Estimation of Carbon and Nitrogen Contents in Forest Ecosystems in the Background Areas of the Russian Arctic (Murmansk Region)
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Carbon and Nitrogen in the Atmospheric Precipitation
3.2. Carbon and Nitrogen in the Soil
3.3. Carbon and Nitrogen in the Soil Water
3.4. Carbon and Nitrogen in the Living Needles and Litter Fall
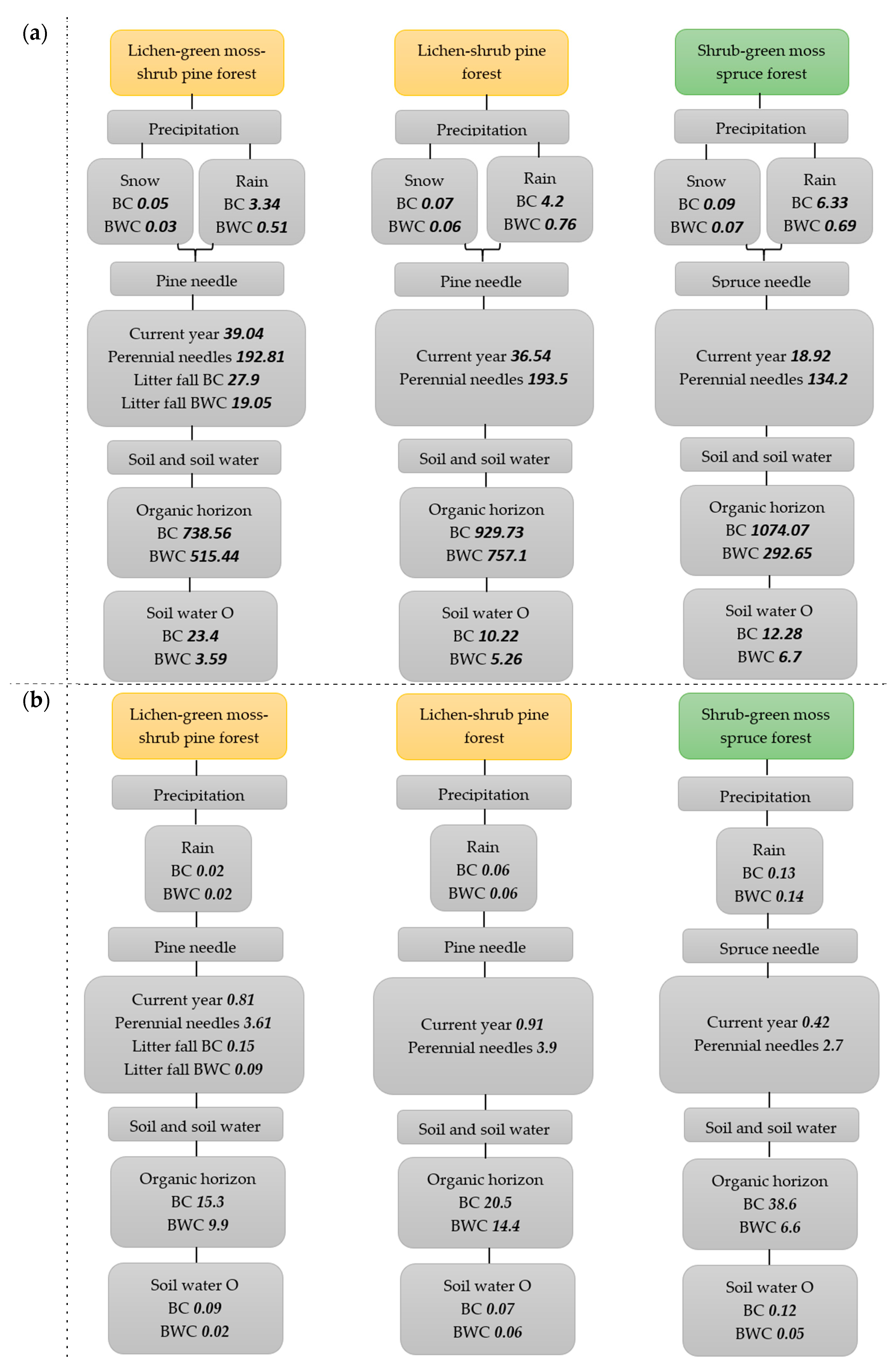
4. Carbon and Nitrogen Contents in Different Components in Northern Taiga Forest Ecosystem
5. Conclusions
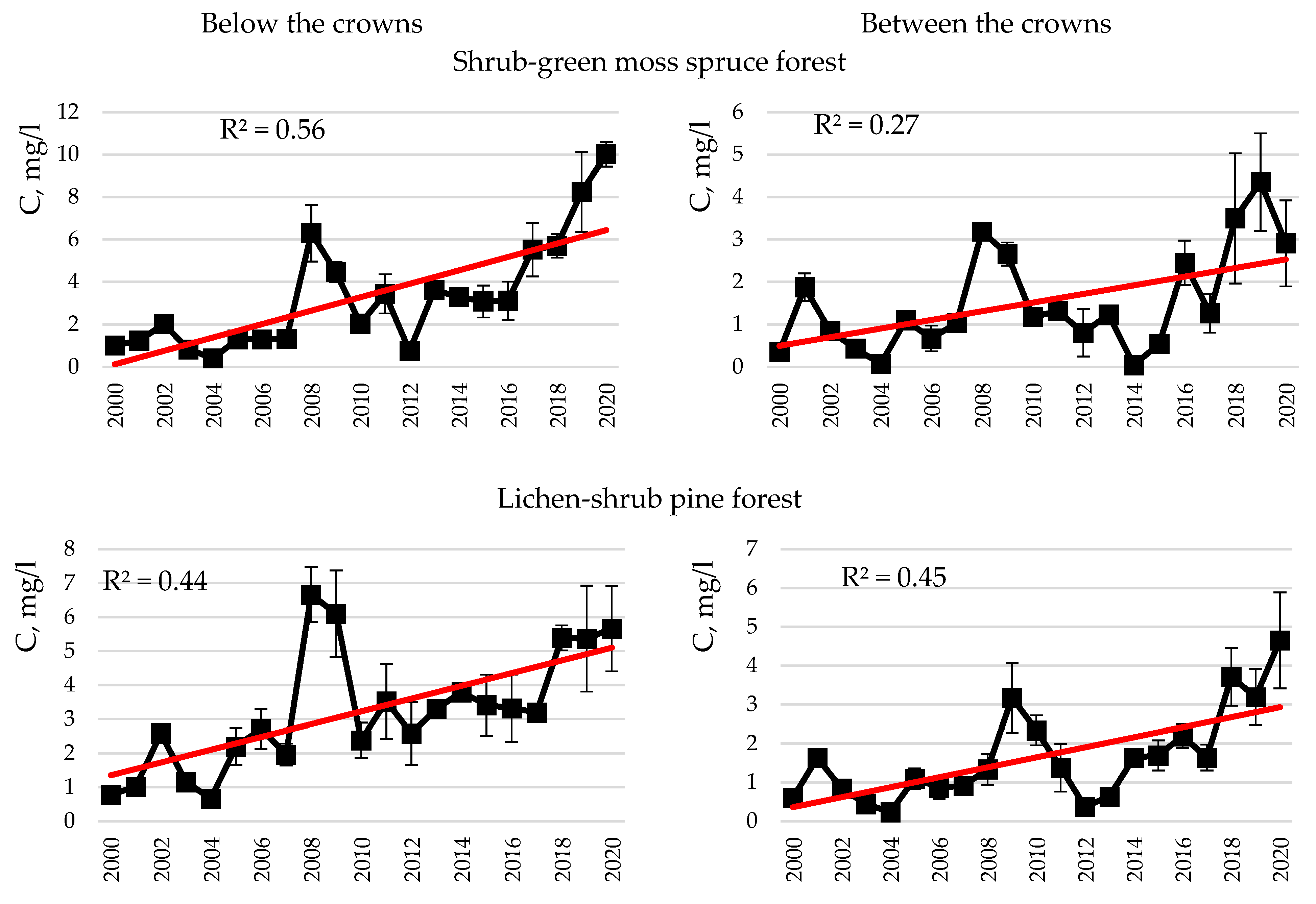
- Carbon and nitrogen in snow and rainwater, as well as atmospheric precipitation of these, were found to be higher below the crowns in spruce and pine forests than between the crowns, which is associated with the wash-off and leaching of elements from the tree crowns. In rainwater in spruce, carbon concentrations and deposition below the tree crowns were higher than those in pine. Increased carbon concentrations in the rain deposition below the crowns in the spruce forest are attributable to a thicker spruce canopy compared to pine. The long-term dynamics of carbon concentrations in snow demonstrated a trend of increasing carbon concentrations in treeless areas, as well as below the crowns in the dwarf shrub-green moss spruce forest and both below and between the crowns in the lichen-shrub pine forest. An increase in carbon concentrations in snow, clearly expressed below the crowns, may be associated with an increase in the number of thaw days in the Murmansk region.
- In spruce and pine forests, a significant decrease was observed in the content of carbon and nitrogen in the soil’s mineral horizons compared to the organic horizon. No significant intrabiogeocoenotic differences in carbon content were found in pine and spruce forest soils. The nitrogen content below the crowns in spruce and pine forests was typically higher than between the crowns. Interbiogeocoenotic differences in carbon content were weakly expressed; in the organic soil horizon, the carbon content was higher in pine compared to spruce, while in the mineral soil horizon, on the contrary, there was a higher carbon content in spruce compared to pine. The nitrogen content below the crowns and between the crowns in the organic and mineral soil horizons in the spruce forest was higher than in the pine forest. This can be explained by the higher content of nitrogen compounds in the atmospheric precipitation in spruce forests compared to pine. The main stocks of soil carbon and nitrogen in northern taiga forests are concentrated below the crowns.
