Response of Seedling Growth Characteristics to Seed Size and Cotyledon Damage in Quercus wutaishanica
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seed Harvesting and Selection
2.2. Seed Sowing and Cotyledon Excision
2.3. Harvesting of Seedlings and Determination of Biomass and Growth Parameters
RSR = (Root dry mass)/(stem and leaf dry mass)
SLA = (Leaf area)/(leaf dry mass (cm2·g−1))
SSL = (shoot length)/(shoot dry mass (cm·g−1))
SRL = (Root length)/(root dry mass (cm·g−1))
2.4. Statistical and Analytical Data
3. Results
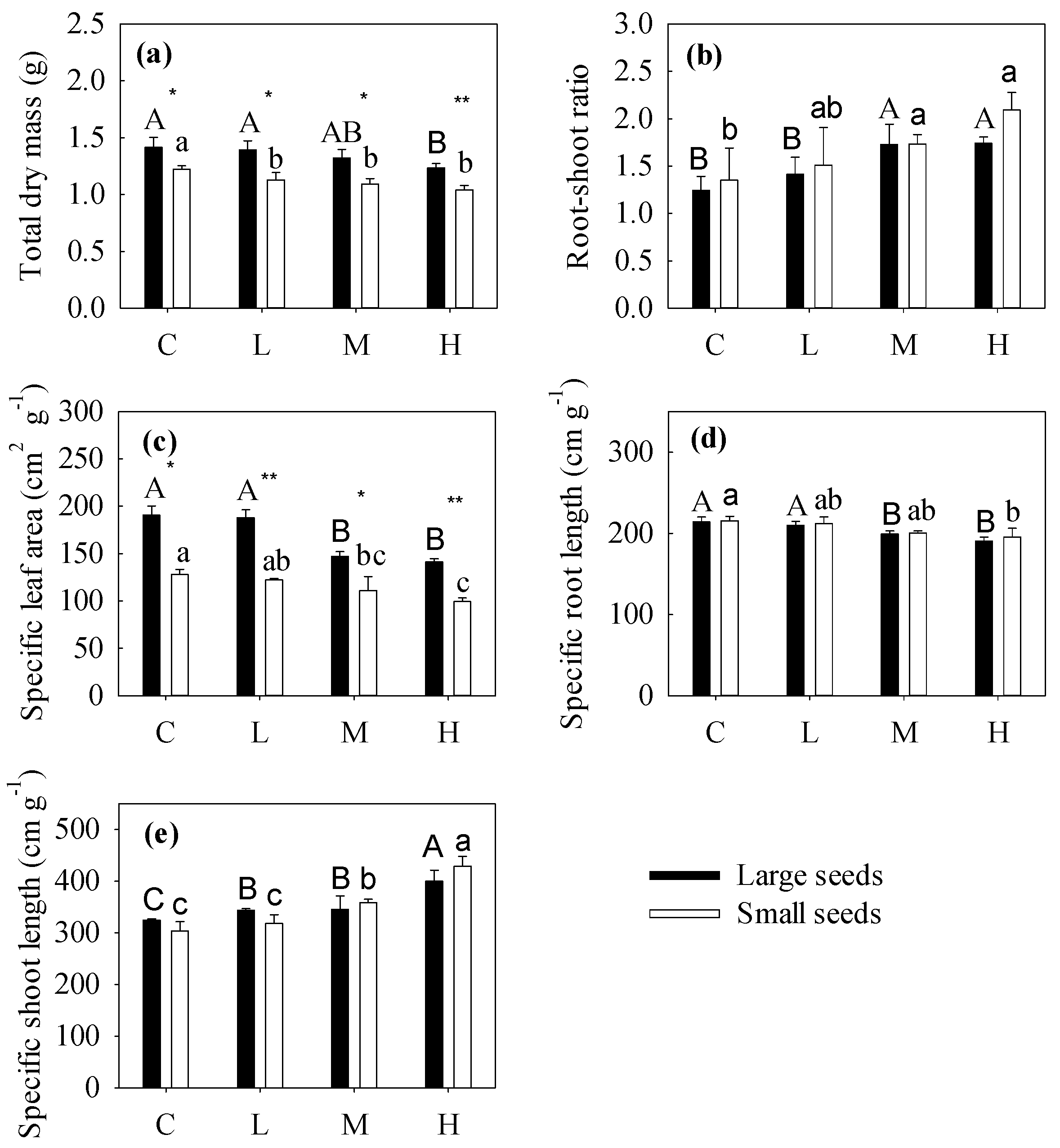
3.1. Effect of Seed Size and Cotyledon Excision on Seedling Growth
3.2. Seedling Growth Response to Seed Size
3.3. Seedling Growth Response to Cotyledon Excision
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.; Ge, J.; Yan, X.; Dayananda, B.; Luo, Y.; Li, J. Frequency-dependent seedling predation by rodents: Growth and survival of Quercus wutaishanica in two habitats. J. Plant Ecol. 2023, 16, rtac086. [Google Scholar] [CrossRef]
- Howe, H.F.; Smallwood, J. Ecology of seed dispersal. Annu. Rev. Ecol. Syst. 1982, 13, 201–228. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, B.; Steele, M.A.; Han, N.; Feng, T.; Wang, J.; Chen, X.; An, X.; Chang, G. Seed traits and rodent community interact to determine seed fate: Evidence from both enclosure and field experiments. Integr. Zool. 2021, 16, 939–954. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Holyoak, M.; Krebs, C.J.; Huang, X. Palatability and profitability of co-occurring seeds alter indirect interactions among rodent-dispersed trees. Integr. Zool. 2022, 17, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Cheng, J.; Yan, X.; Yang, H.; Shen, Y.; Ge, J.; Zhang, M.; Zhang, J.; Xu, Z. Density-Dependent Seed Predation of Quercus wutaishanica by Rodents in Response to Different Seed States. Animals 2023, 13, 1732. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, X.; Dayananda, B.; Luo, Y.; Li, J. Frequency-dependent predation and seedling fate: Effect of forest litter on regeneration of the Quercus wutaishanica seedling. Glob. Ecol. Conserv. 2022, 38, e02233. [Google Scholar] [CrossRef]
- Warpeha, K.M.; Montgomery, B.L. Light and hormone interactions in the seed-to-seedling transition. Environ. Exp. Bot. 2016, 121, 56–65. [Google Scholar] [CrossRef]
- Hanley, M.; Fenner, M.; Whibley, H.; Darvill, B. Early plant growth: Identifying the end point of the seedling phase. New Phytol. 2004, 163, 61–66. [Google Scholar] [CrossRef]
- Hou, X.; Yi, X.; Yang, Y.; Liu, W. Acorn germination and seedling survival of Q. variabilis: Effects of cotyledon excision. Ann. Forest Sci. 2010, 67, 711. [Google Scholar] [CrossRef]
- Yi, X.; Yang, Y. Large acorns benefit seedling recruitment by satiating weevil larvae in Quercus aliena. Plant Ecol. 2010, 209, 291–300. [Google Scholar] [CrossRef]
- Yan, X.; Zhou, L.; Zhang, W.; Zhou, Y. Cotyledon loss and its effects on survival and growth of Quercus wutaishanica seedlings under different densities. Chin. J. Plant Ecol. 2012, 36, 831. [Google Scholar] [CrossRef]
