Fire-Derived Charcoal Attracts Microarthropods in the Litter of Boreal Deciduous Forest
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Litterbag Experiment
2.3. Microarthropod Extraction and Quantification
2.4. Microbial Activity
2.5. Calculations and Statistical Analysis
3. Results
3.1. Water Content Dynamics
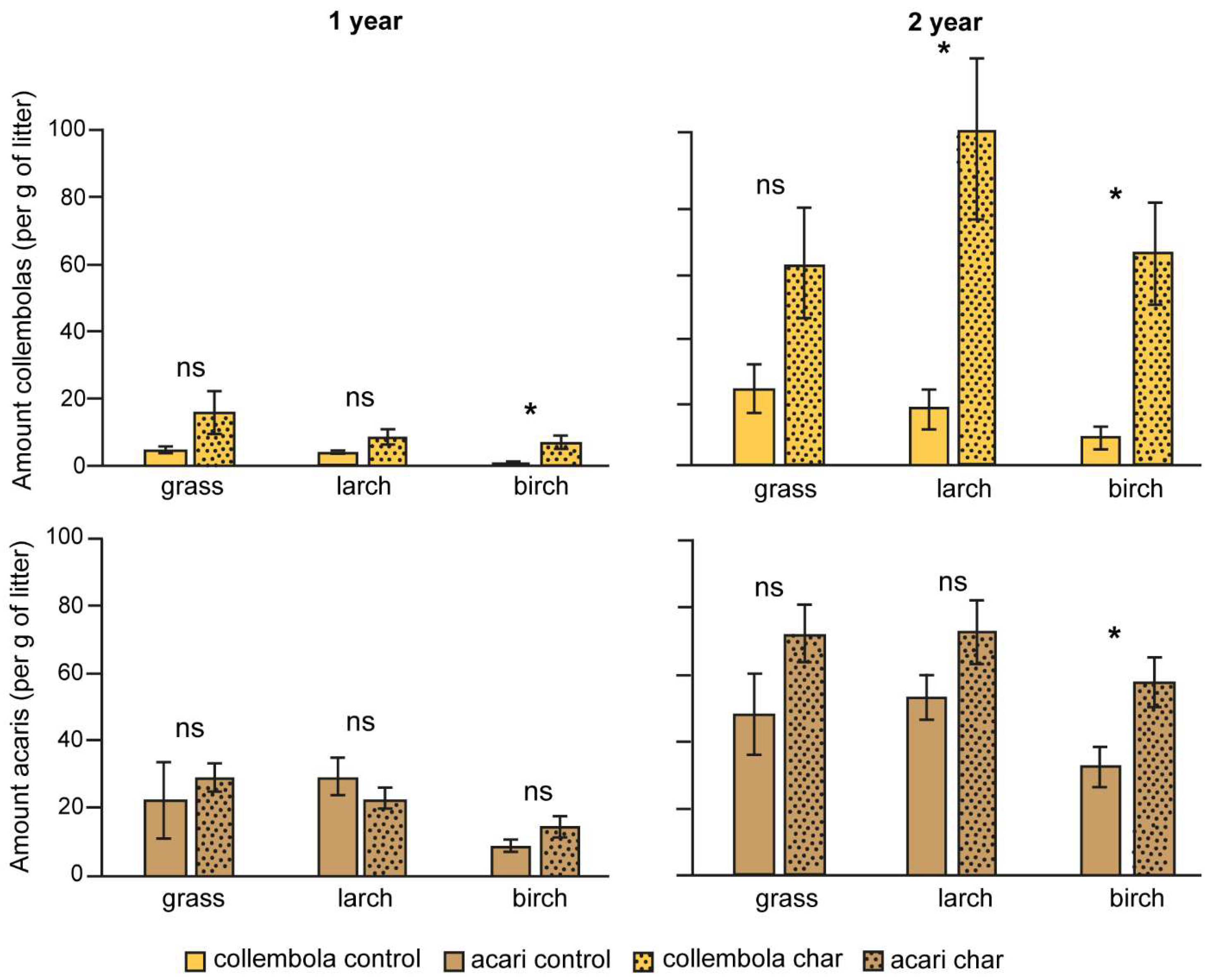
3.2. Abundance of Microarthropods
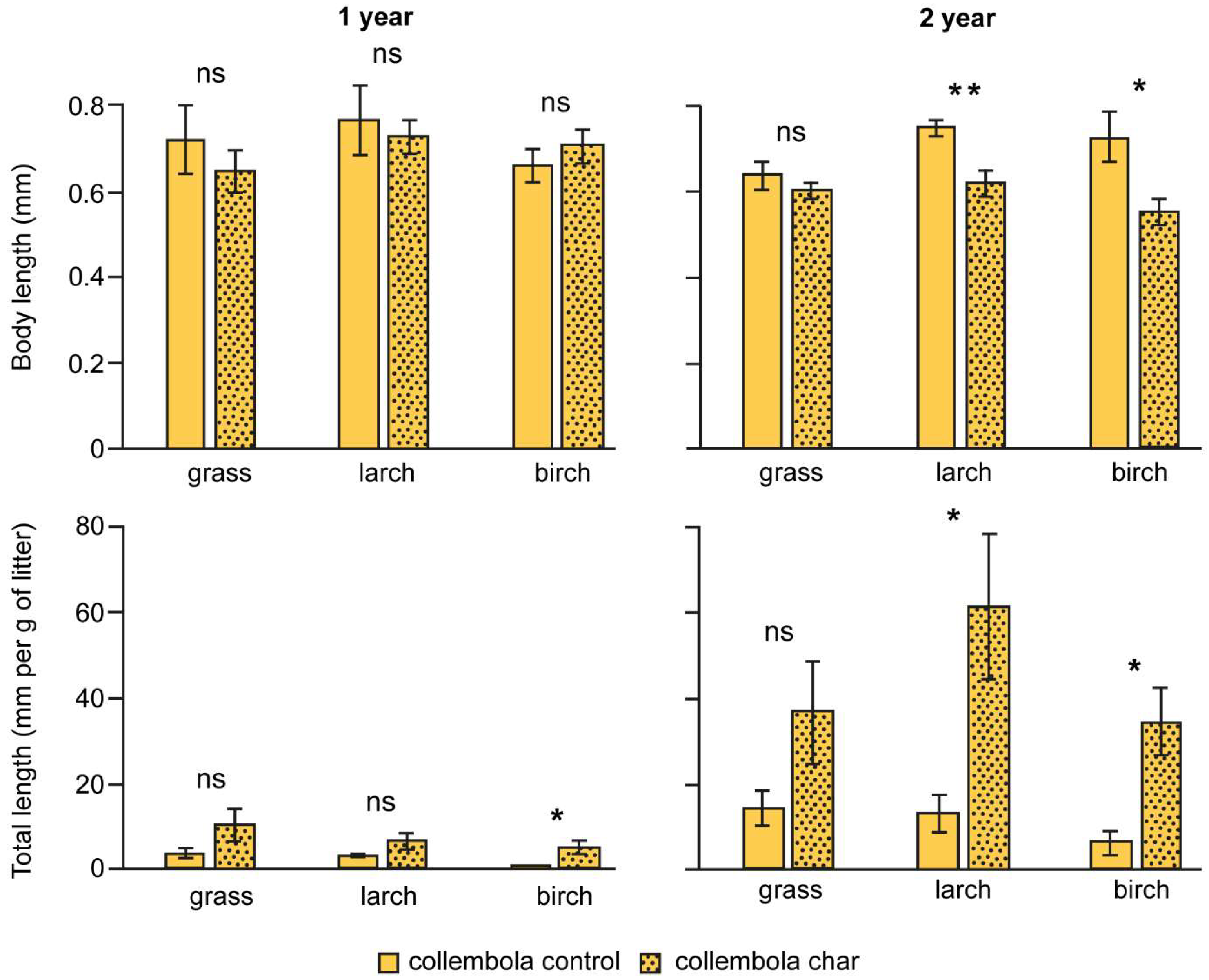
3.3. Collembola Length
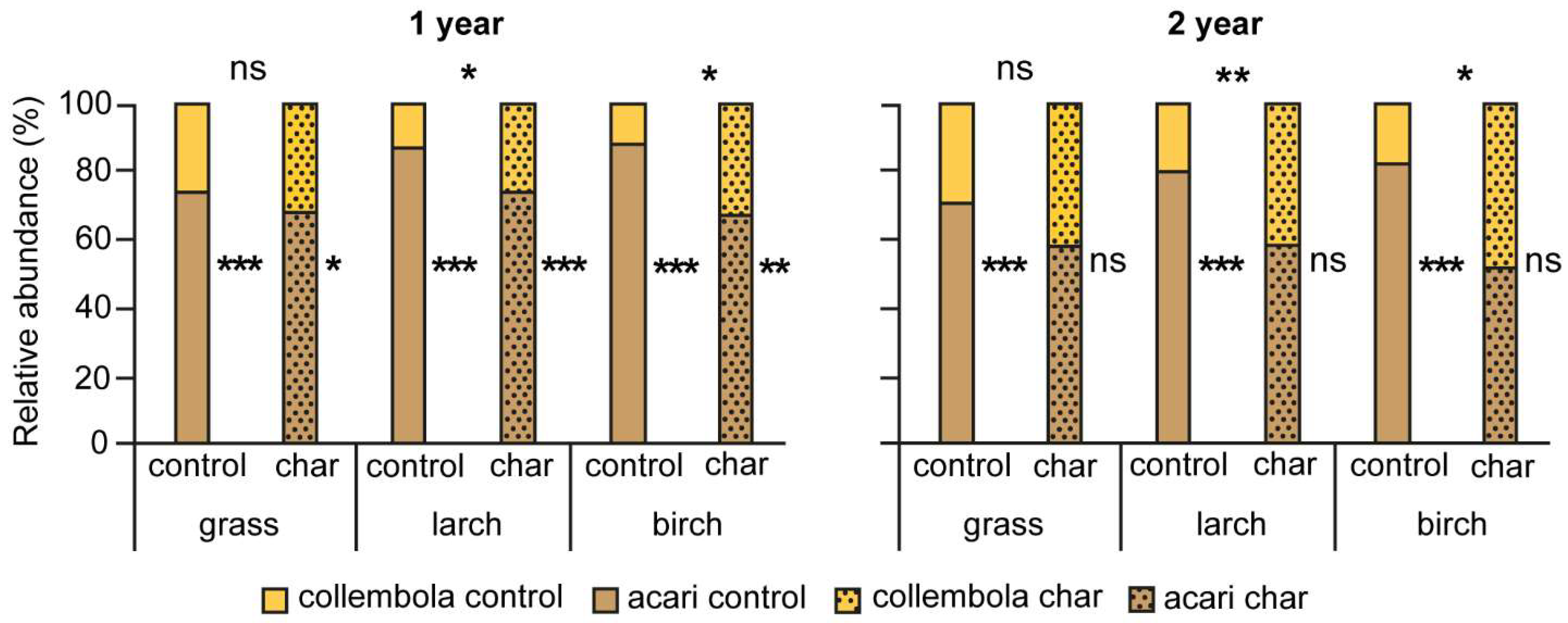
3.4. Proportion of Microarthropods
3.5. Microbial Activity
3.6. Litter Mass Loss
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flannigan, M.; Cantin, A.S.; De Groot, W.J.; Wotton, M.; Newbery, A.; Gowman, L.M. Global Wildland Fire Season Severity in the 21st Century. For. Ecol. Manag. 2013, 294, 54–61. [Google Scholar] [CrossRef]
- Santín, C.; Doerr, S.H.; Preston, C.M.; González-Rodríguez, G. Pyrogenic Organic Matter Production from Wildfires: A Missing Sink in the Global Carbon Cycle. Glob. Chang. Biol. 2015, 21, 1621–1633. [Google Scholar] [CrossRef] [PubMed]
- Seidl, R.; Honkaniemi, J.; Aakala, T.; Aleinikov, A.; Angelstam, P.; Bouchard, M.; Boulanger, Y.; Burton, P.J.; De Grandpré, L.; Gauthier, S.; et al. Globally Consistent Climate Sensitivity of Natural Disturbances across Boreal and Temperate Forest Ecosystems. Ecography 2020, 43, 967–978. [Google Scholar] [CrossRef]
- Wardle, D.A.; Nilsson, M.-C.; Zackrisson, O. Fire-Derived Charcoal Causes Loss of Forest Humus. Science 2008, 320, 629. [Google Scholar] [CrossRef]
- Bryanin, S.; Abramova, E.; Makoto, K. Fire-Derived Charcoal Might Promote Fine Root Decomposition in Boreal Forests. Soil Biol. Biochem. 2018, 116, 1–3. [Google Scholar] [CrossRef]
- Minamino, Y.; Fujitake, N.; Suzuki, T.; Yoshitake, S.; Koizumi, H.; Tomotsune, M. Effect of Biochar Addition on Leaf-Litter Decomposition at Soil Surface during Three Years in a Warm-Temperate Secondary Deciduous Forest, Japan. Sci. Rep. 2019, 9, 16961. [Google Scholar] [CrossRef]
