Abstract
The mycorrhizal type affects the structure and functions of tree roots. Therefore, the mechanical traits of the roots of tree species with different types of mycorrhizal fungi may be linked to different root functional traits. Fine roots, in particular, are closely related to the root structure and are also important for slope protection. However, the relationship among the mycorrhizal types of trees, root mechanical traits, and root structure remains unclear. This study aims to investigate the link between fine root tensile strength (Tr) and root morphological and/or structural traits in temperate trees with different mycorrhizal types. We investigated the seedlings of 15 dominant tree species in the cool temperate forests of northern Japan. For each species, fine root Tr and other five common root morphological and structural traits were measured. There was a significant positive correlation between total fine root biomass and fine root Tr consistently, even in the analysis of mycorrhizal types (arbuscular mycorrhizal (AM), and ectomycorrhizae (EM)). Our findings indicate that the root structural trait is an important driver of fine root Tr, especially for AM and EM species, and suggest that including a plant-mycorrhizal framework in future work offers great potential to improve our understanding of forest restoration.
1. Introduction
Trees significantly contribute to the prevention of shallow landslides by providing root reinforcement for the surface soil [1]. In shallow soils, tree roots penetrate into the soil and anchor the soil into a more stable substrate. Mechanical traits, such as the root tensile force, contribute to the reinforcement and erosion resistance of the root–soil system [2]. Specifically, a higher tensile force allows the roots to fully exert their strength during pull-out and increase soil shear strength. Thus, root tensile strength (Tr) has been studied as an important parameter to evaluate the effect of vegetation slope protection [3,4,5,6,7]. On the other hand, it is laborious to measure the root Tr [8], and large variations in the root Tr exist among tree species [9]. Therefore, for the proper and convenient usage of tree species with a higher root Tr, it is useful to determine powerful predictors of root Tr across a wide range of tree species.
Root morphological traits are known as some of the main predictors of root Tr [2,10]. In particular, root diameter is well known to be an important predictor of root Tr. Root Tr generally decreases with a larger root diameter following a power-law equation [3,11,12]. However, Ghestem et al. [6] showed that the fine root Tr varied by more than an order of magnitude among nine tree species but was not well explained by the variation in fine root diameter. Root gauge length [13] and root topology [14] are also good predictors of fine root Tr. However, these studies have tested the relationship between functional root traits, such as root diameter, and fine root Tr at most for nine species of trees (e.g., [6]). In other words, to our knowledge, previous studies have not identified a link between root morphological and/or structural traits and fine root Tr across a wider range of tree species. Traditionally, coarse roots are considered to have an anchoring function [15], and fine roots are linked to resource acquisition. However, in recent years, the importance of fine roots in maintaining hillside stability via their mechanical force has received increasing attention [1,14,16]. Therefore, to understand the functional plant species that stabilize the hillside slope, for instance, it is important to determine the useful predictors of fine root Tr across a wide range of tree species.
Increasing evidence suggests that simple relationships between morphological and structural traits may need to include the symbiotic relationship between roots and mycorrhizal fungi [17,18,19]. The majority of tree species form symbiotic relationships either with ectomycorrhizae (EM) or arbuscular mycorrhizae (AM), while some species develop symbiotic relationships with both fungal groups (AEM) [20]. The mycorrhizal relationship enhances the acquisition of water and nutrients for the plants by changing the morphology and structure of the roots, and the changes differ in different ways among mycorrhizal types [21,22]. For example, for species with fine roots, AM trees mainly rely on producing more roots to absorb nutrients, whereas EM trees rely on producing more mycorrhizal fungal hyphae [18]. The differences in the nutrient acquisition by mycorrhizal types may predispose AM and EM trees to favor thinner and thicker roots, respectively, leading to large differences in root traits between the two types [19], which had the potential to relate closely with fine root Tr. On the other hand, Chen et al. [23] and Zhang et al. [16] found that inoculation with AM fungi could generally enhance root Tr. The influence of mycorrhizal fungi on root Tr should not be neglected, because of the fundamental (morphological, physiological, biochemical) change in host plants introduced by mycorrhizal fungi [24]. However, to the best of our knowledge, there are few studies that systematically searched for differences in fine root Tr between temperate mycorrhizal fungi-forming trees. Furthermore, an explicit link is lacking between root morphological and/or structural traits and fine root Tr among the contrasting mycorrhizal types. Explaining fine root Tr in terms of mycorrhizal types is useful to understand the species with larger fine root Tr based on the already-existing information of the mycorrhizal type of tree species. The understanding of the relationship between fine root Tr and mycorrhizal types is also useful to select the tree species with larger fine root Tr which is powerful for forest restoration after landslides without labor-intensive measurement of fine root Tr.
