Abstract
Fine roots are the most dynamic and physiologically active components of belowground tree organs. However, much remains unknown regarding the changes in fine root morphological characteristics during mycorrhizal colonization, especially in natural sites. The aim of this study was to analyze seasonal heterogeneity in fine roots and the mycorrhizal colonization of mature white poplar (Populus alba L.) trees under different soil conditions. Two floodplain forests were selected in Central Europe (Poland), which differed in soil moisture and structure. Fine roots were sampled during one growing season from the upper soil layer. Poplars were characterized by dual mycorrhizal colonization on one root system. It was, therefore, possible to investigate the contribution of two mycorrhizal types (arbuscular mycorrhiza—AM; and ectomycorrhiza—ECM) in response to different habitat conditions. The season was shown to be significant for all fine root features, as well as the degree of mycorrhizal colonization. Roots were better adapted to a drier habitat with a greater proportion of sand, mainly due to a reduction in the fine root diameter (FRD), while other root characteristics did not differ significantly. The degree of mycorrhizal colonization (RLC) and the proportion of arbuscular mycorrhizal structures (AM) were significantly and negatively correlated with the soil water content. A mutual competition between arbuscular mycorrhizas and ectomycorrhizas for poplar roots was also observed, particularly with respect to the season, site, and soil moisture. Changing environmental conditions (especially soil moisture) contribute not only to the morphological and functional changes of fine roots but also to changes in the proportion of arbuscular mycorrhiza and ectomycorrhiza. Understanding the mechanisms of adaptation of tree roots to changing environmental conditions is especially important in the context of climate change.
1. Introduction
Ecosystems are constantly modified by changing climatic conditions. Only effective interspecies cooperation can enable survival during unfavorable conditions and habitat adaptation. Long-term soil–water regime changes resulting from climate change could have profound effects, not only on aboveground ecosystem processes and productivity but also on belowground processes. Such belowground effects will subsequently have corresponding impacts on nutrient and carbon cycling in forest ecosystems [1,2,3,4,5]. The ability of forest ecosystems to survive and adapt to climate change depends in part on how plants balance resources among their photosynthetic organs, stems, branches, reproductive organs, and root systems in a complex process that plant physiologists have struggled to understand for quite some time [6,7]. The role of mycorrhizae in adaptation to environmental changes includes the influence of multi-level relationships affecting the circulation of matter and energy. Arbuscular mycorrhizal fungi (AMF) are an ancient clade of mutualists and, by developing within plant cells, allow plants to adapt to stressful terrestrial conditions. Ectomycorrhizal fungi (ECMF) differ completely from AMF as they are evolutionarily younger and have hyphae that wrap around the plant root tips, thereby forming a mantle. ECMF can decompose dead matter more efficiently than AMF [8]. On an ecosystem scale, symbiotic relationships have enormous mutual benefits. The dependence of fine root architecture on the availability of nutrients and water is not yet clear. It is, therefore, necessary to investigate symbiotic relationships that can explain their adaptive abilities, regardless of the genetic background of trees [9]. Mycorrhizae form a very sensitive symbiotic system and are strongly dependent on soil conditions and the growing season. During the vegetation period, interactions conform to site-specific spatial and temporal patterns due to the dynamic plasticity inherent in mycorrhizal symbioses. Several authors describe the annual variability in the symbiotic structures of the same host plants grown in similar environments [10,11].
Poplars are especially interesting as, compared to other trees, their ability to establish symbiotic relationships with both arbuscular and ectomycorrhizal mycorrhizal fungi, which differ in their evolutionary history and morphoanatomical and physiological features [12,13]. The dual AM/ECM colonization of poplar roots was reportedly affected by host genotype and local environmental conditions; however, the mechanisms determining the AM/ECM ratio are poorly understood [14]. Fine tree roots (roots < 2 mm in diameter) are a minor component of the total forest biomass but play a prominent role in forest ecosystem functioning [15,16,17]. The root system of poplars was described by Ruark and Brockheim [18] as a yet largely undefined system. Since then, numerous physiological and morphological characteristics of poplar root systems have been examined, yet researchers still claim that the function and dynamics of poplar roots, as well as their influence on whole-plant growth, remain poorly understood [19,20], especially the fine root fraction.
The rhizosphere is an important, intensive, and biologically active zone influencing soil properties and the buffering capacity of riparian systems. The production and turnover of fine roots can account for over 40% of the total dry-matter production in forest ecosystems [21]. Root production rates and the degree of mycorrhizal colonization are not constant over time; however, distinct periods of increased production and symbiosis appear, thereby leading to seasonal biomass fluctuations in living roots, as well as the presence of mycorrhizal structures [22,23,24]. Nevertheless, we do not yet possess comprehensive knowledge regarding the properties of fine roots and the influence of environmental factors on the structures of poplar roots and mycorrhizal communities, which are crucial for plant nutrition and tolerance towards various abiotic and biotic stress factors [14,25,26]. Understanding the relationship between the functioning of fine roots of mature trees in natural sites, together with the influence of environmental factors affecting the properties of this root fraction and the degree of their mycorrhizal colonization, is critical for predicting the effects of global change [27]. The importance of studying the structure and distribution of fine roots of mature white poplar trees for a comprehensive understanding of the functioning of natural riverside ecosystems and their protection has been described in previous studies [28]. Knowledge of the features of fine roots and the degree of mycorrhizal colonization in the context of the influence of soil factors and seasonality are valuable indicators for the adaptability and plasticity of these trees. Therefore, the aim of this research is to examine and analyze the seasonal dynamics of the fine root traits of the white poplar in natural riparian habitats, together with soil factors modulating the morphology, distribution, and degree of mycorrhizal colonization of fine roots by AM and ECM fungi.
2. Materials and Methods
2.1. Study Sites
This study was conducted in natural riverside forests, which harbored poplar trees, located in the Valley of the Lower Vistula River in Central Poland (53°13′–53°34′ N; 18°16′–18°37′ E). The Vistula, flowing into the catchment area of the Baltic Sea, is one of the largest European rivers and differs from other rivers due to its relatively low degree of regulation. Such a semi-natural character, together with the related changes in the volume of flow, allow for the maintenance of unique forests in the riverbed. The region belongs to a transitional temperate climate zone (which is intermediate between continental and maritime climatic zones). Average monthly temperatures vary from −2 °C in January to 18 °C in July, with a mean annual temperature of 8 °C. The mean annual precipitation is approximately 500 mm (1951–2015) [29].
