Source to Sink of Lignin Phenols in a Subtropical Forest of Southwest China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Sampling
2.2. Lignin Biomarkers
2.3. Analyses of Auxiliary Variables
2.4. Statistical Analyses
3. Results
3.1. Lignin Phenol Contents
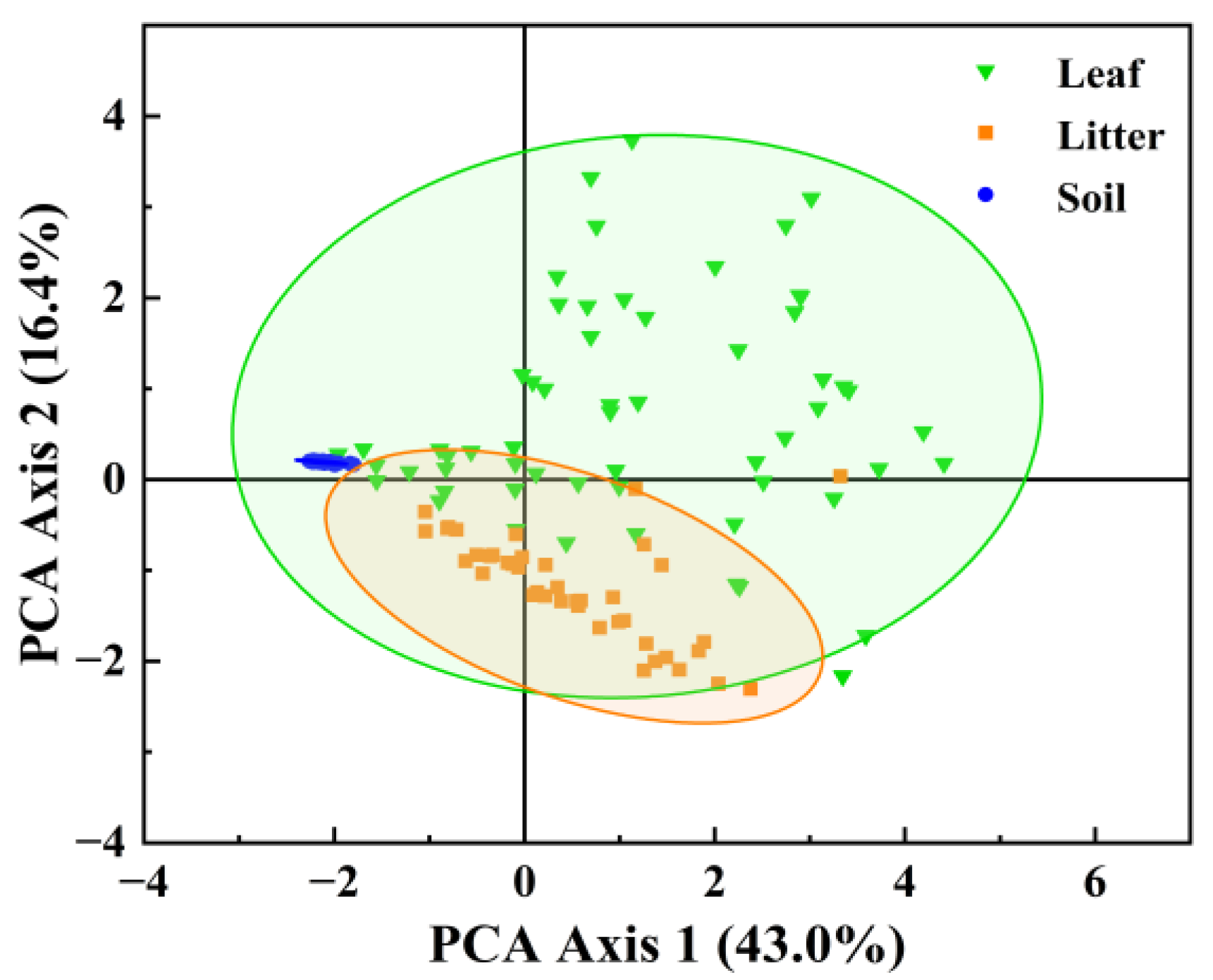
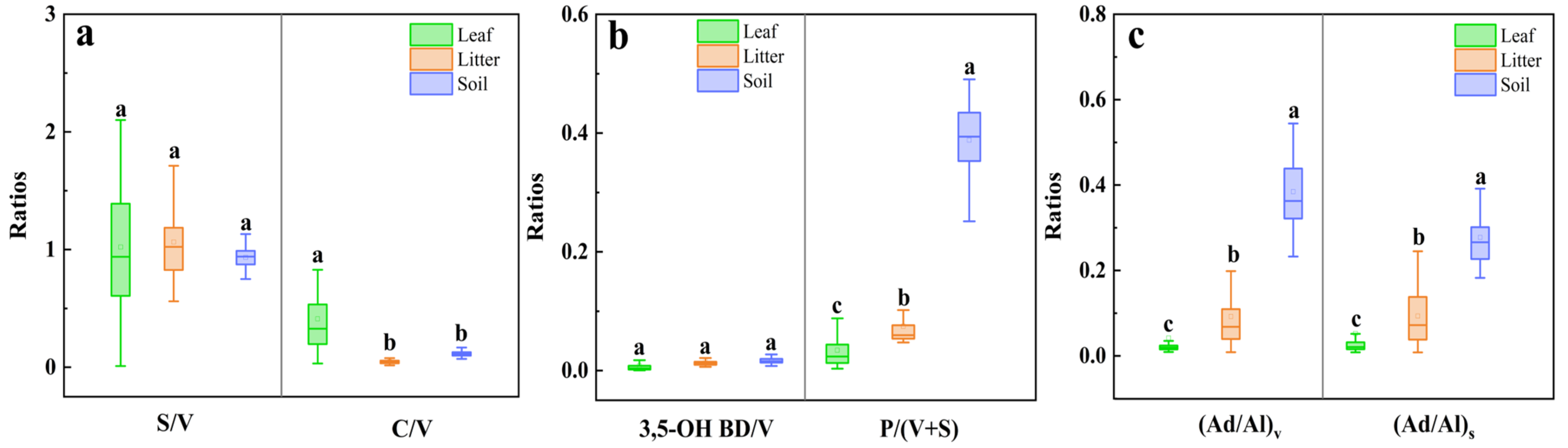
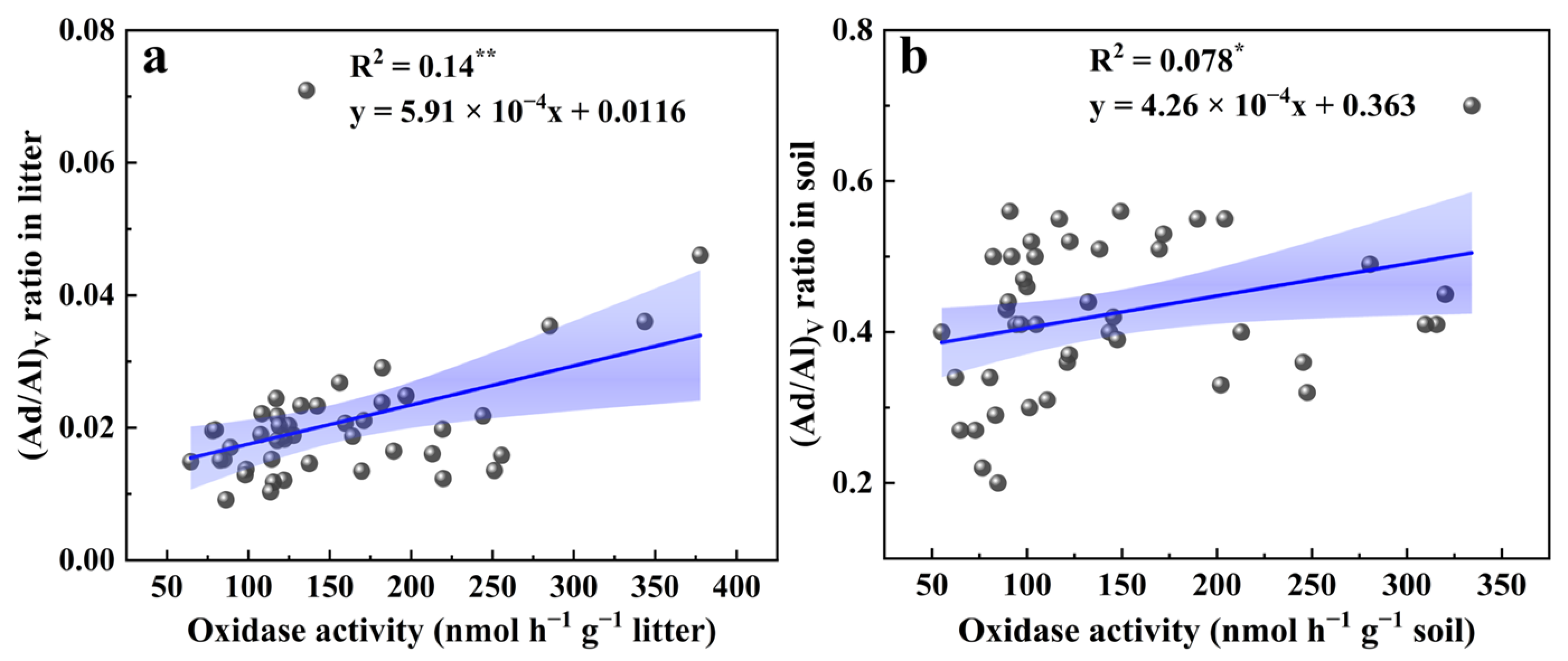
3.2. Lignin Degradation Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Walker, W.S.; Gorelik, S.R.; Cook-Patton, S.C.; Baccini, A.; Farina, M.K.; Solvik, K.K.; Ellis, P.W.; Sanderman, J.; Houghton, R.A.; Leavitt, S.M.; et al. The global potential for increased storage of carbon on land. Proc. Natl. Acad. Sci. USA 2022, 119, e2111312119. [Google Scholar] [CrossRef] [PubMed]
- Bossio, D.A.; Cook-Patton, S.C.; Ellis, P.W.; Fargione, J.; Sanderman, J.; Smith, P.; Wood, S.; Zomer, R.J.; von Unger, M.; Emmer, I.M.; et al. The role of soil carbon in natural climate solutions. Nat. Sustain. 2020, 3, 391–398. [Google Scholar] [CrossRef]
- Zhang, X.M.; Brandt, M.; Yue, Y.M.; Tong, X.W.; Wang, K.L.; Fensholt, R. The Carbon Sink Potential of Southern China After Two Decades of Afforestation. Earth’s Future 2022, 10, e2022EF002674. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Lu, N.; Fu, B.; Wang, S.; Deng, L.; Wang, L. Current and future carbon stocks of natural forests in China. For. Ecol. Manag. 2022, 511, 120137. [Google Scholar] [CrossRef]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Chang. Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef]
- Tamura, M.; Suseela, V.; Simpson, M.; Powell, B.; Tharayil, N. Plant litter chemistry alters the content and composition of organic carbon associated with soil mineral and aggregate fractions in invaded ecosystems. Glob. Chang. Biol. 2017, 23, 4002–4018. [Google Scholar] [CrossRef]
