Investigating the Impact of Garden Plant Smellscapes on Human Well-Being: A Case Study of Pine Forests
Abstract
1. Introduction
1.1. Garden Plants and Their Connections to Human Health
1.2. Health Benefits of Aromas in Garden Plants
1.3. The Concept and Characteristics of a Smellscape
1.4. An Overview of Human Olfactory Perception Assessment Methodologies
1.5. An Overview of Smellscape Evaluation Methods
1.6. A Review of the Impact of Garden Plant Smellscapes on Human Health
1.7. The Purpose and Significance of the Research
2. Materials and Methods
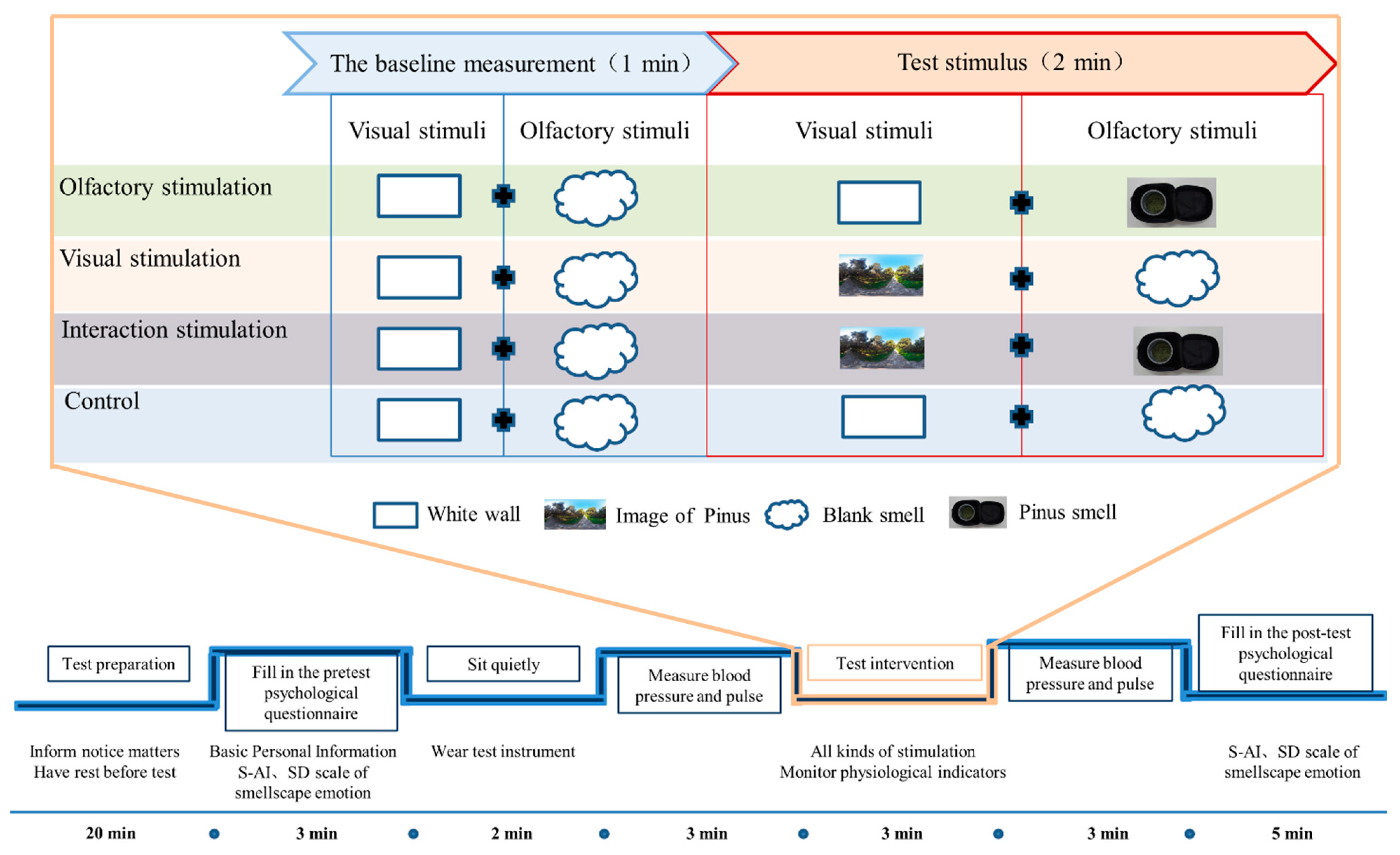
2.1. Research Design
- Impact of plant fragrances on human well-being: The first section explores the effect of pine scent on human physiological and psychological well-being. This experiment simulates the influence of pine aroma, common in garden settings, on various physiological and psychological indicators.
- Influence of visual landscapes: The second section examines the impact of visual landscapes associated with the scent of pine trees. The experimental materials consist of visual images depicting pine tree environments, used to simulate the effects of visual stimuli associated with the aroma of pine trees on various physiological and psychological indicators.
- The combined impact of scent and visual stimulation: This section explores the interactive effects of fragrance from landscape plants with visual stimuli. The experiment simulates the influence of the scent of pine tree and the visual scene of a pine forest on physiological and psychological indicators of the human body.
2.2. Test Site
2.3. Subjects
2.4. Research Methods and Test Materials Collection
2.4.1. Physiological Assessments Methods
2.4.2. Psychological Assessments Methods
2.5. Statistical Analyses
3. Results
3.1. Participant Demographic Analysis
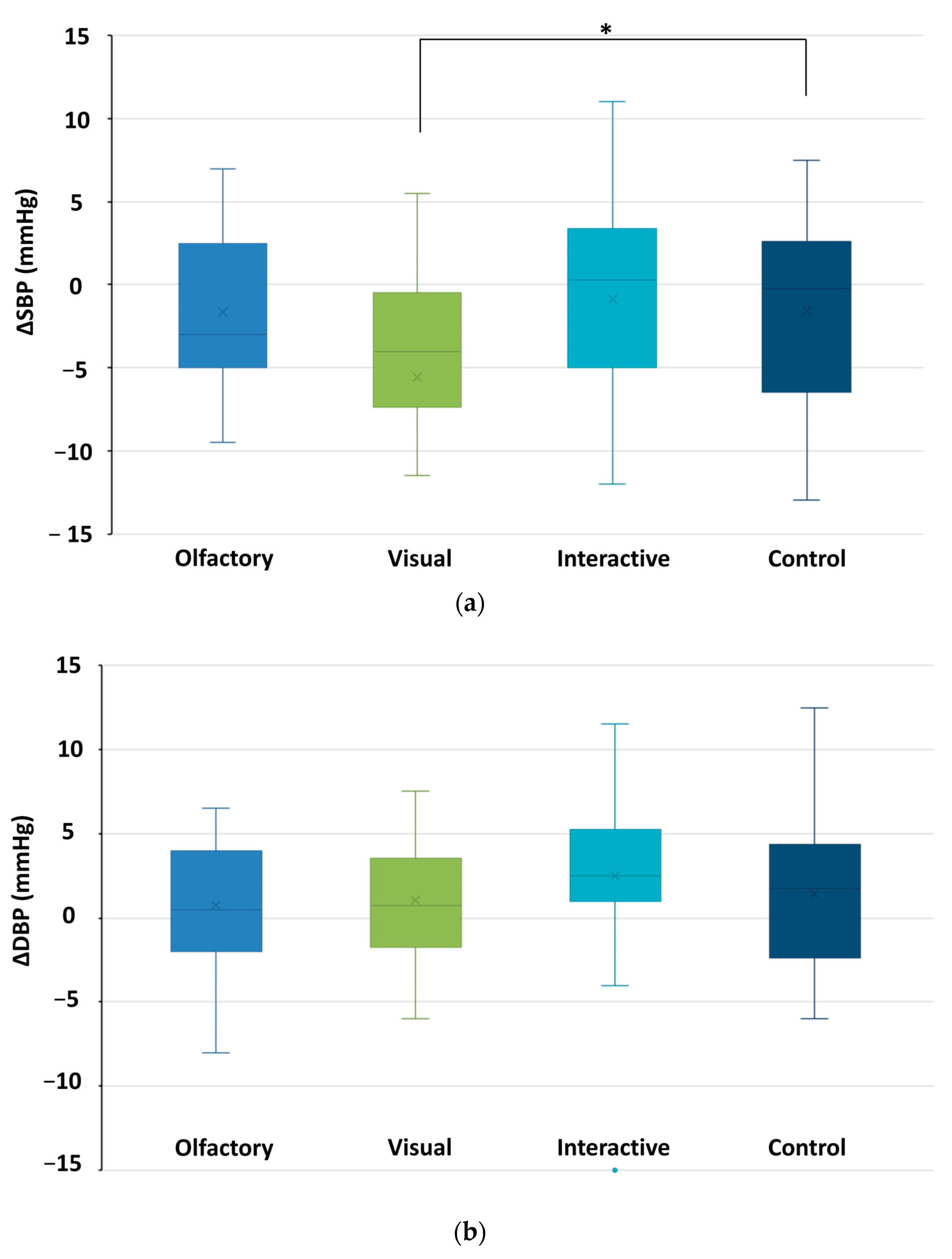
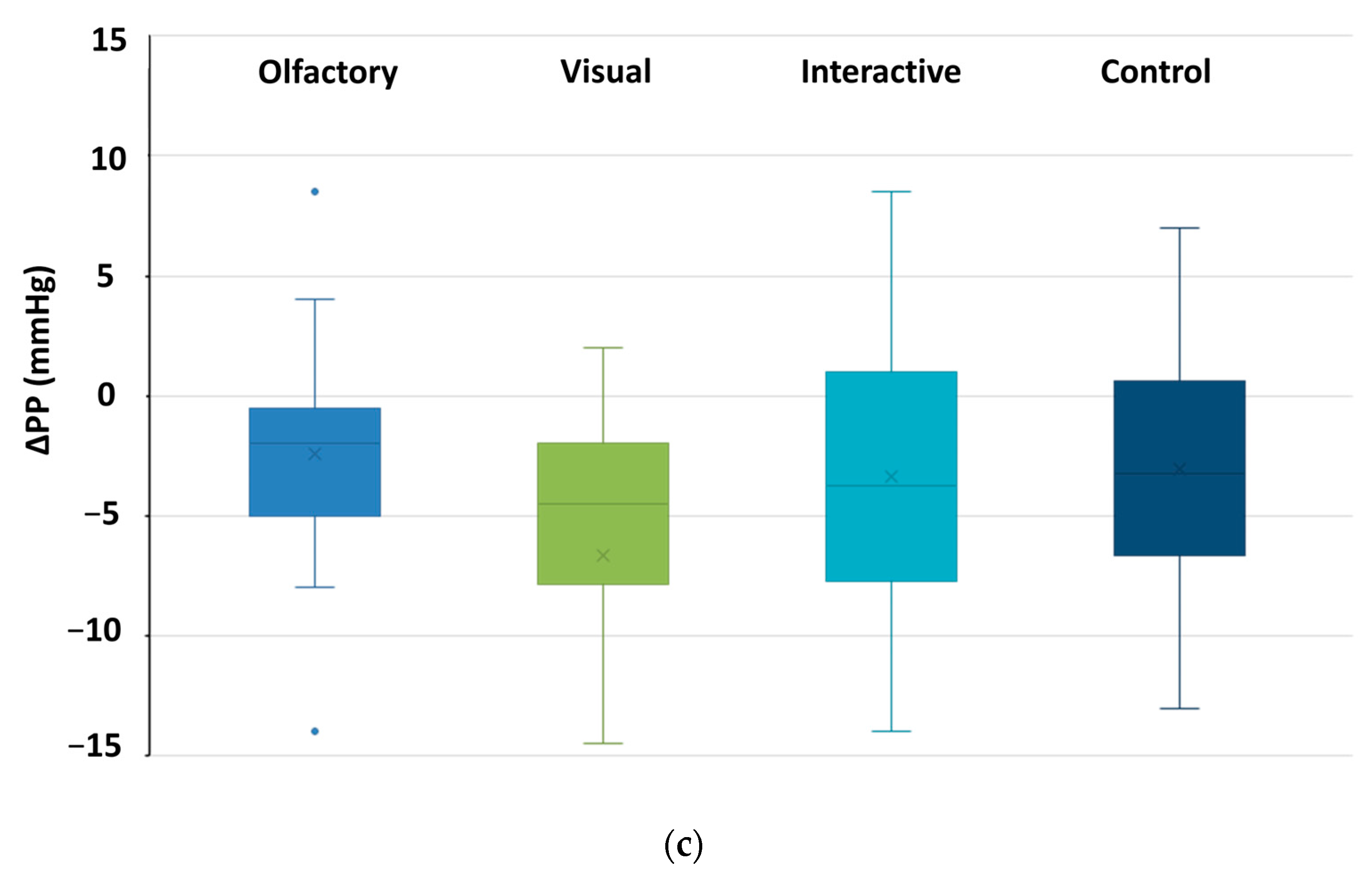
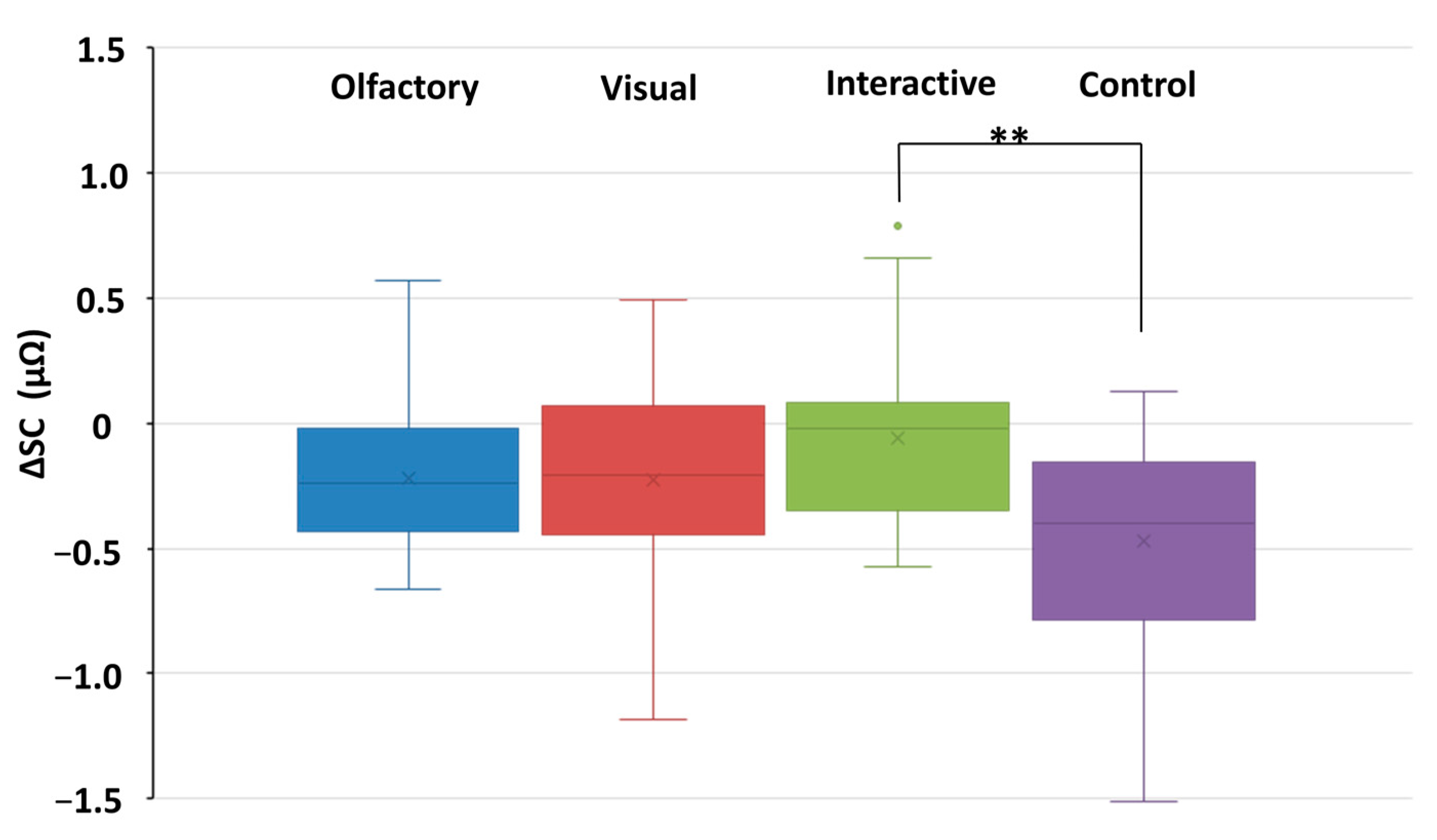
3.2. Changes in Physiological Indicator Data
3.2.1. The Alterations Observed in Autonomic Nervous System Metrics
3.2.2. The Alterations Observed in Central Nervous System Metrics
3.2.3. Differences in Physiological Effects Resulting from Various Types of Stimuli Encountered in Garden Plant Smellscapes
3.3. Changes in Psychological Indicator Data
3.3.1. The Alterations Observed in State Anxiety Scale Scores
3.3.2. The Alterations Observed in Odor Emotion SD Scale Scores
3.3.3. The Diverse Psychological Impacts of Various Stimuli Linked to a Garden’s Smellscape
4. Discussion
4.1. Investigation into the Impact of Olfactory Cues on Physiological Parameters
4.2. Investigation into the Impact of Visual Cues on Physiological Parameters
4.3. Investigation into the Impact of Synchronized Olfactory–Visual Cues on Physiological Response Metrics
4.4. Investigation into the Impact of Olfactory, Visual, and Their Interactions on Psychometric Measures
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lederbogen, F.; Kirsch, P.; Haddad, L.; Streit, F.; Tost, H.; Schuch, P.; Wüst, S.; Pruessner, J.C.; Rietschel, M.; Deuschle, M. City living and urban upbringing affect neural social stress processing in humans. Nature 2011, 474, 498–501. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Zhang, Y.; Yang, X.; Wan, M. Research and construction of healthy landscape of residential green space in Wuhan in the post-epidemic era. Chin. Garden 2021, 37, 14–19. [Google Scholar]
- Selmi, W.; Weber, C.; Rivière, E.; Blond, N.; Mehdi, L.; Nowak, D. Air pollution removal by trees in public green spaces in Strasbourg city, France. Urban For. Urban Green. 2016, 17, 192–201. [Google Scholar] [CrossRef]