Two riverside forest sites with naturally occurring white poplars were selected and differed in soil texture, moisture, and physical and chemical properties. Site 1, Mała Kępa (MK), is located 100 m from the riverbank and is a remnant of a willow–poplar alluvial forest dominated by willows and cultivated exotic hybrid poplars. The site is located on an inner bend where the river deposits its loads, thereby creating sandy areas that are easily permeable, which is unusual for floodplain habitats. Available data indicate that the groundwater level in MK during 1976 was below 200 m; therefore, the maintenance of proper hydrological conditions for the riparian forest is likely related to periodic flooding [30]. However, due to the construction of a dam in 1970, as well as increasing and longer periods of low water levels caused by climate change, flooding occurs less frequently and for shorter periods, which reduces the regenerative capacity of this ecosystem. Site 2, Starogród (STA), is situated 400 m from the river and is characterized by a small difference in height relative to the river level, which translates into prolonged periods of seasonal flooding compared to MK. The short distance between the sites (about 30 km) enabled us to study the impact of soil conditions in similar weather conditions. Site 2 represented a poplar riparian forest composed of trees of different ages, with some individual trees being over 100 years old. The undergrowth was mainly composed of young poplars, with only a small proportion of herbaceous plants.
2.2. Root and Soil Sampling
(1) Soil–root samples were collected from each site during the growing season at four sampling times in 2018 (April, June, August, and October), according to the growing period length for the study area. The samples were taken under the canopy of randomly selected mature white poplars (at approximately 100 cm distance from each tree stem) at three soil depths: 0–10 cm, 10–20 cm, and 20–30 cm. The designated white poplars at MK were located a considerable distance away from the hybrid poplars to ensure a proper origin for the collected roots. Soil–root samples were placed in plastic bags, transported to the laboratory, and stored at 4 °C (for soil moisture measurements) or −20 °C until processing.
(2) The samples were used to examine seasonal changes in fine root features, while their distribution in the soil profile and soil moisture assessment were sampled via coring (with cores being 5.5 cm in diameter and 30 cm in length) and division into 10 cm layers. A total of 144 soil core samples were collected (2 sites × 4 sampling dates × 3 soil depths × 6 samples).
(3) Samples for measuring the dynamics of mycorrhizal colonization were collected using a spade and digging 20 × 20 cm soil blocks from under three poplar trees; a total of 72 samples were collected (2 sites × 4 sampling dates × 3 soil depths × 3 samples).
(4) In April 2018, 18 additional soil samples were collected (2 sites × 3 soil depths × 3 samples) to assess the physico-chemical properties and granulometric composition. Soil–root samples were placed in plastic bags, transported to the laboratory, and stored at 4 °C (for soil moisture measurements) or −20 °C until processing.
2.3. Fine Root Analysis
Roots obtained from soil cores were washed using cold running water on a 1 mm sieve and divided into poplar and other plant roots. The roots of Populus alba were distinguished based on their color, texture, and smell, and fine roots were manually separated from coarse roots based on their diameter (≤2 mm). The prepared roots were scanned carefully to obtain images for determining the total length of all fine roots in a sample (FRL, m m−3) and fine root diameter (FRD, mm) using the radixNova 1.0.1483 program by Cortex Nova. The roots were then wrapped in filter paper, dried at 50 °C for 48 h, and weighed. The specific root length (SRL, m g−1) was calculated by dividing the root length by the root dry mass per soil area to a soil depth of 10 cm (FRB, g m−2).
2.4. Mycorrhizal Colonization Assessment
Roots randomly selected from soil samples were cut into 1 cm fragments, cleared in 10% KOH at 90 °C, bleached in H2O2, and then stained in 0.05% trypan blue at 90 °C according to the Kormanik and McGraw method [31] with modifications in the duration of individual stages to visualize mycorrhizal colonization. The presence of AM fungal structures in the fine roots and ECM fungal mantle on the root surface was evaluated using the intersection method [32] at a 400× magnification, which enabled the proportion of both ECM and AM to be estimated on one root system. This method consists of counting the intersections of previously stained structures of mycorrhizal fungi in the roots and those forming a mantle on the root surface with the line in the microscope eyepiece. For each sample, a minimum of 600-line intersections were analyzed. The percentage of ectomycorrhizal root tips (% ECM) and arbuscular mycorrhizal colonization (% AM) was also measured, as well as the proportion of individual AM structures (vesicles, arbuscules, hyphae). The total mycorrhizal colonization (% ECM + % AM) of the poplar fine roots was determined as the root length colonized (% RLC).
2.5. Soil Analysis
Soil samples were air-dried and sieved through a 2 mm sieve. The total and organic C content was measured using dry combustion. The total N was analyzed according to Kjeldahl’s (1883) method [33]. Ammonium (NH4) and nitrate (NO3)-N were extracted in 0.03 N acetic acid and analyzed with ion-selective electrodes. The total P was measured colorimetrically. Soil-available phosphorus (POlsen) was determined using spectrophotometry according to the method developed by Olsen et al. (1954) [34]. The soil pH was measured in a 1:2.5 soil/water suspension using a standard pH meter. Soil granulometric distributions were determined via sieving and sedimentation. The soil moisture content was measured using the gravimetric method [35].
2.6. Statistical Analysis
Statistical analyses were performed using the software package Statistica 13.3 StatSoft (StatSoft Inc., Tulsa, OK, USA). The effects of the study site on the soil properties and study site and depth on fine root morphological characters and mycorrhizal colonization were analyzed using one-way ANOVAs at a significance level of p < 0.05. The mean values of all characters were separated using Tukey’s honest significance test for an unequal n. A three-way ANOVA was used to examine the significance levels (p < 0.05) of the factors (study site, season, and soil depth) with regard to mycorrhizal colonization parameters (RLC, ECM, AM) and fine root morphological traits (FRL, FRD, SRL, FRB). Pearson’s correlation analysis was performed to analyze the relationships between fine root traits, soil moisture, and the degree of mycorrhizal colonization. Before the analysis, data were checked for normality (Shapiro–Wilk test); homogeneity (Bartlett’s test), and proportional data were transformed according to the Bliss formula [36] x = arcsin√(n%/100) × 180/π, where n% is the percentage value.
3. Results
3.1. Soil Texture
The different soil types were classified as alluvial soils with a silty loam texture in the STA and a sandy loam in MK (Table 1).

Table 1.
Mean values ± SD (n = 3) of soil granulometric composition in two forests in the Vistula River Valley (MK—Mała Kępa, STA—Starogród) in three soil layers (0–10 cm, 10–20 cm, 20–30 cm).