- Craig, M.E.; Geyer, K.M.; Beidler, K.V.; Brzostek, E.R.; Frey, S.D.; Stuart Grandy, A.; Liang, C.; Phillips, R.P. Fast-decaying plant litter enhances soil carbon in temperate forests but not through microbial physiological traits. Nat. Commun. 2022, 13, 1229. [Google Scholar] [CrossRef]
- Angst, G.; Mueller, K.E.; Eissenstat, D.M.; Trumbore, S.; Freeman, K.H.; Hobbie, S.E.; Chorover, J.; Oleksyn, J.; Reich, P.B.; Mueller, C.W. Soil organic carbon stability in forests: Distinct effects of tree species identity and traits. Glob. Chang. Biol. 2019, 25, 1529–1546. [Google Scholar] [CrossRef] [PubMed]
- Angst, G.; Mueller, K.E.; Nierop, K.G.J.; Simpson, M.J. Plant- or microbial-derived? A review on the molecular composition of stabilized soil organic matter. Soil Biol. Biochem. 2021, 156, 108189. [Google Scholar] [CrossRef]
- Jex, C.N.; Pate, G.H.; Blyth, A.J.; Spencer, R.G.M.; Hernes, P.J.; Khan, S.J.; Baker, A. Lignin biogeochemistry: From modern processes to Quaternary archives. Quat. Sci. Rev. 2014, 87, 46–59. [Google Scholar] [CrossRef]
- Moingt, M.; Lucotte, M.; Paquet, S. Lignin biomarkers signatures of common plants and soils of Eastern Canada. Biogeochemistry 2016, 129, 133–148. [Google Scholar] [CrossRef]
- Thevenot, M.; Dignac, M.F.; Rumpel, C. Fate of lignins in soils: A review. Soil Biol. Biochem. 2010, 42, 1200–1211. [Google Scholar] [CrossRef]
- Schmidt, M.W.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kogel-Knabner, I.; Lehmann, J.; Manning, D.A.; et al. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef]
- Feng, X.J.; Simpson, A.J.; Wilson, K.P.; Williams, D.D.; Simpson, M.J. Increased cuticular carbon sequestration and lignin oxidation in response to soil warming. Nat. Geosci. 2008, 1, 836–839. [Google Scholar] [CrossRef]
- Vanholme, R.; Demedts, B.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin biosynthesis and structure. Plant Physiol. 2010, 153, 895–905. [Google Scholar] [CrossRef]