- Liu, L.C.; Seyler, B.; Liu, H.; Feng, C.; Wang, Q.; Li, Y. Biogenic volatile organic compound emission patterns and secondary pollutant formation potentials of dominant greening trees in Chengdu, southwest China. J. Environ. Sci. 2022, 114, 179–193. [Google Scholar] [CrossRef]
- Kaan, I.; Ismail, K.; Ramazan, E.; Hakan, S. Atmospheric Cd, Cr, and Zn. Deposition in Several Landscape Plants in Mersin, Türkiye. Water Air Soil Pollut. 2022, 233, 05607–05608. [Google Scholar]
- Jennifer, D.; Waliczek, T.M.; Zajicek, J.M. The Relationship between Levels of Greenery and Landscaping at Track and Field Sites, Anxiety, and Sports Performance of Collegiate Track and Field Athletes. Hort Technol. 2011, 21, 329–335. [Google Scholar]
- Chris, N.; Alistair, G.; Suyin, L.P.C.; Sanjiana, M.; Mehdi, B.; Jenny, R. Color Aesthetics: A transatlantic comparison of psychological and physiological impacts of warm and cool colors in garden landscapes. Wellbeing Space Soc. 2021, 2, 100038. [Google Scholar]
- Thorpert, P. Green Is Not Just Green-Human Colour Perception in Urban Green Contexts. Doctoral Dissertation, Swedish University of Agricultural Sciences, Alnarp, Sweden, 2019. [Google Scholar]
- Chen, X.; Wang, Y.; Huang, T.; Lin, Z. Research on Digital Experience and Satisfaction Preference of Plant Community Design in Urban Green Space. Land 2022, 11, 1411. [Google Scholar] [CrossRef]
- Kang, N.; Xiu, M.L. Effects of visual characteristics of different plant community types on human psychology. Chin. J. Northwest For. Univ. 2017, 32, 315–320. [Google Scholar]
- Song, R.; Chen, Q.; Zhang, Y.; Jia, Q.A.; He, H.; Gao, T.; Qiu, L. Psychophysiological restorative potential in cancer patients by virtual reality (VR)-based perception of natural environment. Front. Psychol. 2022, 13, 1003497. [Google Scholar] [CrossRef]
- Lee, M.; Kim, E.; Choe, J.; Choi, S.; Ha, S.; Kim, G. Psychological Effects of Green Experiences in a Virtual Environment: A Systematic Review. Forests 2022, 13, 1625. [Google Scholar] [CrossRef]
- Moss, M.; Cook, J.; Wesnes, K.; Duckett, P. Aromas of rosemary and lavender essential oils differentially affect cognition and mood in healthy adults. Int. J. Neurosci. 2003, 113, 15–38. [Google Scholar] [CrossRef]
- Brawley, E.C. Gardens of Memories. Alzhmer’s Care Today 2004, 5, 154–164. [Google Scholar]
- Cohen Mansfield, J.; Werner, P. Outdoor Wandering Parks for Persons with Dementia. Alzheimer Dis. Assoc. Disord. 1999, 13, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Bhat, L.C. Benefits and Attributes of Plants & Aromatherapy within A Healthcare Environment and Their Influence on Healthy & Longevity. Int. J. Complement. Altern. Med. 2017, 8, 13–14. [Google Scholar]
- Cen, C.T.; Lei, Y. Studies on the antidepressant effects of essential oils of Rosemary and lemongrass and in vivo aromas. J. Shanghai JiaoTong Univ. (Agric. Sci.) 2009, 1, 82–85. [Google Scholar]
- Jie, O.Y.; Xiao, D.W.; Bing, Z. Research progress in the application of spice plants. Fragr. Cosmet. 2002, 5, 32–34. [Google Scholar]
- Ming, S.; Ping, L.; Jin, H.L.; Qi, X.Z. Functions and garden applications of aromatic plants. Pract. Technol. For. 2007, 2, 46–47. [Google Scholar]
- Baik, H.J.; Kim, H.J.; Jae, S.Y.; Yi, B.Y. Effects of Fragrance Components of Abies holophylla Max. on Stress Relief and Improvement of Vascular Function. J. People Plants Environ. 2018, 21, 223–232. [Google Scholar] [CrossRef]
- Porteous, J.D. Smellscape. Prog. Hum. Geogr. 1985, 9, 356–378. [Google Scholar] [CrossRef]
- Xiao, J.; Tait, M.; Kang, J. A perceptual model of smellscape pleasantness. Cities 2018, 76, 105–115. [Google Scholar] [CrossRef]
- Young, B.D. Perceiving Smellscapes. Pac. Philos. Q. 2020, 101, 203–223. [Google Scholar] [CrossRef]
- Batty, C. Olfactory Experience I: The Content of Olfactory Experience. Philos. Compass 2010, 5, 1137–1146. [Google Scholar] [CrossRef]
