Diverse Approaches to Insect Control: Utilizing Brassica carinata (A.) Braun and Camelina sativa (L.) Crantz Oil as Modern Bioinsecticides
Abstract
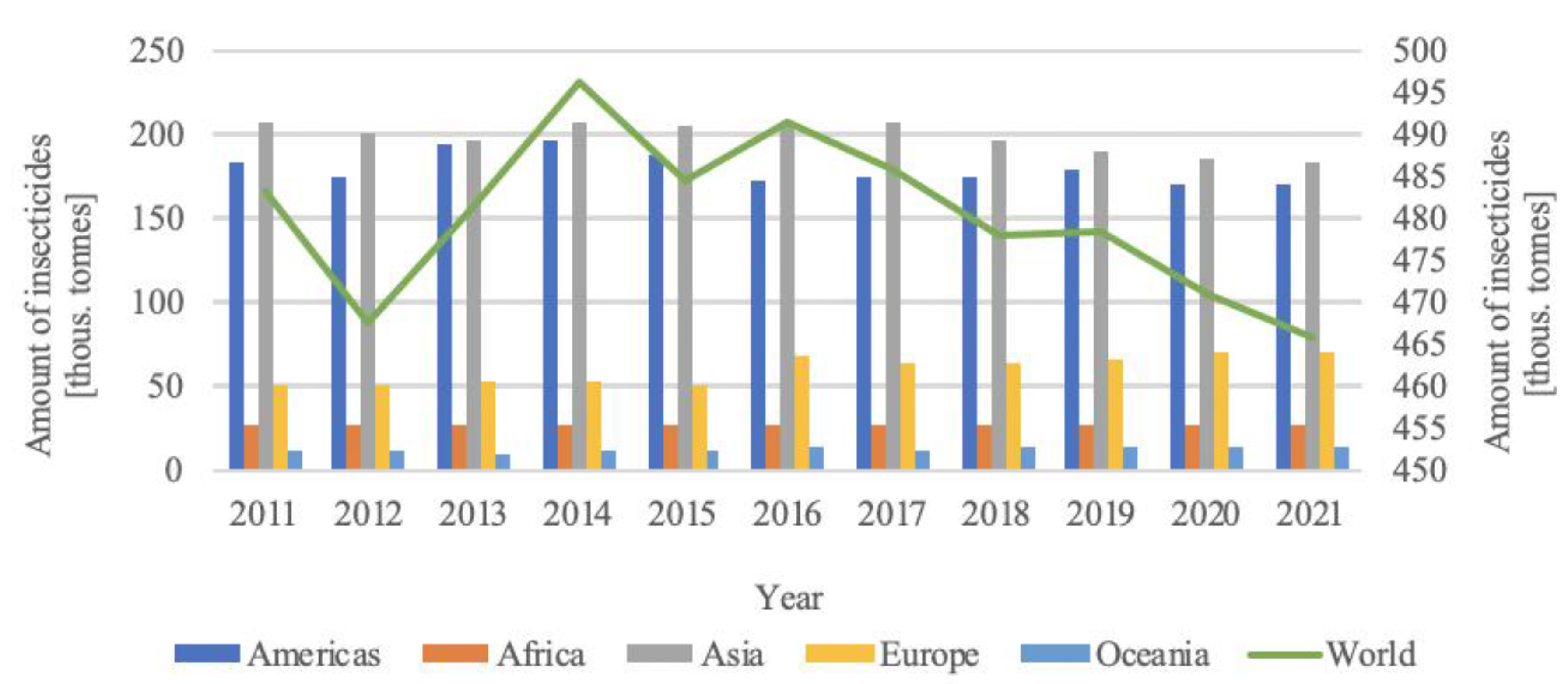
1. Introduction
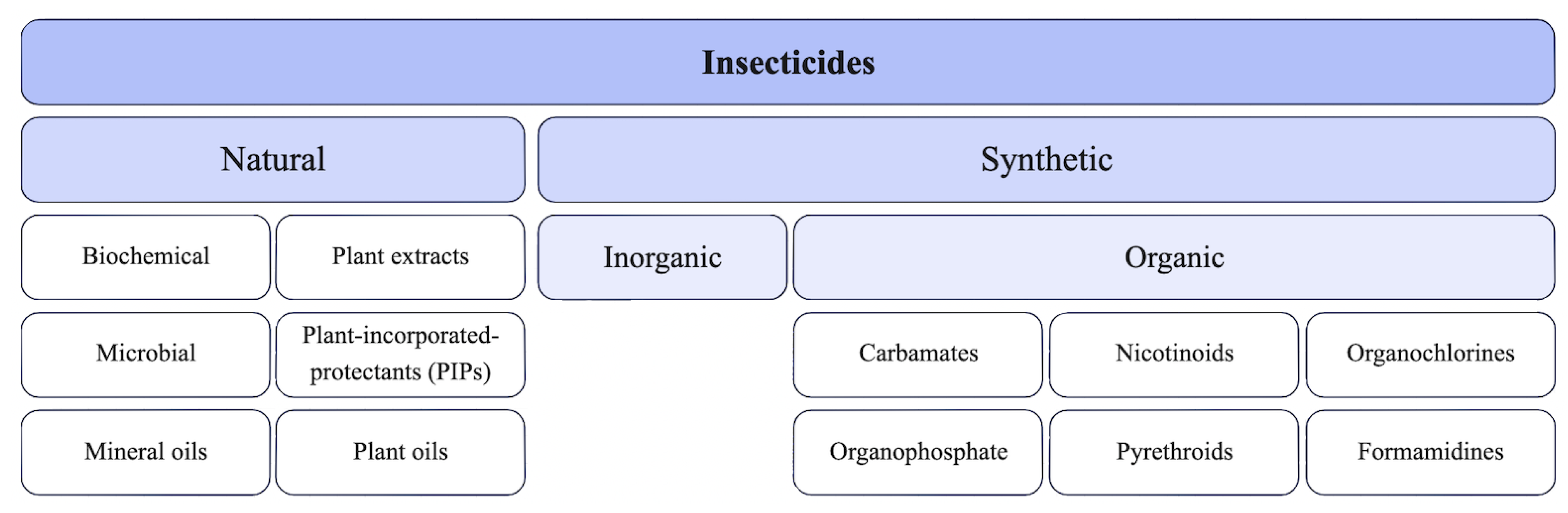
2. Insecticide Characteristics
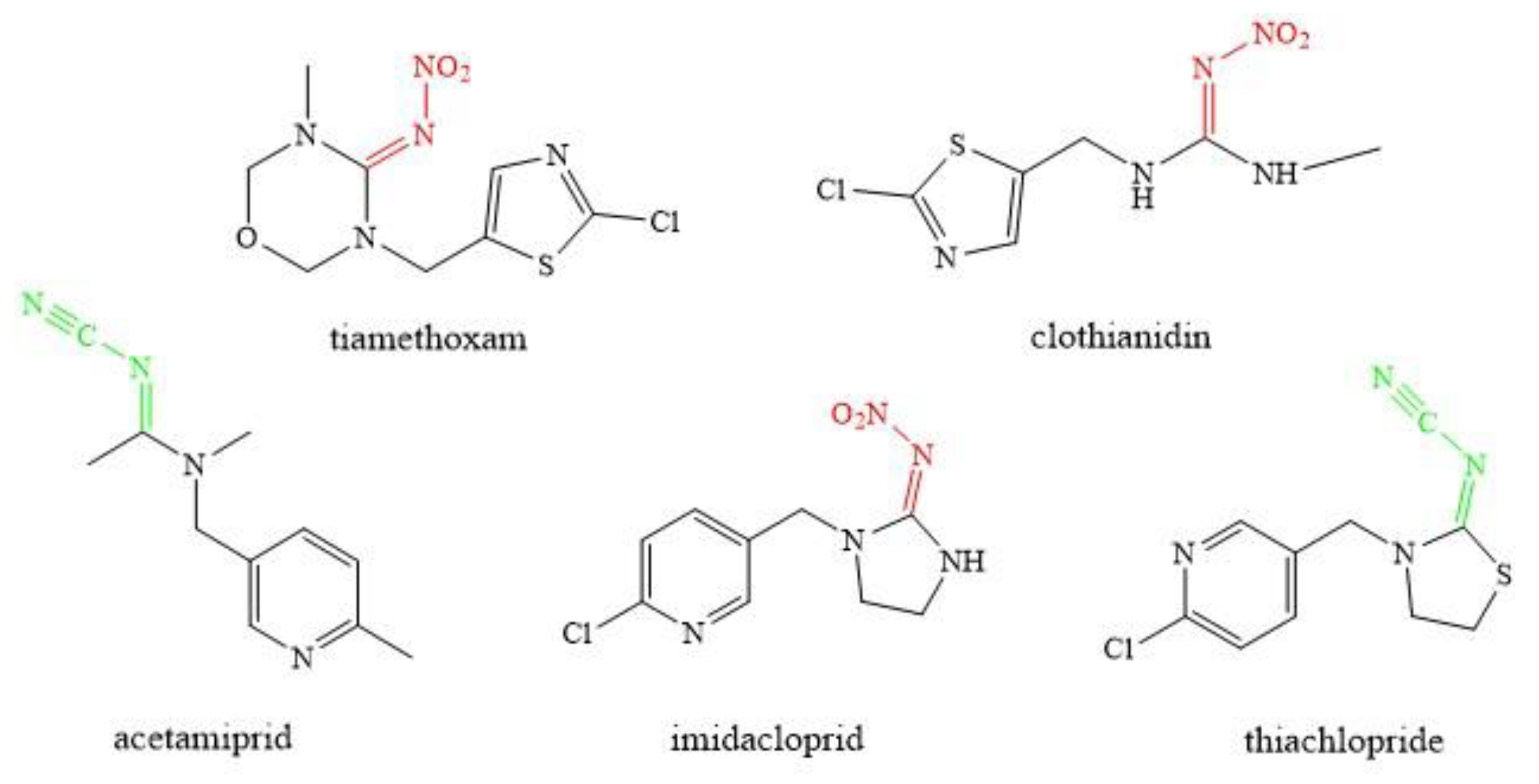
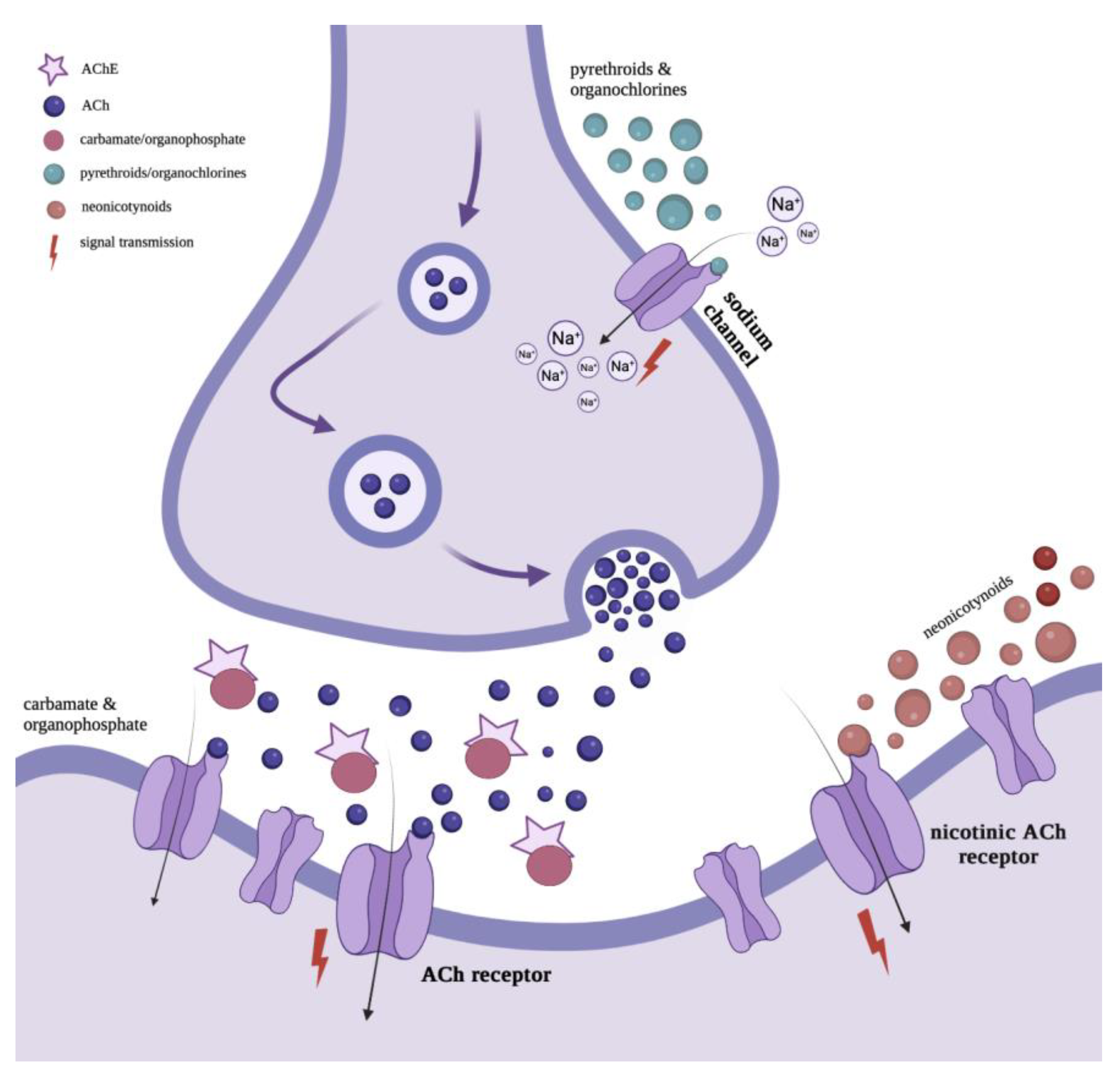
2.1. Characteristics of Neonicotinoids
2.2. Characteristics of Organochlorine Insecticides
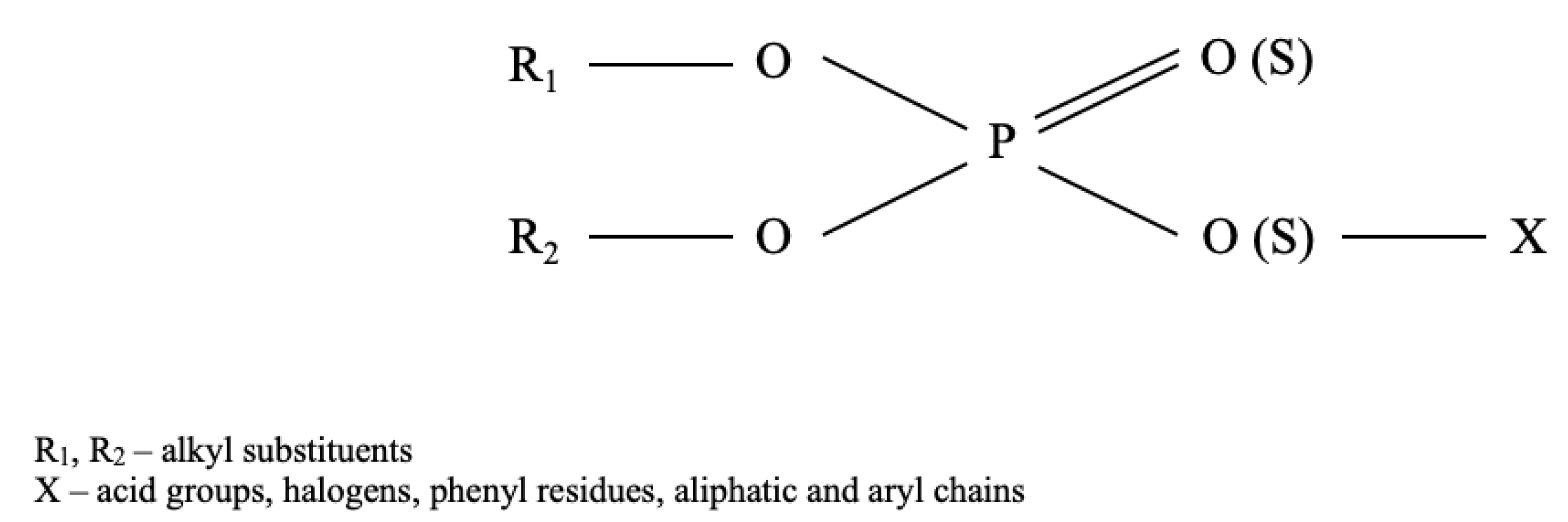
2.3. Characteristics of Organophosphate Insecticides
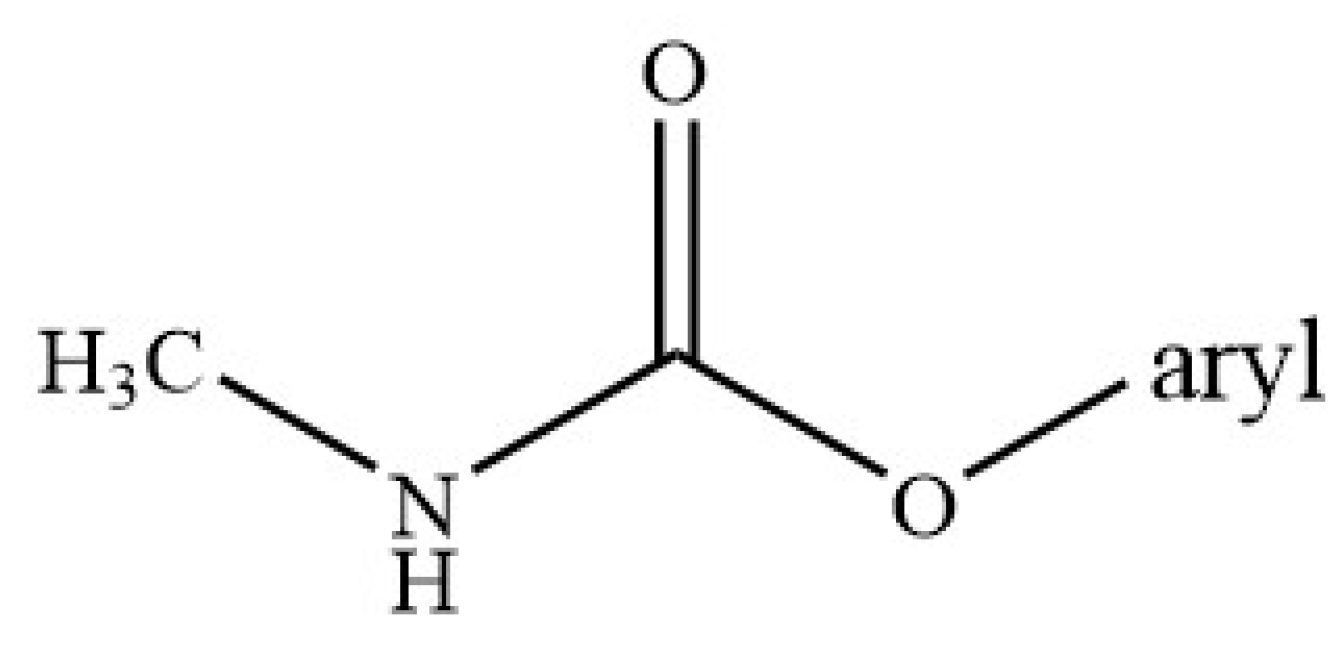
2.4. Characteristics of Carbamates
2.5. Characteristics of Pyrethroids
3. Natural Protective Products for Protecting Plants against Insects
3.1. Mechanical Methods
3.2. Biological Methods
- Augmentative biological control—increasing the density of native or non-native natural enemies through regular releases;
- Conservation biological control—the manipulation of a habitat to increase the reproduction, survival, and effectiveness of natural enemies already present in the affected area;
- Classical biological control (CBC)—the introduction of a natural enemy of native origin to control a pest, which is usually also non-native, to determine whether the population of the natural enemy is sufficient to achieve permanent control of the target pest.
