Abstract
Light is often considered the primary factor leading to the regeneration failure of Korean pines (Pinus koraiensis) under the forest canopy. However, studies on the effect of light on Korean pines mainly focus on the use of an artificial sunshade net to control shade; field studies on the canopy are extremely scarce, and the current experimental results are contradictory. For a deeper understanding of the relationship between light conditions and understory Korean pine trees, the conditions of low, middle, high and full light (control) under the forest were tested at 18 years of age. The photosynthetic pigments, chlorophyll fluorescence, non-structural carbohydrate metabolism, antioxidant enzyme activity, and nutrient concentrations of current-year needles from Korean pine trees were measured. From June to September, light intensity and quality decreased under full light, but following leaf fall, understory light conditions improved slightly. As the light conditions improved, the photosynthetic pigments in the needles decreased, but Car/Chl were highest in the needles under full light. All light conditions had a positive correlation with glucose concentrations and Rubisco activity. Full-light needles had the highest APX activity, DPPH scavenging capacity, and proline concentration, as well as higher NPQ and lower Fv/Fm readings. This indicated that full-light Korean pine trees were stressed and inhibited photosynthesis to some extent, while the understory light environment may alleviate stress. The conservative strategy of storing more starch and using less glucose in understory Korean pine trees may be one of the reasons for the observed differences in growth rates among Korean pine trees under varying light conditions. Overall, this study implies that understory light during the growing season is not always unfavorable to 18-year-old Korean pine trees; this means that 18-year-old Korean pine trees still have shade tolerance to some extent and are capable of living under a canopy of deciduous trees.
1. Introduction
Light is, of course, essential for photosynthesis and supports most life on Earth [1]. However, light intensity and quality are highly variable in space and time and vary depending on the time of day, season, geography, climate, and position of a leaf within a canopy [1,2,3]. Photosynthetic photon flux density (PPFD) and red light:far-red light ratio (R/FR) can be much lower at the bottom of a dense canopy than in areas with full light exposure [4,5].
Any condition or substance that negatively affects or blocks plant metabolism, growth, or development is considered stress [6] and can occur at any stage of plant growth and development [7]. Low light is widely believed to be an all-encompassing abiotic stress that impedes photosynthesis in plants, leading to slower growth rates [3,8,9]. Since ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) is the first enzyme in the Calvin–Benson cycle, the foundation of carbon fixation, a low R/FR may induce excitation imbalances between photosystem II and I, disrupting both the redox chemistry in the transport chain and its coordination with the Calvin-Benson cycle [10,11,12]. Photoinhibition, caused by an excess of light, can hinder plant development, but oxygenic photosynthesis still takes place [3,13]. However, Different types of plants can handle photoinhibition in different ways [14]. When plant species compete for space and limited resources, understanding their relative vulnerability to photoinhibition and their adaptability to diverse light conditions becomes important [15].
Low/high light conditions are both harmful to plants. High light intensity causes photodamage through excessive reactive oxygen species (ROS) or by direct damage of DNA and other cellular compounds through UV-B absorption. Therefore, antioxidant enzymes like SOD, POD, and APX could represent plants’ capacity to adapt to different environments. Meanwhile, low light reduces plants’ capacity for photosynthesis and filters out red and blue light compared to green and far-red light, leading to low R/FR [16].
The research found that supplementary UV-B radiation on Korean pines could enhance their APX and SOD activities, but its defense mechanism is not efficient enough to prevent damage [17]. Li et al. [18] found that the photosynthetic capacity increases with a great change in light intensity.
Chlorophyll, an important pigment involved in photosynthesis, plays a crucial role in absorbing, transmitting, and converting solar energy into electrochemical energy [19]. The rate of light-saturated photosynthesis in shade leaves is lower due to lower levels of photosynthetic enzymes like Rubisco, despite a higher chlorophyll concentration and greater light-capturing capacity due to the bigger antenna size of photosystem II (PSII) [20].
Shade leaves have higher chlorophyll concentrations compared to those grown under sunlight, as found in Hymenaea courbaril L. var. stilbocarpa, Esenbeckia leiocarpa, Cariniana legalis, and Tabebuia roseo-alba [21]. Carotenoids, essential antenna pigments in plants, are utilized to protect photosynthetic components from oxidative stress. Carotenoids can also minimize the damage caused by reactive oxygen species (ROS), which result from an imbalance between the oxidizing and reducing factors commonly experienced by plants under different environmental constraints [22,23]. Studies on Populus tomentosa at the transcriptional level [24] found that oxidative stress inhibited phosphatase activity and led to abnormal mitochondrial function, indicating that oxidative stress causes oxidative damage to plants and thus affects the growth and development process. To mitigate oxidative stress, plants adapt their metabolism to buffer ROS using enzymes such as peroxidase (POD), ascorbate peroxidase (APX), and superoxide dismutase (SOD) as scavengers [25]. Therefore, enhancing the antioxidant defense in plants can effectively increase their tolerance to various stresses.
Non-invasive techniques such as chlorophyll fluorescence offer valuable insights into the photosynthetic apparatus and are widely used to study the impact of stress on photosynthesis [26]. The maximum quantum yield of PSII is represented by Fv/Fm. It indicates the probability that a trapped photon will end up in the reaction center and cause a photochemical event [27]. When exposed to stressors such as excessive light or a low temperature, the photochemical efficiency of photosystem II decreases, as indicated by Fv/Fm [28,29]. Nonphotochemical chlorophyll fluorescence quenching (NPQ) is a process that converts excess absorbed light energy into heat. Studies have shown that Fv/Fm and NPQ decrease as photosynthetically active radiation levels decline [8].
The production of non-structural carbohydrates (NSCs), consisting mainly of soluble sugars and starch, through photosynthesis allows immobile plants to withstand both biotic and abiotic stressors [30,31]. In addition to carbon (C), light also influences the acquisition of macronutrients such as nitrogen (N), phosphorous (P), and potassium (K) in plants [32], which are all essential for plant growth and development [33]. Therefore, understanding the interaction between light, NSCs, and macronutrient acquisition is crucial for optimizing plant growth and productivity.
As a long-lived evergreen species of great value, Korean pine (Pinus koraiensis Sieb. et Zucc.) dominates the natural mixed-broadleaved Korean pine forest, which is of the utmost importance in environmental protection in northern China [34,35,36,37]. Due to heavy exploitation and poor natural regeneration, its area has declined dramatically [35,38]. While significant efforts have been made in recent years to restore its population, the artificial regeneration of Korean pine has been largely unsuccessful [34,35,39,40]. Although the planting of conifers under secondary deciduous forest has been widely accepted and practiced, most studies on Korean pine growth under varying light conditions have focused on seedlings [41,42,43,44,45] and often utilized artificial shading [42,45,46], which may not reflect the realistic natural conditions. Therefore, more on-site research is needed to better understand the effects of light on Korean pine growth and development at different stages of its life cycle, which is essential for developing effective strategies for restoration and conservation.