3.2. Soil Properties
The soils on all study sites were slightly alkaline with pH in a range from 7.62 (STA) at the depth of 0–10 cm to 7.83 in MK at the depth of 20–30 cm. No significant differences between the study sites were found in the concentration of N-NO3, N-NH4, and POlsen. The C-org was significantly higher in STA than in MK but only in the uppermost layer (0–10 cm). The C-tot and the N-tot content was significantly higher in STA than in MK at the depths of 0–10 cm and 20–30 cm. The P-tot content was higher in MK than in STA but only at the 0–10 cm depth; these differences were statistically significant. The soil water content (SWC) was significantly higher in STA than in MK in all the analyzed soil layers (Table 2).

Table 2.
Selected soil properties in two study sites (MK—Mała Kępa, STA—Starogród) in three upper layers of the soil profile (0–10 cm, 10–20 cm, 20–30 cm). Values are means ± SD (n = 3). Different letters indicate means that differ significantly among the study sites and separately for each soil depth (p < 0.05, Tukey’s test).
3.3. Fine Root Morphological Traits and Mycorrhizal Colonization
The study site had a significant effect on FRD and SRL and all mycorrhizal colonization values: % RLC, % ECM, % AM. However, additional differences between the sites in terms of individual depths were visible (Table 3).

Table 3.
Results of three-way ANOVA (F and p-values) on the influence of the study site, soil depth, and season (date of sampling) on various fine root traits (FRL—fine root length; FRD—fine root diameter; FRB—fine root biomass; SRL—specific root length) and mycorrhizal colonization (RLC—Root length colonization; ECM—ectomycorrhizal colonization; AM—arbuscular mycorrhiza colonization).
The study sites MK and STA significantly differed in the fine root diameter at the soil depth of 0–10 cm and 10–20 cm and in the fine root biomass at the soil depth of 20–30 cm. Significantly higher values of the fine root diameter and fine root biomass were observed in STA than in MK. The specific root length was higher in STA at all soil layers, but these differences were not statistically significant. There were no statistically significant differences in the fine root length between STA and MK. The poorer and dryer forest (MK) was characterized by significantly higher values of the % RLC at each soil depth. The % ECM was significantly higher in STA than in MK in deeper soil levels (10–20 cm and 20–30 cm). In the shallow layer (0–10 cm), the % ECM was higher in MK, but the differences were not statistically significant. The % AM values were significantly higher in roots taken from MK than in roots taken from STA at all soil layers (Figure 1). The water conditions of the studied places (SWC) were significantly correlated with SRL (r2 = 0.46, p < 0.001) and negatively with the % RLC (r2 = −0.49, p < 0.001) and % AM (r2 = −0.46, p < 0.001).
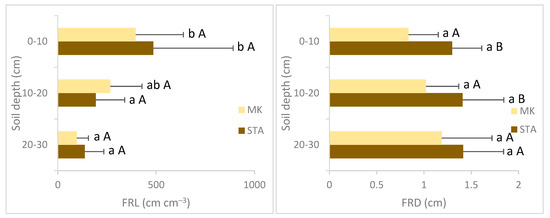
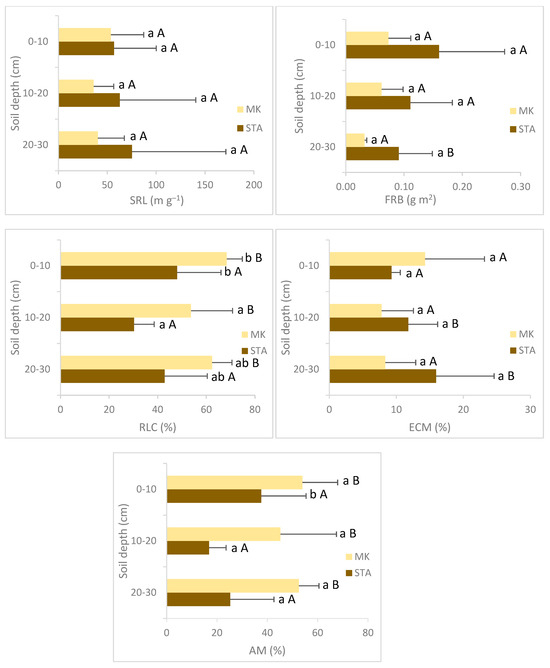
Figure 1.
Mean fine root traits and mycorrhizal colonization for two study sites (MK—Mała Kępa, STA—Starogród) pooled over soil depth. Columns are mean values, and whiskers are standard deviations. Significant differences between the sites at a specific depth are marked with different letters, with a small letter for depths and capital letters for locations (p < 0.05, Tukey’s test).
The depth of the soil had no effect on SRL and % ECM but had an effect on FRL, FRD, FRB, % RLC and % AM (Table 3). In both analyzed sites, the highest fine root length values were observed in the surface layer of the soil (0–10 cm) and decreased with the depth. This soil level differed significantly from the deeper layers. Other morphological features of fine roots (FRD, SRL, FRB) did not change significantly with the soil depth. Both in MK and STA, significantly lower values of the % RLC were observed at the soil depth of 10–20 cm than at the soil depth of 0–10 cm. The ECM colonization did not differ significantly between soil depths. The AM colonization values significantly differed across the soil layers only in STA, where significantly higher AM values were observed in the shallow layer (0–10 cm) compared to deeper depths (10–20 cm and 20–30 cm) (Figure 1, Table 3).
The sampling season had significant effects on all fine root parameters, such as the fine root length, fine root diameter, fine root biomass, and specific root length and mycorrhizal colonization (Table 3). The highest FRL values were observed at both sites at the end of summer (August), which was highly correlated with the biomass of fine roots (r2 = 0.71, p < 0.001), especially in STA, where biomass values were significantly highest in August compared to the other dates. The specific root length was significantly higher in samples taken in April than in other terms, both in MK and STA. Fine root diameter values in spring and summer (April, June, August) were at similar levels in both study sites, while in autumn (October), the average root diameter values in MK decreased significantly, reaching the lowest values, and the opposite process was observed in STA (Figure 2).
