphological and leaf anatomical variability of Crocus cf. heuffelianus Herb. (Iridaceae) populations from the different habitats of the Balkan Peninsula. Turk. J. Bot. 2019, 43, 645–658. [Google Scholar] [CrossRef]
- Kerndorff, H.; Pasche, E.; Harpke, D. The Genus Crocus (Liliiflorae, Iridaceae): Life Cycle, Morphology, Phenotypic Characteristics, and Taxonomically Relevant Parameters. Stapfia 2015, 103, 27–65. [Google Scholar]
- Karaismailoğlu, M.C.; Şik, L.; Gemicioğlu, A.; Erol, O. Seed Structure of Some Taxa of the Genus Crocus L. (Iridaceae) Series Crocus. Turk. J. Bot. 2018, 42, 722–731. [Google Scholar] [CrossRef]
- Song, Y.X.; Peng, S.; Mutie, F.M.; Jiang, H.; Ren, J.; Cong, Y.Y.; Hu, G.W. Evolution and taxonomic significance of seed micromorphology in Impatiens (Balsaminaceae). Front. Plant Sci. 2022, 13, 835943. [Google Scholar] [CrossRef] [PubMed]
- Dyderski, M.K.; Jagodziński, A.M. Impact of invasive tree species on natural regeneration species composition, diversity, and density. Forests 2020, 11, 456. [Google Scholar] [CrossRef]
- Braun-Blanquet, J. Pflanzensoziologie. Grundzüge der Vegetationskunde, 3rd ed.; Springer: Vienna, Austria, 1964. [Google Scholar]
- Di Biase, L.; Tsafack, N.; Pace, L.; Fattorini, S. Ellenberg Indicator Values Disclose Complex Environmental Filtering Processes in Plant Communities along an Elevational Gradient. Biology 2023, 12, 161. [Google Scholar] [CrossRef]
- Ellenberg, H. Zeigerwerte der Gefäßpflanzen Mitteleuropas; Verlag Erich Goltze KG: Göttingen, Germany, 1974. [Google Scholar]
- Cattell, R.B. The Scree Test for the Number of Factors. Multivar. Behav. Res. 1966, 1, 245–276. [Google Scholar] [CrossRef]
- Chudzicki, M. Operat Ekosystemów Leśnych Wielkopolskiego Parku Narodowego—Opis Taksacyjny cz. 2. Projekt Planu Ochrony Wielkopolskiego Parku Narodowego; Biuro Urządzania Lasu i Geodezji Leśnej Oddział w Poznaniu: Poznań, Poland, 2013. [Google Scholar]
- Szafer, W.; Kulczyński, S.; Pawłowski, B. Rośliny Polskie; Volume I-II; PWN: Warsaw, Poland, 1988. [Google Scholar]
- Kornaś, J. Geograficzno-historyczna klasyfikacja roślin synantropijnych. Mater. Zakładu Fitosocjologii Stosow. UW 1968, 25, 33–41. [Google Scholar]
- iNaturalist. Crocus tommasinianus, C. vernus . iNaturalist. Available online: https://www.inaturalist.org (accessed on 23 September 2024).