- Makoto, K.; Koike, T. Charcoal Ecology: Its Function as a Hub for Plant Succession and Soil Nutrient Cycling in Boreal Forests. Ecol. Res. 2021, 36, 4–12. [Google Scholar] [CrossRef]
- DeLuca, T.; Nilsson, M.-C.; Zackrisson, O. Nitrogen Mineralization and Phenol Accumulation along a Fire Chronosequence in Northern Sweden. Oecologia 2002, 133, 206–214. [Google Scholar] [CrossRef]
- DeLuca, T.H.; MacKenzie, M.D.; Gundale, M.J.; Holben, W.E. Wildfire-Produced Charcoal Directly Influences Nitrogen Cycling in Ponderosa Pine Forests. Soil Sci. Soc. Am. J. 2006, 70, 448–453. [Google Scholar] [CrossRef]
- Palviainen, M.; Berninger, F.; Bruckman, V.J.; Köster, K.; De Assumpção, C.R.M.; Aaltonen, H.; Makita, N.; Mishra, A.; Kulmala, L.; Adamczyk, B.; et al. Effects of Biochar on Carbon and Nitrogen Fluxes in Boreal Forest Soil. Plant Soil 2018, 425, 71–85. [Google Scholar] [CrossRef]
- Soong, J.L.; Nielsen, U.N. The Role of Microarthropods in Emerging Models of Soil Organic Matter. Soil Biol. Biochem. 2016, 102, 37–39. [Google Scholar] [CrossRef]
- Makoto, K.; Nemilostiv, Y.; Zyryanova, O.; Kajimoto, T.; Matsuura, Y.; Yoshida, T.; Satoh, F.; Sasa, K.; Koike, T. Regeneration after Forest Fires in Mixed Conifer Broad-Leaved Forests of the Amur Region in Far Eastern Russia: The Relationship between Species Specific Traits Against Fire and Recent Fire Regimes. Eurasian J. For. Res 2007, 10, 51–58. [Google Scholar]
- Gruss, I.; Twardowski, J.P.; Latawiec, A.; Medyńska-Juraszek, A.; Królczyk, J. Risk Assessment of Low-Temperature Biochar Used as Soil Amendment on Soil Mesofauna. Environ. Sci. Pollut. Res. 2019, 26, 18230–18239. [Google Scholar] [CrossRef]
