Exogenous 5-Aminolevulinic Acid Promotes Osmotic Stress Tolerance of Walnuts by Modulating Photosynthesis, Osmotic Adjustment and Antioxidant Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Culture and Treatments
2.2. Measurement of RWC
2.3. Determination of Photosynthetic Gas Exchange Parameters
2.4. Measurement of Chlorophyll Content
2.5. Measurement of Rapid Chlorophyll Fluorescence Characteristics
2.6. Measurement of Physiological and Biochemical Indices
2.7. Real-Time Quantitative PCR Analysis for Gene Expression
2.8. Data Statistical Analysis
3. Results
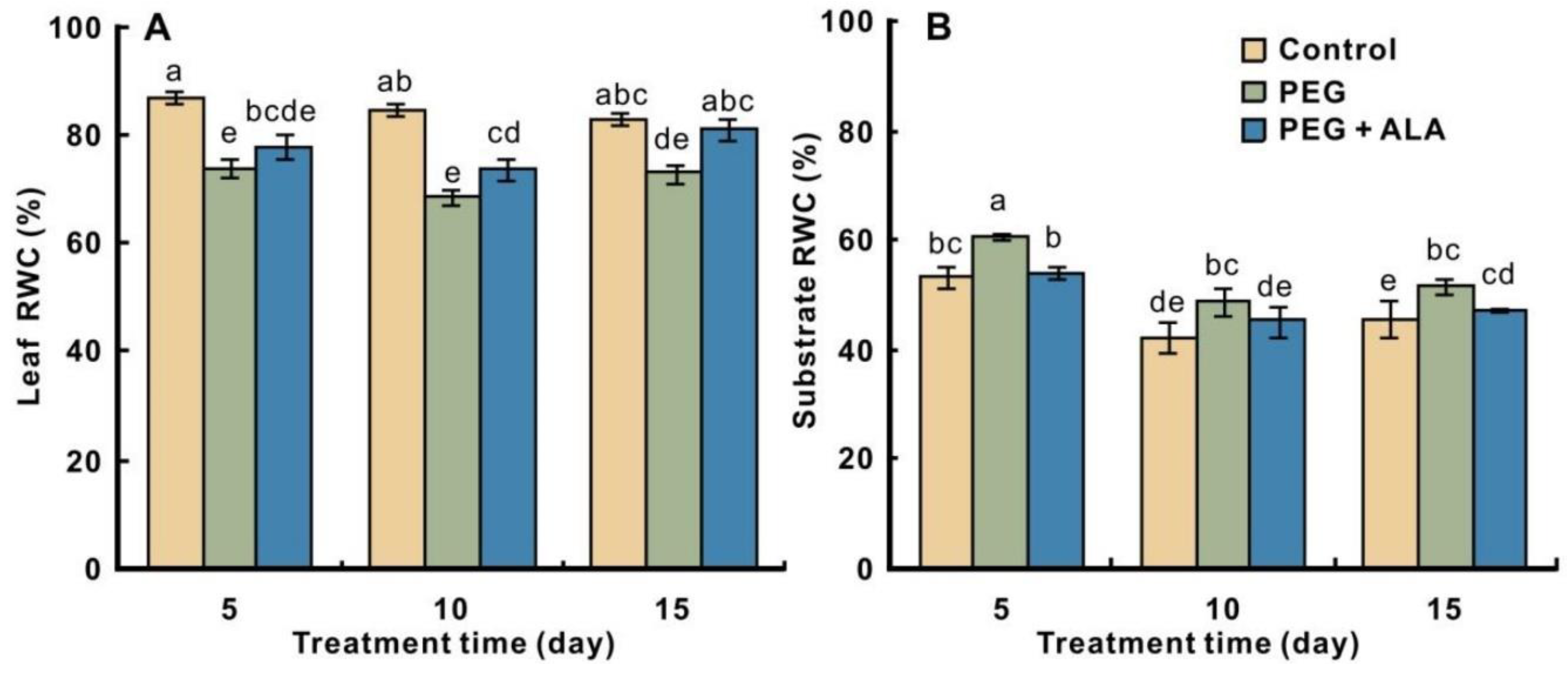
3.1. Effects of ALA on the RWC in Walnut Leaves and Culture Substrates under PEG Stress
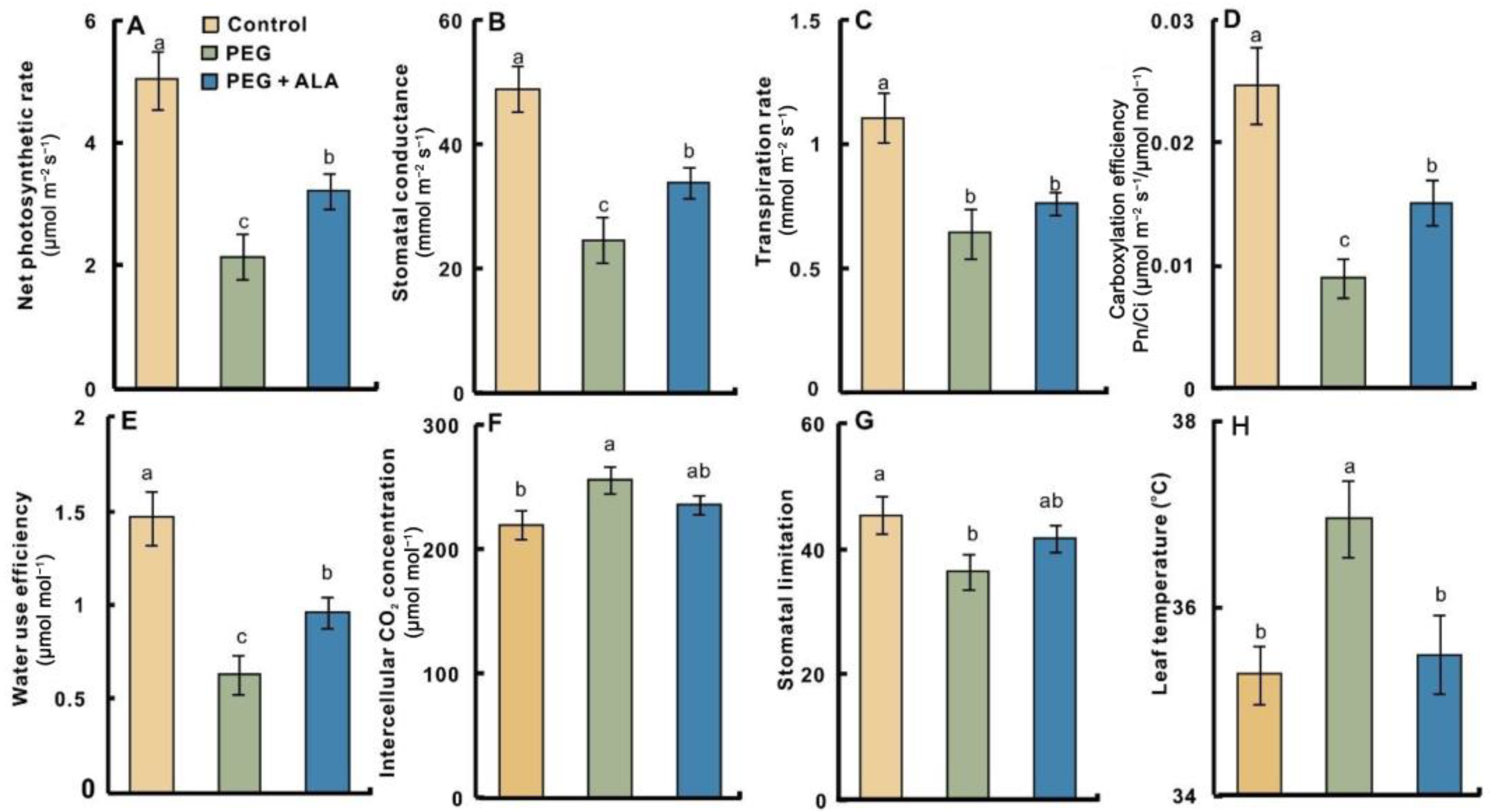
3.2. Effects of ALA on Leaf Photosynthetic Gas Exchanges of Walnut under PEG Stress
3.3. Effects of ALA on the Photochemical Activity of Photosystem Reaction Centers in Walnut Leaves under PEG Stress
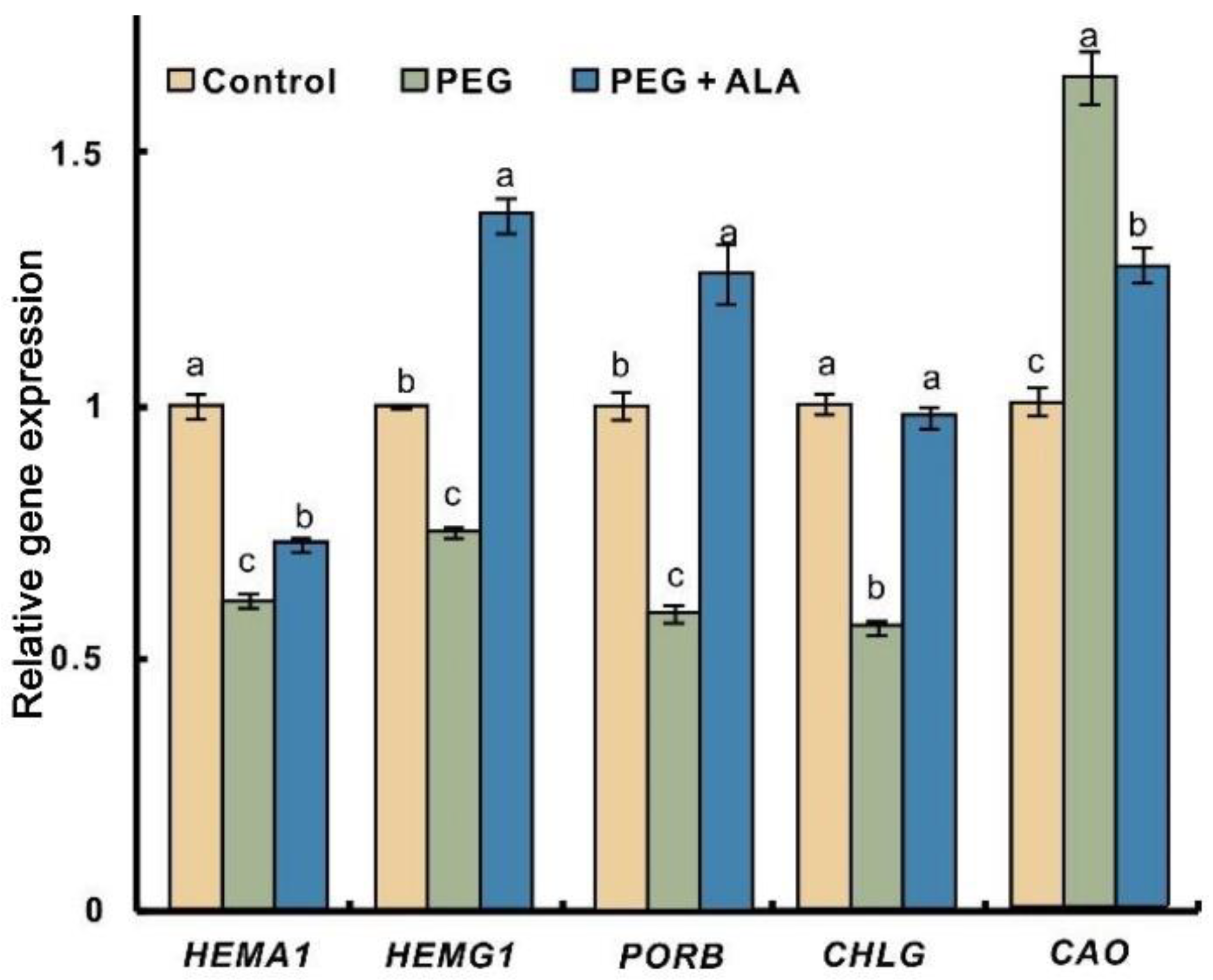
3.4. Effects of ALA on the Chlorophylls in Walnut Leaves under PEG Stress
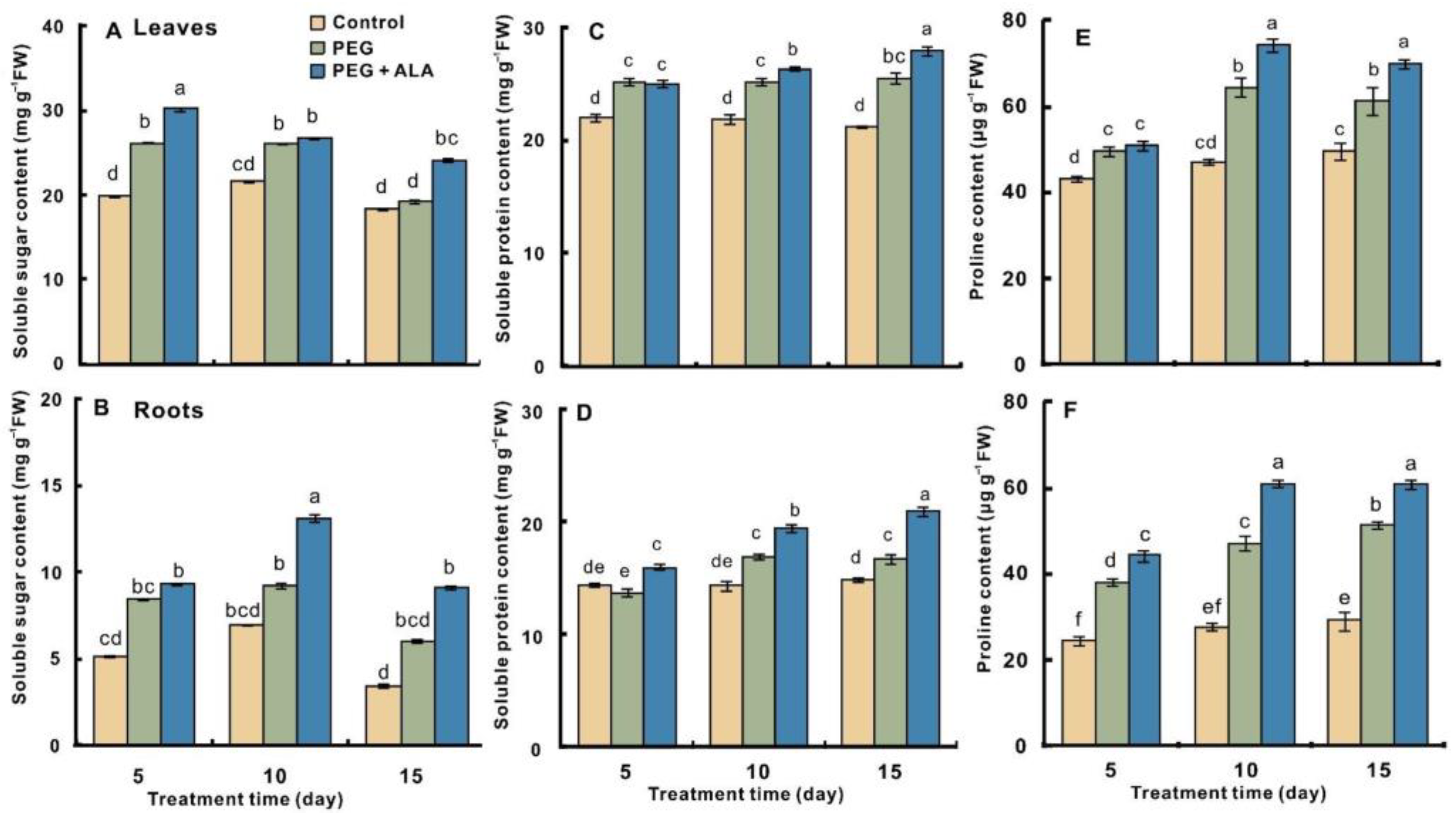
3.5. Effect of ALA on the Osmolyte Content in the Walnuts under PEG Stress
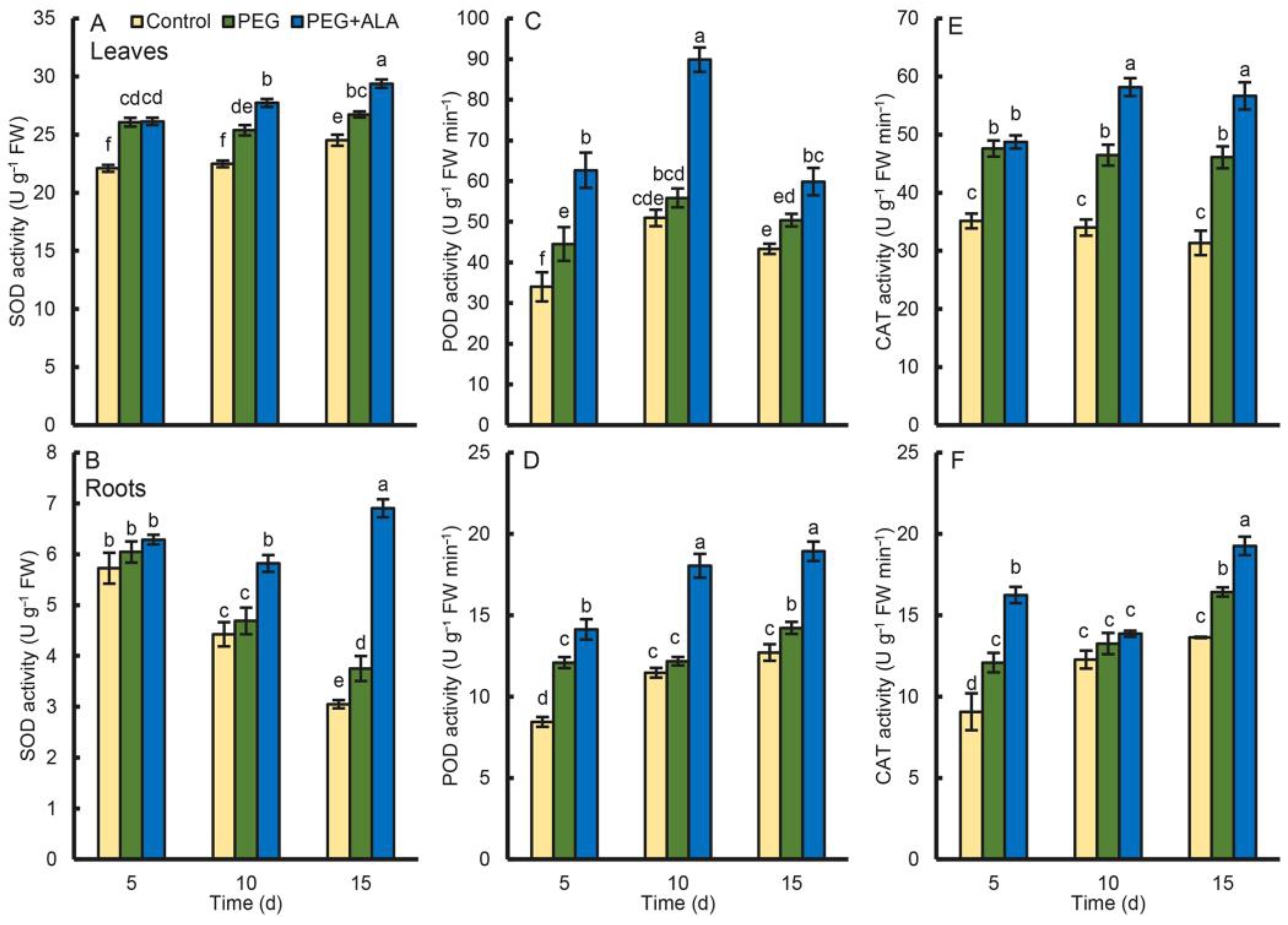
3.6. Effect of ALA on the Antioxidant Enzyme Activity under Osmotic Stress
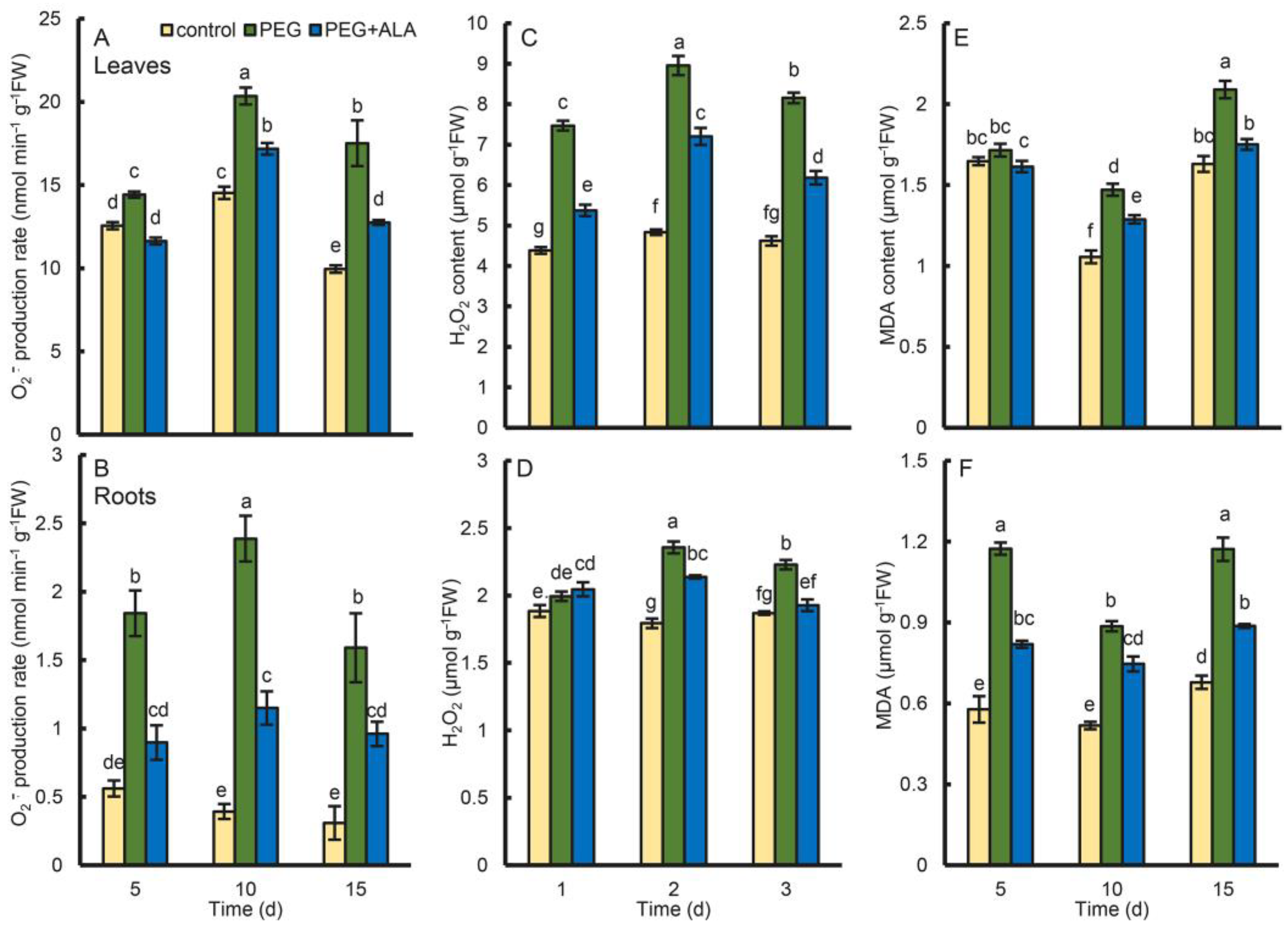
3.7. Effect of ALA on the ROS Content in Walnuts under Osmotic Stress
3.8. ALA Regulates DREB2A Expression of Walnut Plants under Osmotic Stress
3.9. ALA Regulates Expression of PIP and TIP in Walnut Plants under Osmotic Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdallah, I.B.; Tlili, N.; Martinez-Force, E.; Rubio, A.G.P.; Perez-Camino, M.C.; Albouchi, A.; Boukhchina, S. Content of carotenoids, tocopherols, sterols, triterpenic and aliphatic alcohols, and volatile compounds in six walnuts (Juglans regia L.) varieties. Food Chem. 2015, 173, 972–978. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.Q.; Chen, Q.Q.; Yin, Z.Y.; Zhang, J.; Zhang, Y.S.; Xu, Y.M. Application status and fertilization technology of organic fertilizer in normal walnut orchard in the southern Xinjiang. Agric. Sci.-Technol. Comm. 2023, 3, 238–241. [Google Scholar]
- Shi, S.Q. Study on Mechanism of Drought Resistance in Four Species Seedlings. Master’s Thesis, Heibei Agricultural University, Baoding, China, 2003. [Google Scholar]
- Miao, H.X. Study on Response of Characteristics of Six Tree Species to Drought Stress. Master’s Thesis, Shandong Agricultural University, Tai’an, China, 2005. [Google Scholar]
- Wang, Z.Y. Preliminary Studies on Drought Resistance of Different Walnut Cultivars. Master’s Thesis, Northwest A & F University, Xianyang, China, 2014. [Google Scholar]