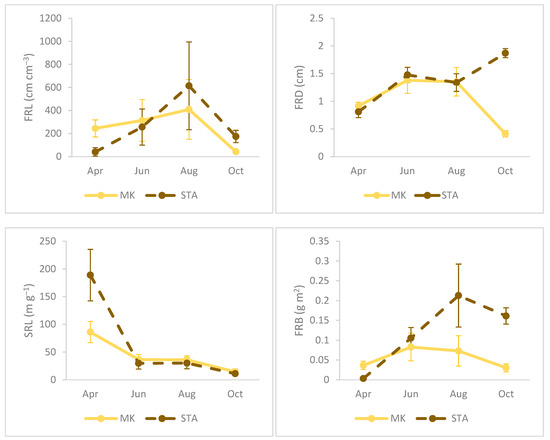
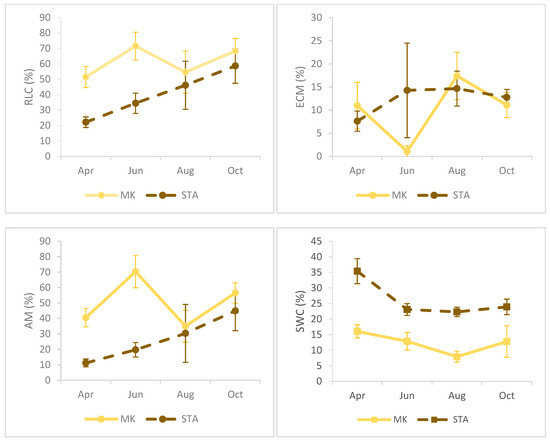
Figure 2.
Seasonal dynamics of fine root traits, mycorrhizal colonization, and soil water content in floodplain forests (MK—Mała Kępa, STA—Starogród). Columns are the mean values, and whiskers are standard deviations.
At the beginning of the growing season (April), high soil moisture at both sites was negatively correlated with the root length (FRL) and fine root biomass (FRB), which could be related to the development and production of new roots at the beginning of the season. However, during sewing and especially at the end of the season, soil moisture significantly influenced FRL, and in October additionally influenced FRD and FRB. The RLC (%) was highly correlated with AM (%) on each of the analyzed sampling dates. In STA, both parameters gradually increased with subsequent months, while, in MK, the general level of colonization (% RLC), as well as the colonization of arbuscular fungi (% AM), varied depending on the sampling date, and the highest significantly different values were observed in June. The % ECM was negatively correlated with % AM at both sites in June and August, but particular differences were visible in MK, where the significantly lowest values were observed in June, which also showed the highest % AM share. In June, there was a positive correlation of ectomycorrhizal colonization (% ECM) with SWC, while the degree of colonization with arbuscular fungi (% AM) in both April and June was negatively correlated with soil moisture (Figure 2, Table 4, Table 5, Table 6 and Table 7).

Table 4.
Pearson correlation matrix of fine root parameters (FRL—fine root length; FRD—fine root diameter; FRB—fine root biomass, SRL—specific root length) mycorrhizal colonization (RLC—root length colonization; ECM—ectomycorrhizal colonization; AM—arbuscular colonization) and SWC (soil water content) for samples taken in April.

Table 5.
Pearson correlation matrix of fine root parameters (FRL—fine root length; FRD—fine root diameter; FRB—fine root biomass, SRL—specific root length) mycorrhizal colonization (RLC—root length colonization; ECM—ectomycorrhizal colonization; AM—arbuscular colonization) and SWC (soil water content) for samples taken in June.

Table 6.
Pearson correlation matrix of fine root parameters (FRL—fine root length; FRD—fine root diameter; FRB—fine root biomass, SRL—specific root length) mycorrhizal colonization (RLC—root length colonization; ECM—ectomycorrhizal colonization; AM—arbuscular colonization) and SWC (soil water content) for samples taken in August.

Table 7.
Pearson correlation matrix of fine root parameters (FRL—fine root length; FRD—fine root diameter; FRB—fine root biomass, SRL—specific root length) mycorrhizal colonization (RLC—root length colonization; ECM—ectomycorrhizal colonization; AM—arbuscular colonization) and SWC (soil water content) for samples taken in October.
4. Discussion
In our study, the greatest impact on the root diameter was at the study site (Table 3). We observed a significant decrease in the fine root diameter at the dry site (MK). Similar trends have been demonstrated in numerous studies [37,38,39] and are related to increases in water and nutrient absorption efficiency. In our study, significant differences in root diameter were present only in October, which may indicate changes in nutrient availability at the end of the growing season since soil moisture was similar throughout the analyzed period (Figure 2). Generally, a poor environment reduces the root diameter [40], especially for low root orders, since the soil moisture level is more important for higher root orders [41]. The lack of significant differences between sites for the remaining analyzed features (FRL, SRL, FRB except for the depth of 20–30 cm) may be related to the low range of soil depths analyzed (0–30 cm). Tan et al. [42] found that the characteristics of fine roots of Populus tomentosa in relation to dry conditions were only significantly different in very deep soil layers (400–600 cm).
Regardless of the different environmental characteristics, the length and biomass of fine roots decreased significantly with the increasing soil depth (Figure 1). These results confirm the distribution of roots typical of poplars, mainly in the upper layers of the soil profile, which has been shown in numerous studies, mainly from plantation crops [42,43], but also in our previous work regarding natural riparian forests [27]. Simultaneously, the distribution of fine roots reflects their absorption and transport functions and is related to decreases in the nutrient pool with increasing soil depth [44].
Soil temperature and moisture are abiotic factors directly correlated with root biomass production and growth [45]. Their fluctuations, characteristic of a temperate climate, influence seasonal differences in the production of fine roots and have been presented in many studies [46,47]. Temperature is particularly important in temperate zones, and an increase in this corresponds to an increase in fine root production. In contrast, soil moisture levels did not show clear seasonal changes and were, therefore, not considered to be a decisive factor [48]. In the current work, we demonstrated gradual root growth in spring, with the highest values occurring at the end of the growing season (August) regardless of location (Figure 2). A similar growth pattern is observed in the studies on various poplar species in plantation crops [47,49], although some results also show a peak in root growth during spring and early summer [24]. The lack of significant differences in the seasonal dynamics of fine root growth at specific localities is related to the short distance between them and, therefore, to similar temperature conditions. The fine root length (FRL) was not correlated with soil water content (SWC), which confirms that soil moisture is not a factor influencing fine root production or that the increased soil moisture stimulated the fine root turnover [50].