- Maaß, S.; Hückelheim, R.; Rillig, M.C. Collembola Laterally Move Biochar Particles. PLoS ONE 2019, 14, e0224179. [Google Scholar] [CrossRef]
- Coleman, D.C.; Callaham, M.A.; Crossley, D.A. Secondary Production. In Fundamentals of Soil Ecology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 77–171. ISBN 978-0-12-805251-8. [Google Scholar]
- Potapov, A.M.; Beaulieu, F.; Birkhofer, K.; Bluhm, S.L.; Degtyarev, M.I.; Devetter, M.; Goncharov, A.A.; Gongalsky, K.B.; Klarner, B.; Korobushkin, D.I.; et al. Feeding Habits and Multifunctional Classification of Soil-associated Consumers from Protists to Vertebrates. Biol. Rev. 2022, 97, 1057–1117. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.J.; Yim, M.H.; Nakane, K. Contribution of Microarthropods to the Decomposition of Needle Litter in a Japanese Cedar (Cryptomeria japonica D. Don) Plantation. For. Ecol. Manag. 2006, 234, 192–198. [Google Scholar] [CrossRef]
- Fujii, S.; Takeda, H. Succession of Soil Microarthropod Communities during the Aboveground and Belowground Litter Decomposition Processes. Soil Biol. Biochem. 2017, 110, 95–102. [Google Scholar] [CrossRef]
- Sánchez-Galindo, L.M.; Sandmann, D.; Marian, F.; Krashevska, V.; Maraun, M.; Scheu, S. Leaf Litter Identity Rather than Diversity Shapes Microbial Functions and Microarthropod Abundance in Tropical Montane Rainforests. Ecol. Evol. 2021, 11, 2360–2374. [Google Scholar] [CrossRef]
- Andrén, O.; Schnürer, J. Barley Straw Decomposition with Varied Levels of Microbial Grazing by Folsomia fimetaria (L.) (Collembola, Isotomidae). Oecologia 1985, 68, 57–62. [Google Scholar] [CrossRef]
- Chamberlain, P.; Mcnamara, N.; Chaplow, J.; Stott, A.; Black, H. Translocation of Surface Litter Carbon into Soil by Collembola. Soil Biol. Biochem. 2006, 38, 2655–2664. [Google Scholar] [CrossRef]
- Yin, R.; Siebert, J.; Eisenhauer, N.; Schädler, M. Climate Change and Intensive Land Use Reduce Soil Animal Biomass via Dissimilar Pathways. eLife 2020, 9, e54749. [Google Scholar] [CrossRef]