- Koutsoklenis, A.; Papadopoulos, K. Olfactory Cues Used for Wayfinding in Urban Environments by Individuals with Visual Impairments. J. Vis. Impair. Blind. 2011, 105, 692–702. [Google Scholar] [CrossRef]
- Young, B.D. Smelling matter. Philos. Psychol. 2016, 129, 520–534. [Google Scholar] [CrossRef]
- Becky, M. Smelling objects. Synthese 2017, 196, 4279–4303. [Google Scholar]
- Zardini, M. Sense of the City: An Alternate Approach to Urbanism. J. Soc. Telegr. Eng. 2005, 5, 67. [Google Scholar]
- Gloor, P. Inputs and Outputs of the Amygdala: What the Amygdala is Trying to Tell the Rest of the Brain; Springer: Berlin/Heidelberg, Germany, 1978; pp. 189–209. [Google Scholar]
- Cabanac, M. What is emotion? Behav. Process. 2002, 60, 69–83. [Google Scholar]
- Kabat-Zinn, J. Smellscape. Mindfulness 2014, 1, 100–101. [Google Scholar] [CrossRef]
- Willander, J.; Larsson, M. Smell Your Way Back to Childhood: Autobiographical Odor Memory. Psychon. Bull. Rev. 2006, 13, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.A.; Stevenson, R.J. The fundamental role of memory in olfactory perception. Trends Neurosci. 2003, 26, 243–247. [Google Scholar] [CrossRef]
- Herz, R.S. Are Odors the Best Cues to Memory? A Cross㎝odal Comparison of Associative Memory Stimulia. Ann. N. Y. Acad. Sci. 1998, 855, 670–674. [Google Scholar] [CrossRef]
- Schooler, H. A Naturalistic Study of Autobiographical Memories Evoked by Olfactory and Visual Cues: Testing the Proustian Hypothesis. Am. J. Psychol. 2002, 115, 21–32. [Google Scholar]
- Engen, T.; Ross, B.M. Long-term memory of odors with and without verbal descriptions. J. Exp. Psychol. 1973, 100, 221. [Google Scholar] [CrossRef]
- Chitty, S.L. The Beast and the Monk: A Life of Charles Kingsley; Hodder and Stoughton: London, UK, 1974; pp. 313–314. [Google Scholar]
- Antje, H.; Henriette, M.; Ilona, C.; Thomas, H. Influence of room fragrance on attention, anxiety and mood. Flavour Fragr. J. 2017, 32, 24–28. [Google Scholar]
- Fletcher, M.L.; Wilson, D.A. Olfactory bulb mitral-tufted cell plasticity: Odorant-specific tuning reflects previous odorant exposure. J. Neurosci. 2003, 23, 6946–6955. [Google Scholar] [CrossRef][Green Version]
- Li, W.; Howard, J.D.; Parrish, T.B.; Gottfried, J.A. Aversive learning enhances perceptual and cortical discrimination of indiscriminable odor cues. Science 2008, 319, 1842–1845. [Google Scholar] [CrossRef]
- Otazu, G.H.; Chae, H.; Davis, M.B.; Albeanu, D.F. Cortical feedback decorrelates olfactory bulb output in awake mice. Neuron 2015, 86, 1461–1477. [Google Scholar] [CrossRef]
- Distel, H.; Ayabe-Kanamura, S.; Margarita, M.; Ina, S.; Sachiko, S.; Robyn, H. Perception of everyday odors—Correlation between intensity, familiarity and strength of hedonic judgement. Chem. Sens. 1999, 24, 191–199. [Google Scholar] [CrossRef]
- Laing, D.G.; Legha, P.K.; Jinks, A.L.; Hutchinson, I. Relationship between molecular structure, concentration and odor qualities of oxygenated aliphatic molecules. Chem. Sens. 2003, 28, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, A.N. What the Nose Knows: The Science of Scent in Everyday Life; Crown Publishers: New York, NY, USA, 2008; p. 141. [Google Scholar]
- Todrank, J. Odors can change preferences for people in photographs: A cross-modal evaluative conditioning study with olfactory USs and visual CSs. Learn. Motiv. 1995, 26, 116–140. [Google Scholar] [CrossRef]
- Thomas, H.; Therese, F.; Daniel, B.; Jonathan, W.; Cornelia, B.H.; Valentin, A.S. The Rewarding Effect of Pictures with Positive Emotional Connotation upon Perception and Processing of Pleasant Odors—An FMRI Study. Front. Neuroanat. 2017, 11, 19. [Google Scholar]