- Yang, G.Y.; Chen, S.W.; Li, D.P.; Gao, X.Q.; Su, L.Y.; Peng, S.B.; Zhai, M.Z. Multiple transcriptional regulation of walnut JrGSTTau1 gene in response to osmotic stress. Physiol. Plant 2019, 166, 748–761. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Razi, K.; Muneer, S. Drought stress-induced physiological mechanisms, signaling pathways and molecular response of chloroplasts in common vegetable crops. Crit. Rev. Biotechnol. 2021, 41, 669–691. [Google Scholar] [CrossRef]
- Mukarram, M.; Choudhary, S.; Kurjak, D.; Petek, A.; Khan, M.M.A. Drought: Sensing, signalling, effects and tolerance in higher plants. Physiol. Plant 2021, 172, 1291–1300. [Google Scholar] [CrossRef]
- Agarwal, P.K.; Jha, B. Transcription factors in plants and ABA dependent and independent abiotic stress signalling. Biol. Plant. 2010, 54, 201–212. [Google Scholar] [CrossRef]
- Chan, Z.L.; Shi, H.T. Improved abiotic stress tolerance of bermudagrass by exogenous small molecules. Plant Signal. Behav. 2015, 10, e991577. [Google Scholar] [CrossRef]
- Tanaka, R.; Tanaka, A. Tetrapyrrole biosynthesis in higher plants. Annu. Rev. Plant Biol. 2007, 58, 321–346. [Google Scholar] [CrossRef]
- Wu, Y.; Liao, W.B.; Dawuda, M.M.; Hu, L.L.; Yu, J.H. 5-Aminolevulinic acid (ALA) biosynthetic and metabolic pathways and its role in higher plants: A review. Plant Growth Regul. 2019, 87, 357–374. [Google Scholar] [CrossRef]
- Akram, N.A.; Ashraf, M. Regulation in plant stress tolerance by a potential plant growth regulator, 5-aminolevulinic acid. J. Plant Growth Regul. 2013, 32, 663–679. [Google Scholar] [CrossRef]
- Rhaman, M.S.; Imran, S.; Karim, M.M.; Chakrobortty, J.; Hasanuzzaman, M. 5-Aminolevulinic acid-mediated plant adaptive responses to abiotic stress. Plant Cell Rep. 2021, 40, 1451–1469. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Z.Y.; Han, S.; Yu, X.; Huang, M.B.; Lian, C.L.; Liu, C.; Yin, W.L.; Xia, X.L. 5-Aminolevulinic acid pretreatment mitigates drought and salt stresses in poplar plants. Forests 2021, 12, 1112. [Google Scholar] [CrossRef]
- Li, D.M.; Zhang, J.; Sun, W.J.; Li, Q.; Dai, A.H.; Bai, J.G. 5-Aminolevulinic acid pretreatment mitigates drought stress of cucumber leaves through altering antioxidant enzyme activity. Sci. Hortic. 2011, 130, 820–828. [Google Scholar] [CrossRef]
- Helaly, M.N.; El-Hoseiny, H.M.; Elsheery, N.I.; Kalaji, H.M.; Santos-Villalobos, S.D.; Wrobel, J.; Hassan, I.F.; Gaballah, M.S.; Abdelrhman, L.A.; Mira, A.M.; et al. 5-Aminolevulinic acid and 24-epibrassinolide improve the drought stress resilience and productivity of banana plants. Plants 2022, 11, 743. [Google Scholar] [CrossRef]
- Liu, D.; Wu, L.T.; Naeem, M.S.; Liu, H.B.; Deng, X.Q.; Xu, L.; Zhang, F.; Zhou, W.J. 5-Aminolevulinic acid enhances photosynthetic gas exchange, chlorophyll fluorescence and antioxidant system in oilseed rape under drought stress. Acta Physiol. Plant. 2013, 35, 2747–2759. [Google Scholar] [CrossRef]