Despite the conventional wisdom that low light conditions in the understory are detrimental to plant growth and development [40], recent studies have suggested that this may not always be the case. Many tree species exhibit improved growth with increasing light supply, similar to the way they respond to increasing water and nutrient supplies [47]. To further investigate this phenomenon, a comprehensive study on the responses of Korean pines to different light conditions was conducted, focusing on 18-year-old Korean pine trees that had adapted to the local environment for an extended period, allowing us to gain insight into the long-term effects of low light exposure. Our hypothesis was that low light conditions in the understory were not always unfavorable for plants. Therefore, we measured and analyzed plant parameters such as chlorophyll and carotenoid concentration, chlorophyll fluorescence, non-structural carbohydrate concentration, antioxidant enzyme activity, and nutrient concentration of 18-year Korean pines under the canopy adapted to the local environment under different light conditions and explored the response mechanism of Korean pine trees under the canopy to different light conditions. The results provide a scientific reference for clarifying the relationship between light conditions and understory Korean pine trees.
2. Materials and Methods
2.1. Study Area
The study site was located at the Taiping working area on the Maoershan Experiment Forest Farm of Northeast Forestry University (127°30′–127°34′ E, 45°21′–45°25′ N; Heilongjiang, China, Figure 1). The climate in this region is characterized as a temperate continental monsoon with a growing season spanning from May to September. The annual mean air temperature is 3 °C, ranging from −19.7 °C in January to 22 °C in July. This area experiences 120–140 frost-free days annually and receives an average precipitation of 600–800 mm, primarily from May to September (80%) [48,49].
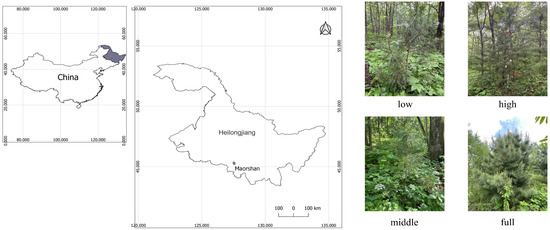
Figure 1.
Study area map (left: pictures) and Korean pine trees under different light conditions (right: photos).
The soil in this region is Hap-Boric Luvisol, composed mainly of dark brown loamy soil (1–10 cm depth) and sandy loam (10–20 cm depth) [50]. The current vegetation comprises plantations and natural secondary forests, which have been shaped by recurrent human disturbances and are dominated by Pinus koraiensis, as well as a variety of broad-leaved species such as Fraxinus mandshurica, Phelodendron amurense, Juglans mandshurica, Betula platyphylla, Quercus mongolica, and Tilia amurensis [48].
2.2. Experimental Design and Sampling
The current study focused on understory Korean pine trees obtained from permanent plots established in 2012. The Korean pine trees were planted in 1989 and replenished in 2003 at micro-sites where Korean pine trees were scarce. The trees used in this experiment were the replenished ones at 18 years old when they were sampled with three replicates (Figure 1). Current-year needles were not fully grown and still had sheaths in June and July, but they were removed throughout the experiment based on their similar age and health side branch to measure physiological and biochemical characteristics.
Low, moderate, and high light conditions corresponded to the opening degrees (K) 1.0, 1.5, and 2.0, respectively [36,37]. Open degree (K) is defined as the sum of the ratio of the distance from the reference tree (here, the planted Korean pine trees) to the nearest neighboring upper trees (here, the upper layer of broadleaved trees) in each quadrat to the height of the neighboring upper trees. The longer the distance of the reference trees to the neighboring broadleaved trees and the lower the height of the upper broadleaved trees, the larger the K value, and the more favorable to the growth of the reference tree [37].
Light conditions had a significant influence on the trees’ morphology. Average (±standard error) tree height and root-collar diameter at the time of measurement were 0.67 (±0.29) m and 2.35 (±0.55) cm, respectively, under low light conditions (L, K = 1.0), and 1.2 (±0.43) m and 3.07 (±0.1.34) cm, respectively, under moderate light (M, K = 1.5) conditions. Average (±standard error) tree height and diameter at breast height (DBH) at the time of measurement were 3.42 (±0.40) m and 2.67 (±0.45) cm, respectively, under high light conditions (H, K = 2.0), and 4.47 (±0.31) m and 10.67 (±0.93) cm, respectively, under full light conditions (F, CK).
Understory Korean pine trees do not develop enough needles until late June, so our sampling time was in late June, July, and early September. August was full of rain and not suitable for sampling. On sunny days, several shoots were cut from the upper half of the mid-third of the crown of trees with current-year needles under different light conditions. Half the needles were removed and placed in liquid nitrogen, while the remaining needles were placed in sterile polythene bags and transported to the laboratory in a box with ice bags (Figure 2). We used random sampling.

Figure 2.
Schematic of the experimental design.
2.3. Light Conditions and Chlorophyll Fluorescence Measurement
Photosynthetic photon flux density (PPFD) was measured under four different light conditions, displayed in units of μmol m−2 s−1 using a Dual Radiation Meter (Spectrum Technologies, Inc., Aurora, IL, USA), and repeated six times for each light condition. Chlorophyll fluorescence and red/far-red light (R/FR) were measured simultaneously. A R/FR light meter was used to determine the R/FR (Spectrum Technologies, Inc., Aurora, IL, USA). A portable pulse-modulated fluorometer (FMS-2, Hansatech, England) was used to measure chlorophyll fluorescence, and needles were enclosed in light-proof clips for 30 min before measurement.
2.4. Photosynthetic Pigment Concentration
For the determination of the concentration of chlorophyll a (Chl a), chlorophyll b (Chl b), and total carotenoid (Car) concentrations, 0.2 g fresh Korean pine needles were used. In brief, they were cut into pieces and soaked in 25 mL extracting solution (ethanol:acetone in 1:1 volumetric fractions) in the dark until the needles turned white. The absorbance of the solution was measured using a UV spectrophotometer (TU-1950) at wavelengths of 663, 646, and 470 nm. The pigment concentration was calculated following the method described by He et al. [51].
2.5. Non-Structural Carbohydrate and Nutrient Concentrations
After being oven-dried at 70 °C until a constant weight was achieved, needle samples were ground using a grinding miller. Next, 0.1 g of the dry needles were ashed at 150 °C, 250 °C, and 350 °C in a muffle furnace with 5 mL of 98% H2SO4 (w/w) for 2 h. The resulting solution was then diluted to 100 mL with deionized water. The potassium concentration was determined using a flame photometer, while the phosphorus concentration was determined using an AutoAnalyzer 3 system (AutoAnalyzer-AA3, Seal Analytical, Norderstedt, Germany). The carbon/nitrogen ratio (C/N) was measured using an elemental analyzer (Elementar, VARIOMacro, Germany). The concentration of non-structural carbohydrates (NSCs, glucose plus starch in this study) was determined using 50 mg of dried samples according to the anthrone method [52].