- Kaneda, S.; Kaneko, N. Influence of Collembola on Nitrogen Mineralization Varies with Soil Moisture Content. Soil Sci. Plant Nutr. 2011, 57, 40–49. [Google Scholar] [CrossRef]
- Vannier, G. The Porosphere as an Ecological Medium Emphasized in Professor Ghilarov’s Work on Soil Animal Adaptations. Biol. Fertil. Soils 1987, 3, 39–44. [Google Scholar] [CrossRef]
- Rakhleeva, A.A.; Semenova, T.A.; Striganova, B.R.; Terekhova, V.A. Dynamics of Zoomicrobial Complexes upon Decomposition of Plant Litter in Spruce Forests of the Southern Taiga. Eurasian Soil Sci. 2011, 44, 38–48. [Google Scholar] [CrossRef]
- Bluhm, C.; Butenschoen, O.; Maraun, M.; Scheu, S. Effects of Root and Leaf Litter Identity and Diversity on Oribatid Mite Abundance, Species Richness and Community Composition. PLoS ONE 2019, 14, e0219166. [Google Scholar] [CrossRef] [PubMed]
- FAO. World Reference Base for Soil Resources 2014: International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; FAO: Rome, Italy, 2014; ISBN 978-92-5-108370-3. [Google Scholar]
- Karavaeva, N.A.; Prokopchuk, V.F. Genesis of Brown Forest Soils in the North of Amur Region and Sakhalin. Eurasian Soil Sci. 2004, 37, 901–910. [Google Scholar]
- Tsibart, A.S.; Gennadiev, A.N. The Influence of Fires on the Properties of Forest Soils in the Amur River Basin (the Norskii Reserve). Eurasian Soil Sci. 2008, 41, 686–693. [Google Scholar] [CrossRef]
- Bryanin, S.V.; Sorokina, O.A. Effect of Soil Properties and Environmental Factors on Chemical Compositions of Forest Soils in the Russian Far East. J. Soils Sediments 2019, 19, 1130–1138. [Google Scholar] [CrossRef]
- Makita, N.; Fujii, S. Tree Species Effects on Microbial Respiration from Decomposing Leaf and Fine Root Litter. Soil Biol. Biochem. 2015, 88, 39–47. [Google Scholar] [CrossRef]