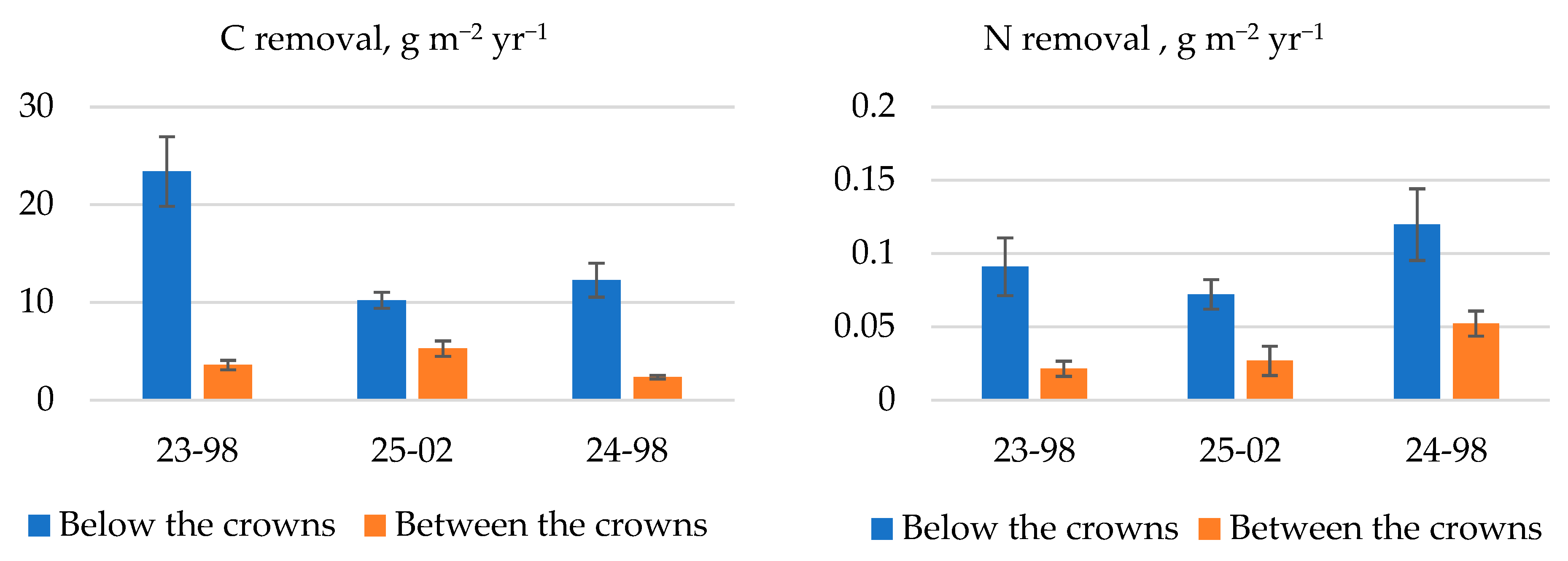
- The concentrations of carbon and nitrogen in the soil water, as well as the removal of these, were typically higher below than between the crowns in the spruce and pine forests. Increased element concentrations in the soil water below the crowns indicate the washout and leaching of element compounds from the tissues of dominant woody plants. In the pine forest, carbon concentrations were usually higher than in the spruce forest, which can be explained by the high carbon content in the organic soil horizon below and between the crowns in the pine forest. The long-term dynamics of carbon concentrations in water from all soil horizons in pine and spruce forests were characterized by significant variability.