The season also had a significant impact on the functional characteristics of fine roots, as determined by SRL (Table 3). Differences between the sites were present only in spring (April), and the sandier site, with a lower total carbon and nitrogen content, was characterized by lower SRL values, while SRL decreased during the growing season when the amount of organic matter supplied was higher (Figure 2). However, research shows an opposite trend in the influence of soil conditions on SRL, where poorer sites with lower soil moisture levels are characterized by thinner and longer roots and, therefore, higher SRL values [51,52,53]. Such an undistinguished relationship may be related to the colonization of roots by arbuscular fungi, which are negatively correlated with SRL. This is related to a change in the strategy of nutrient and water uptake by the roots with a high degree of arbuscular colonization. Similar observations showing the primary role of the extramatrical mycelium in the uptake of phosphorus, with a simultaneous reduction in SRL, were demonstrated by Vanek and Lehmann [54].
This study confirms the dual colonization (ECM and AM) of the fine roots of mature white poplars, although arbuscular mycorrhizae dominated in both sites (Figure S1, Supplementary Materials). On the contrary, our previous studies showed that ectomycorrhizal fungi may predominate over arbuscular mycorrhizal fungi in the colonization of roots from the upper 10 cm of soil under canopies of mature Populus alba trees grown in variable environments [55]. An important factor determining the colonization of poplar roots via ECM and AM fungi is the poplar host genotype; however, environmental conditions can strongly modify the effects of the host plant [14,55,56]. We observed a significantly higher AM at MK (sandy clay) than at STA (silty clay), which indicates the influence of physical soil properties on developmental AMF dynamics. It is commonly believed that the dominance of specific mycorrhizal symbioses on a regional scale result primarily from adaptation to various environments and soil nutrients in particular [57]. Different soil types [58,59] and textures [60,61,62] influence AMF communities and their diversity. Sandy soils can stimulate mycorrhizal colonization by favoring root growth due to greater porosity, while clay soil is less porous and restricts root growth [63]. Paul and Clark [64] explain the greater colonization of the roots of adult trees via ectomycorrhizal fungi due to an increase in the amount of organic matter under the tree canopy due to leaf shedding. ECM fungi prefer organic soils, while AM fungi prefer mineral soils. Arbuscular mycorrhizal fungi are known for their higher tolerance to adverse environmental conditions compared to ectomycorrhizal fungi [65]. During the dual fungal colonization of roots, arbuscular fungi tend to partially migrate into deeper soil layers, characterized by the limited availability of oxygen and nutrients. Ectomycorrhizal fungi predominate on roots in the upper soil layer, characterized by more optimal conditions [13,14]. Wet and poorly aerated types of soil are less stressful for arbuscular fungi than for ectomycorrhizal fungi, which prefer lighter and more well-drained soils. The significant influence of soil moisture on dual colonization has been stressed in various publications, including Lodge [25], Ghering et al. [26], and Querejeta et al. [66]. Generally, the growth of mycorrhizal fungi is inhibited by both low and high water potential [25,67]. Higher arbuscular colonization was recorded in moist soils compared to very dry or flooded soils [25,68,69]. However, the reaction towards varying soil water conditions seems to be species-specific. A variation in the unimodal relationship has often been reported between ECM abundance and soil moisture [26,70]. Within a soil profile, ECM roots were more abundant at shallow depths, and AM roots increased in deeper soil layers [13], suggesting the vertical displacement of mycorrhizae. However, such interactions are not always observed [57], as shown by current research (Figure 1, Table 3). Although we know that ecto- and arbuscular mycorrhizae segregate at the biome scale [71,72,73], the way their presence may shift in a single host species that spans multiple biomes is unclear. This discrepancy is likely a result of the different soil depths sampled. A significantly higher RLC was observed at a depth of 10–20 cm at both our study sites, which was directly correlated with AM. Much of the variation in colonization intensity seems to be related not only to plant and fungal identity but also to seasonality and environmental conditions [74,75,76].
The negative correlation between AM and ECM colonization demonstrated in this study confirmed previous research and may indicate competition between mycorrhizal types, which was suggested by Moyersoen et al. [77]. When interpreting the relationships and mutual proportions of ECM and AM in one root system, one must account for the influence of a set of factors that not only directly influence this relationship but also indirectly affect soil microorganism communities associated with the rhizosphere of trees. Soil bacteria can have profound effects on mycorrhizal establishment. The variety in microbial populations around the mycorrhizal roots of different fungal and plant partners and in different sections of the soil profile has also been assessed [78].
5. Conclusions
A significant positive influence of the soil water content (SWC) on ectomycorrhiza colonization (%ECM) was observed only in the early part of the growing season (June) (Table 5). The growth processes of roots and mycorrhizal fungi require the availability of water present in the soil and carbohydrates transported from the leaves, which are fully developed in June, to the roots. These results indicate that changes in habitat conditions during the growing season result in differences in the dominance of the mycorrhiza type (AM/ECM) in Populus alba trees (Figure 1, Table 3). The lack of relationship between the abundance of ectomycorrhizas and root biomass may be due to the structure of ectomycorrhizas on the roots of white poplars. A thorough morphological and anatomical analysis may clarify this issue (Table 4, Table 5, Table 6 and Table 7). The varied participation of AM and ECM fungi in root colonization in different places and at different depths confirms the benefit of the double colonization of the white poplar.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f15010064/s1, Figure S1: Images of mycorrhizal colonization of white poplar.
Author Contributions
Conceptualization, A.F.-S.; methodology, A.F.-S.; validation, A.F.-S.; formal analysis, A.F.-S. and J.T.-W.; writing—original draft preparation, A.F.-S. and M.K.-S.; writing—review and editing, A.F.-S. and M.K.-S.; visualization, A.F.-S. and J.T.-W.; supervision, A.F.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Science Center, Poland (Grant No. NN 304 0689 33) and by the Polish Minister of Science and Higher Education under the program “Regional Initiative of Excellence” in 2019–2022 (Grant No. 008/RID/2018/19).