- Niu, K.J.; Ma, X.; Liang, G.L.; Ma, H.L.; Jia, Z.F.; Liu, W.H.; Yu, Q.Q. 5-Aminolevulinic acid modulates antioxidant defense systems and mitigates drought-induced damage in Kentucky bluegrass seedlings. Protoplasma 2017, 254, 2083–2094. [Google Scholar] [CrossRef]
- Akram, N.A.; Kausar, S.; Farid, N.; Ashraf, M.; Al-Qurainy, F. 5-Aminolevulinic acid induces regulation in growth, yield and physio-biochemical characteristics of wheat under water stress. Sains Malays. 2018, 47, 661–670. [Google Scholar] [CrossRef]
- Song, J.X.; Anjum, S.A.; Zong, X.F.; Yan, R.; Wang, L.; Yang, A.J.; Ashraf, U.; Zohaib, A.; Lv, J.; Zhang, Y.; et al. Combined foliar application of nutrients and 5-aminolevulinic acid (ALA) improved drought tolerance in Leymus chinensis by modulating its morpho-physiological characteristics. Basic Appl. Ecol. 2017, 68, 474–482. [Google Scholar] [CrossRef]
- Cai, C.; He, S.; An, Y.; Wang, L. Exogenous 5-aminolevulinic acid improves strawberry tolerance to osmotic stress and its possible mechanisms. Physiol. Plant 2020, 168, 948–962. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Miao, M.M.; Wang, C.L. Effects of ALA on photosynthesis, antioxidant enzyme activity, and gene expression, and regulation of proline accumulation in tomato seedlings under NaCl stress. J. Plant Growth Regul. 2015, 34, 637–650. [Google Scholar] [CrossRef]
- Sher, A.; Tahira, A.S.; Sattar, A.; Nawaz, A.; Qayyum, A.; Hussain, S.; Manaf, A. Foliage application of 5-aminolevulinic acid alleviates drought stress in sunflower (Helianthus annuus L.) through improving stay green and antioxidant enzymes activities. Acta Physiol. Plant. 2021, 43, 22. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water culture method for growing plants without soil. Calif. Agric. Exp. Stn. Circ. 1950, 347, 357–359. [Google Scholar]
- Liang, R.L.; Wang, L.J.; Wang, X.Q.; Zhang, J.T.; Gan, X. Effects of Exogenous ALA on leaf photosynthesis, photosynthate transport, and sugar accumulation in Prunus persica L. Forests 2023, 14, 723. [Google Scholar] [CrossRef]
- Khan, W.; Prithiviraj, B.; Smith, D.L. Photosynthetic responses of corn and soybean to foliar application of salicylates. J. Plant Physiol. 2003, 160, 485–492. [Google Scholar] [CrossRef]
- Meher; Shivakrishna, P.; Reddy, K.A.; Rao, D.M. Effect of PEG-6000 imposed drought stress on RNA content, relative water content (RWC), and chlorophyll content in peanut leaves and roots. Saudi J. Biol. Sci. 2018, 25, 285–289. [Google Scholar] [CrossRef]
- Chen, H.; Xu, L.; Li, X.; Wang, D.; An, Y.Y.; Wang, L.J. Effect of 5-aminolevulinic acid on leaves of rhododendron and camphor of cold tolerance. J. Plant Physiol. 2017, 53, 2103–2113. [Google Scholar]