3.3. Bioinsecticides
3.4. Oils as Botanical Pesticides
3.4.1. Essential Oils
3.4.2. Plant Oils
4. New Potential Botanical Insecticides
4.1. Characteristics of Physicochemical Properties of Brassica carinata and Camelina sativa
4.2. Fatty Acid Compositions of Brassica carinata and Camelina sativa
| Camelina sativa | Brassica carinata | |
|---|---|---|
| PUFA [%] | 50.10–72.00 | 17.30–36.90 |
| MUFA [%] | 17.40–41.40 | 52.80–71.00 |
| SFA [%] | 9.10–13.12 | 4.80–11.00 |
| ω-6/ω-3 | 1:1.51–2.87 | 1: 0.80–2.80 |
4.3. Composition of Unsaponifiable Fraction of Brassica carinata and Camelina sativa
4.4. Insecticidal Properties of Compounds Present in the Tested Oils
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Heavilin, J.; Powell, J.; Logan, J.A. Dynamics of Mountain Pine Beetle Outbreaks. In Plant Disturbance Ecology; Elsevier: Amsterdam, The Netherlands, 2007; pp. 527–553. [Google Scholar]
- Zajączkowski, G.; Jabłoński, M.; Jabłoński, T.; Szmidla, H.; Kowalska, A.; Małachowska, J.; Piwnicki, J. Raport o stanie lasów w Polsce 2020. In Państwowe Gospodarstwo Leśne—Lasy Państwowe; Centrum Informacyjne Lasów Państwowych: Warsaw, Poland, 2021; pp. 80–81. [Google Scholar]
- Iqbal, N.; Alvi, A.M.; Saeed, S.; Rashied, A.; Saeed, Q.; Jaleel, W.; Khan, K.A.; Ghramh, H.A. Toxicity and Repellency of Different Insecticides to Odontotermes Obesus (Rambur, 1842) (Blattodea: Termitidae: Macrotermitinae). Turk. J. Entomol. 2019, 43, 241–251. [Google Scholar] [CrossRef]
- Saeed, S.; Naqqash, M.N.; Jaleel, W. Toxicological Studies on Some Important Chemicals against Dysdercus Koenigii Fabr (Hemiptera: Pyrrhocoridae). Pak. J. Zool 2016, 48, 1249–1254. [Google Scholar]
- Oberemok, V.V.; Laikova, K.V.; Gninenko, Y.I.; Zaitsev, A.S.; Nyadar, P.M.; Adeyemi, T.A. A Short History of Insecticides. J. Plant Prot. Res. 2015, 55, 221–226. [Google Scholar] [CrossRef]
- Jaleel, W.; Saeed, Q.; Saeed, S.; Ansari, T.; Naqqash, M.N.; Iqbal, N.; Sial, U. Efficacy and Time Mortality of Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae) by Some Essential Oils through Contact and Fumigant Methods. Appl. Sci. Bus. Econ. 2015, 2, 1–7. [Google Scholar]
- Grdiša, M.; Carović-Stanko, K.; Kolak, I.; Šatović, Z. Morphological and Biochemical Diversity of Dalmatian pyrethrum (Tanacetum cinerariifolium (Trevir.) Sch. Bip.). Agric. Conspec. Sci. 2009, 74, 73–80. [Google Scholar]
- Zubairi, S.I.; Sarmidi, M.R.; Aziz, R.A. A Study of Rotenone from Derris Roots of Varies Location, Plant Parts and Types of Solvent Used. Adv. Environ. Biol. 2014, 8, 445–448. [Google Scholar]
- Dougoud, J.; Toepfer, S.; Bateman, M.; Jenner, W.H. Efficacy of Homemade Botanical Insecticides Based on Traditional Knowledge. A Review. Agron. Sustain. Dev. 2019, 39, 37. [Google Scholar] [CrossRef]
- Jankowska, B.; Wojciechowicz-Żytko, E. Efficacy of Aqueous Extracts of Black alder (Alnus Glutinosa GAERTN.) and Black Elderberry (Sambucus nigra L.) in Reducing the Occurrence of Phyllotreta Spp., Some Lepidopteran Pests and Diamondback Moth Parasitoids on White Cabbage. Pol. J. Entomol. 2016, 85, 377–388. [Google Scholar] [CrossRef]
- Cheraghi Niroumand, M.; Farzaei, M.H.; Karimpour Razkenari, E.; Amin, G.; Khanavi, M.; Akbarzadeh, T.; Shams-Ardekani, M.R. An Evidence-Based Review on Medicinal Plants Used as Insecticide and Insect Repellent in Traditional Iranian Medicine. Iran. Red. Crescent Med. J. 2016, 18, e22361. [Google Scholar] [CrossRef]
- Bate, R. The Rise, Fall, Rise, and Imminent Fall of DDT. Am. Enterp. Inst. Public Policy Res. 2007, 14, 1–9. [Google Scholar]
- Davies, T.G.E.; Field, L.M.; Usherwood, P.N.R.; Williamson, M.S. DDT, Pyrethrins, Pyrethroids and Insect Sodium Channels. IUBMB Life 2007, 59, 151–162. [Google Scholar] [CrossRef]
- Bouwman, H.; Yohannes, Y.B.; Nakayama, S.M.M.; Motohira, K.; Ishizuka, M.; Humphries, M.S.; van der Schyff, V.; du Preez, M.; Dinkelmann, A.; Ikenaka, Y. Evidence of Impacts from DDT in Pelican, Cormorant, Stork, and Egret Eggs from KwaZulu-Natal, South Africa. Chemosphere 2019, 225, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Jampílek, J.; Kráľová, K.; Fedor, P. Bioactivity of Nanoformulated Synthetic and Natural Insecticides and Their Impact on Environment. In Nanopesticides; Springer International Publishing: Cham, Switzerland, 2020; pp. 165–225. [Google Scholar]
- Ansari, I.; El-Kady, M.M.; Arora, C.; Sundararajan, M.; Maiti, D.; Khan, A. Ansari, I.; El-Kady, M.M.; Arora, C.; Sundararajan, M.; Maiti, D.; Khan, A. A Review on the Fatal Impact of Pesticide Toxicity on Environment and Human Health. In Global Climate Change; Elsevier: Amsterdam, The Netherlands, 2021; pp. 361–391. [Google Scholar]
- Watanabe, E. Review on Current Analytical Methods with Chromatographic and Nonchromatographic Techniques for New Generation Insecticide Neonicotinoids. In Insecticides—Advances in Integrated Pest Management; InTech: London, UK, 2012. [Google Scholar]
- Ford, K.A.; Casida, J.E. Comparative Metabolism and Pharmacokinetics of Seven Neonicotinoid Insecticides in Spinach. J. Agric. Food Chem. 2008, 56, 10168–10175. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, P.; Nauen, R. Neonicotinoids-from Zero to Hero in Insecticide Chemistry. Pest Manag. Sci. 2008, 64, 1084–1098. [Google Scholar] [CrossRef] [PubMed]
- Bromilow, R.H.; Chamberlain, K. Principles Governing Uptake and Transport of Chemicals. In Plant Contamination: Modelling and Simulation; Lewis Publishers: London, UK, 1995; pp. 37–64. [Google Scholar]
- Bonmatin, J.-M.; Giorio, C.; Girolami, V.; Goulson, D.; Kreutzweiser, D.P.; Krupke, C.; Liess, M.; Long, E.; Marzaro, M.; Mitchell, E.A.D.; et al. Environmental Fate and Exposure; Neonicotinoids and Fipronil. Environ. Sci. Pollut. Res. 2015, 22, 35–67. [Google Scholar] [CrossRef] [PubMed]
- Tomizawa, M.; Casida, J.E. Neonicotinoid Insecticide Toxicology: Mechanisms of Selective Action. Annu. Rev. Pharmacol Toxicol. 2005, 45, 247–268. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, C.A.; Mineau, P.; Devries, J.H.; Sanchez-Bayo, F.; Liess, M.; Cavallaro, M.C.; Liber, K. Neonicotinoid Contamination of Global Surface Waters and Associated Risk to Aquatic Invertebrates: A Review. Environ. Int. 2015, 74, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Taillebois, E.; Cartereau, A.; Jones, A.K.; Thany, S.H. Neonicotinoid Insecticides Mode of Action on Insect Nicotinic Acetylcholine Receptors Using Binding Studies. Pestic. Biochem. Physiol. 2018, 151, 59–66. [Google Scholar] [CrossRef]