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- McClaugherty, C.A.; Aber, J.D.; Melillo, J.M. The role of fine roots in the organic matter and nitrogen budgets of two forested ecosystems. Ecology 1982, 63, 1481–1490. [Google Scholar] [CrossRef]
- Nadelhoffer, K.J.; Aber, J.D.; Melillo, J.M. Fine roots, net primary production, and soil nitrogen availability: A new hypothesis. Ecology 1985, 66, 1377–1390. [Google Scholar] [CrossRef]
- Joslin, J.D.; Henderson, G. Organic matter and nutrients associated with fine root turnover in a white oak stand. For. Sci. 1987, 33, 330–346. [Google Scholar]
- Hendrick, R.L.; Pregitzer, K.S. Temporal and depth-related patterns of fine root dynamics in northern hardwood forests. J. Ecol. 1996, 84, 167–176. [Google Scholar] [CrossRef]
- Joslin, J.D.; Wolfe, M.H.; Hanson, P.J. Effects of altered water regimes on forest root systems. New Phytol. 2000, 147, 117–129. [Google Scholar] [CrossRef]
- Bradford, K.J.; Hsiao, T.C. Physiological responses to moderate water stress. In Physiological Plant Ecology II: Water Relations and Carbon Assimilation; Springer: Berlin/Heidelberg, Germany, 1982; pp. 263–324. [Google Scholar]
- Kramer, P.J. Carbon dioxide concentration, photosynthesis, and dry matter production. BioScience 1981, 31, 29–33. [Google Scholar] [CrossRef]
- Carteron, A.; Cichonski, F.; Laliberté, E. Ectomycorrhizal Stands Accelerate Decomposition to a Greater Extent than Arbuscular Mycorrhizal Stands in a Northern Deciduous Forest. Ecosystems 2022, 25, 1234–1248. [Google Scholar] [CrossRef]
- Weemstra, M.; Sterck, F.J.; Visser, E.J.W.; Kuyper, T.W.; Goudzwaard, L.; Mommer, L. Fine-root trait plasticity of beech (Fagus sylvatica) and spruce (Picea abies) forests on two contrasting soils. Plant Soil 2017, 415, 175–188. [Google Scholar] [CrossRef]
- Miller, S.P.; Bever, J.D. Distribution of arbuscular mycorrhizal fungi in stands of the wetland grass Panicum hemitomon along a wide hydrologic gradient. Oecologia 1999, 119, 586–592. [Google Scholar] [CrossRef]
- Carvalho, L.M.; Caçador, I.; Martins-Loução, M.A. Temporal and spatial variation of arbuscular mycorrhizas in salt marsh plants of the Tagus estuary (Portugal). Mycorrhiza 2001, 11, 303–309. [Google Scholar] [CrossRef]
- Brundrett, M.; Murase, G.; Kendrick, B. Comparative anatomy of roots and mycorrhizae of common Ontario trees. Can. J. Bot. 1990, 68, 551–578. [Google Scholar] [CrossRef]
- Neville, J.; Tessier, J.L.; Morrison, I.; Scarratt, J.; Canning, B.; Klironomos, J.N. Soil depth distribution of ecto- and arbuscular mycorrhizal fungi associated with Populus tremuloides within a 3-year-ild boreal forest clear-cut. Appl. Soil Ecol. 2002, 19, 209–216. [Google Scholar] [CrossRef]
- Karliński, L.; Rudawska, M.; Kieliszewska-Rokicka, B.; Leski, T. Relationship between genotype and soil environment during colonization of poplar roots by mycorrhizal and endophytic fungi. Mycorrhiza 2010, 20, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Nadelhoffer, K.J.; Raich, J.W. Fine root production estimates and belowground carbon allocation in forest ecosystems. Ecology 1992, 73, 1139–1147. [Google Scholar] [CrossRef]
- Johnson, K.H.; Vogt, K.A.; Clark, H.J.; Schmitz, O.J.; Vogt, D.J. Biodiversity and the productivity and stability of ecosystems. Trends Ecol. Evol. 1996, 11, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Silver, W.L.; Thompson, A.W.; McGroddy, M.E.; Varner, R.K.; Dias, J.D.; Silva, H.; Keller, M. Fine root dynamics and trace gas fluxes in two lowland tropical forest soils. Glob. Change Biol. 2005, 11, 290–306. [Google Scholar] [CrossRef]
- Ruark, G.A.; Bockheim, J.G. Below-ground biomass of 10-, 20-, and 32-year-old: Populus tremuloides in Wisconsin. Pedobiologia 1987, 30, 207–218. [Google Scholar] [CrossRef]
- Pregitzer, K.S.; Friend, A.L. The Structure and Function of Populus Root Systems. In Biology of Populus and Its Implication for Management and Conservation; Stettler, R.F., Ed.; NRC Research Press: Ottawa, ON, Canada, 1996; pp. 331–354. [Google Scholar]
- Brunner, I.; Herzog, C.; Dawes, M.A.; Arend, M.; Sperisen, C. How tree roots respond to drought. Front. Plant Sci. 2015, 6, 547. [Google Scholar] [CrossRef]
- Keyes, M.R.; Grier, C.C. Above-and below-ground net production in 40-year-old Douglas-fir stands on low and high productivity sites. Can. J. For. Res. 1981, 11, 599–605. [Google Scholar] [CrossRef]
- King, J.S.; Pregitzer, K.S.; Zak, D.R. Clonal variation in aboveand belowground growth responses of Populus tremuloides Michaux: Influence of soil warming and nutrient availability. Plant Soil 1999, 217, 119–130. [Google Scholar] [CrossRef]
- Coleman, M.D.; Dickson, R.E.; Isebrands, J.G. Contrasting fine-root production, survival and soil CO2 efflux in pine and poplar plantations. Plant Soil 2000, 225, 129–139. [Google Scholar] [CrossRef]
- Block, R.M.A.; Van Rees, K.C.J.; Knight, J.D. A review of fine root dynamics in Populus plantations. Agrofor. Syst. 2006, 67, 73–84. [Google Scholar] [CrossRef]
- Lodge, D.J. The influence of soil moisture and flooding on formation of VA-endo- and ectomycorrhizae in Populus and Salix. Plant Soil 1989, 117, 243–253. [Google Scholar] [CrossRef]
- Gehring, C.A.; Mueller, R.C.; Whitham, T.G. Environmental and genetic effects on the formation of ectomycorrhizal and arbuscular mycorrhizal associations in cottonwoods. Oecologia 2006, 149, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Cudlin, P.; Kieliszewska-Rokicka, B.; Rudawska, M.; Grebenc, T.; Alberton, O.; Lehto, T.; Bakker, M.R.; Børja, I.; Konôpka, B.; Leski, T.; et al. Fine roots and ectomycorrhizas as indicators of environmental change. Plant Biosyst. 2007, 141, 406–425. [Google Scholar] [CrossRef]