- Hendrix, D.L. Rapid extraction and analysis of non-structural carbohydrates in plant tissues. Crop Sci. 1993, 33, 1301–1311. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Kazemi, N.; Khavari-Nejad, R.A.; Fahimi, H.; Saadatmand, S.; Nejad-Sattari, T. Effects of exogenous salicylic acid and nitric oxide on lipid peroxidation and antioxidant enzyme activities in leaves of Brassica napus L. under nickel stress. Sci. Hortic. 2010, 126, 402–407. [Google Scholar] [CrossRef]
- Zhao, S.; Xu, C.; Zou, Q.; Meng, Q. Improvements of method for measurement of malondialdhyde in plant tissues. Plant Physiol. Comm. 1994, 30, 207–210. (In Chinese) [Google Scholar]
- Uchida, A.; Jagendorf, A.T.; Hibino, T.; Takabe, T.; Takabe, T. Effects of hydrogen peroxide and nitric oxide on both salt and heat stress tolerance in rice. Plant Sci. 2002, 163, 515–523. [Google Scholar] [CrossRef]
- Qian, C.L.; Zhao, Y.Y.; Mi, H.B.; Chen, X.H.; Guo, L.J.; Mao, L.C. Role of antioxidative system during the development and senescence of cucumber fruit. Biol. Plant. 2012, 56, 793–797. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Xiong, J.L.; Wang, H.C.; Tan, X.Y.; Zhang, C.L.; Naeem, M.S. 5-Aminolevulinic acid improves salt tolerance mediated by regulation of tetrapyrrole and proline metabolism in Brassica napus L. seedlings under NaCl stress. Plant Physiol. Biochem. 2018, 124, 88–99. [Google Scholar] [CrossRef]
- Anwar, A.; Yan, Y.; Liu, Y.M.; Li, Y.S.; Yu, X.C. 5-Aminolevulinic acid improves nutrient uptake and endogenous hormone accumulation, enhancing low-temperature stress tolerance in cucumbers. Int. J. Mol. Sci. 2018, 19, 3379. [Google Scholar] [CrossRef] [PubMed]
- Katuwal, K.B.; Rowe, S.; Jespersen, D. The use of 5-aminolevulinic acid to reduce heat-stress-related damages in tall fescue. Crop Sci. 2021, 61, 3206–3218. [Google Scholar] [CrossRef]
- Elansary, H.O.; El-Ansary, D.O.; Al-Mana, F.A. 5-Aminolevulinic acid and soil fertility enhance the resistance of rosemary to Alternaria dauci and Rhizoctonia solani and modulate plant biochemistry. Plants 2019, 8, 585. [Google Scholar] [CrossRef] [PubMed]
- Ostrowska, A.; Biesaga-Koscielniak, J.; Grzesiak, M.T.; Hura, T. Physiological responses of spring wheat to 5-aminolevulinic acid under water stress applied at seedling stage. Cereal Res. Commun. 2019, 47, 32–41. [Google Scholar] [CrossRef]
- Liu, D.; Hu, L.Y.; Ali, B.; Yang, A.G.; Wan, G.L.; Xu, L.; Zhou, W.J. Influence of 5-aminolevulinic acid on photosynthetically related parameters and gene expression in Brassica napus L. under drought stress. Soil Sci. Plant Nutr. 2016, 62, 254–262. [Google Scholar] [CrossRef]
- Tan, S.Y.; Cao, J.; Xia, X.L.; Li, Z.H. Advances in 5-aminolevulinic acid priming to enhance plant tolerance to abiotic stress. Int. J. Mol. Sci. 2022, 23, 702. [Google Scholar] [CrossRef] [PubMed]