To answer these questions, we investigated the relationship among the fine root Tr, mycorrhizal types, and root morphological and structural traits for a total of 15 woody species with different mycorrhizal types in cool temperate forests. Specifically, our objectives were to (1) investigate what predictive relationships exist between root functional traits and Tr at the large species scale; and (2) examine whether the predictive relationships were consistent across mycorrhizal types. The expected results would provide important insights for choosing plant species with high fine root Tr in restoration projects, which is useful for resisting soil erosion in forests.
2. Materials and Methods
2.1. Species Selection and Experimental Design
Fifteen species that are dominant in the cool temperate forests in northern Japan (Hokkaido region) were selected for this experiment (Table 1). The 15 species were classified into three mycorrhizal groups following Makoto et al. [25]: the EM group (Abies sachalinensis Mast., Picea glehnii Mast., Quercus crispula Blume., and Tilia japonica Simonk.), the AM group (Aralia elata Seem., Cercidiphyllum japonicum Sieb. Et Zucc., Fraxinus mandshurica var. Japonica Maxim., Lespedeza bicolor Turcz., Magnolia obovata Thunb., and Ulmus davidiana var. japonica Nakai.), and the AEM group, which include the species that develop a symbiotic relationship with both AM and EM fungi (Alnus hirsuta Turcz., Cerasus sargentii Rehder., Morus australis Poiret., Salix caprea Linn., Sorbus commixta Hedl.). Although the AEM group is less investigated than the other two groups, many of the dominant tree species in our region are categorized into this group, and we should understand their ecosystem function. The selected species are those that (1) are observed in the disturbed soil in the forests of the Hokkaido region, (2) are used by land managers for erosion restoration, and (3) develop different types of mycorrhizae.

Table 1.
Ecological characteristics of the 15 species studied.
One-year-old seedlings were purchased from a seedling company in Hokkaido (Yukijirushi Shubyo Kabushiki Gaisha, Tokyo, Japan). We prepared 6 individuals for each species and planted in the nursery of the Nayoro Research Office of Hokkaido University, located in Nayoro, northern Hokkaido, northern Japan (44°33′N, 142°45′E) (Figure 1). A total of 90 individuals were planted at the beginning of the growing season in 2021, manually managed to ensure survival, and excavated by mid-August 2021 for further analysis, as that is the later part of the most active growing season for woody plants in Hokkaido [25] and the functional traits of the roots can be considered mature at that time. In this area, the average temperature from June to August was 18.3 °C, with a maximum temperature of 36.4 °C in July between 2015 and 2021. The average precipitation was 112.3 mm during the same period. In general, the soil of this area is heavy clay and the parent material is unconsolidated sedimentary rock, and the soil chemical properties at the surface soil are as follows: pH 5.4, total nitrogen content 2.5 g/kg, available potassium contents 0.138 g/kg, available calcium contents 1.97 g/kg, and available magnesium contents 0.373 g/kg [26].
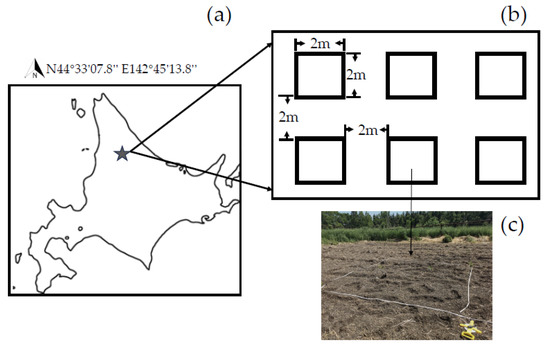
Figure 1.
Experimental nursery and plots used in this study. Note: (a): Locations of nursery in Nayoro, northern Hokkaido; (b): Layout map of 6 plots of 18 m × 12 m nursery; (c): 15 species were randomly planted in each plot.
2.2. Root Traits
Five of the major morphological and structural traits of roots were measured (root morphological traits: average fine root diameter (AD), root tissue density (RTD), specific root length (SRL), and maximum root depth (D); root structural traits: total fine root biomass) [27]. For the trait measurements, each seedling was carefully excavated with a shovel to avoid damage to the roots. First, D (cm), as the deepest soil depth reached by the roots of an individual seedling, was measured on-site with a ruler during the excavation. After the excavation, individual seedlings were grouped into batches and washed gently with water to remove soil particles from the root system. After washing, we separated the aboveground and underground parts of the plant and removed the excess water on the surface of the root system with a rag. Fine roots have been defined as roots ≤ 2 mm in diameter [28]. We separated all the fine roots of the seedlings. Three samples of fine roots were collected randomly from the root system of each individual in order to have representative samples of fine roots in the system. Fine root samples were scanned with a double-lamp bed scanner (GT-X970, Epson, Suwa City, Japan). By using the scanned image, the AD (mm), length (cm), and volume (cm3) of the fine root samples were determined using WinRHIZO Reg (version 2009a, Regent Instrument, Sainte Foy, QC, Canada). Then, all the fine roots of an individual seedling were dried at 70 °C for 48 h and weighed to calculate the total fine root biomass (g). The RTD of fine roots (g/cm3) of an individual seedling was calculated from the ratio of dry mass to root volume of the fine root samples, and SRL (cm/g) was calculated from the ratio of root length to dry mass [27].