- Tuan, Y. Topophilia: A Study of Environmental Perception, Attitudes, and Values; Columbia University Press: New York, NY, USA, 1974; pp. 5–11. [Google Scholar]
- Chen, W.; Chen, K.; Zhou, B.; Zhou, W. Integration of olfactory and sensory information. Sci. Technol. Rev. 2017, 35, 29–36. [Google Scholar]
- Moskowitz, H.R.; Dravnieks, A.; Klarman, L.A. Odor intensity and pleasantness for a diverse set of odorants. Percept. Psychophys. 1976, 19, 122–128. [Google Scholar] [CrossRef]
- Thurstone, L.L. A law of comparative judgment. Psychol. Rev. 1927, 34, 273. [Google Scholar] [CrossRef]
- Peryam, D.R.; Pilgrim, F.J. Hedonic scale method of measuring food preferences. Food Technol. 1957, 11, 9–14. [Google Scholar]
- Stevens, S.S. To honor Fechner and repeal his law. Science 1961, 133, 80–86. [Google Scholar] [CrossRef]
- Buettner, A. (Ed.) Springer Handbook of Odor; Springer: Cham, Switzerland, 2017; pp. 101–102. [Google Scholar]
- McReynolds, P.; Ludwig, K. On the history of rating scales. Personal. Individ. Differ. 1987, 8, 281–283. [Google Scholar] [CrossRef]
- Young, P.T. Constancy of Affective Judgment to Odors. J. Exp. Psychol. 1923, 6, 182. [Google Scholar] [CrossRef]
- Henshaw, V. Urban Smellscapes: Understanding and Designing City Smell Environments; Routledge: London, UK, 2013; p. 42. [Google Scholar]
- Li, X. The Influence of Garden Plant Color on Human Physiology and Psychology; Beijing Forestry University Article: Beijing, China, 2012; p. 37. [Google Scholar]
- McCorry, L.K. Physiology of the autonomic nervous system. Am. J. Pharm. Educ. 2007, 71, 78. [Google Scholar] [CrossRef]
- Matsumoto, T. Aromatic effects of a Japanese citrus fruit—Yuzu (Citrus junos Sieb. ex Tanaka)-on psychoemotional states and autonomic nervous system activity during the menstrual cycle: A single-blind randomized controlled crossover study. Jpn. J. Psychosom. Med. 2017, 57, 292. [Google Scholar] [CrossRef] [PubMed]
- Benarroch, E.E. Primer on the Autonomic Nervous System, 2nd ed.; Academic Press: Cambridge, MA, USA, 2004; pp. 17–19. [Google Scholar]
- Park, B.J.; Tsunetsugu, Y.; Kasetani, T.; Kagawa, T.; Milyazaki, Y. The physiological effects of Shinrin-yoku (taking in the forest atmosphere or forest bathing): Evidence from field experiments in 24 forests across Japan. Environ. Health Prev. Med. 2010, 15, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Harumi, I.; Chorong, S.; Miyazaki, Y. Physiological effect of olfactory stimulation by Hinoki cypress (Chamaecyparis obtusa) leaf oil. J. Physiol. Anthropol. 2015, 34, 1–7. [Google Scholar]
- Tsunetsugu, Y.; Park, B.J.; Miyazaki, Y. Trends in research related to “Shinrin-yoku” (taking in the forest atmosphere or forest bathing) in Japan. Environ. Health Prev. Med. 2010, 15, 27–37. [Google Scholar] [CrossRef]
- Mountz, J.M. Central Nervous System; Springer: New York, NY, USA, 1986; p. 37. [Google Scholar]
- Skingsley, D.R. Introduction to the Central Nervous System; Springer: New York, NY, USA, 2008; p. 53. [Google Scholar]
- Kim, S.M.; Park, S.; Hong, J.W.; Jang, E.J.; Pak, C.H. Psychophysiological effects of orchid and rose fragrances on humans. Hortic. Sci. Technol. 2016, 34, 472–487. [Google Scholar] [CrossRef]
- Sobel, H.N. Prediction Models for the Pleasantness of Binary Mixtures in Olfaction. Chem. Sens. 2008, 33, 599–609. [Google Scholar]
- Katata, K.; Sakai, N.; Doi, K.; Kawamitsu, H.; Fuji, M.; Sugimura, K.; Nibu, K. Functional MRI of regional brain responses to ‘pleasant’ and ‘unpleasant’ odors. Acta Otolaryngol. Suppl. 2009, 129, 85–90. [Google Scholar] [CrossRef]
- Kermen, F.; Chakirian, A.; Sezille, C.; Joussian, P.; Le, G.; Ziessel, A.; Chastrette, M.; Mandairon, N.; Didier, A.; Rouby, C.; et al. Molecular complexity determines the number of olfactory notes and the pleasantness of smells. Sci. Rep. 2011, 1, 11–32. [Google Scholar] [CrossRef]