- Pumpanen, J.; Longdoz, B.; Kutsch, W.L. Field Measurements of Soil Respiration: Principles and Constraints, Potentials and Limitations of Different Methods. In Soil Carbon Dynamics; Kutsch, W.L., Bahn, M., Heinemeyer, A., Eds.; Cambridge University Press: Cambridge, UK, 2010; pp. 16–33. ISBN 978-0-521-86561-6. [Google Scholar]
- Cipola, N.G.; Da Silva, D.D.; Bellini, B.C. Class Collembola. In Thorp and Covich’s Freshwater Invertebrates; Elsevier: Amsterdam, The Netherlands, 2018; pp. 11–55. ISBN 978-0-12-804223-6. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Nakatsuka, H.; Karasawa, T.; Ohkura, T.; Wagai, R. Soil Faunal Effect on Plant Litter Decomposition in Mineral Soil Examined by Two In-Situ Approaches: Sequential Density-Size Fractionation and Micromorphology. Geoderma 2020, 357, 113910. [Google Scholar] [CrossRef]
- Fujii, S.; Berg, M.P.; Cornelissen, J.H.C. Living Litter: Dynamic Trait Spectra Predict Fauna Composition. Trends Ecol. Evol. 2020, 35, 886–896. [Google Scholar] [CrossRef]
- Pollierer, M.M.; Klarner, B.; Ott, D.; Digel, C.; Ehnes, R.B.; Eitzinger, B.; Erdmann, G.; Brose, U.; Maraun, M.; Scheu, S. Diversity and Functional Structure of Soil Animal Communities Suggest Soil Animal Food Webs to Be Buffered against Changes in Forest Land Use. Oecologia 2021, 196, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Kondratova, A.V.; Abramova, E.R.; Bryanin, S.V. Decomposition of Main Litter Types and Nitrogen Release in Post-Fire Larch Forests of the Russian Far East. Contemp. Probl. Ecol. 2021, 14, 171–181. [Google Scholar] [CrossRef]
- Castracani, C.; Maienza, A.; Grasso, D.A.; Genesio, L.; Malcevschi, A.; Miglietta, F.; Vaccari, F.P.; Mori, A. Biochar–Macrofauna Interplay: Searching for New Bioindicators. Sci. Total Environ. 2015, 536, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Guidi, C.; Frey, B.; Brunner, I.; Meusburger, K.; Vogel, M.E.; Chen, X.; Stucky, T.; Gwiazdowicz, D.J.; Skubała, P.; Bose, A.K.; et al. Soil Fauna Drives Vertical Redistribution of Soil Organic Carbon in a Long-term Irrigated Dry Pine Forest. Glob. Chang. Biol. 2022, 28, 3145–3160. [Google Scholar] [CrossRef] [PubMed]