2.3. Root Tensile Strength
The water content of fine roots strongly affects the root Tr [29]. To counteract this water content-dependent change in Tr (which we do not intend to focus on in our study), representative fine root pieces were put into a 15% alcohol solution in order to prevent root decomposition and refrigerated at 4 °C before measuring [30,31], and 6 pieces of fine roots for each seedling were stored. The root Tr was measured by a tearing and punching test machine (Kanagawa Giken, Odawara, Kanagawa, Japan) connected to a force gauge (DS2-50N, Imada, Toyohashi, Aichi, Japan). The precision of the force gauge was 0.01 kN. To ensure that the test roots did not slip, we stuck sandpaper in the clips [32]. A tensile force was applied to the root samples at a loading rate of 50 mm/min, and the maximum value of the digital display was recorded, which was the maximum external force required to break the root samples. Following Ji et al. [33], only samples that fractured in the middle third of the root length between the clamps were considered successful, which could ensure the root fracture was due to tensile force and not induced by the stress concentration proximal to the clips, and data from the successful tests were used for the analysis. Finally, there were 82 fine roots Tr data for the AM species group, 41 for the EM species group, and 71 for the AEM species group. After the tensile test, the root diameter was measured at the fracture, and the measurement was taken as the average between the diameter of the two separated parts with a digital Vernier caliper with a 0.01 mm accuracy. The diameter of each part was the mean of at least two measurements of height and width of each part to avoid the case that the root is not perfectly circular. The over-bark diameter of the tested root pieces varied between 0.1 and 1.18 mm, and the length of each piece was at least 15 times its diameter. The Tr at the break was calculated as the maximum force required to cause root failure divided by the root cross-sectional area (CSA) at the point of failure [5].
2.4. Statistical Analysis
Trait differences between species and mycorrhizal types were investigated with ANOVA and Duncan tests. A Kolmogorov–Smirnov test was used to test the normality of all the original data before proceeding with ANOVA. When the data did not follow a normal distribution, the data were log-transformed before analysis to make the data meet the analysis requirements. Statistical significance was recognized when p < 0.05. To check the relationship between the fine root Tr and other root traits, we employed a generalized linear mixed model (GLMM). Before performing the model, all data were standardized and variance inflation factors (VIFs) were calculated to detect collinearity among predictor variables. Root functional traits were set as the fixed factors, and species identity and the location of the plots (where seedlings were planted) were set as the random factors. All analyses were carried out with SPSS (version 27 for Windows), and figure preparation was carried out using Origin 9.0 software (OriginLab Corporation, Northampton, MA, USA).
3. Results
3.1. Fine Root Tr and 5 Root Traits
There was a significant difference in the fine root Tr among mycorrhizal types (F = 7.088, p < 0.01, ANOVA) (Figure 2 and Table 2). The fine root Tr of the EM species group was significantly different from that of the AM and AEM species groups (Figure 2 and Table 2). Across the 3 species groups, the mean fine root Tr ranged from a minimum of 12.88 MPa in the EM species group to a maximum of 32.32 MPa in the AEM species group (Figure 2, Supplementary Table S1). The fine root Tr differed significantly among species (F = 11.77, p < 0.01, ANOVA) (Table 2); M. australis showed the highest fine root Tr, while M. obovata showed the lowest value (Supplementary Figure S1). All measured root traits except for D and SRL were significantly different among species and were significantly different among mycorrhizal types (Table 2).
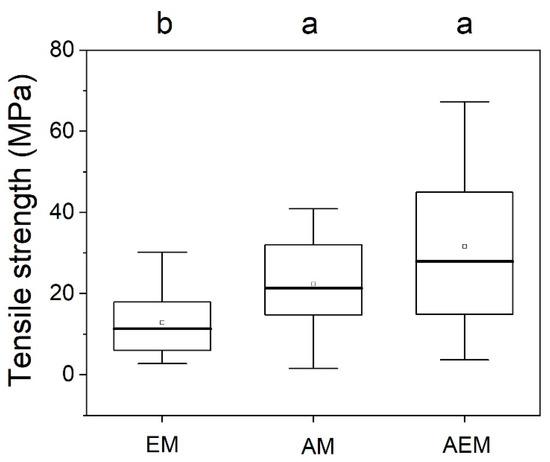
Figure 2.
Fine root Tr of different mycorrhizal types (EM: ectomycorrhizae, AM: arbuscular mycorrhizae, AEM: dual mycorrhizae). Different lowercase letters indicate significant differences among mycorrhizal types (p < 0.05) according to Duncan tests.

Table 2.
ANOVA results for root functional traits of the 15 studied species. Significance levels: ns nonsignificant, * p < 0.05, ** p < 0.01, *** p < 0.001.