- Verebová, V.; Staničová, J. The Effect of Neonicotinoid Insecticides on the Structure and Stability of Bio-Macromolecules. In Insecticides [Working Title]; IntechOpen: London, UK, 2021. [Google Scholar]
- de Lima e Silva, C.; Brennan, N.; Brouwer, J.M.; Commandeur, D.; Verweij, R.A.; van Gestel, C.A.M. Comparative Toxicity of Imidacloprid and Thiacloprid to Different Species of Soil Invertebrates. Ecotoxicology 2017, 26, 555–564. [Google Scholar] [CrossRef]
- Brandt, A.; Gorenflo, A.; Siede, R.; Meixner, M.; Büchler, R. The Neonicotinoids Thiacloprid, Imidacloprid, and Clothianidin Affect the Immunocompetence of Honey Bees (Apis mellifera L.). J. Insect. Physiol. 2016, 86, 40–47. [Google Scholar] [CrossRef]
- Abdourahime, H.; Anastassiadou, M.; Arena, M.; Auteri, D.; Barmaz, S.; Brancato, A.; Brocca, D.; Bura, L.; Carrasco Cabrera, L.; Chiusolo, A.; et al. Peer Review of the Pesticide Risk Assessment of the Active Substance Thiacloprid. EFSA J. 2019, 17. [Google Scholar] [CrossRef]
- Taiwo, A.M. A Review of Environmental and Health Effects of Organochlorine Pesticide Residues in Africa. Chemosphere 2019, 220, 1126–1140. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.-T. Organochlorine Insecticides. In Encyclopedia of Toxicology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 711–713. [Google Scholar]
- Jayaraj, R.; Megha, P.; Sreedev, P. Review Article. Organochlorine Pesticides, Their Toxic Effects on Living Organisms and Their Fate in the Environment. Interdiscip Toxicol. 2016, 9, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Grimalt, J.O.; van Drooge, B.L.; Ribes, A.; Vilanova, R.M.; Fernandez, P.; Appleby, P. Persistent Organochlorine Compounds in Soils and Sediments of European High Altitude Mountain Lakes. Chemosphere 2004, 54, 1549–1561. [Google Scholar] [CrossRef] [PubMed]
- Olisah, C.; Okoh, O.O.; Okoh, A.I. Occurrence of Organochlorine Pesticide Residues in Biological and Environmental Matrices in Africa: A Two-Decade Review. Heliyon 2020, 6, e03518. [Google Scholar] [CrossRef] [PubMed]
- Bogdanik, T.; Brzeziński, J.; Chmielnicka, J.; Jacyszyn, K.; Jodynis-Liebert, J.; Kozłowski, J.; Krechniak, J.; Ludwicki, J.K.; Mrozikiewicz, A.; Sapota, A.; et al. Toksykologia Szczegółowa Pestycydów. In Toksykologia; Wydawnictwo Lekarskie PZWL: Warsaw, Poland, 2002; pp. 615–648. [Google Scholar]
- Zhang, H.B.; Luo, Y.M.; Zhao, Q.G.; Wong, M.H.; Zhang, G.L. Residues of Organochlorine Pesticides in Hong Kong Soils. Chemosphere 2006, 63, 633–641. [Google Scholar] [CrossRef]
- El-Shahawi, M.S.; Hamza, A.; Bashammakh, A.S.; Al-Saggaf, W.T. An Overview on the Accumulation, Distribution, Transformations, Toxicity and Analytical Methods for the Monitoring of Persistent Organic Pollutants. Talanta 2010, 80, 1587–1597. [Google Scholar] [CrossRef]
- Coats, J.R. Mechanisms of Toxic Action and Structure-Activity Relationships for Organochlorine and Synthetic Pyrethroid Insecticides. Environ. Health Perspect 1990, 87, 255–262. [Google Scholar] [CrossRef]
- Le Goff, G.; Giraudo, M. Effects of Pesticides on the Environment and Insecticide Resistance. In Olfactory Concepts of Insect Control—Alternative to insecticides; Springer International Publishing: Cham, Switzerland, 2019; pp. 51–78. [Google Scholar]
- Casida, J.E.; Quistad, G.B. Organophosphate Toxicology: Safety Aspects of Nonacetylcholinesterase Secondary Targets. Chem. Res. Toxicol. 2004, 17, 983–998. [Google Scholar] [CrossRef]
- Horsak, R.D.; Bedient, P.B.; Hamilton, M.C.; Thomas, F. Ben Pesticides. Environ. Forensics Contam. Specif. Guide 1964, 143–165. [Google Scholar] [CrossRef]
- Eddleston, M.; Gunnell, D.; Karunaratne, A.; De Silva, D.; Sheriff, M.H.R.; Buckley, N.A. Epidemiology of Intentional Self-Poisoning in Rural Sri Lanka. Br. J. Psychiatry 2005, 187, 583–584. [Google Scholar] [CrossRef] [PubMed]
- Eddleston, M.; Buckley, N.A.; Eyer, P.; Dawson, A.H. Management of Acute Organophosphorus Pesticide Poisoning. Lancet 2008, 371, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Voorhees, J.R.; Rohlman, D.S.; Lein, P.J.; Pieper, A.A. Neurotoxicity in Preclinical Models of Occupational Exposure to Organophosphorus Compounds. Front. Neurosci. 2017, 10, 590. [Google Scholar] [CrossRef] [PubMed]
- Lukaszewicz-Hussain, A. Role of Oxidative Stress in Organophosphate Insecticide Toxicity—Short Review. Pestic. Biochem. Physiol. 2010, 98, 145–150. [Google Scholar] [CrossRef]
- Rezg, R.; Mornagui, B.; El-Fazaa, S.; Gharbi, N. Organophosphorus Pesticides as Food Chain Contaminants and Type 2 Diabetes: A Review. Trends Food Sci. Technol. 2010, 21, 345–357. [Google Scholar] [CrossRef]
- Eddleston, M. Novel Clinical Toxicology and Pharmacology of Organophosphorus Insecticide Self-Poisoning. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 341–360. [Google Scholar] [CrossRef]
- Kaushal, J.; Khatri, M.; Arya, S.K. A Treatise on Organophosphate Pesticide Pollution: Current Strategies and Advancements in Their Environmental Degradation and Elimination. Ecotoxicol. Environ. Saf. 2021, 207, 111483. [Google Scholar] [CrossRef]
- Moser, V.C.; McDaniel, K.L.; Phillips, P.M.; Lowit, A.B. Time-Course, Dose-Response, and Age Comparative Sensitivity of N-Methyl Carbamates in Rats. Toxicol. Sci. 2010, 114, 113–123. [Google Scholar] [CrossRef]
- Silberman, J.; Taylor, A. Carbamate Toxicity; StatPearls Publishing: Treasure Island, FL, ISA, 2022. [Google Scholar]
- Gammon, D.W.; Liu, Z.; Chandrasekaran, A.; El-Naggar, S.F.; Kuryshev, Y.A.; Jackson, S. Pyrethroid Neurotoxicity Studies with Bifenthrin Indicate a Mixed Type I/II Mode of Action. Pest Manag. Sci. 2019, 75, 1190–1197. [Google Scholar] [CrossRef]
- Shafer, T.J.; Meyer, D.A.; Crofton, K.M. Developmental Neurotoxicity of Pyrethroid Insecticides: Critical Review and Future Research Needs. Environ. Health Perspect 2005, 113, 123–136. [Google Scholar] [CrossRef]
- Wolansky, M.J.; Harrill, J.A. Neurobehavioral Toxicology of Pyrethroid Insecticides in Adult Animals: A Critical Review. Neurotoxicol. Teratol. 2008, 30, 55–78. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Wang, Y.; Wang, H.; Li, J.; Xu, P. Bioaccumulation and Enantioselectivity of Type I and Type II Pyrethroid Pesticides in Earthworm. Chemosphere 2016, 144, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cheng, F.; Wei, Y.; Lydy, M.J.; You, J. Global Occurrence of Pyrethroid Insecticides in Sediment and the Associated Toxicological Effects on Benthic Invertebrates: An Overview. J. Hazard Mater. 2017, 324, 258–271. [Google Scholar] [CrossRef] [PubMed]