- The carbon content in living Picea obovata and Pinuss ylvestris needles and Pinus Sylvestris needle litter had minor variability; no significant interbiogeocoenotic and age differences were found. The nitrogen content in the current year needles was typically higher than that in the perennial needles and was significantly reduced in brown needles and needle litter.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kuznetsov, M.A. Pools and fluxes of organic carbon in the system soil-phytocenosis of bilberry-sphagnum spruce forest in the middle taiga zone of the Komi republic. Vestnic IB Komi SC UrB RAS 2014, 5, 17–19. (In Russian) [Google Scholar]
- Bobkova, K.S.; Osipov, A.F. Carbon cycling in system phytocenosis-soil in bilberry-sphagnum pine forests of the middle taiga (republic of Komi). Contemp. Probl. Ecol. 2012, 2, 11–18. [Google Scholar]
- Osipov, A.F. Organic carbon stocks and fluxes in the ecosystem of a mature blueberry pine forest of the middle taiga. Sib. For. J. 2017, 2, 70–80. (In Russian) [Google Scholar] [CrossRef]
- Bakhmet, O.N. Carbon Deposits in Soils of Pine and Spruce Forests of Karelia. Contemp. Probl. Ecol. 2018, 11, 697–703. [Google Scholar] [CrossRef]
- Menyailo, O.V.; Matvienko, A.I.; Makarov, M.I.; Cheng, S.K. Role of nitrogen in the regulation of the carbon cycle in forest ecosystems. Contemp. Probl. Ecol. 2018, 2, 143–159. (In Russian) [Google Scholar] [CrossRef]
- Murillo, J.C.R. Temporal Variations in the Carbon Budget of Forest Ecosystems in Spain. Ecol. Appl. 1997, 7, 461–469. [Google Scholar] [CrossRef]
- Marty, C.; Houle, D.; Courchesne, F.; Gagnon, C. Soil C:N ratio is the main driver of soil δ15N in cold and N-limited eastern Canadian forests. Catena 2019, 172, 285–294. [Google Scholar] [CrossRef]
- Schulte-Uebbing, L.; de Vries, W. Global-scale impacts of nitrogen deposition on tree carbon sequestration in tropical, temperate, and boreal forests: A meta-analysis. Glob. Chang. Biol. 2018, 24, e416–e431. [Google Scholar] [CrossRef]
- Lukina, N.V.; Nikonov, V.V. Biogeochemical Cycles in the Northern forests Subjected to Air Pollution; KNC RAN: Apatity, Russia, 1996; Volume 1. (In Russian) [Google Scholar]
- Bobkova, K.S.; Galenko, E.P.; Zaboeva, I.V.; Torlopova, N.V.; Ivasishina, N.A.; Kuzin, S.N.; Martynjuk, Z.P.; Zagirova, S.V.; Tuzhilkina, V.V.; Robakidze, E.A.; et al. Process of Bioproduction in Forest Ecosystems of the North; Nauka: Saint Petersburg, Russia, 2001; ISBN 5-02-026154-8. (In Russian)
- Manakov, K.N. Productivity and biological cycle in pine forests. In Biological Productivity and Exchange at the Forest Biogeocenosis of Kola Peninsula; Kol. Fil. AN SSSR: Apatity, Russia, 1978. (In Russian) [Google Scholar]
- Nikonov, V.V. Formation of Soils on the Northern Tree Line of Pine Biogeocoenoses; Nauka: Leningrad, Russia, 1987. (In Russian)
- Nikonov, V.V.; Pereversev, V.N. Formation of Soils in the Kola Subarctic; Nauka: Leningrad, Russia, 1989. (In Russian)
- Albrektson, A. Needle litterfall in stands of Pinus sylvestris L. in Sweden, in relation to site quality, stand age, and latitude. Scand. J. For. Res. 1988, 3, 333–342. [Google Scholar] [CrossRef]