- Frymark-Szymkowiak, A.; Kieliszewska-Rokicka, B. The Fine Root Distribution and Morphology of Mature White Poplar in Natural Temperate Riverside Forests under Periodically Flooded or Dry Hydrological Conditions. Forests 2023, 14, 223. [Google Scholar] [CrossRef]
- Wójcik, G.; Marciniak, K. Climate. In Nature of the Kujawsko-Pomorskie Voivodeship; Przystalski, A., Ed.; Kujawsko-Pomorskie Voivodeship Office, Voivodeship Nature Conservation: Bydgoszcz, Poland, 2001; pp. 23–32. [Google Scholar]
- Figaj, J.; Stecki, Z. From studies on the adaptation of poplars to various site conditions. Arbor. Kórn. 1976, 21, 213–256. [Google Scholar]
- Kormanik, P.P.; McGraw, A.C. Quantification of vesicular-arbuscular mycorrhizae in plant roots. In Methods and Principles of Mycorrhizal Research; Schenck, N.C., Ed.; American Phytopathological Society: St. Paul, MN, USA, 1982; pp. 37–45. [Google Scholar]
- McGonigle, T.P.; Miller, M.H.; Evans, D.G.; Fairchild, G.L.; Swan, J.A. A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef]
- Kjeldahl, J. A New Method for the Determination of Nitrogen in Organic Matter. Z. Anal. Chem. 1883, 22, 366–382. [Google Scholar] [CrossRef]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; U.S. Department of Agriculture: Washington, DC, USA, 1954; p. 19. [Google Scholar]
- Hausenbuiller, R. Soil Science Principles and Practice, 4th ed.; Wm. C. Brown Co.: Dubuque, IA, USA, 1975; p. 90. [Google Scholar]
- Snedecor, W.; Cochran, W.G. Statistical Methods, 6th ed.; The Iowa State University Press: Iowa City, IA, USA, 1976. [Google Scholar]
- Wen, X.; Wang, X.; Ye, M.; Liu, H.; He, W.; Wang, Y.; Li, T.; Zhao, K.; Hou, G.; Chen, G.; et al. Response strategies of fine root morphology of Cupressus funebris to the different soil environment. Front. Plant Sci. 2022, 13, 1077090. [Google Scholar] [CrossRef]
- Ostonen, I.; Lõhmus, K.; Helmisaari, H.-S.; Truu, J.; Meel, S. Fine root morphological adaptations in Scots pine, Norway spruce and silver birch along a latitudinal gradient in boreal forests. Tree Physiol. 2007, 27, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, P.S.; Bauerle, T.L.; Häberle, K.H.; Blaschke, H.; Brunner, I.; Matyssek, R. Fine-root traits reveal contrasting ecological strategies in European beech and Norway spruce during extreme drought. Front. Plant Sci. 2020, 11, 1211. [Google Scholar] [CrossRef] [PubMed]
- Hogan, J.A.; Valverde-Barrantes, O.J.; Tang, W.; Ding, Q.; Xu, H.; Baraloto, C. Evidence of elemental homeostasis in fine root and leaf tissues of samplings across a fertility gradient in tropical montane forest in Hainan, China. Plant Soil 2021, 460, 625–646. [Google Scholar] [CrossRef]
- Li, T.; Ren, J.; He, W.; Wang, Y.; Wen, X.; Wang, X.; Ye, M.; Chen, G.; Zhao, K.; Hou, G.; et al. Anatomical structure interpretation of the effect of soil environment on fine root function. Front. Plant Sci. 2022, 13, 993127. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Yu, W.; Liu, Y.; Gou, Y.; Liu, N.; Fu, H.; Di, N.; Duan, J.; Li, X.; Xi, B. Response of Fine-Root Traits of Populus tomentosa to Drought in Shallow and Deep Soil. Forests 2023, 14, 951. [Google Scholar] [CrossRef]
- Di, N.; Liu, Y.; Mead, D.J.; Xie, Y.; Jia, L.; Xi, B. Root-system characteristic of plantation-grow Populus tomentosa adapted to seasonal fluctuation in the groundwater table. Trees 2018, 32, 137–149. [Google Scholar] [CrossRef]
- Ostonen, I.; Helmisaari, H.; Borken, W.; Tedersoo, L.; Kukumägi, M.; Bahram, M.; Lindroos, A.; Nöjd, P.; Uri, V.; Merilä, P.; et al. Fine root foraging strategies in Norway spruce forests across a European climate gradient. Glob. Change Biol. 2011, 17, 3620–3632. [Google Scholar] [CrossRef]
- McCormack, M.L.; Guo, D. Impacts of environmental factors on fine root lifespan. Front. Plant Sci. 2014, 5, 205. [Google Scholar] [CrossRef] [PubMed]
- Ruess, R.W.; Cleve, K.V.; Yarie, J.; Viereck, L.A. Contributions of fine root production and turnover to the carbon and nitrogen cycling in taiga forests of the Alaskan interior. Can. J. For. Res. 1996, 26, 1326–1336. [Google Scholar] [CrossRef]
- Kern, C.C.; Friend, A.L.; Johnson, J.M.-F.; Coleman, M.D. Fine root dynamics in a developing Populus deltoides plantation. Tree Physiol. 2004, 24, 651–660. [Google Scholar] [CrossRef]
- Abramoff, R.Z.; Finzi, A.C. Are above- and below- ground phenology in sync? New Phytol. 2015, 205, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Block, R.M.A. Fine Root Dynamics and Carbon Sequestration in Juvenile Hybrid Poplar Plantation in Saskatchewan, Canada. Master’s Thesis, University of Saskatchewan, Saskatoon, SK, Canada, 2004. [Google Scholar]
- Zhang, X.; Xing, Y.; Yan, G.; Han, S.; Wang, Q. Effects of precipitation change on fine root morphology and dynamics at a global scale: A meta-analysis. Can. J. Soil Sci. 2019, 99, 1–11. [Google Scholar] [CrossRef]
- Ostonen, I.; Püttsepp, Ü.; Biel, C.; Alberton, O.; Bakker, M.R.; Lõhmus, K.; Majdi, H.; Metcalfe, D.; Olsthoorn, A.F.M.; Pronk, A.; et al. Specific root length as an indicator of environmental change. Plant Biosyst. 2007, 141, 426–442. [Google Scholar] [CrossRef]
- Kramer-Walter, K.R.; Bellingham, P.J.; Millar, T.R.; Smissen, R.D.; Richardson, S.J.; Laughlin, D.C. Root traits are multidimensional: Specific root length is independent from root tissue density and the plant economic spectrum. J. Ecol. 2016, 104, 1299–1310. [Google Scholar] [CrossRef]