- Feng, X.; Feakins, S.J.; Liu, Z.; Ponton, C.; Wang, R.Z.; Karkabi, E.; Galy, V.; Berelson, W.M.; Nottingham, A.T.; Meir, P.; et al. Source to sink: Evolution of lignin composition in the Madre de Dios River system with connection to the Amazon basin and offshore. J. Geophys. Res. Biogeosci. 2016, 121, 1316–1338. [Google Scholar] [CrossRef]
- Otto, A.; Simpson, M.J. Evaluation of CuO oxidation parameters for determining the source and stage of lignin degradation in soil. Biogeochemistry 2006, 80, 121–142. [Google Scholar] [CrossRef]
- He, M.; Zhao, R.; Tian, Q.; Huang, L.; Wang, X.; Liu, F. Predominant effects of litter chemistry on lignin degradation in the early stage of leaf litter decomposition. Plant Soil 2019, 442, 453–469. [Google Scholar] [CrossRef]
- Wang, X.; Tian, Q.; Li, Q.; Liao, C.; He, M.; Liu, F. Lignin characteristics in soil profiles in different plant communities in a subtropical mixed forest. J. Plant Ecol. 2018, 11, 560–568. [Google Scholar] [CrossRef]
- Brock, O.; Kooijman, A.; Nierop, K.G.J.; Muys, B.; Vancampenhout, K.; Jansen, B. Disentangling the effects of parent material and litter input chemistry on molecular soil organic matter composition in converted forests in Western Europe. Org. Geochem. 2019, 134, 66–76. [Google Scholar] [CrossRef]
- Dao, T.T.; Gentsch, N.; Mikutta, R.; Sauheitl, L.; Shibistova, O.; Wild, B.; Schnecker, J.; Barta, J.; Capek, P.; Gittel, A.; et al. Fate of carbohydrates and lignin in north-east Siberian permafrost soils. Soil Biol. Biochem. 2018, 116, 311–322. [Google Scholar] [CrossRef]
- Jiang, Z.; Lian, Y.; Qin, X. Rocky desertification in Southwest China: Impacts, causes, and restoration. Earth-Sci. Rev. 2014, 132, 1–12. [Google Scholar] [CrossRef]
- Du, H.; Hu, F.; Zeng, F.; Wang, K.; Peng, W.; Zhang, H.; Zeng, Z.; Zhang, F.; Song, T. Spatial distribution of tree species in evergreen-deciduous broadleaf karst forests in southwest China. Sci. Rep. 2017, 7, 15664. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, C.; Chen, H.; Yue, Y.; Zhang, W.; Zhang, M.; Qi, X.; Fu, Z. Karst landscapes of China: Patterns, ecosystem processes and services. Landsc. Ecol. 2019, 34, 2743–2763. [Google Scholar] [CrossRef]