- Chen, B.; Yu, J.; Tang, Y.; Zhang, C.; Chen, R.; Xu, F. A comparative analysis of electromyography biofeedback electrical stimulation therapy for stroke patients with shoulder joint subluxation at different stimulation sites and directions. Chin. Gener. Pract. 2020, 23, 540–546. [Google Scholar]
- Yamaguchi, M.; Kanemori, T.; Kanemaru, M.; Mizuno, Y.; Yoshida, H. Correlation of Stress and Salivary Amylase Activity. Jpn. J. Med. Electron. Biol. Eng. 2001, 39, 234–239. [Google Scholar]
- Wang, D.; Wang, X.; Ma, H. Handbook of Psychological Health Assessment (Revised Edition). Beijing Chin. J. Psychol. Health 1999, 2, 77–79. [Google Scholar]
- Jo, H.; Rodiek, S.; Fujii, E.; Miyazaki, Y.; Park, B.J. Physiological and Psychological Response to Floral Scent. HortScience 2013, 48, 82–88. [Google Scholar] [CrossRef]
- Jo, H.; Fujii, E.; Cho, T. An experimental study on physiological and psychological effects of pine scent. Methods Inform. Med. 2010, 38, 1–10. [Google Scholar]
- Morita, E.; Fukuda, S.; Nagano, J.; Hamajima, N.; Yamamoto, H.; Iwai, Y.; Nakashima, T.; Ohira, H.; Shirakawa, T. Psychological effects of forest environments on healthy adults: Shinrinyoku (forest-air bathing, walking) as a possible method of stress reduction. Public Health 2007, 121, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Ikei, H.; Song, C.; Miyazaki, Y. Effects of olfactory stimulation by α-pinene on autonomic nervous activity. J. Wood Sci. 2016, 62, 568–572. [Google Scholar] [CrossRef]
- Sandusky, A.; Parducci, A. Pleasantness of odors as a function of the immediate stimulus context. Psychon. Sci. 1965, 3, 321–322. [Google Scholar] [CrossRef]
- Igarashi, M.; Yamamoto, T.; Lee, J.; Song, C.; Ikei, H.; Miyazaki, Y. Effects of stimulation by three-dimensional natural images on prefrontal cortex and autonomic nerve activity: A comparison with stimulation using two-dimensional images. Cogn. Process. 2014, 15, 551–556. [Google Scholar] [CrossRef]
- Song, C.; Ikei, H.; Miyazaki, Y. Physiological effects of forest-related visual, olfactory, and combined stimuli on humans: An additive combined effect. Urban For. Urban Green. 2019, 44, 126437. [Google Scholar] [CrossRef]
- Sun, M.; Kim, H. Progress in Research on the Impact of Japanese Plant Odors on Human Health. World For. Res. 2020, 5, 108–112. [Google Scholar]
- Huo, X. Analysis of Volatile Components from Four Aromatic Plants and Their Intervention Effects on Human Health. Master’s Thesis, Zhejiang Agriculture and Forestry University, Zhejiang, China, 2019. [Google Scholar]
- Fang, M. A Study on the Influence of Floral Fragrance and Environmental Sound on Psychological Well-Being. Ph.D. Thesis, National Taiwan University, Taiwan, China, 2016. [Google Scholar]
- Ba, M.; Kang, J. A laboratory study of the sound-odour interaction in urban environments. Build. Environ. 2019, 147, 314–326. [Google Scholar] [CrossRef]
- Hozumi, H.; Hasegawa, S.; Tsunenari, T.; Snapei, N.; Arashina, Y.; Takahashi, K.; Konnno, A.; Chida, E.; Tomimatsu, S. Aromatherapies using Osmanthus fragrans oil and grapefruit oil are effective complementary treatments for anxious patients undergoing colonoscopy: A randomized controlled study. Complement. Ther. Med. 2017, 34, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Jin, H. Mei, the Cultural Significance of Osmanthus and Magnolia: A Study on their Aroma Substances and Impact on Human Health. PhD Thesis, Beijing Forestry University, Beijing, China, 2003. [Google Scholar]
- Jia, M. A Study on Volatile Compounds of Several Fragrant Plants in Rehabilitation Landscape and Their Impact on Human Health. Master’s Thesis, Zhejiang Agriculture and Forestry University, Zhejiang, China, 2017. [Google Scholar]