- Shakirova, F.M.; Sakhabutdinova, A.R.; Bezrukova, M.V.; Fatkhutdinova, R.A.; Fatkhutdinova, D.R. Changes in the hormonal status of wheat seedlings induced by salicylic acid and salinity. Plant Sci. 2003, 164, 317–322. [Google Scholar] [CrossRef]
- Blum, A. Osmotic adjustment is a prime drought stress adaptive engine in support of plant production. Plant Cell Environ. 2017, 40, 4–10. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- McDowell, N.G.; Sevanto, S. The mechanisms of carbon starvation: How, when, or does it even occur at all? New Phytol. 2010, 186, 264–266. [Google Scholar] [CrossRef]
- Galmes, J.; Medrano, H.; Flexas, J. Photosynthetic limitations in response to water stress and recovery in Mediterranean plants with different growth forms. New Phytol. 2007, 175, 81–93. [Google Scholar] [CrossRef]
- Bousba, R.; Ykhlef, N.; Djekoun, A. Water use efficiency and flat leaf photosynthetic in response to water deficit of durum wheat (Triticum durum Desf). World J. Agric.Sci. 2009, 5, 609–616. [Google Scholar]
- Schansker, G.; Srivastava, A.; Govindjee; Strasser, R.J. Characterization of the 820-nm transmission signal paralleling the chlorophyll a fluorescence rise (OJIP) in pea leaves. Funct. Plant Biol. 2003, 30, 785–796. [Google Scholar] [CrossRef]
- Ceppi, M.G.; Oukarroum, A.; Cicek, N.; Strasser, R.J.; Schansker, G. The IP amplitude of the fluorescence rise OJIP is sensitive to changes in the photosystem I content of leaves: A study on plants exposed to magnesium and sulfate deficiencies, drought stress and salt stress. Physiol. Plant 2012, 144, 277–288. [Google Scholar] [CrossRef]
- Zivcak, M.; Kalaji, H.M.; Shao, H.B.; Olsovska, K.; Brestic, M. Photosynthetic proton and electron transport in wheat leaves under prolonged moderate drought stress. J. Photochem. Photobiol. B-Biol. 2014, 137, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wei, S.; Wang, J.; Su, X.; Zhao, H. Exogenous application of 5-aminolevulinic acid on wheat seedlings under drought stress enhances the transcription of psbA and psbD genes and improves photosynthesis. Braz. J. Bot. 2018, 41, 275–285. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Guisse, B.; Srivastava, A.; Strasser, R.J. Effects of high temperature and water stress on the polyphasic chlorophyll a fluorescence transient of potato leaves. In Proceedings of the Xth International Photosynthesis Congress, Montpllier, France, 20–25 August 1995; pp. 913–916. [Google Scholar]
- Phour, M.; Ghai, A.; Rose, G.; Dhull, N.; Sindhu, S.S. Role of aminolevulinic acid in stress adaptation and crop productivity. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1516–1524. [Google Scholar] [CrossRef]
- Han, R.H.; Gao, G.J.; Li, Z.D.; Dong, Z.X.; Guo, Z.F. Effects of exogenous 5-aminolevulinic acid on seed germination of alfalfa (Medicago varia Martyn.) under drought stress. Grassl. Sci. 2018, 64, 100–107. [Google Scholar] [CrossRef]