- Wolf, L.; Hainsworth, F.R.; Mercier, T.; Benjamin, R. Seed size variation and pollinator uncertainty in Ipomopsis aggregata (Polemoniaceae). J. Ecol. 1986, 74, 361–371. [Google Scholar] [CrossRef]
- Venable, D.L. Size-number trade-offs and the variation of seed size with plant resource status. Am. Nat. 1992, 140, 287–304. [Google Scholar] [CrossRef]
- Henery, M.L.; Westoby, M. Seed mass and seed nutrient content as predictors of seed output variation between species. Oikos 2001, 92, 479–490. [Google Scholar] [CrossRef]
- Parciak, W. Environmental variation in seed number, size, and dispersal of a fleshy-fruited plant. Ecology 2002, 83, 780–793. [Google Scholar] [CrossRef]
- Sober, V.; Ramula, S. Seed number and environmental conditions do not explain seed size variability for the invasive herb Lupinus polyphyllus. Plant Ecol. 2002, 214, 883–892. [Google Scholar] [CrossRef]
- Milberg, P.; Lamont, B.B. Seed/cotyledon size and nutrient content play a major role in early performance of species on nutrient-poor soils. New Phytol. 1997, 137, 665–672. [Google Scholar] [CrossRef]
- Giertych, M.J.; Suszka, J. Consequences of cutting off distal ends of cotyledons of Quercus robur acorns before sowing. Ann. For. Sci. 2011, 68, 433–442. [Google Scholar] [CrossRef]
- Yi, X.; Wang, Z.; Liu, C.; Liu, G.; Zhang, M. Acorn cotyledons are larger than their seedlings’ need: Evidence from artificial cutting experiments. Sci. Rep. 2015, 5, 8112. [Google Scholar] [CrossRef]
- Lebrija-Trejos, E.; Reich, P.B.; Hernández, A.; Wright, S.J. Species with greater seed mass are more tolerant of conspecific neighbours: A key driver of early survival and future abundances in a tropical forest. Ecol. Lett. 2016, 19, 1071–1080. [Google Scholar] [CrossRef]
- Jørgensen, M.S.; Labouriau, R.; Olesen, B. Seed size and burial depth influence Zostera marina L. (eelgrass) seed survival, seedling emergence and initial seedling biomass development. PLoS ONE 2019, 14, e0215157. [Google Scholar] [CrossRef]
- Perea, R.; San Miguel, A.; Gil, L. Leftovers in seed dispersal: Ecological implications of partial seed consumption for oak regeneration. J. Ecol. 2011, 99, 194–201. [Google Scholar] [CrossRef]
- Yan, X.; Fang, S.; Shi, C.; Chou, Z.; Zhou, Y. Effects of simulated cotyledon predation on the seed germination and early seedling growth of Quercus wutaishanica. Chin. J. Ecol. 2014, 33, 973–981. [Google Scholar]
- Zhang, H.; Chen, Y.; Zhang, Z. Differences of dispersal fitness of large and small acorns of Liaodong oak (Quercus liaotungensis) before and after seed caching by small rodents in a warm temperate forest, China. For. Ecol. Manag. 2008, 255, 1243–1250. [Google Scholar] [CrossRef]
- Bolker, B.M.; Brooks, M.E.; Clark, C.J.; Geange, S.W.; Poulsen, J.R.; Henry, H.M. Generalized linear mixed models: A practical guide for ecology and evolution. Trends Ecol. Evol. 2019, 24, 127–135. [Google Scholar] [CrossRef]