3.2. Relationships between Fine Root Tr and 5 Root Traits
In the GLMM analysis for all 15 species, there were no multicollinearity issues among the explanatory variables, and the total fine root biomass showed a significant positive correlation with fine root Tr (Figure 3, p = 0.013). The fine root Tr of the mycorrhizal groups showed a significant relationship with the root structural trait. There was no root trait was excluded from the model due to multicollinearity issues, and the fine root Tr of AM and EM species both showed a significant positive correlation with total fine root biomass (Figure 4, p = 0.029; Figure 5, p = 0.016). For the AEM species, there was no significant relationship between the fine root Tr and root traits.
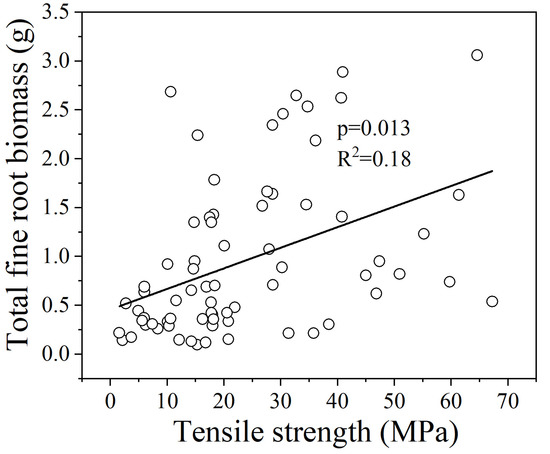
Figure 3.
Relationships between the fine root Tr and total fine root biomass of 15 species. The statistical significance for the relationship between the two parameters is shown as the p value based on the GLMM analysis.
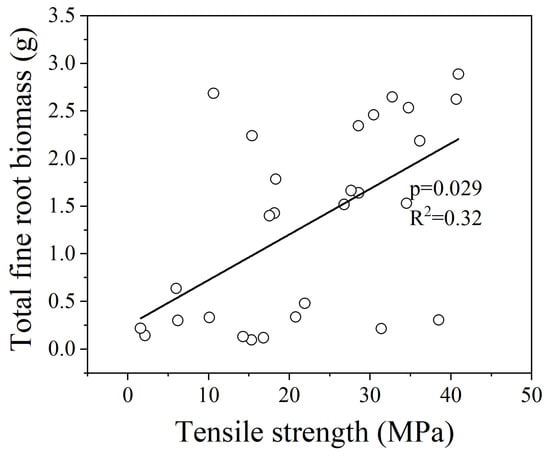
Figure 4.
Relationships between the fine root Tr and total fine root biomass of AM species. The statistical significance for the relationship between the two parameters is shown as the p value based on the GLMM analysis.
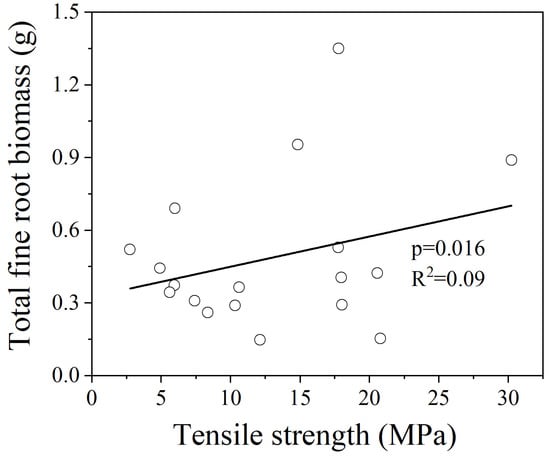
Figure 5.
Relationships between the fine root Tr and total fine root biomass of EM species. The statistical significance for the relationship between the two parameters is shown as the p value based on the GLMM analysis.
4. Discussion
4.1. Determinants among Mycorrhizal Groups
Previous studies suggested a predictive relationship between Tr and root diameter [3,11,12]. We found that total fine root biomass can also be used as a strong predictor of fine root Tr (Figure 3, Figure 4 and Figure 5). Although the mycorrhizal type is known to be linked to the differences in fine root functional traits (e.g., root nitrogen content and root tissue density [34]), to the best of our knowledge, this is the first study that demonstrates the difference in fine root Tr among tree species with different types of mycorrhizal symbiosis.
A smaller diameter has been reported to cause a stronger fine root Tr in many studies [5,10,13]. In our study, when we performed GLMM analysis of average fine root diameter and fine root Tr, although the average root diameter significantly affected the fine root Tr of 15 species (Supplementary Figure S2a, p = 0.015), such an effect did not appear in the three mycorrhizal types (Supplementary Figure S2b–d). This suggests that, at finer taxonomic scales, fine root diameter does not always have a general effect on fine root Tr. This result is consistent with those of several previous studies [6,14], which reported that the diameter alone was insufficient to explain the variation in fine root Tr, although the determinant factor was not well discussed. The total fine root biomass might be an important alternative predictive factor for fine root Tr (Figure 3, Figure 4 and Figure 5, Supplementary Table S1). Fine roots play a vital role in energy and material flow in plants [28], and their growth is an important strategy for nutrient acquisition in trees [35]. It has been suggested that the nitrogen concentration of fine root tissue is higher in soil patches where nutrient availability is greater [36]. A certain increase in nutrients, especially nitrogen, can lead to a higher cellulose content in fine roots [37], which results in a higher Tr. Therefore, we propose that individuals with high total fine root biomass have large fine root Tr because of their high nutrient availability.