- Chrustek, A.; Hołyńska-Iwan, I.; Dziembowska, I.; Bogusiewicz, J.; Wróblewski, M.; Cwynar, A.; Olszewska-Słonina, D. Current Research on the Safety of Pyrethroids Used as Insecticides. Medicina B Aires 2018, 54, 61. [Google Scholar] [CrossRef]
- Burns, C.J.; Pastoor, T.P. Pyrethroid Epidemiology: A Quality-Based Review. Crit. Rev. Toxicol. 2018, 48, 297–311. [Google Scholar] [CrossRef]
- Bradberry, S.M.; Cage, S.A.; Proudfoot, A.T.; Vale, J.A. Poisoning Due to Pyrethroids. Toxicol. Rev. 2005, 24, 93–106. [Google Scholar] [CrossRef]
- Karuppuchamy, P.; Venugopal, S. Integrated Pest Management. In Ecofriendly Pest Management for Food Security; Elsevier: Amsterdam, The Netherlands, 2016; pp. 651–684. [Google Scholar]
- Stenberg, J.A. A Conceptual Framework for Integrated Pest Management. Trends Plant Sci. 2017, 22, 759–769. [Google Scholar] [CrossRef]
- Jeffers, A.H.; Chong, J.-H. Biological Control Strategies in Integrated Pest Management (IPM) Programs. Clemson SC Clemson Coop. Ext. Land Grant Press Clemson Ext. 2021, 1111, 1–9. [Google Scholar]
- Nuruzzaman, M.; Liu, Y.; Rahman, M.M.; Dharmarajan, R.; Duan, L.; Uddin, A.F.M.J.; Naidu, R. Nanobiopesticides: Composition and Preparation Methods. In Nano-Biopesticides Today and Future Perspectives; Elsevier: Amsterdam, The Netherlands, 2019; pp. 69–131. [Google Scholar]
- Kumar, J.; Ramlal, A.; Mallick, D.; Mishra, V. An Overview of Some Biopesticides and Their Importance in Plant Protection for Commercial Acceptance. Plants 2021, 10, 1185. [Google Scholar] [CrossRef]
- Wattimena, C.M.; Latumahina, F.S. Effectiveness of Botanical Biopesticides with Different Concentrations of Termite Mortality. J. Belantara 2021, 4, 66–74. [Google Scholar] [CrossRef]
- Fenibo, E.O.; Ijoma, G.N.; Matambo, T. Biopesticides in Sustainable Agriculture: A Critical Sustainable Development Driver Governed by Green Chemistry Principles. Front Sustain. Food Syst. 2021, 5, 619058. [Google Scholar] [CrossRef]
- Ruiu, L. Microbial Biopesticides in Agroecosystems. Agronomy 2018, 8, 235. [Google Scholar] [CrossRef]
- Ram, K.; Singh, R. Efficacy of Different Fungicides and Biopesticides for the Management of Lentil Wilt (Fusarium oxysporum f. Sp. Lentis). J. AgriSearch 2021, 8, 55–58. [Google Scholar] [CrossRef]
- Mishra, J.; Dutta, V.; Arora, N.K. Biopesticides in India: Technology and Sustainability Linkages. 3 Biotech 2020, 10, 210. [Google Scholar] [CrossRef]
- Mangesh, P.M. Distribution of Vip Genes, Protein Profiling and Determination of Entomopathogenic Potential of Local Isolates of Bacillus thuringiensis. Bt Res. 2013, 4. [Google Scholar] [CrossRef]
- Parker, K.M.; Sander, M. Environmental Fate of Insecticidal Plant-Incorporated Protectants from Genetically Modified Crops: Knowledge Gaps and Research Opportunities. Environ. Sci. Technol. 2017, 51, 12049–12057. [Google Scholar] [CrossRef]
- Wei, J.-Z.; O’Rear, J.; Schellenberger, U.; Rosen, B.A.; Park, Y.-J.; McDonald, M.J.; Zhu, G.; Xie, W.; Kassa, A.; Procyk, L.; et al. A Selective Insecticidal Protein from Pseudomonas mosselii for Corn Rootworm Control. Plant Biotechnol. J. 2018, 16, 649–659. [Google Scholar] [CrossRef]
- Magierowicz, K.; Górska-Drabik, E.; Golan, K. Effects of Plant Extracts and Essential Oils on the Behavior of Acrobasis advenella (Zinck.) Caterpillars and Females. J. Plant Dis. Prot. 2020, 127, 63–71. [Google Scholar] [CrossRef]
- Abdelatti, Z.A.S.; Hartbauer, M. Plant Oil Mixtures as a Novel Botanical Pesticide to Control Gregarious locusts. J. Pest Sci. 2020, 93, 341–353. [Google Scholar] [CrossRef]
- Fahn, A. Structure and Function of Secretory Cells; Academic Press: London, UK, 2000; pp. 37–75. [Google Scholar]
- El Asbahani, A.; Miladi, K.; Badri, W.; Sala, M.; Addi, E.H.A.; Casabianca, H.; El Mousadik, A.; Hartmann, D.; Jilale, A.; Renaud, F.N.R.; et al. Essential Oils: From Extraction to Encapsulation. Int. J. Pharm. 2015, 483, 220–243. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils—Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [PubMed]
- Boussaada, O.; Chemli, R. Chemical Composition of Essential Oils from Flowers, Leaves and Peel of Citrus aurantium L. Var. Amara from Tunisia. J. Essent. Oil Bear. Plants 2006, 9, 133–139. [Google Scholar] [CrossRef]
- Bora, H.; Kamle, M.; Mahato, D.K.; Tiwari, P.; Kumar, P. Citrus Essential Oils (CEOs) and Their Applications in Food: An Overview. Plants 2020, 9, 357. [Google Scholar] [CrossRef] [PubMed]
- Roman, P. History, Presence and Perspective of Using Plant Extracts as Commercial Botanical Insecticides and Farm Products for Protection against Insects—A Review. Plant Prot. Sci. 2016, 52, 229–241. [Google Scholar] [CrossRef]
- Huang, Y.; Ho, S.-H.; Lee, H.-C.; Yap, Y.-L. Insecticidal Properties of Eugenol, Isoeugenol and Methyleugenol and Their Effects on Nutrition of Sitophilus Zeamais Motsch. (Coleoptera: Curculionidae) and Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). J. Stored Prod. Res. 2002, 38, 403–412. [Google Scholar] [CrossRef]
- Adedire, C.O.; Obembe, O.M.; Akinkurolere, R.O.; Oduleye, S.O. Response of Callosobruchus maculatus (Coleoptera: Chrysomelidae: Bruchinae) to Extracts of Cashew Kernels. J. Plant Dis. Prot. 2011, 118, 75–79. [Google Scholar] [CrossRef]
- Ileke, K.D.; Olotuah, O.F. Bioactivity of Anacardium occidentale (L) and Allium sativum (L) Powders and Oils Extracts against Cowpea Bruchid, Callosobruchus maculatus (Fab.) [Coleoptera: Chrysomelidae]. Int. J. Biol. 2011, 4. [Google Scholar] [CrossRef]
- Isman, M.B. Bioinsecticides Based on Plant Essential Oils: A Short Overview. Z. Für Naturforschung C 2020, 75, 179–182. [Google Scholar] [CrossRef]
- Ibrahim, S.S. Essential Oil Nanoformulations as a Novel Method for Insect Pest Control in Horticulture. In Horticultural Crops; IntechOpen: London, UK, 2020. [Google Scholar]
- Fazolin, M.; Estrela, J.L.V.; Medeiros, A.F.M.; da Silva, I.M.; Gomes, L.P.; de Silva, M.S.F. Synergistic Potential of Dillapiole-Rich Essential Oil with Synthetic Pyrethroid Insecticides against Fall Armyworm. Ciência Rural. 2016, 46, 382–388. [Google Scholar] [CrossRef]
- Teke, M.A.; Mutlu, Ç. Insecticidal and Behavioral Effects of Some Plant Essential Oils against Sitophilus Granarius L. and Tribolium castaneum (Herbst). J. Plant Dis. Prot. 2021, 128, 109–119. [Google Scholar] [CrossRef]