- Huang, Y.; Ma, Y.; Zhao, K.; Niklaus, P.A.; Schmid, B.; He, J.-S. Positive effects of tree species diversity on litterfall quantity and quality along a secondary successional chronosequence in a subtropical forest. J. Plant Ecol. 2017, 10, 28–35. [Google Scholar] [CrossRef]
- Wang, S.; El Moujahid, L.; Le Roux, X.; Michalet, S.; Bellvert, F.; Weigelt, A.; Poly, F. Effect of plant diversity on the diversity of soil organic compounds. PLoS ONE 2017, 12, e0170494. [Google Scholar] [CrossRef]
- Liu, X.; Trogisch, S.; He, J.S.; Niklaus, P.A.; Bruelheide, H.; Tang, Z.; Erfmeier, A.; Scherer-Lorenzen, M.; Pietsch, K.A.; Yang, B.; et al. Tree species richness increases ecosystem carbon storage in subtropical forests. Proc. Biol. Sci. 2018, 285, 20181204. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. World Reference Base for Soil Resources 2014: International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports; FAO: Rome, Italy, 2014; Volume 106, pp. 12–21. [Google Scholar]
- Du, H.; Peng, W.X.; Song, T.Q.; Zeng, F.P.; Wang, K.L.; Song, M.; Zhang, H. Spatial pattern of woody plants and their environmental interpretation in the karst forest of southwest China. Plant Biosyst. 2015, 149, 121–130. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Hu, G.; Zhu, J.-D.; Luo, D.-H.; Ni, J. Spatial patterns and interspecific associations of dominant tree species in two old-growth karst forests, SW China. Ecol. Res. 2010, 25, 1151–1160. [Google Scholar] [CrossRef]
- Qian, Z.; Gu, R.; Gao, K.; Li, D. High plant species diversity enhances lignin accumulation in a subtropical forest of southwest China. Sci. Total Environ. 2023, 865, 161113. [Google Scholar] [CrossRef] [PubMed]
- Hedges, J.I.; Mann, D.C. The characterization of plant tissues by their lignin oxidation products. Geochim. Cosmochim. Acta 1979, 43, 1803–1807. [Google Scholar] [CrossRef]