4.2. Variation among Mycorrhizal Groups
We also found that the difference in fine root Tr existed not only between mycorrhizal types but also within mycorrhizal types. The plasticity in fine root Tr within each mycorrhizal group was greater than 50% (Supplementary Table S2). For this within-mycorrhizal group variation, fine root biomass was an important predictor of the variation in fine root Tr of 15 species, AM and EM mycorrhizal types (Figure 3, Figure 4 and Figure 5). Unlike the results of many previous studies (e.g., [6]), it was interesting to see that, rather than the morphological traits, structural traits were important predictors of the variation in fine root Tr within each group. As previously described, fine roots are the main organ that plants use to uptake nutrients from the soil [28], and a larger fine root biomass might represent the amount of nutrients that a plant can uptake, potentially resulting in a higher Tr. About AEM species, it is difficult to further discuss the underlying mechanism affecting the fine root Tr of this mycorrhizal group because trait-based functional studies are scarce, and even the definition of AEM species is debated [38].
To our surprise, the fine root Tr of the AM species group was significantly higher than that of the EM species group in our study (Figure 2, Supplementary Table S1). On the other hand, we also found that the total fine root biomass of AM species group was significantly higher than that of the EM species group (Supplementary Table S1). This also supports our previous finding that the structural trait is an important determinant of fine root Tr. Furthermore, the difference in lignin and cellulose contents could be possible parameters driving the differential fine root Tr among mycorrhizal groups. The colonization of AM fungi on fine roots increases the proportions of cellulose [23], and cellulose contributes to the increase in root Tr [5]. However, this issue is beyond the scope of this study, and further investigation of the difference in lignin and cellulose content in fine roots among mycorrhizal types could be helpful to determine the underlying mechanisms of fine root Tr divergence among mycorrhizal types in detail.
5. Conclusions
The importance of mycorrhizal types in influencing plant growth performance and ecosystem processes, for example, root respiration [34] and soil organic matter [39], has received much attention. However, it is unclear whether mycorrhizal types could also affect root trait–Tr relationships. In this study, we sought to assess morphological and structural traits on a more precise scale to better link them with the fine root Tr of 3 mycorrhizal types. Total fine root biomass had a significantly positive correlation with the fine root Tr of tree species and was a good predictor even across different mycorrhizal types. Compared with morphological traits, the root structural trait could better predict the fine root Tr of the mycorrhizal types in this study. Through detailed analysis of root samples, characteristics in predictors of fine root Tr among mycorrhizal types were detected that would have been overlooked in the more rapid analysis of bulked samples.
It is well known that most plants and nearly all species used for eco-engineering measures have a symbiotic relationship with mycorrhizal fungi [40]. Previous studies have shown that mycorrhizal fungi can improve a plant’s ability to overcome periods controlled by strong (growth) limiting factors [24], promote plant growth and survival, and significantly increase the root network of fungal host plants [41,42], thus indirectly increasing soil stability. On the other hand, fungi, through their filamentous growth forms and giant mycelial networks, entangle loose soil particles and solidify them by producing “sticky” metabolites such as polysaccharides and hydrophobic compounds, thereby achieving aggregation stability [43,44,45]. Furthermore, the use of mycorrhizal fungi and the symbiosis of certain root species lead to an increase in the root Tr, which enhances mechanical reinforcement [16,23]. Much of the existing research and experience tends to emphasize the role of mycorrhizal fungal communities in restoring degraded ecosystems [46,47,48]. The findings of the present study provide the evidence for convenient selection of temperate trees, especially at the seedling stage, with larger fine root Tr which is useful to be planted for the restoration after landslides; namely, AM and AEM trees. Future work should be conducted to explore functional consequences of differential mycorrhizal type and their slope protection with special reference to fine root Tr.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f14081542/s1, Figure S1. Fine root Tr (MPa) for 15 cool-temperate woody species in Hokkaido. Values are means ± SEM. Different letters show statistically significant differences among the species (Duncan tests, P < 0.05). Figure S2. Relationships between fine root Tr and average fine root diameter of 15 species (a), AM species (b), EM species (c), and AEM species (d). The statistical significance for the relationship between the two parameters is shown as the p value based on the GLMM analysis. Table S1. The values for functional traits of the 15 species studied. Values are mean. When letters differ, differences are significant between groups using a Duncan test (p < 0.05). Table S2. Summary of the traits of 15 cool-temperate woody species in Hokkaido.