- Sona, B.; Loos, H.M. The smell of wood and its impact on physiological responses. Int. J. Psychophysiol. 2018, 131, S40. [Google Scholar]
- Kim, D.S.; Goo, Y.M.; Cho, J.; Lee, J.; Lee, D.Y.; Sin, S.M.; Shin, E.C. Effect of Volatile Organic Chemicals in Chrysanthemum indicum Linné on Blood Pressure and Electroencephalogram. Molecules 2018, 23, 2063. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, L.; Xie, Y.; Xu, J.; Liu, Y. The Effects of Aromatherapy and Music Intervention on Pain and Anxiety in Breast Cancer Patients During the Perioperative Period. J. Cent. South Univ. (Med. Ed.) 2018, 43, 656–661. [Google Scholar]
- Wang, C.; Wang, Z.; Zhuang, G.; Jin, C. The Effects of Auricular Acupuncture Combined with Aromatherapy on Stress Response in Colorectal Cancer Surgical Patients. Chin. Nurs. J. 2013, 48, 623–625. [Google Scholar]
- Biswas, D.; Labrecque, L.I.; Lehmann, D.R. Effects of Sequential Sensory Cues on Food Taste Perception: Cross odal Interplay Between Visual and Olfactory Stimuli. J. Consum. Psychol. 2021, 31, 746–764. [Google Scholar] [CrossRef]
- Sabiniewicz, A.; Schaefer, E.; Cagdas, G.; Cedric, M.; Moustafa, B.; Nadejda, K.; Gabriele, N.; Thomas, H. Smells Influence Perceived Pleasantness but Not Memorization of a Visual Virtual Environment. i-Perception 2021, 12, 2041669521989731. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, R.R.; Koenig, J.; Wilker, F.W.; Hillecke, T.K.; Thayer, J.F. Event-related response in skin conductance to six musical emotions—A replication study. In Psychophysiology; Wiley: Hoboken, NJ, USA, 2014; pp. 51–57. [Google Scholar]
- Fu, H.; Niu, J.; Wu, Z.; Cheng, B.; Guo, X.; Zuo, J. Exploration of public stereotypes of supply-and-demand characteristics of recycled water infrastructure—Evidence from an event-related potential experiment in Xi’an, China. J. Environ. Manag. 2022, 322, 116103. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.; Li, S.H.; Li, F.H. Study on the effect of landscape on human psychology. Chin. Landsc. Archit. 2008, 7, 69–72. [Google Scholar]
- Du, R.; Mehmood, R.M.; Lee, H.J. Alpha activity during emotional experience revealed by ERSP. J. Internet Technol. 2014, 15, 775–782. [Google Scholar]
- Yu, C.; Wang, M. Survey of emotion recognition methods using EEG information. Cogn. Robot 2022, 2, 132–146. [Google Scholar] [CrossRef]
- Jin, H.X. The Material Basis of Culture and Fragrance of Prunus mume and Osmanthus fragrans and Its Effect on Human Health. Ph.D. Thesis, Beijing Forestry University, Beijing, China, 2003. [Google Scholar]
- Lee, S.J.; Kim, H.; Oh, B.K.; Choi, H.; Lee, J.Y.; Lee, S.H.; Kim, B.J.; Kim, B.S.; Kang, J.H.; Kang, J.; et al. Association of inter-arm systolic blood pressure differences with arteriosclerosis and athero-sclerosis: A cohort study of 117,407 people. Atherosclerosis 2022, 58, 34219–34224. [Google Scholar]
- Wen, C.; Jiang, C.; Wu, Y.; Zhou, H. Comparison of the effects of square dancing and brisk walking on cardiovascular function in middle-aged and elderly women. Chin. J. Henan Normal Univ. (Nat. Sci. Ed.) 2019, 48, 109–117. [Google Scholar]
- Zheng, H. Environmental Quality Evaluation of Green Olfactory in Beijing. Ph.D. Thesis, Beijing Forestry University, Beijing, China, 2002. [Google Scholar]
- Kang, J.; Zhang, M. Semantic differential analysis of the soundscape in urban open public spaces. Build. Environ. 2010, 45, 150–157. [Google Scholar] [CrossRef]
- Lee, J.; Park, B.J.; Tsunetsugu, Y.; Ohira, T.; Kagawa, T.; Milyazaki, Y. Effect of forest bathing on physiological and psychological responses in young Japanese male subjects. Public Health 2011, 125, 93–100. [Google Scholar] [CrossRef]