- Khani, A.; Rahdari, T. Chemical Composition and Insecticidal Activity of Essential Oil from Coriandrum Sativum Seeds against Tribolium Confusum and Callosobruchus Maculatus. ISRN Pharm. 2012, 2012, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.L.; Chu, S.S.; Liu, Q.R. Chemical Composition and Insecticidal Activity against Sitophilus Zeamais of the Essential Oils of Artemisia Capillaris and Artemisia Mongolica. Molecules 2010, 15, 2600–2608. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Pavela, R.; Cianfaglione, K.; Sender, J.; Danuta, U.; Maślanko, W.; Canale, A.; Barboni, L.; Petrelli, R.; Zeppa, L.; et al. Ascaridole-Rich Essential Oil from Marsh Rosemary (Ledum palustre) Growing in Poland Exerts Insecticidal Activity on Mosquitoes, Moths and Flies without Serious Effects on Non-Target Organisms and Human Cells. Food Chem. Toxicol. 2020, 138, 111184. [Google Scholar] [CrossRef] [PubMed]
- Sehari, N.H.; Hellal, B.; Sehari, M.; Maatoug, M. Insecticide Effect of Pennyroyal and Rosemary Essential Oils on the Rice Weevil. Ukr. J. Ecol. 2018, 8, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Yoon, J.; Tak, J.-H. Synergistic Mechanism of Insecticidal Activity in Basil and Mandarin Essential Oils against the Tobacco Cutworm. J. Pest Sci. 2021, 94, 1119–1131. [Google Scholar] [CrossRef]
- Quesada, C.R.; Sadof, C.S. Efficacy of Horticultural Oil and Insecticidal Soap against Selected Armored and Soft Scales. Horttechnology 2017, 27, 618–624. [Google Scholar] [CrossRef]
- Piccinini, E.; Ferrari, V.; Campanelli, G.; Fusari, F.; Righetti, L.; Pagnotta, E.; Lazzeri, L. Effect of Two Liquid Formulations Based on Brassica Carinata Co-Products in Containing Powdery Mildew on Melon. Ind. Crops Prod. 2015, 75, 48–53. [Google Scholar] [CrossRef]
- Siegwart, M.; Graillot, B.; Blachere Lopez, C.; Besse, S.; Bardin, M.; Nicot, P.C.; Lopez-Ferber, M. Resistance to Bio-Insecticides or How to Enhance Their Sustainability: A Review. Front Plant Sci. 2015, 6, 381. [Google Scholar] [CrossRef]
- Kilani-Morakchi, S.; Morakchi-Goudjil, H.; Sifi, K. Azadirachtin-Based Insecticide: Overview, Risk Assessments, and Future Directions. Front. Agron. 2021, 3, 676208. [Google Scholar] [CrossRef]
- Muhammad, A.; Kashere, M.A. Neem, Azadirachta indica L. (A. Juss): An Eco-Friendly Botanical Insecticide for Managing Farmers’ Insects Pest Problems—A Review. Fudma J. Sci. 2021, 4, 484–491. [Google Scholar] [CrossRef]
- Vollmann, J.; Eynck, C. Camelina as a Sustainable Oilseed Crop: Contributions of Plant Breeding and Genetic Engineering. Biotechnol. J. 2015, 10, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Rajpurohit, B.; Singha, P. Camelina (Camelina sativa) Seed. In Oilseeds: Health Attributes and Food Applications; Springer: Singapore, 2021; pp. 455–471. [Google Scholar]
- Kramer, J.K.G.; Sauer, F.D.; Wolynetz, M.S.; Farnworth, E.R.; Johnston, K.M. Effects of Dietary Saturated Fat on Erucic Acid Induced Myocardial Lipidosis in Rats. Lipids 1992, 27, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Paula., E.M.; da Silva, L.G.; Brandao, V.L.N.; Dai, X.; Faciola, A.P. Feeding Canola, Camelina, and Carinata Meals to Ruminants. Animals 2019, 9, 704. [Google Scholar] [CrossRef] [PubMed]
- Lietzow, J. Biologically Active Compounds in Mustard Seeds: A Toxicological Perspective. Foods 2021, 10, 2089. [Google Scholar] [CrossRef]
- Schulmeister, T.M.; Ruiz-Moreno, M.; Silva, G.M.; Garcia-Ascolani, M.; Ciriaco, F.M.; Henry, D.D.; Lamb, G.C.; Dubeux, J.C.B.; Dilorenzo, N. Evaluation of Brassica Carinata Meal as a Protein Supplement for Growing Beef Heifers1,2. J. Anim. Sci. 2019, 97, 4334–4340. [Google Scholar] [CrossRef]
- Yadav, S.; Teng, P.-Y.; Choi, J.; Singh, A.K.; Kim, W.K. Nutrient Profile and Effects of Carinata Meal as Alternative Feed Ingredient on Broiler Performance, Tight Junction Gene Expression and Intestinal Morphology. Poult. Sci. 2022, 101, 101411. [Google Scholar] [CrossRef]
- Seepaul, R.; Marois, J.; Small, I.M.; George, S.; Wright, D.L. Carinata Dry Matter Accumulation and Nutrient Uptake Responses to Nitrogen Fertilization. Agron. J. 2019, 111, 2038–2046. [Google Scholar] [CrossRef]
- Bashyal, M.; Mulvaney, M.J.; Lee, D.; Wilson, C.; Iboyi, J.E.; Leon, R.G.; Landry, G.M.; Boote, K.J. Brassica carinata Biomass, Yield, and Seed Chemical Composition Response to Nitrogen Rates and Timing on Southern Coastal Plain Soils in the United States. GCB Bioenergy 2021, 13, 1275–1289. [Google Scholar] [CrossRef]
- Iboyi, J.E.; Mulvaney, M.J.; Balkcom, K.S.; Seepaul, R.; Bashyal, M.; Perondi, D.; Leon, R.G.; Devkota, P.; Small, I.M.; George, S.; et al. Tillage System and Seeding Rate Effects on the Performance of Brassica carinata. GCB Bioenergy 2021, 13, 600–617. [Google Scholar] [CrossRef]
- Gugel, R.K.; Falk, K.C. Agronomic and Seed Quality Evaluation of Camelina sativa in Western Canada. Can. J. Plant Sci. 2006, 86, 1047–1058. [Google Scholar] [CrossRef]
- Hossain, Z.; Johnson, E.N.; Wang, L.; Blackshaw, R.E.; Gan, Y. Comparative Analysis of Oil and Protein Content and Seed Yield of Five Brassicaceae Oilseeds on the Canadian Prairie. Ind. Crops Prod. 2019, 136, 77–86. [Google Scholar] [CrossRef]
- Mondor, M.; Hernández-Álvarez, A.J. Camelina sativa Composition, Attributes, and Applications: A Review. Eur. J. Lipid Sci. Technol. 2022, 124, 2100035. [Google Scholar] [CrossRef]
- Terpinc, P.; Čeh, B.; Ulrih, N.P.; Abramovič, H. Studies of the Correlation between Antioxidant Properties and the Total Phenolic Content of Different Oil Cake Extracts. Ind. Crops Prod. 2012, 39, 210–217. [Google Scholar] [CrossRef]
- Abdelazim Mohdaly, A.A.; Ramadan, M.F. Characteristics, Composition and Functional Properties of Seeds, Seed Cake and Seed Oil from Different Brassica Carinata Genotypes. Food Biosci. 2022, 48, 100752. [Google Scholar] [CrossRef]
- Negash, Y.A.; Amare, D.E.; Bitew, B.D.; Dagne, H. Assessment of Quality of Edible Vegetable Oils Accessed in Gondar City, Northwest Ethiopia. BMC Res. Notes 2019, 12, 793. [Google Scholar] [CrossRef]
- Mohdaly, A.A.A.; Hassan, M.M.S.; Mahmoud, A.A.; Hafez, N. Physicochemical and Quality Characteristics of Canola Seed Oils from Different Genotypes. Egypt. J. Food 2017, 44, 55–67. [Google Scholar]
- Piravi-vanak, Z.; Azadmard-Damirchi, S.; Kahrizi, D.; Mooraki, N.; Ercisli, S.; Savage, G.P.; Rostami Ahmadvandi, H.; Martinez, F. Physicochemical Properties of Oil Extracted from Camelina (Camelina sativa) Seeds as a New Source of Vegetable Oil in Different Regions of Iran. J. Mol. Liq. 2022, 345, 117043. [Google Scholar] [CrossRef]
- Perera, D.N.; Hewavitharana, G.G.; Navaratne, S.B. Determination of Physicochemical and Functional Properties of Coconut Oil by Incorporating Bioactive Compounds in Selected Spices. J. Lipids 2020, 2020, 1–11. [Google Scholar] [CrossRef]