Author Contributions
Conceptualization, K.M.; Investigation, R.Z.; Methodology, R.Z.; Data curation, R.Z.; Visualization, R.Z.; Writing—original draft, R.Z.; Writing—review & editing, K.M.; Funding acquisition, K.M.; Supervision, K.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by JSPS grant in aid basic study B (19H02986 to M.K.).
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We thank Toshiya Yoshida for the selection of research species, and Tomoya Kawakami and Haruka Kobayashi for help in maintaining the seedlings in the nursery. We sincerely thank Akira. S. Mori for the usage of the device to measure tensile strength of the plants.
Conflicts of Interest
The authors declare no conflict of interest relevant to the content of this manuscript.
References
- Stokes, A.; Atger, C.; Bengough, A.G.; Fourcaud, T.; Sidle, R.C. Desirable plant root traits for protecting natural and engineered slopes against landslides. Plant Soil 2009, 324, 1–30. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, H.; He, B.H.; Yan, H.; Liu, X.H.; Qiang, J.J. Root tensile strength of terrace hedgerow plants in the karst trough valleys of SW China: Relation with root morphology and fiber content. Int. Soil. Water Consev. 2022, 10, 677–686. [Google Scholar] [CrossRef]
- Abdi, E.; Majnounian, B.; Rahimi, H.; Zobeiri, M. Distribution and tensile strength of Hornbeam (Carpinus betulus) roots growing on slopes of Caspian Forests, Iran. J. For. Res. 2009, 20, 105–110. [Google Scholar] [CrossRef]
- De Baets, S.; Poesen, J.; Reubens, B.; Wemans, K.; De Baerdemaeker, J.; Muys, B. Root tensile strength and root distribution of typical Mediterranean plant species and their contribution to soil shear strength. Plant Soil 2008, 305, 207–226. [Google Scholar] [CrossRef]
- Genet, M.; Stokes, A.; Salin, F.; Mickovski, S.B.; Fourcaud, T.; Dumail, J.F.; Beek, R.V. The influence of cellulose content on tensile strength in tree roots. Plant Soil 2005, 278, 1–9. [Google Scholar] [CrossRef]
- Ghestem, M.; Cao, K.F.; Ma, W.Z.; Rowe, N.; Leclerc, R.; Gadenne, C.; Stokes, A. A framework for identifying plant species to be used as ‘ecological engineers’ for fixing soil on unstable slopes. PLoS ONE 2014, 9, e95876. [Google Scholar] [CrossRef]
- Mahannopkul, K.; Jotisankasa, A. Influence of root suction on tensile strength of Chrysopogon zizanioides roots and its implication on bioslope stabilization. J. Mt. Sci. 2019, 16, 275–284. [Google Scholar] [CrossRef]
- Giadrossich, F.; Schwarz, M.; Cohen, D.; Cislaghi, A.; Vergani, C.; Hubble, T.; Phillips, C.; Stokes, A. Methods to measure the mechanical behaviour of tree roots: A review. Ecol. Eng. 2017, 109, 256–271. [Google Scholar] [CrossRef]
- Bischetti, G.B.; Chiaradia, E.A.; Simonato, T.; Speziali, B.; Vitali, B.; Vullo, P.; Zocco, A. Root strength and root area of forest species in Lombardy (Northern Italy). Plant Soil 2005, 278, 11–22. [Google Scholar] [CrossRef]
- Zhang, C.B.; Chen, L.H.; Jiang, J. Why fine tree roots are stronger than thicker roots: The role of cellulose and lignin in relation to slope stability. Geomorphology 2014, 206, 196–202. [Google Scholar] [CrossRef]
- Burylo, M.; Rey, F.; Mathys, N.; Dutoit, T. Plant root traits affecting the resistance of soils to concentrated flow erosion. Earth Surf. Process Landf. 2012, 37, 1463–1470. [Google Scholar] [CrossRef]
- Lee, J.T.; Chu, M.Y.; Lin, Y.S.; Kung, K.N.; Lin, W.C.; Lee, M.J. Root Traits and Biomechanical Properties of Three Tropical Pioneer Tree Species for Forest Restoration in Landslide Areas. Forests 2020, 11, 179. [Google Scholar] [CrossRef]
- Zhang, C.B.; Chen, L.H.; Jiang, J.; Zhou, S. Effects of gauge length and strain rate on the tensile strength of tree roots. Trees 2012, 26, 1577–1584. [Google Scholar] [CrossRef]
- Mao, Z.; Wang, Y.; McCormack, M.L.; Rowe, N.; Deng, X.B.; Yang, X.D.; Xia, S.E.; Nespoulous, J.; Sidle, R.C.; Guo, D.L.; et al. Mechanical traits of fine roots as a function of topology and anatomy. Ann. Bot. 2018, 122, 1103–1116. [Google Scholar] [CrossRef]