2.6. Enzyme Activity, DPPH Scavenging Capacity, Proline, and ABA Concentration
For specific methods in determining superoxide dismutase (SOD) and peroxidase (POD) activity and free radical (1,1-diphenyl-2-picrylhydrazyl, DPPH) scavenging capacity in Korean pine needles, please refer to the kit instructions purchased from Suzhou Comin Biotechnology Co., Ltd., Suzhou, China, No. 88, Shengbang Road, Qiandeng. Town Measurements of the activity of Rubisco, GSH-PX, and APX and concentration of Proline and ABA were made using ELISA kits and followed the manufacturers’ instructions.
2.7. Data Analysis
The study was conducted with three replicates for all parameters, except for PPFD and R/FR, which used six replications for each light condition. Mean values ± standard deviation (SD) of the triplicates were calculated for all parameters and analyzed using IBM SPSS Statistics software (SPSS Inc., Chicago, IL, USA) version 26 through analysis of variance (ANOVA). Duncan’s test was used as a post-test to determine differences among the mean values, with results deemed significant at p < 0.05. Pearson’s correlation coefficient was used to conduct a correlation analysis of PPFD, R/FR, and physiological and biochemical traits. Graphs were generated using the ggplot2 and ggbreak [53] and other packages in R.
3. Results
3.1. Dynamics of PPFD and R/FR under Different Light Conditions throughout the Research Period
PPFD and R/FR were higher under full light compared to the other three light conditions. Under the understory light conditions, PPFD and R/FR were highest under high light (H) conditions, followed by moderate light (M) and low light (L) conditions, consistent with our classification. PPFD and R/FR significantly differed (p < 0.0001) across three sampling periods (Figure 3). Understory PPFD ranged from 6 to 68, while PPFD under full light (F) conditions ranged from 679 to 1681, with understory PPFD being less than 1% of the F conditions. Understory R/FR ranged from 0.176 to 0.796, while R/FR under F conditions ranged from 0.871 to 1.241.

Figure 3.
Relationship between PPFD (A) and R/FR (B) in each month, measured under four light conditions. Breaks in PPFD: 70-720. L is the abbreviation of low light; M denotes moderate light; H denotes high light; F denotes full light. **** p < 0.0001; 6, 7, and 9 represent June, July, and September, respectively.
Under F conditions, PPFD continuously decreased over time. In September, PPFD was lower than that in July and June, by 47.15% and 40.58%, respectively. The PPFD of the three understory light conditions all decreased from June to July and then increased from July to September. Understory R/FR in September was higher than that in June within each light condition by 4.02%, 9.73%, and 57.06% for L, M, and H conditions, respectively. Understory R/FR increased over time, with the slope of R/FR generally increasing with increasing light conditions (Figure S1).
3.2. Effects of Light on Photosynthetic Pigment Concentration, Chlorophyll Fluorescence, and Rubisco Activity
Under F conditions, needles had the lowest concentrations of Chl a, Chl b, and Car compared to the other three light conditions (Table 1). However, they had the highest Car/Chl at all sampling times. For Chl a, the concentration of needles under L and H conditions first increased and then decreased, while that of needles under M and F conditions kept increasing. Low-light needles had the highest Chl b concentration in June and July, and the highest Car concentration at all sampling times. In September, the Car/Chl of understory needles was significantly (p < 0.05) higher than that in June by 13.45%, 12.5%, and 14.59% for L, M and H conditions, respectively. In June, the Fv/Fm readings of needles under L, M, and H conditions were significantly (p < 0.05) higher than those under F condition, by 3.88%, 3.60%, and 4.44%, respectively (Table 1). In July, low-light needles had significantly (p < 0.05) lower Fv/Fm readings than the other three light conditions. However, in September, there was no significant difference in Fv/Fm values among the four light conditions. The Fv/Fm of needles under F conditions increased by 0.84% and 2.72% in July and September, respectively, compared to those in June. In June and September, Fv/Fm of needles under low light was significantly (p < 0.05) higher than that in July, by 4.76% and 5.33%, respectively. Needles under F conditions had the highest NPQ among the four light conditions, and NPQ increased from June to September (Table 1). In September, the NPQ of needles under L conditions was significantly (p < 0.05) higher than that in July and June by 37.93% and 57.44%, respectively. Additionally, Rubisco activity decreased with decreasing light at each sampling time. In September, understory needles had lower Rubisco activity than in June, while full-light needles had higher Rubisco activity in September than in June by 4.42%.

Table 1.
Comparison of the means of photosynthetic pigment concentration, chlorophyll fluorescence, and Rubisco activity of Korean pine needles under the four light conditions.
3.3. Effects of Light on NSC and Nutrient Variation
Within each sampling time, glucose concentrations in needles increased with increasing R/FR, particularly in June and July, with a statistical significance of less than 0.001. Starch concentrations significantly increased with increasing R/FR in June (p < 0.001) but decreased significantly (p < 0.05) in September (Figure 4). Starch concentrations in needles significantly decreased over time (except in L conditions), while glucose concentrations significantly (p < 0.001) increased within each light condition. The slope of glucose concentrations in understory needles decreased with increasing light conditions, and glucose concentrations in needles under different light conditions differed significantly (p < 0.01) at each sampling time.

Figure 4.
The correlations between glucose (A,C) and starch (B,D) were measured in Korean pine needles with R/FR and sampling time. L, M, H, and F represent low light, moderate light, high light, and full light conditions, respectively. ** p < 0.01, *** p < 0.001, **** p < 0.0001, ns was omitted; 6, 7, and 9 represent June, July, and September, respectively.
The C/N of needles differed significantly (p < 0.0001) among different light conditions within each sampling time (Figure 5). The C/N values of understory needles increased over time, and the slope decreased with increasing light conditions.
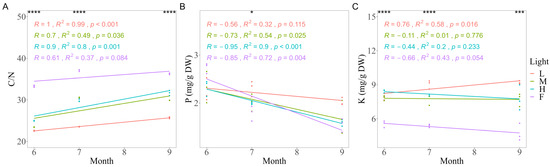
Figure 5.
The correlations of C/N (A), P (B), and K (C) concentrations were measured in needles of Korean pine trees for a month under different light conditions at three sampling times. L is the abbreviation of low light, M for moderate light, H for high light, and F for full light. * p < 0.05 *** p < 0.001, **** p < 0.0001, ns was omitted; 6, 7, and 9 represent June, July, and September, respectively.
Phosphorus (P) concentrations in needles significantly decreased with time (p < 0.05), except for those under L conditions. Potassium (K) concentrations in needles differed significantly (p < 0.001) among different light conditions at each sampling time.
3.4. Effects of Light on Proline and ABA Concentration, Enzyme Activity, and DPPH Scavenging Capacity
The concentration of proline in needles under F conditions showed a significant (p < 0.05) increase each time (Figure 6). ABA concentration in needles differed significantly (p < 0.01) among the different light conditions at each sampling time. In needles under F conditions, the concentration of ABA significantly increased with time (p < 0.001). Conversely, in needles under L and M conditions, the concentration of ABA decreased significantly (p < 0.05) with time, and the slope of the ABA concentration with time decreased with increasing light.