- Pedersen, L.B.; Bille-Hansen, J. A comparison of litterfall and element fluxes in even aged Norway spruce, sitka spruce and beech stands in Denmark. For. Ecol. Manag. 1999, 114, 55–70. [Google Scholar] [CrossRef]
- Berg, B. Litter decomposition and organic matter turnover in northern forest soils. For. Ecol. Manag. 2000, 133, 13–22. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, Y.; Xin, J.; Tang, G.; Song, T.; Wang, Y.; Li, X.; Wu, F. Study on dissolved organic carbon in precipitation in Northern China. Atmos. Environ. 2010, 44, 2350–2357. [Google Scholar] [CrossRef]
- Lavorivska, L.; Boyer, E.W.; De Walle, D.R. Atmospheric deposition of organic carbon via precipitation. Atmos. Environ. 2016, 146, 153–163. [Google Scholar] [CrossRef]
- Derome, J.; Lindroos, A.-J. Effects of heavy metal contamination on macronutrient availability and acidification parameters in forest soil in the vicinity of the Harjavalta Cu-Ni smelter, SW Finland. Environ. Pollut. 1998, 99, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Yashin, I.M.; Raskatov, V.A.; Shishov, L.L. Water Migration of Chemical Elements in Soil Cover; MSHA: Moscow, Russia, 2003; ISBN 5-94327-144-9. (In Russian)
- Kalbitz, K.; Solinger, S.; Park, J.H.; Michalzik, B.; Matzner, E. Controls on the dynamics of dissolved organic matter in soils: A review. Soil Sci. 2000, 165, 277–304. [Google Scholar] [CrossRef]
- Kaiser, K.; Guggenberger, G.; Zech, W. Sorption of DOM and DOM fractions to forest soils. Geoderma 1996, 74, 281–303. [Google Scholar] [CrossRef]
- Karavanova, E.I. Dissolved organic matter: Fractional composition and sorbability by the soil solid phase (Review of literature). Eurasian Soil Sci. 2013, 46, 833–844. [Google Scholar] [CrossRef]
- Camino-Serrano, M.; Gielen, B.; Luyssaert, S.; Ciais, P.; Vicca, S.; Guenet, B.; Vos, B.D.; Cools, N.; Ahrens, B.; Altaf Arain, M.; et al. Linking variability in soil solution dissolved organic carbon to climate, soil type, and vegetation type. Glob. Biogeochem. Cycles 2014, 28, 497–509. [Google Scholar] [CrossRef]
- Camino-Serrano, M.; Graf Pannatier, E.; Vicca, S.; Luyssaert, S.; Jonard, M.; Ciais, P.; Guenet, B.; Gielen, B.; Peñuelas, J.; Sardans, J.; et al. Trends in soil solution dissolved organic carbon (DOC) concentrations across European forests. Biogeosciences 2016, 13, 5567–5585. [Google Scholar] [CrossRef]
- Sultanbaeva, R.R.; Koptsik, G.N.; Smirnova, I.E. Input and migration of soluble organic carbon in soils of forest ecosystems of the broad-leaved-coniferous forest subzone. Mosc. Univ. Soil Sci. Bull. 2015, 4, 37–42. (In Russian) [Google Scholar] [CrossRef]
- Kuznetsova, A.I.; Lukina, N.V.; Orlova, M.A.; Teben’kova, D.N. Comparative assessment of the size of carbon removal with soil water in taiga and coniferous-broadleaved forests. In Carbon Accumulation in Forest Soils and Successional Status of Forests; Lukina, N.V., Ed.; Tovarishestvonauchnihizdaniy KMK: Moscow, Russia, 2018; pp. 140–146. ISBN 978-5-907099-47-0. (In Russian) [Google Scholar]
- Lukina, N.V.; Orlova, M.A.; Teben’kova, D.N.; Ershov, V.V.; Gorbacheva, T.T.; Isaeva, L.G. Assessment of soil water composition in the northern taiga coniferous forests of background territories in the industrially developed region. Eurasian Soil Sci. 2018, 51, 277–289. [Google Scholar] [CrossRef]