- Bhagawati, S.; Medhi, B.K.; Bhattacharjee, S.; Mishra, H. Diversity and Density of Collembola as Influenced by Soil Physico-Chemical Properties in Fallow Land Ecosystem of Assam, India. J. Environ. Biol. 2020, 41, 1626–1631. [Google Scholar] [CrossRef]
- Potapov, A.V.; Pollierer, M.M.; Salmon, S.; Šustr, V.; Chen, T.-W. Multidimensional Trophic Niche Revealed by ComplementaryApproaches: Gut Content, Digestive Enzymes, Fatty Acids and Stable Isotopes in Collembola. J. Anim. Ecol. 2021, 90, 1919–1933. [Google Scholar] [CrossRef]
- Jaafar, N.M.; Clode, P.L.; Abbott, L.K. Microscopy observations of habitable space in biochar for colonization by fungal hyphae from soil. J. Integr. Agric. 2014, 13, 483–490. [Google Scholar] [CrossRef]
- Frey, S.D.; Elliott, E.T.; Paustian, K.; Peterson, G.A. Fungal Translocation as a Mechanism for Soil Nitrogen Inputs to Surface Residue Decomposition in a No-Tillage Agroecosystem. Soil Biol. Biochem. 2000, 32, 689–698. [Google Scholar] [CrossRef]
- Prescott, C.E.; Vesterdal, L. Decomposition and Transformations along the Continuum from Litter to Soil Organic Matter in Forest Soils. For. Ecol. Manag. 2021, 498, 119522. [Google Scholar] [CrossRef]
- Ganihar, S.R. Biomass Estimates of Terrestrial Arthropods Based on Body Length. J. Biosci. 1997, 22, 219–224. [Google Scholar] [CrossRef]
- Gruner, D. Regressions of Length and Width to Predict Arthropod Biomass in the Hawaiian Islands. Pac. Sci. 2003, 57, 325–336. [Google Scholar] [CrossRef]