- Fort, F.; Freschet, G.T. Plant ecological indicator values as predictors of fine-root trait variations. J. Ecol. 2020, 108, 1565–1577. [Google Scholar] [CrossRef]
- Vanek, S.J.; Lehmann, J. Phosphorus availability to beans via interactions between mycorrhizas and biochar. Plant Soil 2015, 395, 105–123. [Google Scholar] [CrossRef]
- Tyburska, J.; Frymark-Szymkowiak, A.; Kulczyk-Skrzeszewska, M.; Kieliszewska-Rokicka, B. Mycorrhizal status of forest trees grown in urban and rural environments in Poland. Ecol. Quest. 2013, 18, 49–57. [Google Scholar] [CrossRef][Green Version]
- Bainard, L.D.; Klironomos, J.N.; Gordon, A.M. Arbuscular mycorrhizal fungi in tree-based intercropping systems: A review of their abundance and diversity. Pedobiologia 2011, 54, 57–61. [Google Scholar] [CrossRef]
- Karst, J.; Franklin, J.; Simeon, A.; Light, A.; Bennett, J.A.; Erbilgin, N. Assessing the dual-mycorrhizal status of a widespread tree species as a model for studies on stand biogeochemistry. Mycorrhiza 2021, 31, 313–324. [Google Scholar] [CrossRef]
- Oehl, F.; Laczko, E.; Bogenrieder, A.; Stahr, K.; Bösch, R.; van der Heijden, M.; Sieverding, E. Soil type and land use intensity determine the composition of arbuscular mycorrhizal fungal communities. Soil Biol. Biochem. 2010, 42, 724–738. [Google Scholar] [CrossRef]
- Tran, C.T.; Watts-Williams, S.J.; Smernik, R.J.; Cavagnaro, T.R. Root and arbuscular mycorrhizal effects on soil nutrient loss are modulated by soil texture. Appl. Soil. Ecol. 2021, 167, 104097. [Google Scholar] [CrossRef]
- Vieira, L.C.; Silva, D.K.A.D.; Escobar, I.E.C.; Silva, J.M.D.; Moura, I.A.D.; Oehl, F.; Silva, G.A.D. Changes in an arbuscular mycorrhizal fungi community along an environmental gradient. Plants 2020, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Stephen, D. Comparative Effects of Soil Nutrient Status and Texture on Mycorrhiza-Legume Base Cropping System in Two Soil Types of Nigeria. Crop Sci. 2022, 3, 6–15. [Google Scholar]
- Moebius-Clune, D.J.; Moebius-Clune, B.N.; van Es, H.M.; Pawlowska, T.E. Arbuscular mycorrhizal fungi associated with a single agronomic plant host across the landscape: Community differentiation along a soil textural gradient. Soil Biol. Biochem. 2013, 64, 191–199. [Google Scholar] [CrossRef]
- Carrenho, R.; Trufem, S.F.B.; Bononi, V.L.R.; Silva, E.S. The effect of different soil properties on arbuscular mycorrhizal colonization of peanuts, sorghum and maize. Acta Bot. Bras. 2007, 21, 723–730. [Google Scholar] [CrossRef]
- Paul, E.A.; Clark, F.E. Soil Microbiology and Biochemistry; Academic Press: San Diego, CA, USA, 1996; p. 340. [Google Scholar]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: San Diego, CA, USA, 2010. [Google Scholar]
- Querejeta, J.; Egerton-Warburton, L.M.; Allen, M.F. Topographic position modulates the mycorrhizal response of oak trees to interannual rainfall variability. Ecology 2009, 90, 649–662. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Tang, M.; Chen, H.; Zhang, Q.; Feng, X. Effects of two Glomus species on the growth and physiological performance of Sophora davidii seedlings under water stress. New For. 2013, 44, 399–408. [Google Scholar] [CrossRef]
- Miller, S.P.; Sharitz, R.R. Manipulation of flooding and arbuscular mycorrhiza formation influences growth and nutrition of two semiaquatic grass species. Funct. Ecol. 2000, 14, 738–748. [Google Scholar] [CrossRef]
- Entry, J.A.; Rygiewicz, P.T.; Watrud, L.S.; Donnelly, P.K. Influence of adverse soil conditions on the formation and function of arbuscular mycorrhizas. Adv. Environ. Res. 2002, 7, 123–138. [Google Scholar] [CrossRef]
- Teste, F.P.; Jones, M.D.; Dickie, I.A. Dual-mycorrhizal plants: Their ecology and relevance. New Phytol. 2020, 225, 1835–1851. [Google Scholar] [CrossRef]
- Fahey, C.; Bell, F.W.; Antunes, P.M. Effects of dual mycorrhizal inoculation on Pinus strobus seedlings are influenced by soil resource availability. Plant Soil 2022, 479, 607–620. [Google Scholar] [CrossRef]
- Soudzilovskaia, N.A.; van Bodegom, P.M.; Terrer, C.; Zelfde, M.V.T.; McCallum, I.; Luke McCormack, M.; Tedersoo, L. Global mycorrhizal plant distribution linked to terrestrial carbon stocks. Nat. Commun. 2019, 10, 5077. [Google Scholar] [CrossRef] [PubMed]
- Steidinger, B.S.; Crowther, T.W.; Liang, J.; Van Nuland, M.E.; Werner, G.D.; Reich, P.B.; Peay, K.G. Climatic controls of decomposition drive the global biogeography of forest-tree symbioses. Nature 2019, 569, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Van Nuland, M.E.; Ke, P.J.; Wan, J.; Peay, K.G. Mycorrhizal nutrient acquisition strategies shape tree competition and coexistence dynamics. J. Ecol. 2023, 111, 564–577. [Google Scholar] [CrossRef]
- Maltz, M.R.; Treseder, K.K. Sources of inocula influence mycorrhizal colonization of plants in restoration projects: A meta-analysis. Restor. Ecol. 2015, 23, 625–634. [Google Scholar] [CrossRef]
- Hoeksema, J.D.; Bever, J.D.; Chakraborty, S.; Chaudhary, V.B.; Gardes, M.; Gehring, C.A.; Zee, P.C. Evolutionary history of plant hosts and fungal symbionts predicts the strength of mycorrhizal mutualism. Commun. Biol. 2018, 1, 116. [Google Scholar] [CrossRef]
- Moyersoen, B.; Fitter, A.H.; Alexander, I.J. Spatial distribution of ectomycorrhizas and arbuscular mycorrhizas in Korup National Park rain forest, Cameroon, in relation to edaphic parameters. New Phytol. 1998, 139, 311–320. [Google Scholar] [CrossRef]
- Tarkka, M.; Nehls, U.; Hampp, R. Physiology of ectomycorrhiza (ECM). In Progress in Botany: Genetics Physiology Systematics Ecology; Springer: Berlin/Heidelberg, Germany, 2005; pp. 247–276. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).