- Yang, H.; Irudayaraj, J. Comparison of Near-Infrared, Fourier Transform-Infrared, and Fourier Transform-Raman Methods for Determining Olive Pomace Oil Adulteration in Extra Virgin Olive Oil. J. Am. Oil. Chem. Soc. 2001, 78, 889. [Google Scholar] [CrossRef]
- Pulassery, S.; Abraham, B.; Ajikumar, N.; Munnilath, A.; Yoosaf, K. Rapid Iodine Value Estimation Using a Handheld Raman Spectrometer for On-Site, Reagent-Free Authentication of Edible Oils. ACS Omega 2022, 7, 9164–9171. [Google Scholar] [CrossRef] [PubMed]
- Agregán, R.; Lorenzo, J.M.; Munekata, P.E.S.; Dominguez, R.; Carballo, J.; Franco, D. Assessment of the Antioxidant Activity of Bifurcaria Bifurcata Aqueous Extract on Canola Oil. Effect of Extract Concentration on the Oxidation Stability and Volatile Compound Generation during Oil Storage. Food Res. Int. 2017, 99, 1095–1102. [Google Scholar] [CrossRef]
- Medeiros de Azevedo, W.; Ferreira Ribeiro de Oliveira, L.; Alves Alcântara, M.; Tribuzy de Magalhães Cordeiro, A.M.; Florentino da Silva Chaves Damasceno, K.S.; Kelly de Araújo, N.; Fernandes de Assis, C.; Sousa Junior, F.C. de Physicochemical Characterization, Fatty Acid Profile, Antioxidant Activity and Antibacterial Potential of Cacay Oil, Coconut Oil and Cacay Butter. PLoS ONE 2020, 15, e0232224. [Google Scholar] [CrossRef]
- Baeten, V.; Dardenne, P.; Aparicio, R. Interpretation of Fourier Transform Raman Spectra of the Unsaponifiable Matter in a Selection of Edible Oils. J. Agric. Food Chem. 2001, 49, 5098–5107. [Google Scholar] [CrossRef]
- Farhoosh, R.; Tavassoli-Kafrani, M.H.; Sharif, A. Antioxidant Activity of the Fractions Separated from the Unsaponifiable Matter of Bene Hull Oil. Food Chem. 2011, 126, 583–589. [Google Scholar] [CrossRef]
- Cardeno, A.; Sanchez-Hidalgo, M.; Aparicio-Soto, M.; Alarcón-de-la-Lastra, C. Unsaponifiable Fraction from Extra Virgin Olive Oil Inhibits the Inflammatory Response in LPS-Activated Murine Macrophages. Food Chem. 2014, 147, 117–123. [Google Scholar] [CrossRef]
- Prevc, T.; Cigić, B.; Vidrih, R.; Poklar Ulrih, N.; Šegatin, N. Correlation of Basic Oil Quality Indices and Electrical Properties of Model Vegetable Oil Systems. J. Agric. Food Chem. 2013, 61, 11355–11362. [Google Scholar] [CrossRef]
- Belayneh, H.D.; Wehling, R.L.; Cahoon, E.; Ciftci, O.N. Extraction of Omega-3-Rich Oil from Camelina Sativa Seed Using Supercritical Carbon Dioxide. J. Supercrit Fluids 2015, 104, 153–159. [Google Scholar] [CrossRef]
- Abramović, H.; Abram, V. Physico-Chemical Properties, Composition and Oxidative Stability of Camelina Sativa Oil. Food Technol. Biotechnol. 2005, 43, 63–70. [Google Scholar]
- Righini, D.; Zanetti, F.; Monti, A. The Bio-Based Economy Can Serve as the Springboard for Camelina and Crambe to Quit the Limbo. OCL 2016, 23, D504. [Google Scholar] [CrossRef]
- Nagao, K.; Yanagita, T. Medium-Chain Fatty Acids: Functional Lipids for the Prevention and Treatment of the Metabolic Syndrome. Pharmacol. Res. 2010, 61, 208–212. [Google Scholar] [CrossRef]
- Kurasiak-Popowska, D.; Ryńska, B.; Stuper-Szablewska, K. Analysis of Distribution of Selected Bioactive Compounds in Camelina Sativa from Seeds to Pomace and Oil. Agronomy 2019, 9, 168. [Google Scholar] [CrossRef]
- Sharafi, Y.; Majidi, M.M.; Goli, S.A.H.; Rashidi, F. Oil Content and Fatty Acids Composition in Brassica Species. Int. J. Food Prop. 2015, 18, 2145–2154. [Google Scholar] [CrossRef]
- Toncea, I.; Necseriu, D.; Prisecaru, T.; Balint, L.N.; Ghilvacs, M.; Popa, M. The Seed’s and Oil Composition of Camelia—First Romanian Cultivar of Camelina (Camelina sativa, L. Crantz). Rom. Biotechnol. Lett. 2013, 18, 85948–88602. [Google Scholar]
- Jadhav, A.; Marillia, E.F.; Babic, V.; Giblin, M.E.; Cahoon, E.B.; Kinney, A.J.; Mietkiewska, E.; Brost, J.M.; Taylor, D.C. Production of 22:2Δ5, Δ13 and 20:1Δ5 in Brassica carinata and Soybean Breeding Lines via Introduction of Limnanthes Genes. Mol. Breed. 2005, 15, 157–167. [Google Scholar] [CrossRef]
- Abramovič, H.; Butinar, B.; Nikolič, V. Changes Occurring in Phenolic Content, Tocopherol Composition and Oxidative Stability of Camelina Sativa Oil during Storage. Food Chem. 2007, 104, 903–909. [Google Scholar] [CrossRef]
- Günç Ergönül, P.; Aksoylu Özbek, Z. Identification of Bioactive Compounds and Total Phenol Contents of Cold Pressed Oils from Safflower and Camelina Seeds. J. Food Meas. Charact. 2018, 12, 2313–2323. [Google Scholar] [CrossRef]
- Velasco, L.; García-Navarro, E.; Pérez-Vich, B.; Fernández-Martínez, J.M. Selection for Contrasting Tocopherol Content and Profile in Ethiopian Mustard. Plant Breeding 2013, 132, 694–700. [Google Scholar] [CrossRef]
- Shukla, V.K.S.; Dutta, P.C.; Artz, W.E. Camelina Oil and Its Unusual Cholesterol Content. J. Am. Oil. Chem. Soc. 2002, 79, 965–969. [Google Scholar] [CrossRef]
- Ratusz, K.; Symoniuk, E.; Wroniak, M.; Rudzińska, M. Bioactive Compounds, Nutritional Quality and Oxidative Stability of Cold-Pressed Camelina (Camelina sativa L.) Oils. Appl. Sci. 2018, 8, 2606. [Google Scholar] [CrossRef]
- Raczyk, M.; Popis, E.; Kruszewski, B.; Ratusz, K.; Rudzińska, M. Physicochemical Quality and Oxidative Stability of Linseed (Linum usitatissimum) and Camelina (Camelina sativa) Cold-pressed Oils from Retail Outlets. Eur. J. Lipid Sci. Technol. 2016, 118, 834–839. [Google Scholar] [CrossRef]
- MarszaŁkiewicz, S.; Siger, A.; Radziejewska-Kubzdela, E.; Ratusz, K.; Rudzińska, M. The Physicochemical Properties of Cold-Pressed Camelina Seed Oils. Nauka Przyr. Technol. 2017, 11, 235–244. [Google Scholar] [CrossRef]
- Park, I.-K.; Lee, H.-S.; Lee, S.-G.; Park, J.-D.; Ahn, Y.-J. Insecticidal and Fumigant Activities of Cinnamomum Cassia Bark-Derived Materials against Mechoris ursulus (Coleoptera: Attelabidae). J. Agric. Food Chem. 2000, 48, 2528–2531. [Google Scholar] [CrossRef]
- Murillo, M.C.Á.; Suarez, L.E.C.; Salamanca, J.A.C. Insecticidal Activity against Spodoptera frugiperda (Lepidoptera: Noctuidae) of Metabolites Isolated from the Aerial Part of Piper septuplinervium (Miq.) C. DC. and Inflorescences of Piper subtomentosum Trel. & Yunck. (Piperaceae). Quim Nova 2014, 37. [Google Scholar] [CrossRef]
- Nobsathian, S.; Ruttanaphan, T.; Bullangpoti, V. Insecticidal Effects of Triterpene Glycosides Extracted from Holothuria atra (Echinodermata: Holothuroidea) against Spodoptera litura (Lepidoptera: Noctuidae). J. Econ. Entomol. 2019, 112, 1683–1687. [Google Scholar] [CrossRef] [PubMed]