- Harpaz-Saad, S.; Azoulay, T.; Arazi, T.; Ben-Yaakov, E.; Mett, A.; Shiboleth, Y.M.; HoRtensteiner, S.; Gidoni, D.; Gal-On, A.; Eyal, G.Y. Chlorohyllase is a rate-limiting enzyme in chlorophyll catabolism and is posttranslationally regulated. Plant Cell 2007, 19, 1007–1022. [Google Scholar] [CrossRef]
- Shalygo, N.; Czarnecki, O.; Peter, E.; Grimm, B. Expression of chlorophyll synthase is also involved in feedback-control of chlorophyll biosynthesis. Plant Mol. Biol. 2009, 71, 425–436. [Google Scholar] [CrossRef]
- Hirashima, M.; Satoh, S.; Tanaka, R.; Tanaka, A. Pigment shuffling in antenna systems achieved by expressing prokaryotic chlorophyllide a oxygenase in Arabidopsis. J. Biol. Chem. 2006, 281, 15385–15393. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Pei, Z.F.; Naeem, M.S.; Ming, D.F.; Liu, H.B.; Khan, F.; Zhou, W.J. 5-Aminolevulinic acid activates antioxidative defence system and seedling growth in Brassica napus L. under water-deficit stress. J. Agron. Crop Sci. 2011, 197, 284–295. [Google Scholar] [CrossRef]
- Rasheed, R.; Yasmeen, H.; Hussain, I.; Iqbal, M.; Parveen, A. Exogenously applied 5-aminolevulinic acid modulates growth, secondary metabolism and oxidative defense in sunflower under water deficit stress. Physiol. Mol. Biol. Plants 2020, 26, 489–499. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Kaushik, D.; Aryadeep, R. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar]
- Scandalios, J.G. The rise of ROS. Trends Biochem. Sci. 2002, 27, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Boguszewska, D.; Grudkowska, M.; Zagdanska, B. Drought-responsive antioxidant enzymes in potato (Solanum tuberosum L.). Potato Res. 2010, 53, 373–382. [Google Scholar] [CrossRef]
- Benesova, M.; Hola, D.; Fischer, L.; Jedelsky, P.L.; Hnilicka, F.; Wilhelmova, N.; Rothova, O.; Kocova, M.; Prochazkova, D.; Honnerova, J.; et al. The physiology and proteomics of dought tolerance in maize: Early stomatal closure as a cause of lower tolerance to short-term dehydration? PLoS ONE 2012, 7, e3801. [Google Scholar] [CrossRef]
- Sairam, R.K.; Srivastava, G.C.; Agarwal, S.; Meena, R.C. Differences in antioxidant activity in response to salinity stress in tolerant and susceptible wheat genotypes. Biol. Plant. 2005, 49, 85–91. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C. Proline accumulation in plants: A review. Amino Acids 2008, 35, 753–759. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Soualiou, S.; Duan, F.Y.; Li, X.; Zhou, W.B. Crop production under cold stress: An understanding of plant responses, acclimation processes, and management strategies. Plant Physiol. Biochem. 2022, 190, 47–61. [Google Scholar] [CrossRef]