- Chang, L.; Sun, X.; Wang, B.; Gao, M.; Chen, L.; Liang, A.; Wu, D. Green More than Brown Food Resources Drive the Effect of Simulated Climate Change on Collembola: A Soil Transplantation Experiment in Northeast China. Geoderma 2021, 392, 115008. [Google Scholar] [CrossRef]
- Tan, Y.; Yang, K.; Xu, Z.; Zhang, L.; Li, H.; You, C.; Tan, B. The Contributions of Soil Fauna to the Accumulation of Humic Substances during Litter Humification in Cold Forests. Forests 2022, 13, 1235. [Google Scholar] [CrossRef]

| Grass | Larch | Birch | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Year | Control | Char | p-Value | Control | Char | p-Value | Control | Char | p-Value |
| remaining mass, g | |||||||||
| 1 | 66.9 ± 1.4 | 67.7 ± 0.6 | 0.91 | 83.4 ± 2.3 | 76.8 ± 1.2 | 0.04 | 74.0 ± 1.5 | 70.0 ± 1.3 | 0.2 |
| 2 | 54.2 ± 1.4 | 48.8 ± 2.8 | 0.32 | 50.8 ± 3.9 | 51.1 ± 4.4 | 0.92 | 48.5 ± 3.1 | 50.1 ± 3.2 | 0.8 |
| CO2, nmol g−1 s−1 | |||||||||
| 1 | 2.86 ± 0.67 | 1.52 ± 0.12 | 0.08 | 2.45 ± 0.54 | 2.34 ± 0.21 | 0.85 | 2.67 ± 0.29 | 2.35 ± 0.23 | 0.42 |
| 2 | 7.05 ± 1.64 | 6.76 ± 0.76 | 0.87 | 4.84 ± 0.53 | 5.76 ± 0.56 | 0.26 | 5.68 ± 0.66 | 5.32 ± 0.86 | 0.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kondratova, A.; Bryanin, S. Fire-Derived Charcoal Attracts Microarthropods in the Litter of Boreal Deciduous Forest. Forests 2023, 14, 1432. https://doi.org/10.3390/f14071432
Kondratova A, Bryanin S. Fire-Derived Charcoal Attracts Microarthropods in the Litter of Boreal Deciduous Forest. Forests. 2023; 14(7):1432. https://doi.org/10.3390/f14071432
Chicago/Turabian StyleKondratova, Anjelica, and Semyon Bryanin. 2023. "Fire-Derived Charcoal Attracts Microarthropods in the Litter of Boreal Deciduous Forest" Forests 14, no. 7: 1432. https://doi.org/10.3390/f14071432
APA StyleKondratova, A., & Bryanin, S. (2023). Fire-Derived Charcoal Attracts Microarthropods in the Litter of Boreal Deciduous Forest. Forests, 14(7), 1432. https://doi.org/10.3390/f14071432