- Shivashankar, S.; Sumathi, M. Gallic Acid Induces Constitutive Resistance against Bactrocera Dorsalis Infestation in Mango Fruit by Its Dual Action. Pestic Biochem. Physiol. 2022, 188, 105268. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhang, Q.; Qu, C.; Wang, Q.; Wang, J.; Luo, C. Toxicity, Baseline of Susceptibility, Detoxifying Mechanism and Sublethal Effects of Chlorogenic Acid, a Potential Botanical Insecticide, on Bemisia Tabaci. Front. Plant Sci. 2023, 14, 1150853. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Fang, Y.; Li, L.; Zhang, L.; Gao, S.; Wang, R.; Wang, J. The Insecticidal Effect of the Botanical Insecticide Chlorogenic Acid on Mythimna separata (Walker) Is Related to Changes in MsCYP450 Gene Expression. Front. Plant Sci. 2022, 13, 1015095. [Google Scholar] [CrossRef]
- Summers, C.B.; Felton, G.W. Prooxidant Effects of Phenolic Acids on the Generalist Herbivore Helicoverpa zea (Lepidoptera: Noctuidae): Potential Mode of Action for Phenolic Compounds in Plant Anti-Herbivore Chemistry. Insect. Biochem. Mol. Biol. 1994, 24, 943–953. [Google Scholar] [CrossRef]
- Joshi, R.S.; Wagh, T.P.; Sharma, N.; Mulani, F.A.; Sonavane, U.; Thulasiram, H.V.; Joshi, R.; Gupta, V.S.; Giri, A.P. Way toward “Dietary Pesticides”: Molecular Investigation of Insecticidal Action of Caffeic Acid against Helicoverpa Armigera. J. Agric. Food Chem. 2014, 62, 10847–10854. [Google Scholar] [CrossRef]
- Punia, A.; Singh, V.; Thakur, A.; Chauhan, N.S. Impact of Caffeic Acid on Growth, Development and Biochemical Physiology of Insect Pest, Spodoptera litura (Fabricius). Heliyon 2023, 9, e14593. [Google Scholar] [CrossRef]
- Herrera-Mayorga, V.; Guerrero-Sánchez, J.A.; Méndez-álvarez, D.; Paredes-Sánchez, F.A.; Rodríguez-Duran, L.V.; Niño-García, N.; Paz-González, A.D.; Rivera, G. Insecticidal Activity of Organic Extracts of Solidago Graminifolia and Its Main Metabolites (Quercetin and Chlorogenic Acid) against Spodoptera Frugiperda: An In Vitro and In Silico Approach. Molecules 2022, 27, 3325. [Google Scholar] [CrossRef] [PubMed]
- Samuel, R.; Pathalam, G.; Babu, V.; Kamaraj, R.; Subramanian, M.; Antony, S.; Sanmugapriya, N.K.; Palaniswamy, S.; Savarimuthu, I. Biocontrol Efficacy of Apigenin Isolated from Anisomeles indica (L.) Kuntze against Immature Stages of Culex Quinquefasciatus (Say, 1823) and Its in Silico Studies. Biocatal Agric. Biotechnol. 2023, 48, 102637. [Google Scholar] [CrossRef]
- Lahcene, S.; Taibi, F.; Mestar, N.; Ali Ahmed, S.; Boumendjel, M.; Ouafi, S.; Houali, K. Insecticidal Effects of the Olea Europaea Subsp. Laperrinei Extracts on the Flour Pyralid Ephestia Kuehniella. Cell Mol. Biol. 2018, 64, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Goławska, S.; Łukasik, I.; Goławski, A.; Kapusta, I.; Janda, B. Alfalfa (Medicago sativa L.) Apigenin Glycosides and Their Effect on the Pea Aphid (Acyrthosiphon pisum). Pol. J. Environ. Stud. 2010, 19, 913–919. [Google Scholar]


| Plant | Insect | ||||
|---|---|---|---|---|---|
| Family | Name | Major Constituent(s) | Order | Name | Ref. |
| Apiaceae | Foeniculum vulgare | estragole | Coleoptera | Tribolium castaneum | [85] |
| Coriandrum sativum | linalol, geranyl acetate | Tribolium confusum | [86] | ||
| Callosobruchus maculatus | |||||
| Asteraceae | Artemisia capillaris | 1,8-cineole, germacrene D, camphor | Sitophilus zemais | [87] | |
| Artemisia mongolica | α-pinene, germacrene D, γ-terpinene | ||||
| Echinacea purpurea | β-cubebene, caryophyllene | Sitophilus granarius | [85] | ||
| Ericaceae | Ledum palustre | ascaridole, p-cymene | Diptera | Culex quinquefasciatus | [88] |
| Musca domestica | |||||
| Lepidoptera | Spodoptera littoralis | ||||
| Lamiaceae | Romarinus officianlis | 1,8-cineole, geraniol | Coleoptera | Sitophilus oryzae | [89] |
| Ocimum basilicum | methyl cinnamate, linalool, eucalyptol | Sitophilus granarius | [85] | ||
| Lepidoptera | Spodoptera litura | [90] | |||
| Lamiales | Mentha pulegium | pulegone, β-pinene, linalol, eucalyptol | Coleoptera | Sitophylus oryzae | [89] |
| Parameter | Camelina sativa | Brassica carinata |
|---|---|---|
| Specific gravity (25 °C) | 0.91–0.92 | 0.90–0.95 |
| Refractive index (20 °C) | 1.47 | 1.47 |
| Iodine value (g I2/100 g oil) | 143.18–162.26 | 90.00–113.00 |
| Saponification value (mg KOH/g oil) | 178.60–187.80 | 129.00–154.0 |
| Unsaponifiable fraction (%) | 0.54–0.87 | 4.20–6.60 |
| Peroxide value (meq O2/kg oil) | 0.89–3.47 | 4.10–9.10 |
| p-Anisidine value | 0.22–1.48 | 3.10–6.30 |
| TOTOX number | 2.16–8.10 | 11.40–24.50 |
| Fatty Acids [%] | Camelina sativa | Brassica carinata |
|---|---|---|
| C12:0 Lauric | 0.04–0.05 | – |
| C14:0 Myristic | 0.13–0.16 | 0.02–0.08 |
| C16:0 Palmitic | 5.10–6.59 | 2.30–4.10 |
| C16:1 Palmitoleic | 0.10–0.14 | 0.04–0.95 |
| C17:0 Margaric | 0.06–0.10 | 0.01–3.20 |
| C18:0 Stearic | 2.19–3.42 | 0.73–3.17 |
| C18:1n9 cis Oleic | 14.90–20.47 | 7.00–29.50 |
| C18:1n9 trans Elaidic | – | 0.03–7.60 |
| C18:2n6 cis Linoleic | 16.00–22.40 | 9.10–21.79 |
| C18:3n3 Linolenic | 28.00–50.30 | 4.70–19.30 |
| C20:0 Arachidic | 1.37–1.80 | 0.50–1.20 |
| C20:1n11 cis Paillinic | – | 1.30–11.00 |
| C20:1n9 Eicosenoic | 11.51–17.50 | 0.55–5.10 |
| C20:2n6 Eicosadienoic | 0.30–2.00 | 1.00–1.07 |
| C20:3n3 Eicosatrienoic | 1.14–3.10 | – |
| C20:5n3 Eicosapentaenoic | – | 0.20–1.70 |
| C22:0 Behenic acid | 0.27–0.80 | 0.25–1.41 |
| C22:1n9 Eruic | 1.52–4.23 | 20.1–56.3 |
| C22:2n6 Docosadienoic | 0.09–0.17 | – |
| C22:4n6 Docosatetraenoic | – | 0.68–2.0 |
| C24:0 Lignoceric | 0.13–0.20 | – |
| C24:1n9 Nervonic | 0.42–0.90 | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rzyska, K.; Stuper-Szablewska, K.; Kurasiak-Popowska, D. Diverse Approaches to Insect Control: Utilizing Brassica carinata (A.) Braun and Camelina sativa (L.) Crantz Oil as Modern Bioinsecticides. Forests 2024, 15, 105. https://doi.org/10.3390/f15010105
Rzyska K, Stuper-Szablewska K, Kurasiak-Popowska D. Diverse Approaches to Insect Control: Utilizing Brassica carinata (A.) Braun and Camelina sativa (L.) Crantz Oil as Modern Bioinsecticides. Forests. 2024; 15(1):105. https://doi.org/10.3390/f15010105
Chicago/Turabian StyleRzyska, Katarzyna, Kinga Stuper-Szablewska, and Danuta Kurasiak-Popowska. 2024. "Diverse Approaches to Insect Control: Utilizing Brassica carinata (A.) Braun and Camelina sativa (L.) Crantz Oil as Modern Bioinsecticides" Forests 15, no. 1: 105. https://doi.org/10.3390/f15010105
APA StyleRzyska, K., Stuper-Szablewska, K., & Kurasiak-Popowska, D. (2024). Diverse Approaches to Insect Control: Utilizing Brassica carinata (A.) Braun and Camelina sativa (L.) Crantz Oil as Modern Bioinsecticides. Forests, 15(1), 105. https://doi.org/10.3390/f15010105