- Ashraf, U.; Mahmood, S.; Anjum, S.A.; Abbas, R.N.; Rasul, F.; Iqbal, J.; Mo, Z.W.; Tang, X.R. Exogenous gamma-aminobutyric acid application induced modulations in the performance of aromatic rice under lead toxicity. Front. Plant Sci. 2022, 13, 933694. [Google Scholar] [CrossRef]
- Yang, Y.X.; Xia, J.X.; Fang, X.; Jia, H.R.; Wang, X.C.; Lin, Y.L.; Liu, S.Y.; Ge, M.Q.; Pu, Y.F.; Fang, J.G.; et al. Drought stress in ‘Shine Muscat’ grapevine: Consequences and a novel mitigation strategy-5-aminolevulinic acid. Front. Plant Sci. 2023, 14, 1129114. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.L.; Wu, Q.; Zheng, D.A.F.; Feng, N.J.; Liang, X.L.; Liu, M.L.; Li, Y.; Mou, B.M. Plant growth regulators enhance saline-alkali tolerance by upregulating the levels of antioxidants and osmolytes in soybean seedlings. J. Plant Growth Regul. 2022, 41, 3218–3232. [Google Scholar] [CrossRef]
- Mahawar, L.; Ramasamy, K.P.; Suhel, M.; Prasad, S.M.; Zivcak, M.; Brestic, M.; Rastogi, A.; Skalicky, M. Silicon nanoparticles: Comprehensive review on biogenic synthesis and applications in agriculture. Environ. Res. 2023, 232, 116292. [Google Scholar] [CrossRef] [PubMed]



| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| 18S | GGTCAATCTTCTCGTTCCCTT | TCGCATTTCGCTACGTTCTT |
| HEMA1 | GTGGGAAAGCGTGTTAGGA | GCATGTGAGGATTCAGGGA |
| HEMG1 | CTTCTTTCGCACCCAATGTC | TTCAACCCACTATCCACCAC |
| CHLG | TAATGATTGGTATGACCGAGAA | GCTTTAGAGGTGGAGCAGAGTA |
| PORB | AACATTTGCTTCCCTTTACC | CACTTTCCGAGCCTTATCTG |
| PIP1;1 | GATTGTTGGCACTTTCGTT | ATGGCTCTTATGACTATCTGGT |
| PIP1;3 | GCTGCTCAGACAGACAAAGAC | AACAAGAAGGTAGCCACGAA |
| PIP1;4 | ATACAAGAGCCAGACCGACAA | CCTTCACAAGCCCACATCC |
| PIP1;5 | GGGAACTAACTTTGTGAACC | TAGACAGCAGCAAGAGCAG |
| TIP120 | GCGAACGAACTTTGTGAACGC | TAAAGAGGAGCAAGAGCAC |
| TIP1;1 | CTTACTGATAATGGGTCAACGA | TCACAATCTCCAGAACTACCG |
| TIP2;1 | TTGCTGGTGTTGGTTCTGC | CAAGGCTGTGGATGGGAAT |
| TIP1;2 | CTCACAGACAAAGGCTCCACT | AAGAAGCAGGCAAGCAACG |
| DREB1 | AAGGGATGTATGAAAGGTAAGG | CAGGAGTTGCCACTGAAGAC |
| DREB2 | AAGGTGAGGAGGCAGACGAT | TGGCTGGTAATGTATTTGAAGG |
| DREB3 | GCATCCAGCGACTTCCAAC | GCGGCGTAACATCGTCATC |
| DREB4 | GACCTGGCAACTCCACAACG | AGCACCATCACGAAATCCTT |
| DREB5 | GAATGGCTAAACTACTCCTCC | CAACCAAACCCTTGTCCTAT |
| DREB6 | GAGCAACGAATGGCTAAACT | CAACCAAACCCTTGTCCTAT |
| DREB7 | GTAAGTGGGTTGCGGAAAT | CCCGGAGTGGTTGAATGTAG |
| DREB8 | AGGTCCAACAAGAGGCAAAG | GATAAGCATCTGGTCCATACA |
| DREB9 | CCGCAAGATTGATACTGACA | GAACCTATTTCTCCCTTTACCT |
| Fluorescence Parameters | Control | PEG | PEG + ALA | Fluorescence Parameters | Control | PEG | PEG + ALA |
|---|---|---|---|---|---|---|---|
| Fo (×103) | 7.65 ± 0.11 c | 9.09 ± 0.33 a | 8.34 ± 0.12 b | φDo | 0.16 ± 0.00 b | 0.21 ± 0.01 a | 0.18 ± 0.01 b |
| FK (×103) | 20.20 ± 0.37 c | 22.47 ± 0.35 a | 20.55 ± 0.52 b | Wk | 0.45 ± 0.01 ab | 0.49 ± 0.03 a | 0.41 ± 0.02 b |
| FJ (×103) | 30.87 ± 0.43 a | 30.32 ± 0.68 a | 30.11 ± 0.53 a | Mo | 1.25 ± 0.03 b | 1.60 ± 0.07 a | 1.28 ± 0.04 b |