- Pregitzer, K.S. Fine roots of trees-a new perspective. New Phytol. 2002, 154, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Q.; Liu, Z.K.; Chen, H.; Tang, M. Symbiosis of arbuscular mycorrhizal fungi and Robinia pseudoacacia L. improves root tensile strength and soil aggregate stability. PLoS ONE 2016, 11, e0153378. [Google Scholar] [CrossRef] [PubMed]
- Kubisch, P.; Hertel, D.; Leuschner, C. Do ectomycorrhizal and arbuscular mycorrhizal temperate tree species systematically differ in root order-related fine root morphology and biomass? Front. Plant Sci. 2015, 6, 64. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.L.; Koide, R.T.; Adams, T.S.; De Forest, J.L.; Cheng, L.; Eissenstat, D.M. Root morphology and mycorrhizal symbioses together shape nutrient foraging strategies of temperate trees. Proc. Natl. Acad. Sci. USA 2016, 113, 8741–8746. [Google Scholar] [CrossRef]
- Ma, Z.Q.; Guo, D.L.; Xu, X.L.; Bardgett, R.D.; Eissenstat, D.M.; McCormack, M.L.; Hedin, L.O. Evolutionary history resolves global organization of root functional traits. Nature 2018, 555, 94–97. [Google Scholar] [CrossRef]
- Karst, J.; Franklin, J.; Simeon, A.; Light, A.; Bennett, J.A.; Erbilgin, N. Assessing the dual-mycorrhizal status of a widespread tree species as a model for studies on stand biogeochemistry. Mycorrhiza 2021, 31, 313–324. [Google Scholar] [CrossRef]
- Cheng, L.; Chen, W.L.; Adams, T.S.; Wei, X.; Li, L.; McCormack, M.L.; DeForest, J.L.; Koide, R.T.; Eissenstat, D.M. Mycorrhizal fungi and roots are complementary in foraging within nutrient patches. Ecology 2016, 97, 2815–2823. [Google Scholar] [CrossRef] [PubMed]
- Püschel, D.; Bitterlich, M.; Rydlová, J.; Jansa, J. Facilitation of plant water uptake by an arbuscular mycorrhizal fungus: A Gordian knot of roots and hyphae. Mycorrhiza 2020, 30, 299–313. [Google Scholar] [CrossRef]
- Chen, X.W.; Kang, Y.; So, P.S.; Ng, C.W.W.; Wong, M.H. Arbuscular mycorrhizal fungi increase the proportion of cellulose and hemicellulose in the root stele of vetiver grass. Plant Soil 2018, 425, 309–319. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: London, UK, 2008; ISBN 978-0-12-370526-6. [Google Scholar]
- Makoto, K.; Wilson, S.D.; Sato, T.; Blume-Werry, G.; Cornelissen, J.H.C. Synchronous and asynchronous root and shoot phenology in temperate woody seedlings. Oikos 2020, 129, 643–650. [Google Scholar] [CrossRef]
- Nakamura, Y.; Fujikawa, T.; Fujita, M. Long-term changes in the soil properties and the soil macrofauna and mesofauna of an agricultural field in northern Japan during transition from chemical-intensive farming to nature farming. J. Crop Prod. 2001, 3, 63–75. [Google Scholar] [CrossRef]
- Freschet, G.T.; Pagès, L.; Iversen, C.M.; Comas, L.H.; Rewald, B.; Roumet, C.; Klimešová, J.; Zadworny, M.; Poorter, H.; Postma, J.A.; et al. A starting guide to root ecology: Strengthening ecological concepts and standardising root classification, sampling, processing and trait measurements. New Phytol. 2021, 232, 973–1122. [Google Scholar] [CrossRef] [PubMed]
- McCormack, M.L.; Dickie, I.A.; Eissenstat, D.M.; Fahey, T.J.; Fernandez, C.W.; Guo, D.L.; Helmisaari, H.S.; Hobbie, E.A.; Iversen, C.M.; Jackson, R.B.; et al. Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytol. 2015, 207, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Hales, T.C.; Cole-Hawthorne, C.; Lovell, L.; Evans, S. Assessing the accuracy of simple field based root strength measurements. Plant Soil 2013, 372, 553–565. [Google Scholar] [CrossRef]
- Böhm, W. Methods of Studying Root Systems; Durham, V.D.B., Athens, F.G., Wtirzburg, O.L.L., Oak Ridge, J.S.O., Eds.; Springer: Berlin/Heidelberg, Germany, 1979; ISBN 978-3-642-67284-2. [Google Scholar]
- Mattia, C.; Bischetti, G.B.; Gentile, F. Biotechnical characteristics of root systems of typical Mediterranean species. Plant Soil 2005, 278, 23–32. [Google Scholar] [CrossRef]
- Docker, B.B.; Hubble, T.C.T. Quantifying root-reinforcement of river bank soils by four Australian tree species. Geomorphology 2008, 100, 401–418. [Google Scholar] [CrossRef]