Figure 6.
The correlations between proline (A) and ABA (B) concentrations were measured in needles of Korean pine trees for a month. L is the abbreviation for low light, M for moderate light, H for high light, and F for full light. * p < 0.05, ** p < 0.01, ns was omitted; 6, 7, and 9 represent June, July, and September, respectively.
In terms of antioxidant enzymes, full-light needles had the highest SOD activity in June and July (Table 2). In September, low-light needles had the highest SOD activity. The POD activity of needles kept increasing over the sampling times, with high-light needles exhibiting the highest activity among the four light conditions. Light enhanced APX activity in Korean pine needles, with a unimodal change in APX activity across the sampling time for each light condition.

Table 2.
Comparison of the means of antioxidant enzyme activities and DPPH scavenging capacity in Korean pine needles under four light conditions.
The GSH-PX activity of needles under L and M conditions was significantly (p < 0.05) higher than that under H and F conditions in July, but this trend was reversed in September. The GSH-PX activity of needles under all light conditions first increased and then decreased, dropping below the first sampling time. The GSH-PX activity of needles under H conditions decreased over the three sampling stages.
Light and time had a remarkable impact on the DPPH free-radical scavenging capacity. Needles under F conditions exhibited a consistently high DPPH scavenging capacity compared to those under the other three light conditions at each sampling time, with significant differences (p < 0.05). Furthermore, the DPPH scavenging capacity of understory needles showed a steady increase over time.
4. Discussion
4.1. Dynamics of Light Affect Plant Photosynthetic Responses
Light availability is a pivotal factor in forest ecosystems, with significant variations observed between the understory and outer regions of the canopy, primarily due to the earth’s rotation and wind-induced leaf movements [54]. These sampled trees have adapted to their environment [35,37], and earlier experiments showed that light plays a crucial role in their growth [36], as evidenced by their DBH. In September, as deciduous trees began to defoliate, R/FR increased under the canopy. This finding aligns with previous research indicating that leaf-off periods in the spring and autumn provide more light in the understory than the summer [55]. These periods may thus be crucial for plant growth and survival.
During heavy shading time, photosynthesis is also important, and chlorophyll is indispensable for photosynthesis [19]. Previous research on six-year-old Korean pine trees found a significant (p < 0.05) correlation between needle chlorophyll concentration and light, with chlorophyll levels increasing as light levels increased [45]. However, in this experiment, needles grown under L conditions exhibited higher concentrations of Chl a, Chl b, and carotenoids (Table 1), which was consistent with another study on four-year-old P. koraiensis seedlings shaded by black nets [46]. In shade-tolerant species, chlorophyll concentrations tend to increase with decreasing light exposure to enhance light harvesting [56]. The same pattern has also been observed in Heptacodium miconioides [57] and holly seedlings [58]. A reduction in Chl concentration has been proposed as an indicator of chlorophyll destruction through excessive irradiation [59].
Carotenoids play an important role in plant adaptation to fluctuating environments and could be influenced by reactive oxygen species/redox status to work as stress signals that may be involved in feedback controls [60]. Moreover, they are light-collecting pigments and photoprotectors in photosynthesis. In our results, Car/Chl was significantly and positively related to June and July light, but in September full-light needles stayed high, which indicated that full light may sometimes act as a stress to Korean pine trees.
Rubisco enables net CO2 assimilation in photosynthesis and requires large investments in nitrogen [61], which is in accordance with our results that in full-light needles, C/N was higher due to more investments in Rubisco synthesis and higher Rubisco activity. Understory needles of Korean pines tended to have higher Rubisco activity in June and July (except low-light needles), while full-light needles had higher Rubisco activity in September, which also implied that September was more suitable for full-light trees than June and July.
After being intercepted by a leaf and other photosynthetic organs, light has three fates: chlorophyll a fluorescence, dissipation (i.e., heat or non-photochemical quenching), and photosynthesis; a change in one of these will result in a change in the other two [62,63]. In this study, Korean pine needles exposed to F conditions exhibited low Fv/Fm values, indicating a less active photosynthetic apparatus compared to those under lower light conditions [64], which was attributed to the effects of full-light exposure.
Nonphotochemical chlorophyll fluorescence quenching (NPQ) is a process in which the excess absorbed light energy is dissipated into heat [65]. An experiment demonstrated that NPQ increased rapidly before stabilizing when the light shifted from darkness to high levels [1], highlighting its significance. In this study, the NPQ of full-light needles was significantly (p < 0.05) higher than that of lower-light needles, consistent with a previous study on other evergreen species [66]. The effects of light and time × light on NPQ and Fv/Fm were both significant (p < 0.05, Table 3). In September, the similar Fv/Fm values among the four light conditions indicated that the plants were able to maintain a consistent level of photosynthetic efficiency, regardless of the quantity or quality of light they received. An experiment using simulated sunflecks discovered that the lower NPQ that was found under a low-light intensity increased the maximum photochemical quantum efficiency of PSII under light in shade-tolerant species Panax notoginseng [67], indicating that the lower NPQ readings in Korean pine needles were not necessarily detrimental. Although needles under F conditions may exhibit lower photosynthetic pigment concentrations, they may have a higher photoprotection ability, as indicated by the highest Car/Chl and NPQ values. These findings suggest that 18-year-old Korean pine trees in the current study have still some shade-tolerant capacity and that F conditions, possibly combined with the high temperatures during the growing season, might impose stress on Korean pine trees.

Table 3.
Pearson correlation coefficients between PPFD and R/FR and other parameters at different sampling times.
4.2. NSC Concentrations in Korean Pine Needles Varied under Different Light Conditions
Non-structural carbohydrates (NSCs), mainly sugars and starch, are involved in energy metabolism, nutrient acquisition, and defense mechanisms [68], and are essential to the growth and survival of plants [69]. For instance, research on Arabidopsis suggested that a low light intensity reduced carbohydrate concentration, thus partly prolonging the juvenile vegetative phase [70]. This phenomenon implies that understory Korean pine trees, which have lower NSC concentrations, might require a longer time to enter the reproductive phase. Abiotic stressors such as drought, salinity, chilling, freezing, low nutrient availability, and oxygen stress have been found to increase the concentration of soluble sugars in plants [71], which also indicate that Korean pine trees growing under F conditions might be experiencing stress in July. The concentrations of glucose in Korean pine needles were found to be significantly (p < 0.05) associated with PPFD and R/FR, except in September, when the relationship was only significant with PPFD (Table 3).