- Ershov, V.V.; Isaeva, L.G.; Gorbacheva, T.T.; Lukina, N.V.; Orlova, M.A.; Smirnov, V.E. Assessment of Soil-Water Composition Dynamics in the North Taiga Forests upon the Reduction of Industrial Air Pollution by Emissions of a Copper-Nickel Smelter. Contemp. Probl. Ecol. 2019, 12, 97–108. [Google Scholar] [CrossRef]
- ICP Forests. Forest Monitoring Methodology under the International Program ICP Forests; ICP Forests: Moscow, Russia, 2008; 46p. [Google Scholar]
- Belov, N.P. Soils of the Murmansk Region; Belov, N.P., Baranovskaja, A.V., Eds.; Nauka: Moscow, Russia, 1969. (In Russian)
- Chertov, O.G.; Men’shikova, G.P. Changes in forest soils under the influence of acid precipitation. Izv. AN SSSR. Ser. Biol. 1983, 6, 110–115. (In Russian) [Google Scholar]
- Fedorets, N.G.; Bakhmet, O.N. Peculiarities of soil and soil cover formation in the Karelia—Kola region. Proc. Kar. RC RAS 2016, 12, 39–51. (In Russian) [Google Scholar] [CrossRef][Green Version]
- Pereverzev, V.N.; Alekseeva, N.S. Organic Matter in Soils of the Kola Peninsula; Nauka: Leningrad, Russia, 1980. (In Russian)
- Derome, J.; Niska, K.; Lindroos, A.-J.; Valikangas, P. The Ion Balance Monitoring Plot Network. The Lapland Forest Damage Project; Russian-Finnish Cooperation Report; Rovaniemi Research Station, The Finnish Forest Research Institute: Rovaniemi, Finland, 1993; pp. 49–57. [Google Scholar]
- Lukina, N.V.; Nikonov, V.V. Nutritional Regime of Northern Taiga Forests (Natural Regularities and Pollution-Inducted Changes); KNC RAN: Apatity, Russia, 1998. (In Russian) [Google Scholar]
- Maher, W.; Krikowa, F.; Wruck, D.; Louie, H.; Nguyen, T.; Huang, W.Y. Determination of total phosphorus and nitrogen in turbid waters by oxidation with alkaline potassium peroxodisulfate and low pressure microwave digestion, autoclave heating or the use of closed vessels in a hot water bath: Comparison with Kjeldahl digestion. Anal. Chim. Acta 2002, 463, 283–293. [Google Scholar] [CrossRef]
- Ershov, V.V.; Lukina, N.V.; Orlova, M.A.; Zukert, N.V. Dynamics of snowmelt water composition in conifer forests Exposed to Airborne Industrial Pollution. Russ. J. Ecol. 2016, 47, 46–52. [Google Scholar] [CrossRef]
- Fedorets, N.G.; Bakhmet, O.N.; Medvedeva, M.V.; Novikov, S.G.; Tkachenko, U.N.; Solodovnikov, A.N. Heavy Metals in Soils of Karelia; Karelian Research Centre of the RAS: Petrozavodsk, Russia, 2015; ISBN 978-5-9274-0674-6. (In Russian) [Google Scholar]
- Scharlemann, J.P.; Tanner, E.V.; Hiederer, R.; Kapos, V. Global soil carbon: Understanding and managing the largest terrestrial carbon pool. Carbon Manag. 2014, 5, 81–91. [Google Scholar] [CrossRef]
- Artemkina, N.A.; Orlova, M.A.; Lukina, N.V. Micromosaic structure of vegetation and variability of the chemical composition of l layer of the litter in dwarf shrub–green moss spruce forests of the northern taiga. Contemp. Probl. Ecol. 2018, 11, 754–761. [Google Scholar] [CrossRef]
- Kovda, V.A. Soil Cover Biogeochemistry; Zonn, S.V., Ed.; Nauka: Moscow, Russia, 1985. (In Russian)