- Ji, J.N.; Kokutse, N.; Genet, M.; Fourcaud, T.; Zhang, Z.Q. Effect of spatial variation of tree root characteristics on slope stability. A case study on Black Locust (Robinia pseudoacacia) and Arborvitae (Platycladus orientalis) stands on the Loess Plateau, China. Catena 2012, 92, 139–154. [Google Scholar] [CrossRef]
- Gao, J.; Zhou, M.Y.; Shao, J.J.; Zhou, G.Y.; Liu, R.Q.; Zhou, L.G.; Liu, H.T.; He, Y.H.; Chen, Y.; Zhou, X.H.; et al. Fine root trait-function relationships affected by mycorrhizal type and climate. Geoderma 2021, 394, 115011. [Google Scholar] [CrossRef]
- Ma, X.M.; Zhu, B.; Nie, Y.X.; Liu, Y.; Kuzyakov, Y. Root and mycorrhizal strategies for nutrient acquisition in forests under nitrogen deposition: A meta-analysis. Soil. Biol. Biochem. 2021, 163, 108418. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Z.C.; Ma, X.H.; Jin, G.Q. Foraging ability and growth performance of four subtropical tree species in response to heterogeneous nutrient environments. J. For. Res. 2010, 15, 91–98. [Google Scholar] [CrossRef]
- Zhao, J.J.; Gong, L. Response of fine root carbohydrate content to soil nitrogen addition and its relationship with soil factors in a Schrenk (Picea schrenkiana) forest. J. Plant Growth Regul. 2021, 40, 1210–1221. [Google Scholar] [CrossRef]
- Teste, F.P.; Jones, M.D.; Dickie, I.A. Dual-mycorrhizal plants: Their ecology and relevance. New Phytol. 2020, 225, 1835–1851. [Google Scholar] [CrossRef] [PubMed]
- Craig, M.E.; Turner, B.L.; Liang, C.; Clay, K.; Johnson, D.J.; Phillips, R.P. Tree mycorrhizal type predicts within-site variability in the storage and distribution of soil organic matter. Glob. Chang. Biol. 2018, 24, 3317–3330. [Google Scholar] [CrossRef]
- Graf, F.; Frei, M. Soil aggregate stability related to soil density, root length, and mycorrhiza using site-specific Alnus incana and Melanogaster variegatus s.l. Ecol. Eng. 2013, 57, 314–323. [Google Scholar] [CrossRef]
- Bast, A.; Wilcke, W.; Graf, F.; Lüscher, P.; Gärtner, H. Does mycorrhizal inoculation improve plant survival, aggregate stability, and fine root development on a coarse-grained soil in an alpine eco-engineering field experiment? J. Geophys. Res.-Biogeo. 2016, 121, 2158–2171. [Google Scholar] [CrossRef]
- Berta, G.; Trotta, A.; Fusconi, A.; Hooker, J.E.; Munro, M.; Atkinson, D.; Giovannetti, M.; Morini, S.; Fortuna, P.; Tisserant, B.; et al. Arbuscular mycorrhizal induced changes to plant growth and root system morphology in Prunus cerasifera. Tree Phys. 1995, 15, 281–293. [Google Scholar] [CrossRef]
- Caesar-Ton That, T.C.; Shelver, W.L.; Thorn, R.G.; Cochran, V.L. Generation of antibodies for soil aggregating basidiomycete detection as an early indicator of trends in soil quality. Appl. Soil. Ecol. 2001, 18, 99–116. [Google Scholar] [CrossRef]
- Mankel, A.; Krause, K.; Kothe, E. Identification of a hydrophobin gene that is developmentally regulated in the ectomycorrhizal fungus Tricholoma terreum. Appl. Environ. Microbiol. 2002, 68, 1408–1413. [Google Scholar] [CrossRef] [PubMed]
- Tagu, D.; De Bellis, R.; Balestrini, R.; De Vries, O.M.H.; Piccoli, G.; Stocchi, V.; Bonfante, P.; Martin, F. Immunolocalization of hydrophobin HYDPt-1 from the ectomycorrhizal basidiomycete Pisolithus tinctorius during colonization of Eucalyptus globulus roots. New Phytol. 2008, 149, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Caravaca, F.; Barea, J.M.; Palenzuela, J.; Figueroa, D.; Alguacil, M.M.; Roldán, A. Establishment of shrub species in a degraded semiarid site after inoculation with native or allochthonous arbuscular mycorrhizal fungi. Appl. Soil. Ecol. 2003, 22, 103–111. [Google Scholar] [CrossRef]
- Chaudhary, V.B.; Bowker, M.A.; O’Dell, T.E.; Grace, J.B.; Redman, A.E.; Rillig, M.C.; Johnson, N.C. Untangling the biological contributions to soil stability in semiarid shrublands. Ecol. Appl. 2009, 19, 110–122. [Google Scholar] [CrossRef]
- King, E.G.; Hobbs, R.J. Identifying linkages among conceptual models of ecosystem degradation and restoration: Towards an integrative framework. Restor. Ecol. 2006, 14, 369–378. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).