The decline in needle starch concentrations under full light is most pronounced among the four light treatments (Figure 4), likely due to the larger size and greater sink capacity. The simultaneous increase in glucose content over time partly indicates the immediacy of starch utilization, suggesting that, even under conditions of sufficient light, more starch is used to foster growth rather than being stored. This differential strategy for utilizing NSC may contribute to the superior growth of plants under full light compared to those growing in the understory [30]. The relatively stable starch concentration may serve as a long-term carbon storage mechanism that allows for Korean pine trees to withstand periods of low light availability and to continue to grow at a slower pace. Additionally, lower glucose concentrations may be indicative of reduced photosynthetic activity under L conditions. These findings highlight the importance of considering the trade-offs between growth and resource allocation strategies when examining plant responses to light availability.
The positive correlation between starch and PPFD in June, which was negative in September, showed a strategy of growth and survival trade-off. When needles were not mature, more starch was stored as the light increased, while in September, as the temperature went down, plants had to prepare for winter, and starch declined as light increased. In other words, low-light needles acted more rapidly than full-light ones.
Here, we only examined NSC concentrations in needles (source); those in sink tissues (stems, roots, and others) were not included. The R/FR and PPFD meters can only represent a certain time period, but light conditions change rapidly and unpredictably. Sunflecks are also crucial in plants, particularly for those under the canopy [5,67,72], as light is never constant for them.
4.3. Anti-Stress Responses in Korean Pine Needles under Different Light Conditions
Reactive oxygen species (ROS) are highly reactive and toxic agents that can cause oxidative damage to cells. To counteract the harmful effects of excessive ROS, a large number of ROS-detoxifying enzymes such as SOD, POD, and APX are required [73]. As understory needles were not fully developed in June and July, the activity of SOD was similar in June and July. However, full-light needles had the highest SOD activity in June and July, which indicated that full light may exert stress on them. Moreover, APX activity and DPPH-scavenging capacity, as well as proline concentration, were constantly higher in needles under full light at all three sampling times. There were also strong correlations between PPFD, R/FR, and DPPH-scavenging capacity, C/N, and K concentrations throughout all sampling stages (Table 3). Notably, shade leaves had higher mass-based concentrations of P and K compared to sun leaves in July and September, as previously reported by Marler and Krishnapillai [74]. Furthermore, a recent study demonstrated that shade-tolerant species exhibited higher survival rates, which were associated with higher leaf K content [3].
The increase in proline has been seen in response to biotic stressors as well as abiotic conditions such as drought, high salt, high light, UV irradiation, heavy metals, and oxidative stress [75]. Numerous reviews have underscored the protective role of proline accumulation in stressed plants [76,77]. It has been demonstrated that proline serves as a molecular chaperone that can preserve the integrity of proteins and improve the functions of certain enzymes. Some examples of these functions include protecting nitrate reductase from heavy metal and osmotic stress, stabilizing ribonucleases and proteases when exposed to arsenate, and preventing protein aggregation and stabilizing M4 lactate dehydrogenase at high temperatures [78,79,80]. Multiple investigations have found that proline has antioxidant properties, including the ability to quench singlet oxygen and scavenge ROS [81,82]. In addition to scavenging ROS, proline pretreatment reduces Hg2+ toxicity in rice (Oryza sativa) [83]. Proline in isolated thylakoid membranes protects PSII against the destructive effects of singlet oxygen and hydroxyl radicals [84].
ABA is commonly known as the “stress hormone” that responds to a variety of environmental stresses, including both biotic and abiotic stress [85]. In tomato leaves, shade conditions have been shown to increase ABA levels [86], whereas full light reduces ABA accumulation in barley leaves [87], which was also observed in our June and July experiments. However, an experiment found that low temperatures and short-day lengths in autumn could result in an increase in ABA levels in leaf tissue [88]. In September, there were significant (p < 0.05) correlations between ABA concentration and light (PPFD and R/FR, Table 3). Proline, another stress-related compound that accumulates in many plant species in response to environmental stress [89], was also found to increase in barley leaves under full light exposure [88], and our findings were consistent with this (Figure 6). The increase in proline and ABA concentration in full-light needles over time suggested that they were under some kind of stress, which may be linked to light quality.
5. Conclusions
Our study highlighted the crucial role of light conditions in determining leaf physiology and plant responses to stress. Contrary to common belief, our results showed that the low light conditions experienced by understory Korean pine trees during the growing season are not necessarily detrimental to their health. Understory Korean pine trees possessed less Chl and lower Rubisco activity. These trees also had lower total NSC concentrations compared to those grown in full light and had a more conservative strategy of storing starch and having lower glucose concentrations, which might contribute to the slower growth rates of Korean pine trees under low light conditions compared to those under full light conditions. Furthermore, our findings suggested that full-light conditions do not always benefit plants. While they tended to invest more starch in growth, they also experienced a high DPPH scavenging capacity during the growing season and accumulated more defense compounds such as proline, ABA, and carotenoids (higher Car/Chl), and their capacity of photosynthesis was inhibited to some extent, which could be indicated by lower Fv/Fm and higher NPQ readings. Overall, our results demonstrate the complex and multifaceted nature of plant responses to light conditions and emphasize the need for a more nuanced understanding of the role of light in plant ecology. However, it is still unknown at which specific light intensity the growth and development of the understory Korean pine trees are promoted (or inhibited), so further and more specific research is needed in the future.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f14071333/s1, Figure S1: The correlations between light conditions (PPFD and R/FR) and Month. L is the abbreviation of low light, M for moderate light, H for high light, and F for full light. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, ns was omitted.
Author Contributions
X.M., B.L. and H.S. conceived the whole idea; B.L. and W.L. performed the experiment and did the statistical analyses, created figures, and wrote the entire manuscript; S.S. worked on the manuscript revision; H.W., P.Z. and H.S. worked on the final revision. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Nature Science Foundation of China (Grant No. 31972950) and the Heilongjiang Touyan Innovation Team Program (Technology Development Team for High-efficient Silviculture of Forest Resources).