- Krause, J. Niskie, Ale Pięknie Kwitnące Rośliny Cebulowe. Krokus, Szafirek; PWRiL: Warszawa, Poland, 1987. [Google Scholar]
- Knebel, C.H. Rośliny Cebulowe; Klub dla Ciebie: Warsaw, Poland, 2006. [Google Scholar]
- Wendebourg, T. Zwiebelpflanzen für den Garten; Verlag Eugen Ulmer: Stuttgart, Germany, 2004. [Google Scholar]
- Adams, K. Krokusy, Tulipany, Lilie; Świat Książki: Warsaw, Poland, 2014. [Google Scholar]
- Johansson, B.G. Crocus på Gotland. Gotlands Bot. Förening 1991, 11, 28–32. [Google Scholar]
- Wall, S.V.; Longland, W.S. Diplochory and the evolution of seed dispersal. In Seed Fate: Predation, Dispersal and Seedling Establishment; Forget, P.M., Lambert, J.E., Hulme, P.E., Vander Wall, S.B., Eds.; CABI Publishing: Wallingford, UK, 2005; pp. 297–314. [Google Scholar] [CrossRef]
- Gorb, E.; Gorb, S. Seed Dispersal by Ants in a Deciduous Forest Ecosystem: Mechanisms, Strategies, Adaptations; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003. [Google Scholar]
- Peruzzi, L.; Carta, A. Crocus ilvensis sp. nov. (Sect. Crocus, Iridaceae), Endemic to Elba Island (Tuscan Archipelago, Italy). Nord. J. Bot. 2011, 29, 6–13. [Google Scholar] [CrossRef]
- Biały, K. The Effect of Boar (Sus scrofa) Rooting on the Distribution of Organic Matter in Soil Profiles and the Development of Wood Anemone (Anemone nemorosa L.) in the Oak-Hornbeam Stand (Tilio-Carpinetum) in the Białowieza Primeval Forest. Forestry 1996, 38, 77–88. [Google Scholar] [CrossRef]
- Faliński, J.B. Interaction: Wild Boar Rooting—Participation of Geophytes in the Herb Layer of Oak-Linden-Hornbeam Forest. Phytocoennosis 2002, 5, 70. [Google Scholar]
- Podgórski, T.; Lusseau, D.; Scandura, M.; Sönnichsen, L.; Jędrzejewska, B. Long-Lasting, Kin-Directed Female Interactions in a Spatially Structured Wild Boar Social Network. PLoS ONE 2014, 9, e99875. [Google Scholar] [CrossRef] [PubMed]
- Faliński, J.B. Stadia Neofityzmu i Stosunek Neofitów do Innych Komponentów Zbiorowiska. Mater. Zakładu Fitosocjologii Stosow. Uniw. Warsz. 1968, 25, 15–31. [Google Scholar]
- Faliński, J.B. Inwazje w Świecie Roślin: Mechanizmy, Zagrożenia, Projekt Badań. Phytocoen. N.S. 2004, 16, 1–32. [Google Scholar]
- Piskorz, R.; Klimko, M. Współwystępowanie Niecierpka Drobnokwiatowego Impatiens parviflora DC. i wybranych roślin lasu dębowo-grabowego w Wielkopolskim Parku Narodowym. Sylwan 2007, 151, 43–58. [Google Scholar]
- Rice, E.L. Allelopathy; Acad. Press: New York, NY, USA, 1984. [Google Scholar]
- Wink, M. Evolution of Secondary Metabolites from an Ecological and Molecular Phylogenetic Perspective. Phytochemistry 2003, 64, 3–19. [Google Scholar] [CrossRef]
- Barkhordari, K.; Sorooshzadeh, A.; Mokhtassi, B.A. Allelopathic Effect of Extraction Solution of Leaves and Corms of Saffron (Crocus sativus) in Phenological Stages on Seed Germination of Jimson Weed (Datura stramonium). Modares J. Biotechnol. 2018, 9, 233–239. [Google Scholar]
- Mikolajchuk, V.; Panfilova, A.; Korkhova, M.; Drobitko, A. Allelopathic Activity of Water-Soluble and Volatile Secretions of Crocus sativus L. (Iridaceae) Flowers in the Northern Black Sea Region. J. Biotechnol. 2022, 9, 233–239. [Google Scholar] [CrossRef]
- Hosseini, M.; Rizvi, S.J.H. A Preliminary Investigation on Possible Role of Allelopathy in Saffron (Crocus sativus L.). In II International Symposium on Saffron Biology and Technology; Kafi, M., Koocheki, A., Eds.; ISHS: Leuven, Belgium, 2006; pp. 75–79. [Google Scholar]
- Feizi, H.; Salari, A.; Gharar, F. Study of the Allelopathic Effect of Saffron (Crocus sativus L.) Organs’ Aqueous Extract on the Seed Germination and Seedling Growth of Sugar Beet and Safflower at Different Concentrations. J. Med. Spice Plants 2018, 22, 156–161. [Google Scholar]
- Zhang, Z.; Liu, Y.; Yuan, L.; Weber, E.; van Kleunen, M. Effect of Allelopathy on Plant Performance: A Meta-Analysis. Ecol. Lett. 2021, 24, 348–362. [Google Scholar] [CrossRef]
- Dolatyari, A.; Abolhasani, M.T.; Ardalani, F.; Rukšāns, J. A taxonomic revision of the genus Crocus (Iridaceae) in Iran. Nord. J. Bot. 2024, e04270. [Google Scholar] [CrossRef]
- Fenner, M.; Thompson, K. The Ecology of Seeds; Cambridge University Press: Cambridge, UK, 2005; Available online: https://www.bsbi.org/ (accessed on 17 October 2014).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).