- Osgood, C.; Suci, G.; Tannenbaum, P. The Measurement of Meaning; University of Illinois Press: Urbana, IL, USA, 1957; pp. 51–53. [Google Scholar]
- Doty, R.L. An examination of relationships between the pleasantness, intensity, and concentration of 10 odorous stimuli. Percept. Psychophys. 1975, 17, 492–496. [Google Scholar] [CrossRef]
- Critchley, H.D. Electrodermal responses: What happens in the brain. Neuroscience 2002, 8, 132–142. [Google Scholar]
- Baer, T.; Coppin, G.; Porchero, C.; Cayeux, I.; Sander, D.; Delplanque, S. “Dior, J’adore”: The role of contextual information of luxury on emotional responses to perfumes. Food Qual. Prefer 2017, 69, 36–43. [Google Scholar] [CrossRef]
- Fan, T.T.; Yao, L.; Li, Y.L.; Pan, X.H.; Li, Y.H. Acute anti-anxiety effects of aroma substances from three species of aromatic plants. Chin. J. Shanghai Jiaotong Univ. (Agric. Sci. Ed.) 2017, 35, 24–30. [Google Scholar]
- Lorig, T.S.; Schwartz, G.E. Brain and odor: I. Alteration of human EEG by odor administration. Psychobiology 1988, 16, 281–284. [Google Scholar] [CrossRef]
- Kim, Y.; Watanuki, S. Characteristics of electroencephalographic responses induced by a pleasant and an unpleasant odor. J. Physiol. Anthropol. Appl. Hum. Sci. 2003, 22, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiang, M.; Huang, Y.; Sheng, Z.; Huang, X.; Lin, W.; Lv, B. Physiological and psychological effects of watching videos of different durations showing urban bamboo forests with varied structures. Int. J. Environ. Res. Public Health 2020, 17, 3434. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Q. Effects of garden plant smell on human health. Chin. Sci. Silvae Sin. 2023, 59, 100–116. [Google Scholar]
- Tong, N.; Kuang, S. Gender differences in visual system. Prog. Biochem. Biophys. 2021, 48, 779–787. [Google Scholar]
- Buettner, A. Springer Handbook of Odor, Middle Section; Science Press: Beijing, China, 2019; pp. 699–700. [Google Scholar]
- Zhang, Z.; Li, X.; Pan, H.W. Evaluation of plant landscape in Shenzhen park green space using AHP method and human physiological and psychological indicators. Chin. J. Beijing For. Univ. (Soc. Sci. Ed.) 2011, 10, 30–37. [Google Scholar]
- Kritsidima, M.; Newton, T.; Asimakopoulou, K. The effects of lavender scent on dental patient anxiety levels: A cluster randomised-controlled trial. Commun. Dent. Oral Epidemiol. 2010, 38, 83–87. [Google Scholar] [CrossRef]
- Karaman, T.; Karaman, S.; Dogru, S.; Tapar, H.; Sahin, A.; Suren, M.; Arici, S.; Kaya, Z. Evaluating the efficacy of lavender aromatherapy on peripheral venous cannulation pain and anxiety: A prospective, randomized study. Complement. Ther. Clin. Pract. 2016, 23, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Wohlwill, W.F. The concept of nature: A psychologist’s view. In Human Behavior and the Environment, 6th ed.; Altman, I., Wohlwill, F., Eds.; Behavior and the Natural Environment, Plenum: New York, NY, USA, 1983; pp. 5–37. [Google Scholar]
- Shankar, M.U.; Levitan, C.A.; Spence, C. Grape expectations: The role of cognitive influences in color–flavor interactions. Conscious. Cognit. 2010, 19, 380–390. [Google Scholar] [CrossRef]
- Gottfried, J.A.; Dolan, R.J. The nose smells what the eye sees: Crossmodal visual facilitation of human olfactory perception. Neuron 2003, 39, 375–386. [Google Scholar] [CrossRef]
- Carmichael, S.T.; Price, J.L. Sensory and premotor connections of the orbital and medial prefrontal cortex of macaque monkeys. J. Comp. Neurol. 1995, 363, 642–664. [Google Scholar] [CrossRef]
- Rolls, E.T.; Baylis, L.L. Gustatory, olfactory, and visual convergence within the primate orbitofrontal cortex. J. Neurosci. Off. J. Soc. Neurosci. 1994, 14, 5437–5452. [Google Scholar] [CrossRef] [PubMed]




| Basic Information | O | V | O and V | C | |