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors would like to thank Maoershan Experimental Forest Farm of Northeast Forestry University for providing access to the study area for this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Han, J.M.; Gu, L.H.; Warren, J.M.; Guha, A.; Mclennan, D.A.; Zhang, W.F.; Zhang, Y.L. The roles of photochemical and non-photochemical quenching in regulating photosynthesis depend on the phases of fluctuating light conditions. Tree Physiol. 2022, 42, 848–861. [Google Scholar] [CrossRef] [PubMed]
- Murchie, E.H.; Niyogi, K.K. Manipulation of photoprotection to improve plant photosynthesis. Plant Physiol. 2011, 155, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes, L.; Moreno, F. Trait coordination at leaf level explains the resistance to excess light stress in shade-tolerant tropical tree species. Tree Physiol. 2022, 42, 1325–1336. [Google Scholar] [CrossRef] [PubMed]
- Chazdon, R.L. Fetcher N Photosynthetic light environments in a lowland tropical rain forest in Costa Rica. J. Ecol. 1984, 72, 553–564. [Google Scholar] [CrossRef]
- Way, D.A. Pearcy RW Sunflecks in trees and forests: From photosynthetic physiology to global change biology. Tree Physiol. 2012, 32, 1066–1081. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Vegetation stress: An introduction to the stress concept in plants. J. Plant Physiol. 1996, 148, 4–14. [Google Scholar] [CrossRef]
- Vishwakarma, K.; Upadhyay, N.; Kumar, N.; Yadav, G.; Singh, J.; Mishra, R.K.; Kumar, V.; Verma, R.; Upadhyay, R.G.; Pandey, M.; et al. Abscisic acid signaling and abiotic stress tolerance in plants: A review on current knowledge and future prospects. Front. Plant Sci. 2017, 8, 161. [Google Scholar] [CrossRef]
- Song, X.H.; Li, H. Effects of building shade on photosynthesis and chlorophyll fluorescence of Euonymus fortunei. Acta Ecol. Sin. 2016, 36, 350–355. [Google Scholar] [CrossRef]
- Hitsuma, G.; Han, Q.; Chiba, Y. Photosynthesis and growth of Thujopsis dolabrata var. hondai seedlings in the understory of trees with various phenologies. J. For. Res. 2017, 17, 156–163. [Google Scholar] [CrossRef]
- Allen, J.F. Cyclic. pseudocyclic and noncyclic photophosphorylation: New links in the chain. Trends Plant Sci. 2003, 8, 15–19. [Google Scholar] [CrossRef]
- Dietzel, L.; Bräutigam, K.; Pfannschmidt, T. Photosynthetic acclimation: State transitions and adjustment of photosystem stoichiometry—Functional relationships between short-term and long-term light quality acclimation in plants. FEBS J. 2008, 275, 1080–1088. [Google Scholar] [CrossRef]
- Schreier, T.B.; Hibberd, J.M. Variations in the Calvin-Benson cycle: Selection pressures and optimization? J. Exp. Bot. 2019, 70, 1697–1701. [Google Scholar] [CrossRef]
- Raven, J.A. The cost of photoinhibition. Physiol. Plantarum. 2011, 142, 87–104. [Google Scholar] [CrossRef]
- Kitao, M.; Yoneda, R.; Tobita, H.; Matsumoto, Y.; Maruyama, Y.; Arifin, A.; Asan, A.; Muhamad, M. Susceptibility to photoinhibition in seedlings of six tropical fruit tree species native to Malasia following transplantation to a degraded land. Trees 2006, 20, 601–610. [Google Scholar] [CrossRef]
- Krause, G.H.; Koroleva, O.Y.; Dalling, W.; Winter, K. Acclimation of tropical tree seedlings to excessive light in simulated tree-fall gaps. Plant Cell Environ. 2001, 24, 1345–1352. [Google Scholar] [CrossRef]
- Fankhauser, C.; Batschauer, A. Shadow on the Plant: A Strategy to Exit. Cell 2016, 164, 15–17. [Google Scholar] [CrossRef]
- Zu, Y.G.; Wei, X.X.; Yu, J.H.; Li, D.W.; Pang, H.H.; Tong, L. Responses in the physiology and biochemistry of Korean pine (Pinus koraiensis) under supplementary UV-B radiation. Photosynthetica 2011, 49, 448. [Google Scholar] [CrossRef]
- Li, X.N.; Wang, Y.H.; Yang, Z.H.; Liu, T.; Mu, C.C. Photosynthesis adaption in Korean pine to gap size and position within Populus davidiana forests in Xiaoxing’anling, China. J. For. Res. 2022, 33, 1517–1527. [Google Scholar] [CrossRef]
- Wang, P.; Grimm, B. Connecting chlorophyll metabolism with accumulation of the photosynthetic apparatus. Trends Plant Sci. 2021, 26, 484–495. [Google Scholar] [CrossRef]
- Osmond, C.B. What is photoinhibition? Some insights from comparison of shade and sun plants. In Photoinhibition of Photosynthesis: From Molecular Mechanisms to the Field; Baker, N.R., Bowyer, J.R., Eds.; BIOS Scientific Publishers: Oxford, UK, 1994; pp. 1–24. [Google Scholar]
- Favaretto, V.F.; Martinez, C.A.; Soriani, H.H.; Furriel, R.P. Differential responses of antioxidant enzymes in pioneer and late-successional tropical tree species grown under sun and shade conditions. Environ. Exp. Bot. 2011, 70, 20–28. [Google Scholar] [CrossRef]
- Bian, Z.H.; Yang, Q.C.; Liu, W.K. Effects of light quality on the accumulation of phytochemicals in vegetables produced in controlled environments: A review. J. Sci. Food Agric. 2015, 95, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Potters, G.; Horemans, N.; Jansen, M.A.K. The cellular redox state in plant stress biology—A charging concept. Plant Physiol. Bioch. 2010, 48, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Fen, Y.; Hai, L. Effects of oxidative stress on growth and development of suspension cells of Populus tomentosa by transcriptome analysis. J. Beijing For. Univ. 2019, 41, 90–98. [Google Scholar]
- Asada, K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Guidi, L.; Lo Piccolo, E.; Landi, M. Chlorophyll fluorescence, photoinhibition and abiotic stress: Does it make any difference the fact to be a C3 or C4 species? Front. Plant Sci. 2019, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Stirbet, A.; Govindjee. On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and Photosystem II: Basics and applications of the OJIP fluorescence transient. J. Photoch. Photobio. B 2011, 104, 236–257. [Google Scholar] [CrossRef]
- Aranda, I.; Castro, L.; AlÍa, R.; Pardos, J.A.; Gil, L. Low temperature during winter elicits differential responses among populations of the Mediterranean evergreen cork oak (Quercus suber). Tree Physiol. 2005, 25, 1085–1090. [Google Scholar] [CrossRef]
- Öquist, G.; Wass, R. A portable, microprocessor operated instrument for measuring chlorophyll fluorescence kinetics in stress physiology. Physiol. Plantarum. 1988, 73, 211–217. [Google Scholar] [CrossRef]