| FI (×103) | 43.67 ± 0.69 a | 40.67 ± 1.26 b | 42.66 ± 0.70 ab | ETo/CSo (×102) | 27.20 ± 0.56 ab | 26.63 ± 1.17 b | 29.43 ± 0.56 a |
| Fm (×103) | 47.96 ± 0.68 a | 43.39 ± 1.38 b | 46.48 ± 0.82 a | RC/CSo (×102) | 29.83 ± 0.49 ab | 28.05 ± 0.83 b | 30.85 ± 0.73 a |
| Fv (×103) | 40.33 ± 0.62 a | 34.43 ± 1.57 b | 38.26 ± 0.77 a | PIabs | 1.85 ± 0.09 a | 0.98 ± 0.12 b | 1.58 ± 0.10 a |
| φPo | 0.84 ± 0.00 a | 0.79 ± 0.01 b | 0.82 ± 0.00 a | PItotal | 0.63 ± 0.03 a | 0.26 ± 0.03 c | 0.49 ± 0.3 b |
| φEo | 0.36 ± 0.01 a | 0.30 ± 0.01 b | 0.35 ± 0.01 a | VPSI (×10−4) | 13.5 ± 0.30 b | 10.90 ± 0.83 c | 15.60 ± 0.44 a |
| φRo | 0.09 ± 0.00 a | 0.06 ± 0.01 b | 0.08 ± 0.01 a | VPSII-PSI (×10−5) | 2.85 ± 0.24 a | 2.05 ± 0.16 c | 1.75 ± 0.27 b |
| Time (d) | Treatment | Chlorophyll a (mg g−1 FW) | Chlorophyll b (mg g−1 FW) | Total Chlorophyll (mg g−1 FW) | Chlorophyll a/b |
|---|---|---|---|---|---|
| 5 | Control | 1.23 ± 0.05 a | 0.65 ± 0.03 ab | 1.88 ± 0.08 a | 1.91 ± 0.01 a |
| PEG | 0.48 ± 0.04 de | 0.33 ± 0.03 e | 0.71 ± 0.07 de | 1.45 ± 0.06 d | |
| PEG + ALA | 0.94 ± 0.10 b | 0.53 ± 0.05 c | 1.47 ± 0.06 b | 1.74 ± 0.03 b | |
| 10 | Control | 1.36 ± 0.08 a | 0.72 ± 0.04 a | 2.08 ± 0.12 a | 1.88 ± 0.01 a |
| PEG | 0.38 ± 0.02 ef | 0.33 ± 0.01 e | 0.71 ± 0.03 ef | 1.15 ± 0.03 f | |
| PEG + ALA | 0.68 ± 0.01 c | 0.42 ± 0.01 d | 1.10 ± 0.01 c | 1.60 ± 0.00 c | |
| 15 | Control | 0.99 ± 0.05 b | 0.61 ± 0.04 bc | 1.60 ± 0.09 b | 1.64 ± 0.02 c |
| PEG | 0.26 ± 0.02 f | 0.25 ± 0.02 e | 0.51 ± 0.04 f | 1.02 ± 0.01 g | |
| PEG + ALA | 0.57 ± 0.04 cd | 0.43 ± 0.02 d | 1.00 ± 0.06 cd | 1.31 ± 0.02 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, Y.; Liu, C.; Wei, B.; Zhang, J.; An, Y.; Wang, L. Exogenous 5-Aminolevulinic Acid Promotes Osmotic Stress Tolerance of Walnuts by Modulating Photosynthesis, Osmotic Adjustment and Antioxidant Systems. Forests 2023, 14, 1789. https://doi.org/10.3390/f14091789
Zhong Y, Liu C, Wei B, Zhang J, An Y, Wang L. Exogenous 5-Aminolevulinic Acid Promotes Osmotic Stress Tolerance of Walnuts by Modulating Photosynthesis, Osmotic Adjustment and Antioxidant Systems. Forests. 2023; 14(9):1789. https://doi.org/10.3390/f14091789
Chicago/Turabian StyleZhong, Yan, Changzhou Liu, Bo Wei, Jianting Zhang, Yuyan An, and Liangju Wang. 2023. "Exogenous 5-Aminolevulinic Acid Promotes Osmotic Stress Tolerance of Walnuts by Modulating Photosynthesis, Osmotic Adjustment and Antioxidant Systems" Forests 14, no. 9: 1789. https://doi.org/10.3390/f14091789
APA StyleZhong, Y., Liu, C., Wei, B., Zhang, J., An, Y., & Wang, L. (2023). Exogenous 5-Aminolevulinic Acid Promotes Osmotic Stress Tolerance of Walnuts by Modulating Photosynthesis, Osmotic Adjustment and Antioxidant Systems. Forests, 14(9), 1789. https://doi.org/10.3390/f14091789