|---|---|---|---|---|---|
| Quantity (Pct.) | Quantity (Pct.) | Quantity (Pct.) | Quantity (Pct.) | ||
| Gender | male | 7 (30%) | 6 (25%) | 2 (8%) | 6 (25%) |
| female | 16 (70%) | 18 (75%) | 22 (92%) | 18 (75%) | |
| Sample size | 23 | 24 | 24 | 24 |
| Type of Stimulus (Independent Variable) | Dependent Variable (Unit) | Before | During | T | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| O | SBP (mm Hg) | 107.72 | 11.89 | 106.04 | 11.66 | 1.637 |
| V | 104.96 | 11.75 | 99.38 | 9.92 | 2.508 * | |
| O and V | 106.75 | 9.98 | 105.85 | 7.98 | 0.566 | |
| C | 106.54 | 9.15 | 104.94 | 7.10 | 1.406 | |
| O | DBP (mm Hg) | 63.63 | 7.24 | 64.35 | 7.40 | −0.895 |
| V | 60.56 | 4.52 | 61.63 | 4.21 | −1.555 | |
| O and V | 63.38 | 6.05 | 65.88 | 7.03 | −2.445 * | |
| C | 61.00 | 7.93 | 62.46 | 5.85 | −1.421 | |
| O | PP (mm Hg) | 44.09 | 8.36 | 41.70 | 8.88 | 2.597 * |
| V | 44.40 | 10.03 | 39.78 | 6.43 | 3.077 ** | |
| O and V | 43.38 | 7.40 | 39.98 | 4.40 | 2.838 ** | |
| C | 45.54 | 6.84 | 42.48 | 5.15 | 2.899 ** | |
| O | P (bpm) | 73.63 | 8.94 | 75.48 | 7.24 | −2.116 * |
| V | 71.63 | 8.87 | 72.60 | 8.92 | −1.074 | |
| O and V | 74.58 | 8.14 | 74.35 | 9.43 | 0.239 | |
| C | 74.38 | 10.47 | 74.40 | 7.86 | −0.21 | |
| O | SC (µΩ) | 2.12 | 1.46 | 1.89 | 1.34 | 2.241 * |
| V | 3.22 | 2.45 | 2.99 | 2.28 | 2.757 * | |
| O and V | 1.72 | 1.43 | 1.66 | 1.28 | 0.865 | |
| C | 3.35 | 2.05 | 2.88 | 2.06 | 3.284 * | |
| O | α waves (µV) | 16.49 | 8.96 | 11.36 | 5.40 | 3.521 ** |
| V | 13.96 | 7.12 | 16.13 | 8.12 | −1.912 | |
| O and V | 22.08 | 19.79 | 21.98 | 14.57 | 0.059 | |
| C | 19.78 | 17.16 | 14.60 | 10.52 | 2.486 * | |
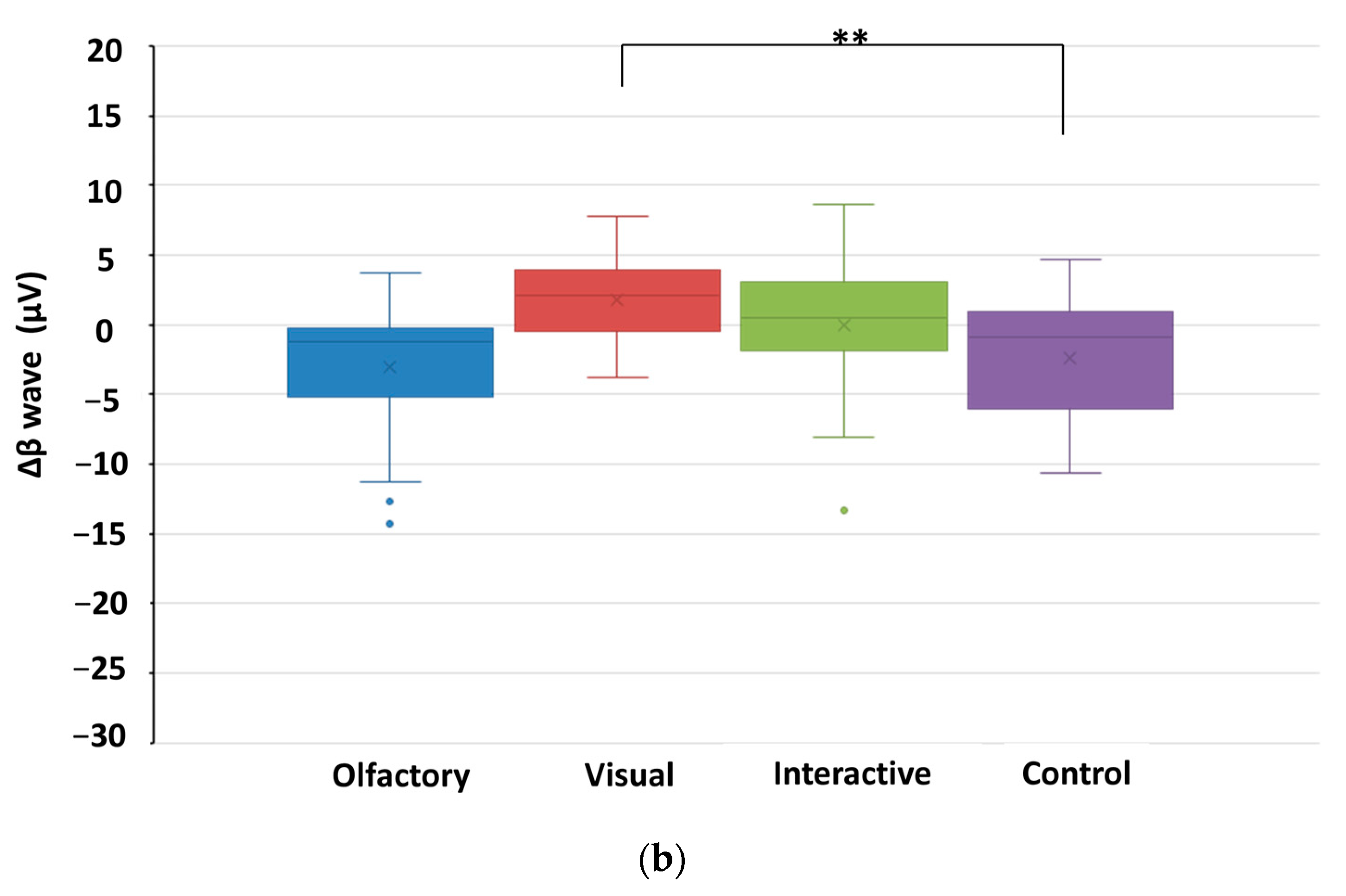
| O | β waves (µV) | 11.42 | 6.06 | 8.47 | 5.10 | 2.954 ** |
| V | 9.07 | 5.32 | 10.89 | 5.14 | −2.940 ** | |
| O and V | 15.91 | 12.46 | 15.88 | 10.49 | 0.033 | |
| C | 13.37 | 12.65 | 10.11 | 9.15 | 2.537 * | |
| P (LSD) | ||||
|---|---|---|---|---|
| O | V | O and V | ||
| SC | C | -- | -- | 0.009 |
| α waves | O | -- | -- | 0.017 |
| C | -- | 0.005 | -- | |
| β waves | V | 0.000 | -- | 0.031 |
| C | -- | 0.002 | -- | |
| Type of Stimulus (Independent Variable) | Dependent Variable (Unit) | Before | During | T | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
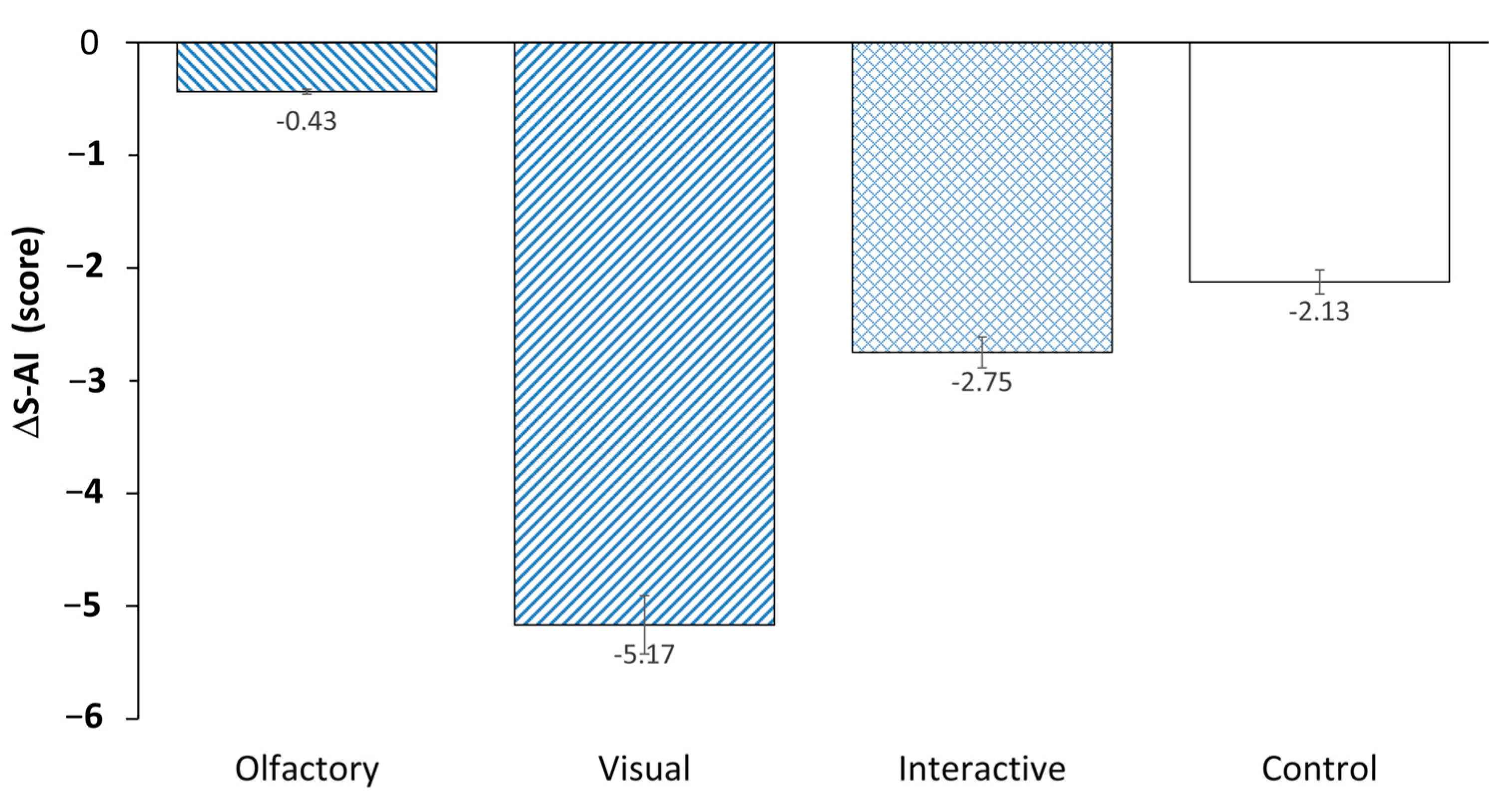
| O | S-AI (score) | 34.09 | 7.68 | 33.65 | 7.85 | 0.270 |
| V | 36.13 | 7.55 | 30.96 | 4.59 | 3.234 ** | |
| O and V | 34.25 | 5.89 | 31.50 | 7.96 | 1.862 | |
| C | 35.33 | 7.12 | 33.21 | 7.96 | 1.596 | |
| Sig. (Double Tail) | Z | |||
|---|---|---|---|---|
| C | O | O and V | ||
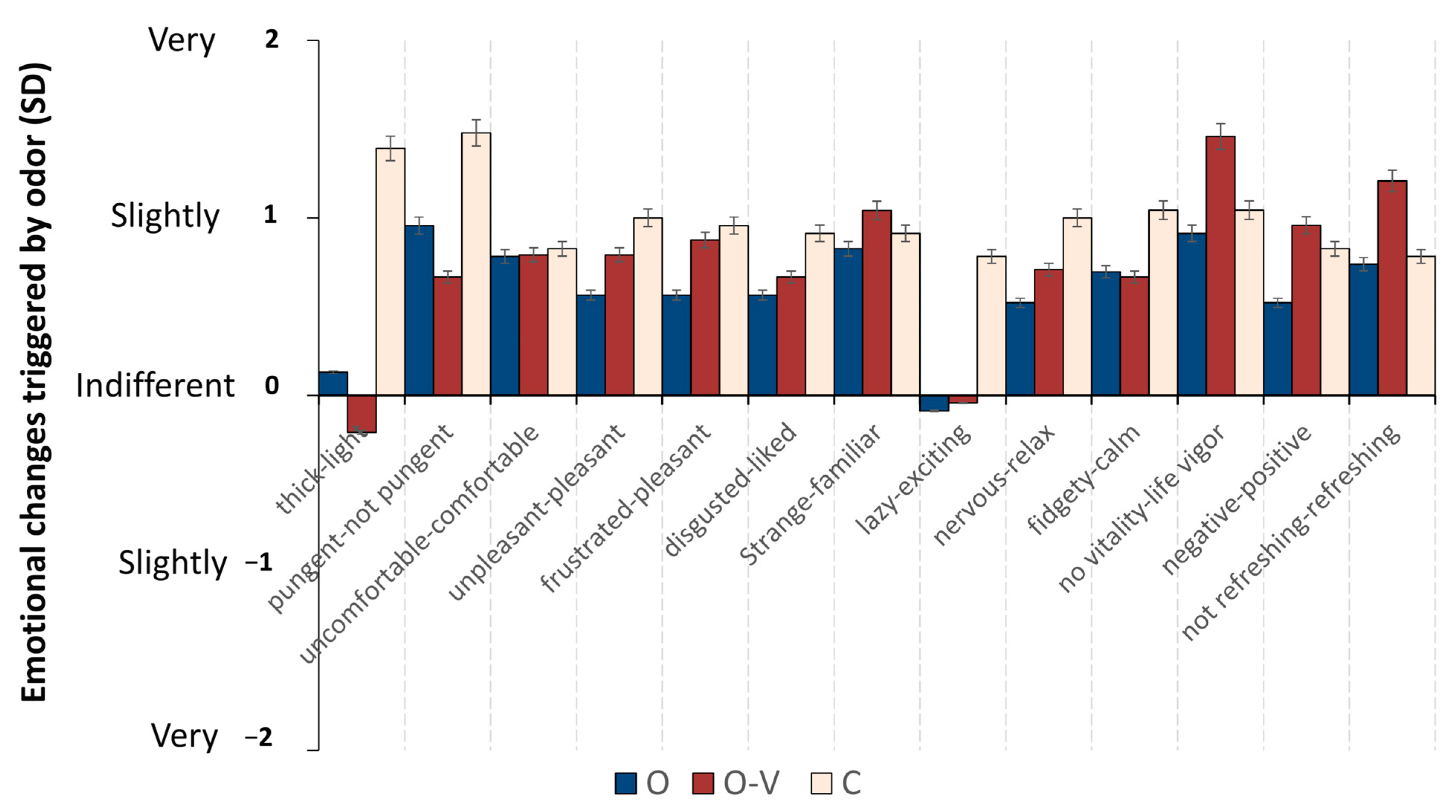
| “thick–light” | C | -- | −3.196 | −4.073 |
| O and V | 0.000 | -- | -- | |
| O | 0.001 | -- | -- | |
| “lazy–exciting” | C | -- | −2.383 | −2.318 |
| O | 0.017 | -- | -- | |
| O and V | 0.020 | -- | -- | |
| “no vitality–life vigor” | O | -- | -- | −1.973 |
| O and V | -- | 0.049 | -- | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zhang, Q. Investigating the Impact of Garden Plant Smellscapes on Human Well-Being: A Case Study of Pine Forests. Forests 2024, 15, 1794. https://doi.org/10.3390/f15101794
Zhang X, Zhang Q. Investigating the Impact of Garden Plant Smellscapes on Human Well-Being: A Case Study of Pine Forests. Forests. 2024; 15(10):1794. https://doi.org/10.3390/f15101794
Chicago/Turabian StyleZhang, Xinguo, and Qixiang Zhang. 2024. "Investigating the Impact of Garden Plant Smellscapes on Human Well-Being: A Case Study of Pine Forests" Forests 15, no. 10: 1794. https://doi.org/10.3390/f15101794
APA StyleZhang, X., & Zhang, Q. (2024). Investigating the Impact of Garden Plant Smellscapes on Human Well-Being: A Case Study of Pine Forests. Forests, 15(10), 1794. https://doi.org/10.3390/f15101794