- Dietze, M.C.; Sala, A.; Carbone, M.S.; Czimczik, C.I.; Mantooth, J.A.; Richardson, A.D.; Vargas, R. Nonstructural carbon in woody plants. Annu. Rev. Plant Biol. 2014, 65, 667–687. [Google Scholar] [CrossRef]
- Richardson, A.D.; Carbone, M.S.; Huggett, B.A.; Furze, M.E.; Czimczik, C.I.; Walker, J.C.; Xu, X.M.; Schaberg, P.G.; Murakami, P. Distribution and mixing of old and new nonstructural carbon in two temperate trees. New Phytol. 2015, 206, 590–597. [Google Scholar] [CrossRef]
- Xing, K.X.; Zhao, M.F.; Niinemets, Ü.; Niu, S.L.; Tian, J.; Jiang, Y.; Chen, H.Y.H.; White, P.J.; Guo, D.L.; Ma, Z.Q. Relationships between leaf carbon and macronutrients across woody species and forest ecosystems highlight how carbon is allocated to leaf structural function. Front. Plant Sci. 2021, 12, 674932. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.F.; Wu, W.H. Potassium and phosphorus transport and signaling in plants. J. Integr. Plant Biol. 2021, 63, 34–52. [Google Scholar] [CrossRef]
- Li, J.Q.; Li, J.W. Regeneration and restoration of broad-leaved Korean pine forests in Lesser Xing’an Mountains of Northeast China. Acta Ecol. Sin. 2003, 23, 1268–1277. [Google Scholar]
- Shen, H.L.; Zhang, Q.; Fan, S.H.; Zhao, K.Z.; Yang, W.H. Influence of community structural characteristics of natural secondary forest on the growth of the young trees of Pinus koraiensis. For. Res. 2004, 17, 610–615. (In Chinese) [Google Scholar]
- Shen, H.L.; Cong, J.; Zhang, P.; Zhang, Q.; Fang, S.H.; Yang, W.H.; Liu, S.R. Effect of opening degree regulation on diameter and height increment and aboveground biomass of Korean pine trees planted under secondary forest. Chin. J. Appl. Ecol. 2011, 22, 2781–2791. (In Chinese) [Google Scholar]
- Cong, J.; Shen, H.L.; Yang, W.H.; Fan, S.H.; Zhang, Q. Effect of microenvironmental quantitative regulation on growth of Korean pine trees planted under secondary forest. J. For. Res. 2011, 22, 175–181. [Google Scholar] [CrossRef]
- Chen, X.W.; Li, B.L.; Lin, Z.S. The acceleration of succession for the restoration of the mixed-broadleaved Korean pine forests in Northeast China. For. Ecol. Manag. 2003, 177, 503–514. [Google Scholar] [CrossRef]
- Shen, H.L.; Wang, L.; Lin, C.X.; Cong, J.; Yang, W.H.; Zhang, P.; Zhang, Q.; Fang, S.H. Effect of community structure regulation on upper-layer broad-leaved trees in a mixed forest of planted Korean pine and naturally-regenerated broad-leaved trees. Sci. Silvae Sin. 2014, 50, 22–30. (In Chinese) [Google Scholar]
- Zhang, M.; Zhu, J.J.; Li, M.C.; Zhang, G.Q.; Yan, Q.L. Different light acclimation strategies of two coexisting tree species seedlings in a temperate secondary forest along five natural light levels. For. Ecol. Manag. 2013, 306, 234–242. [Google Scholar] [CrossRef]
- Li, Y.X.; Zhang, X.X.; Cai, K.W.; Zhang, Q.H.; Jiang, L.P.; Li, H.X.; Lv, Y.Z.; Qu, G.Z.; Zhao, X. Y Comparative transcriptomic and metabolic analyses reveal the coordinated mechanisms in Pinus koraiensis under different light stress conditions. Int. J. Mol. Sci. 2022, 23, 9556. [Google Scholar] [CrossRef]
- Makoto, K.; Koike, T. Effects of nitrogen supply on photosynthetic and anatomical changes in current-year needles of Pinus koraiensis seedlings grown under two irradiances. Photosynthetica 2007, 45, 99–104. [Google Scholar] [CrossRef]
- Sun, Y.R.; Zhu, J.J.; Sun, O.J.X.; Yan, Q.L. Photosynthetic and growth responses of Pinus koraiensis seedlings to canopy openness: Implications for the restoration of mixed-broadleaved Korean pine forests. Environ. Exp. Bot. 2016, 129, 118–126. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, Q.J.; Xu, Z.Z.; Du, W.X.X.; Yu, J.; Meng, S.W.; Zhou, H.; Qin, L.H.; Shah, S. How can the shade intolerant Korean pine survive under dense deciduous canopy? Forest Ecol. Manag. 2020, 457, 117735. [Google Scholar] [CrossRef]
- Zhu, J.J.; Wang, K.; Sun, Y.R.; Yan, Q.L. Response of Pinus koraiensis seedling growth to different light conditions based on the assessment of photosynthesis in current and one-year-old needles. J. For. Res. 2014, 25, 53–62. [Google Scholar] [CrossRef]
- Li, J.; Ding, D.X.; Li, N.H.; Xie, J.M.; Yu, J.H.; Lyv, J.; Bakpa, E.P.; Zhang, J.; Wang, C.; Zhang, J.F. Melatonin enhances the low-temperature combined low-light tolerance of pepper (Capsicum annuum L.) seedlings by regulating photosynthesis, carotenoid, and hormone metabolism. Environ. Exp. Bot. 2022, 199, 104868. [Google Scholar] [CrossRef]
- Binkley, D.; Adams, M.; Fredericksen, T.; Laclau, J.P.; Mäkinen, H.H.; Prescott, C. Editors’ note. For. Ecol. Manag. 2015, 349, 1–3. [Google Scholar] [CrossRef]
- Yuan, D.Y.; Zhu, L.J.; Cherubini, P.; Li, Z.S.; Zhang, Y.D.; Wang, X.C. Species-specific indication of 13 tree species growth on climate warming in temperate forest community of northeast China. Ecol. Indic. 2021, 133, 108389. [Google Scholar] [CrossRef]
- Wu, H.B.; Yin, D.S.; Salomón, R.L.; Rodríguez-Calcerrada, J.; Zhang, J.Y.; Zhang, P.; Shen, H.L. Cone-bearing branches of Pinus koraiensis are not carbon autonomous during cone development. Forests 2021, 12, 1257. [Google Scholar] [CrossRef]
- Yang, K.; Zhu, J.J.; Gu, J.C.; Yu, L.Z.; Wang, Z.Q. Changes in soil phosphorus fractions after 9 years of continuous nitrogen addition in a Larix gmelinii plantation. Ann. For. Sci. 2014, 72, 435–442. [Google Scholar] [CrossRef]
- He, J.; Xü, X.; Li, S.H.; Mi, H.L.; Zhang, Y.P.; Zhao, T.C.; Ma, Y.M. Effects of water stress on photosynthetic pigment in leaves and chlorophyll fluorescence of Cynanchum komarovii. Acta Bot. Boreali-Occident. Sin. 2004, 24, 1594–1598. (In Chinese) [Google Scholar]
- Hansen, J.; Møller, I.B. Percolation of starch and soluble carbohydrates from plant tissue for quantitative determination with anthrone. Anal. Biochem. 1975, 68, 87–94. [Google Scholar] [CrossRef]
- Xu, S.; Chen, M.; Feng, T.; Zhan, L.; Zhou, L.; Yu, G. Use ggbreak to effectively utilize plotting space to deal with large datasets and outliers. Front. Genet. 2021, 12, 774–846. [Google Scholar] [CrossRef]
- Valladares, F.; Niinemets, U. The Architecture of Plant Crowns: From Design Rules to Light Capture and Performance. In Handbook of Functional Plant Ecology, 2nd ed.; Pugnaire, F.I., Valladares, F., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 101–149. [Google Scholar]