- Moles, A.T.; Westoby, M. Seedling survival and seed size: A synthesis of the literature. J. Ecol. 2004, 92, 372–383. [Google Scholar] [CrossRef]
- Kang, P.; Zhang, Z.; Zhang, J.; Luo, Y.; Deng, X.; Zhou, L.; Yan, X. Density dependence of cotyledon loss of Quercus wutaishanica seedlings germinated from different sized seeds and the responses of seedling growth. Chin. J. Ecol. 2021, 40, 3098–3106. [Google Scholar]
- Stanton, M.L. Seed variation in wild radish: Effect of seed size on components of seedling and adult fitness. Ecology 1984, 65, 1105–1112. [Google Scholar] [CrossRef]
- Perez-Harguindeguy, N.; Diaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.; Cornwell, W.; Craine, J.; Gurvich, D. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- Díaz, S.; Kattge, J.; Cornelissen, J.H.; Wright, I.J.; Lavorel, S.; Dray, S.; Reu, B.; Kleyer, M.; Wirth, C.; Colin Prentice, I. The global spectrum of plant form and function. Nature 2016, 529, 167–171. [Google Scholar] [CrossRef]
- Jiang, X.; Li, G.; Shi, W.; Zhao, K.; Li, C. Effects of Cotyledon Loss Intensity and Time on Seedling Growth Status and Reserves Translocation in Quercus variabilis. Chin. For. Sci. 2018, 54, 56–64. [Google Scholar]

| Growth Parameters | Seed Size | Cotyledon Excision | Seed Size × Cotyledon | |||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| Shoot height | 70.84 | <0.001 | 11.53 | 0.003 | 0.28 | 0.600 |
| Basal stem diameter | 22.86 | <0.001 | 8.56 | 0.009 | 3.58 | 0.074 |
| Leaf number | 45.28 | <0.001 | 3.69 | 0.081 | 2.7 | 0.118 |
| Leaf area | 67.03 | <0.001 | 4.91 | 0.039 | 0.71 | 0.411 |
| Total dry mass | 52.78 | <0.001 | 11.35 | 0.003 | 0.28 | 0.601 |
| Root-shoot ratio | 1.52 | 0.233 | 9.45 | 0.007 | 0.03 | 0.867 |
| Specific leaf area | 65.40 | <0.001 | 14.08 | 0.002 | 0.71 | 0.409 |
| Specific root length | 0.04 | 0.843 | 4.75 | 0.043 | 0.69 | 0.410 |
| Specific shoot length | 0.22 | 0.640 | 9.71 | 0.005 | 0.62 | 0.438 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.; Zhang, J.; Yan, X.; Zhang, M.; Wei, S.; Yang, H.; Shen, Y.; Zhang, J.; Cheng, J. Response of Seedling Growth Characteristics to Seed Size and Cotyledon Damage in Quercus wutaishanica. Forests 2023, 14, 1905. https://doi.org/10.3390/f14091905
Luo Y, Zhang J, Yan X, Zhang M, Wei S, Yang H, Shen Y, Zhang J, Cheng J. Response of Seedling Growth Characteristics to Seed Size and Cotyledon Damage in Quercus wutaishanica. Forests. 2023; 14(9):1905. https://doi.org/10.3390/f14091905
Chicago/Turabian StyleLuo, Yonghong, Jinfeng Zhang, Xingfu Yan, Min Zhang, Shuhua Wei, Hui Yang, Yan Shen, Jinbao Zhang, and Jiming Cheng. 2023. "Response of Seedling Growth Characteristics to Seed Size and Cotyledon Damage in Quercus wutaishanica" Forests 14, no. 9: 1905. https://doi.org/10.3390/f14091905
APA StyleLuo, Y., Zhang, J., Yan, X., Zhang, M., Wei, S., Yang, H., Shen, Y., Zhang, J., & Cheng, J. (2023). Response of Seedling Growth Characteristics to Seed Size and Cotyledon Damage in Quercus wutaishanica. Forests, 14(9), 1905. https://doi.org/10.3390/f14091905