- Feng, X.; Hills, K.M.; Simpson, A.J.; Whalen, J.K.; Simpson, M.J. The role of biodegradation and photo-oxidation in the transformation of terrigenous organic matter. Org. Geochem. 2011, 42, 262–274. [Google Scholar] [CrossRef]
- Datta, R.; Kelkar, A.; Baraniya, D.; Molaei, A.; Moulick, A.; Meena, R.; Formanek, P. Enzymatic Degradation of Lignin in Soil: A Review. Sustainability 2017, 9, 1163. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L. Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil Biol. Biochem. 2010, 42, 391–404. [Google Scholar] [CrossRef]
- Dittmar, T.; Lara, R.J. Molecular evidence for lignin degradation in sulfate-reducing mangrove sediments (Amazônia, Brazil). Geochim. Cosmochim. Acta 2001, 65, 1417–1428. [Google Scholar] [CrossRef]
- Dickens, A.F.; Gudeman, J.A.; Gélinas, Y.; Baldock, J.A.; Tinner, W.; Hu, F.S.; Hedges, J.I. Sources and distribution of CuO-derived benzene carboxylic acids in soils and sediments. Org. Geochem. 2007, 38, 1256–1276. [Google Scholar] [CrossRef]
- Hernes, P.J.; Kaiser, K.; Dyda, R.Y.; Cerli, C. Molecular Trickery in Soil Organic Matter: Hidden Lignin. Environ. Sci. Technol. 2013, 47, 9077–9085. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, Z.; Fan, Z.; Peng, W.; Du, H.; Hu, P. Source to Sink of Lignin Phenols in a Subtropical Forest of Southwest China. Forests 2023, 14, 1701. https://doi.org/10.3390/f14091701
Qian Z, Fan Z, Peng W, Du H, Hu P. Source to Sink of Lignin Phenols in a Subtropical Forest of Southwest China. Forests. 2023; 14(9):1701. https://doi.org/10.3390/f14091701
Chicago/Turabian StyleQian, Zongyao, Zi Fan, Wanxia Peng, Hu Du, and Peilei Hu. 2023. "Source to Sink of Lignin Phenols in a Subtropical Forest of Southwest China" Forests 14, no. 9: 1701. https://doi.org/10.3390/f14091701
APA StyleQian, Z., Fan, Z., Peng, W., Du, H., & Hu, P. (2023). Source to Sink of Lignin Phenols in a Subtropical Forest of Southwest China. Forests, 14(9), 1701. https://doi.org/10.3390/f14091701