- Sukhareva, T.A.; Lukina, N.V. Mineral composition of assimilative organs of conifers after reduction of atmospheric pollution in the Kola Peninsula. Russ. J. Ecol. 2014, 45, 95–102. [Google Scholar] [CrossRef]
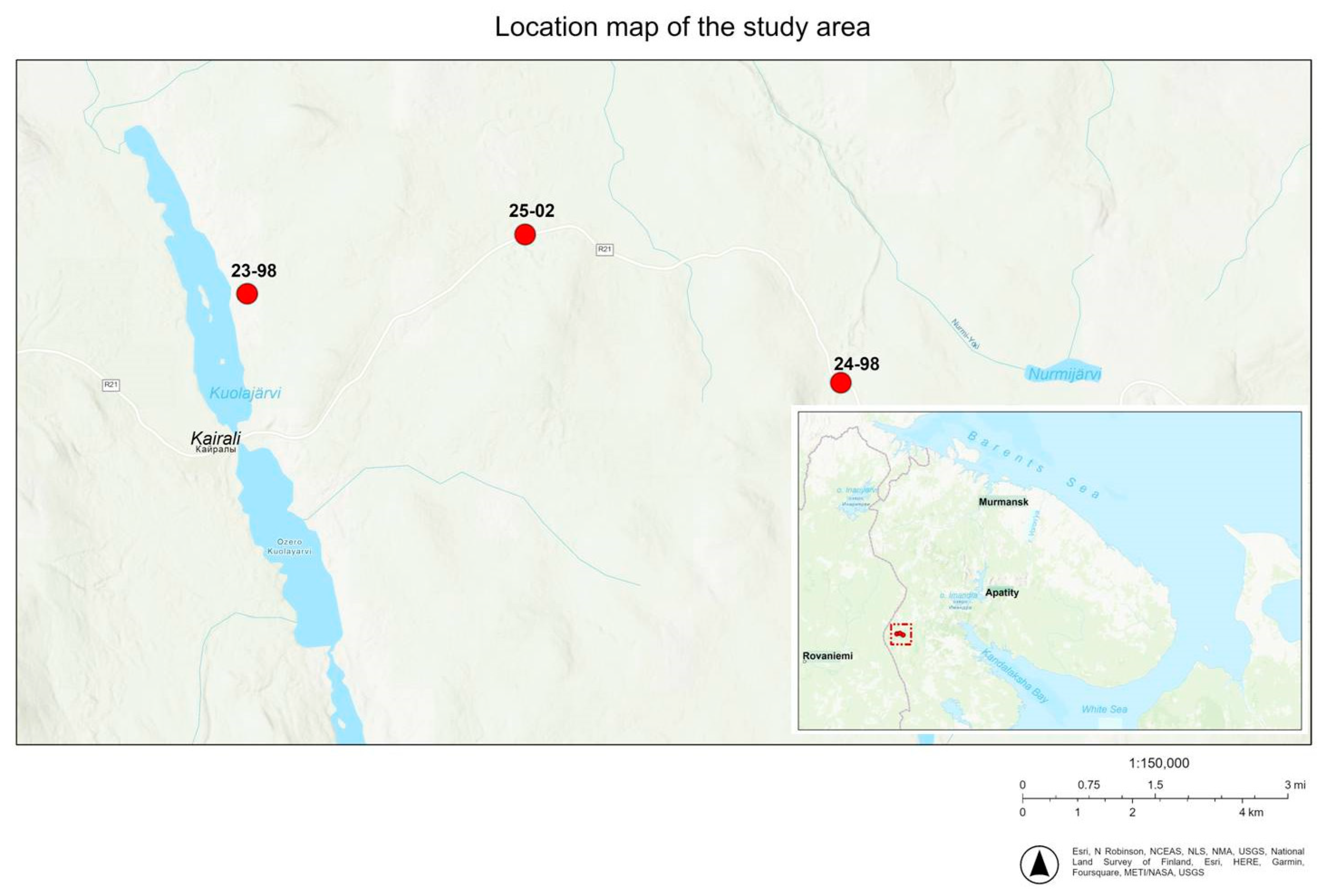




| PMP | Coordinates | ASL | Stand Composition | Stand Age, Years | Average Diameter, cm | Number of Trees, pcs/ha | Average Height, m | Stand Completeness (Relative) | Tree Stand, Cover, % |
|---|---|---|---|---|---|---|---|---|---|
| 23-98 | N 66,95245 E 29,60692 | 225 | 10P | 160–180 | 15,1 | 2000 | 12.2 | 0.4 | 55–75 |
| 25-02 | N 66,96195 E 29,72147 | 297 | 9P1B | 160–180 | 18.9 | 1800 | 15.4 | 0.4 | 55–75 |
| 24-98 | N 66,93890 E 29,85465 | 327 | 7S3B | 200–220 | 18.0 | 1400 | 14.1 | 0.5 | 35–85 |
| PMP | BGC Type | Element | Snow | Rain | ||
|---|---|---|---|---|---|---|
| Below | Between | Below | Between | |||
| 23-98 | lichen-green moss-shrub pine forest | C | 2.69 | 1.17 | 57.88 | 4.66 |
| 0.32 | 0.15 | 2.68 | 0.34 | |||
| N | ND | ND | 0.51 | 0.23 | ||
| 0.18 | 0.03 | |||||
| 25-02 | lichen-shrub pine forest | C | 4.39 | 2.85 | 45.06 | 4.92 |
| 0.43 | 0.36 | 1.79 | 0.26 | |||
| N | ND | ND | 0.53 | 0.43 | ||
| 0.09 | 0.11 | |||||
| 24-98 | spruce forest lichen-shrub green moss | C | 3.30 | 1.57 | 91.32 | 5.37 |
| 0.38 | 0.19 | 5.39 | 0.30 | |||
| N | ND | ND | 0.47 | 0.36 | ||
| 0.04 | 0.07 | |||||
| Open area | wetland | Open area | ||||
| C | 2.65 | 4.27 | ||||
| 0.58 | 0.33 | |||||
| N | ND | 0.32 | ||||
| 0.07 | ||||||
| PMP | BGC Type | Soil Horizon | OL | OF | OH | E | BH | C Horizon |
|---|---|---|---|---|---|---|---|---|
| 23-98 | Lichen-green moss-shrub pine forest | Below the Crowns | ||||||
| C | 50.92 | 49.74 | 42.56 | 0.37 | 0.72 | ND | ||
| 1.42 | 1.58 | 2.51 | 0.07 | 0.05 | ||||
| N | 1.08 | 1.17 | 0.82 | 0.02 | 0.05 | ND | ||
| 0.03 | 0.05 | 0.09 | 0.003 | 0.003 | ||||
| C/N | 47.48 | 42.85 | 55.49 | 15.43 | 16.27 | ND | ||
| 1.48 | 1.63 | 5.69 | 1.84 | 1.44 | ||||
| Between the crowns | ||||||||
| C | 48.94 | 49.86 | 44.53 | 0.43 | 0.71 | 0.32 | ||
| 1.40 | 1.60 | 1.60 | 0.10 | 0.10 | 0.09 | |||
| N | 0.79 | 1.01 | 0.95 | 0.02 | 0.05 | 0.02 | ||
| 0.07 | 0.05 | 0.03 | 0.003 | 0.01 | 0.004 | |||
| C/N | 63.34 | 50.13 | 46.92 | 16.09 | 15.89 | 16.72 | ||
| 4.26 | 2.76 | 1.87 | 2.18 | 1.42 | 6.01 | |||
| 25-02 | Lichen-shrub pine forest | Below the crowns | ||||||
| C | 49.17 | 46.74 | 40.71 | 0.25 | 0.52 | ND | ||
| 1.72 | 3.00 | 3.64 | 0.04 | 0.08 | ||||
| N | 1.13 | 1.15 | 0.96 | 0.02 | 0.03 | ND | ||
| 0.11 | 0.07 | 0.09 | 0.002 | 0.003 | ||||
| C/N | 47.37 | 40.80 | 43.58 | 14.63 | 20.05 | ND | ||
| 5.90 | 2.27 | 3.55 | 2.73 | 2.14 | ||||
| Between the crowns | ||||||||
| C | 49.88 | 46.23 | 39.97 | 0.46 | 0.83 | 0.24 | ||
| 1.64 | 2.04 | 3.04 | 0.08 | 0.18 | 0.04 | |||
| N | 0.90 | 1.11 | 0.89 | 0.02 | 0.04 | 0.01 | ||
| 0.04 | 0.10 | 0.08 | 0.003 | 0.01 | 0.001 | |||
| C/N | 56.13 | 44.11 | 46.01 | 22.71 | 20.68 | 18.58 | ||
| 3.82 | 5.65 | 4.42 | 4.83 | 2.41 | 5.06 | |||
| 24-98 | dwarf shrub-green moss spruce forest | Below the crowns | ||||||
| C | 47.57 | 43.48 | 34.35 | 0.33 | 1.15 | ND | ||
| 1.43 | 1.47 | 4.76 | 0.06 | 0.16 | ||||
| N | 1.52 | 1.69 | 1.29 | 0.03 | 0.07 | ND | ||
| 0.07 | 0.05 | 0.20 | 0.002 | 0.01 | ||||
| C/N | 31.79 | 25.91 | 27.15 | 12.37 | 16.73 | ND | ||
| 2.28 | 1.04 | 0.78 | 2.21 | 0.95 | ||||
| Between the crowns | ||||||||
| C | 49.89 | 41.47 | 16.41 | 0.34 | 1.37 | 0.39 | ||
| 1.47 | 1.55 | 3.90 | 0.05 | 0.43 | 0.03 | |||
| N | 1.68 | 1.12 | 0.62 | 0.02 | 0.07 | 0.03 | ||
| 0.06 | 0.13 | 0.09 | 0.001 | 0.02 | 0.004 | |||
| C/N | 29.89 | 39.74 | 25.99 | 16.24 | 17.55 | 15.92 | ||
| 1.15 | 5.03 | 3.25 | 2.70 | 1.31 | 2.06 | |||
| PMP | BGC Type | Element | O | E+B | BC |
|---|---|---|---|---|---|
| 23-98 | lichen-green moss-shrub pine forest | Below | |||
| C | 175.85 | ND | ND | ||
| 10.01 | |||||
| N | 0.68 | ||||
| 0.10 | |||||
| Between | |||||
| C | 36.69 | 16.87 | ND | ||
| 1.54 | 5.38 | ||||
| N | 0.25 | ND | |||
| 0.02 | |||||
| 25-02 | lichen-shrub pine forest | Below | |||
| C | 82.75 | 50.34 | 27.12 | ||
| 4.17 | 1.77 | 2.66 | |||
| N | 0.65 | 0.41 | 0.41 | ||
| 0.07 | 0.06 | 0.12 | |||
| Between | |||||
| C | 43.89 | 31.85 | 29.70 | ||
| 2.83 | 1.39 | 2.01 | |||
| N | 0.67 | 0.41 | 0.31 | ||
| 0.06 | 0.05 | 0.04 | |||
| 24-98 | shrub-green moss spruce forest | Below | |||
| C | 84.92 | 52.34 | 23.64 | ||
| 4.63 | 5.44 | 2.75 | |||
| N | 0.87 | 0.36 | 0.25 | ||
| 0.15 | 0.04 | 0.04 | |||
| Between | |||||
| C | 47.47 | 23,6 | 13.56 | ||
| 2.70 | 1.69 | 1.3 | |||
| N | 0.48 | 0.27 | ND | ||
| 0.04 | 0.05 | ||||
| PMP | BGC Type | Element | Current Year | 1 Year | Perennial Needles | Brown Needles | Litter |
|---|---|---|---|---|---|---|---|
| 23-98 | lichen-green moss-shrub pine forest | C | 55.25 | 55.28 | 55.54 | 53.61 | 56.91 |
| 0.97 | 1.01 | 1.56 | 1.05 | 0.77 | |||
| N | 1.16 | 1.09 | 1.00 | 0.45 | 0.32 | ||
| 0.04 | 0.04 | 0.04 | 0.02 | 0.02 | |||
| 25-02 | lichen-shrub pine forest | C | 51.71 | 56.24 | 55.01 | 56.28 | ND |
| 1.57 | 1.61 | 1.20 | 0.80 | ||||
| N | 1.30 | 1.14 | 1.09 | 0.41 | ND | ||
| 0.04 | 0.03 | 0.03 | 0.01 | ||||
| 24-98 | shrub-green moss spruce forest | C | 53.83 | 52.45 | 53.32 | ND | ND |
| 1.23 | 1.10 | 1.16 | |||||
| N | 1.20 | 1.16 | 0.96 | ND | ND | ||
| 0.03 | 0.04 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ershov, V.; Sukhareva, T.; Ryabov, N.; Ivanova, E.; Shtabrovskaya, I. Estimation of Carbon and Nitrogen Contents in Forest Ecosystems in the Background Areas of the Russian Arctic (Murmansk Region). Forests 2024, 15, 29. https://doi.org/10.3390/f15010029
Ershov V, Sukhareva T, Ryabov N, Ivanova E, Shtabrovskaya I. Estimation of Carbon and Nitrogen Contents in Forest Ecosystems in the Background Areas of the Russian Arctic (Murmansk Region). Forests. 2024; 15(1):29. https://doi.org/10.3390/f15010029
Chicago/Turabian StyleErshov, Vyacheslav, Tatyana Sukhareva, Nickolay Ryabov, Ekaterina Ivanova, and Irina Shtabrovskaya. 2024. "Estimation of Carbon and Nitrogen Contents in Forest Ecosystems in the Background Areas of the Russian Arctic (Murmansk Region)" Forests 15, no. 1: 29. https://doi.org/10.3390/f15010029
APA StyleErshov, V., Sukhareva, T., Ryabov, N., Ivanova, E., & Shtabrovskaya, I. (2024). Estimation of Carbon and Nitrogen Contents in Forest Ecosystems in the Background Areas of the Russian Arctic (Murmansk Region). Forests, 15(1), 29. https://doi.org/10.3390/f15010029