- Consrabel, A.J.; Lieffers, V.J. Seasonal patterns of light transmission through boreal mixedwood canopies. Can. J. For. Res 1996, 26, 1008–1014. [Google Scholar] [CrossRef]
- Valladares, F.; Niinemets, Ü. Shade tolerance, a key plant feature of complex nature and consequences. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 237–257. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Chen, C.; Jin, Z.X.; Yang, Z.N.; Li, Y.L. Leaf anatomy, photosynthesis, and chloroplast ultrastructure of Heptacodium miconioides seedlings reveal adaptation to light environment. Environ. Exp. Bot. 2022, 195, 104780. [Google Scholar] [CrossRef]
- Valladares, F.; Arrieta, S.; Aranda, I.; Lorenzo, D.; Sánchez-Gómez, D.; Tena, D.; Suárez, F.; Pardos, J.A. Shade tolerance, photoinhibition sensitivity and phenotypic plasticity of Ilex aquifolium in continental Mediterranean sites. Tree Physiol. 2005, 25, 1041–1052. [Google Scholar] [CrossRef]
- Griffin, J.J.; Ranney, T.G.; Pharr, D.M. Photosynthesis, chlorophyll fluorescence, and carbohydrate concentration of Illicium taxa grown under varied irradiance. J. Amer. Soc. Hort. Sci. 2004, 129, 46–53. [Google Scholar] [CrossRef]
- Fanciullino, A.L.; Bidel, L.P.; Urban, L. Carotenoid responses to environmental stimuli: Integrating redox and carbon controls into a fruit model. Plant Cell Environ. 2014, 37, 273–289. [Google Scholar] [CrossRef]
- Carmo-Silva, E.; Scales, J.C.; Madgwick, P.J.; Parry, M.A. Optimizing Rubisco and its regulation for greater resource use efficiency. Plant Cell Environ. 2015, 38, 1817–1832. [Google Scholar] [CrossRef]
- Banks, J.M. Continuous excitation chlorophyll fluorescence parameters: A review for practitioners. Tree Physiol. 2017, 37, 1128–1136. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Linkosalo, T.; Heikkinen, J.; Pulkkinen, P.; Mäkipää, R. Fluorescence measurements show stronger cold inhibition of photosynthetic light reactions in Scots pine compared to Norway spruce as well as during spring compared to autumn. Front. Plant Sci. 2014, 5, 264. [Google Scholar] [CrossRef] [PubMed]
- Ruban, A.V. Nonphotochemical chlorophyll fluorescence quenching: Mechanism and effectiveness in protecting plants from photodamage. Plant Physiol. 2016, 170, 1903–1916. [Google Scholar] [CrossRef] [PubMed]
- Wyka, T.; Robakowski, P.; Żytkowiak, R. Acclimation of leaves to contrasting irradiance in juvenile trees differing in shade tolerance. Tree Physiol. 2007, 27, 1293–1306. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Zhang, Q.H.; Shuang, S.P.; Cun, Z.; Wu, H.M.; Chen, J.W. The responses of light reaction of photosynthesis to dynamic sunflecks in a typically shade-tolerant species Panax notoginseng. Front. Plant Sci. 2021, 12, 718981. [Google Scholar] [CrossRef]
- Hartmann, H.; Trumbore, S. Understanding the roles of nonstructural carbohydrates in forest trees—From what we can measure to what we want to know. New Phytol. 2016, 211, 386–403. [Google Scholar] [CrossRef]
- Yan, L.; Zhang, Z.; Jin, G.; Liu, Z. Variations of leaf nonstructural carbohydrates in an evergreen coniferous species: Needle age and phenology dominate over life history. Ecol. Indic. 2022, 136, 108685. [Google Scholar] [CrossRef]
- Xu, M.L.; Hu, T.Q.; Poethig, R.S.S. Low light intensity delays vegetative phase change. Plant Physiol. 2021, 187, 1177–1188. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Akram, N.A.; Ashraf, M. Osmoprotection in plants under abiotic stresses: New insights into a classical phenomenon. Planta 2019, 251, 3. [Google Scholar] [CrossRef]
- Chazdon, R.L.; Pearcy, R.W. The importance of sunflecks for forest understory plants. BioScience 1991, 41, 760–766. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Breusegem, F.V. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef]
- Marler, T.E.; Krishnapillai, M.V. Incident light and leaf age influence leaflet element concentrations of Cycas micronesica trees. Horticulturae 2019, 5, 58. [Google Scholar] [CrossRef]
- Das, S.K.; Patra, J.K.; Thatoi, H. Antioxidative response to abiotic and biotic stresses in mangrove plants: A review. Int. Rev. Hydrobiol. 2016, 101, 3–19. [Google Scholar] [CrossRef]
- Shafi, A.; Zahoor, I.; Mushtaq, U. Proline Accumulation and Oxidative Stress: Diverse roles and Mechanism of Tolerance and Adaptation under Salinity Stress. Salt Stress, Microbes, and Plant Interactions: Mechanisms and Molecular Approaches; Springer: Berlin/Heidelberg, Germany, 2019; Volume 2, pp. 269–300. [Google Scholar]
- Forlani, G.; Trovato, M.; Funck, D.; Signorelli, S. Regulation of proline accumulation and its molecular and physiological functions in stress defence. In Osmoprotectant-Mediated Abiotic Stress Tolerance in Plants: Recent Advances and Future Perspectives; Springer: Berlin/Heidelberg, Germany, 2019; pp. 73–97. [Google Scholar]
- Rajendrakumar, C.S.V.; Reddy, B.V.B.; Reddy, A.R. Proline-protein interactions: Protection of structural and functional integrity of M4 lactate dehydrogenase. Biochem. Biophys. Res. Commun. 1994, 201, 957–963. [Google Scholar] [CrossRef]
- Sharma, P.; Dubey, R.S. Modulation of nitrate reductase activity in rice seedlings under aluminium toxicity and water stress: Role of osmolytes as enzyme protectant. J. Plant Physiol. 2005, 162, 854–864. [Google Scholar] [CrossRef]
- Mishra, S.; Dubey, R.S. Inhibition of ribonuclease and protease activities in arsenic exposed rice seedlings: Role of proline as enzyme protectant. J. Plant Physiol. 2006, 163, 927–936. [Google Scholar] [CrossRef]
- Smirnoff, N.; Cumbes, Q.J. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry 1989, 28, 1057–1060. [Google Scholar] [CrossRef]
- Matysik, J.; Bhalu, A.B.; Mohanty, P. Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants. Curr. Sci. 2002, 82, 525–532. [Google Scholar]
- Wang, F.J.; Zeng, B.; Sun, Z.X.; Zhu, C. Relationship between proline and Hg2+-induced oxidative stress in a tolerant rice mutant. Arch. Environ. Contam. Toxicol. 2009, 56, 723–731. [Google Scholar] [CrossRef]
- Alia, P.S.; Mohanty, P. Involvement of proline in protecting thylakoid membranes against free radical-induced photodamage. J. Photochem. Photobiol. 1997, 38, 253–257. [Google Scholar] [CrossRef]
- Yang, C.W.; Li, L. Hormonal regulation in shade avoidance. Front. Plant Sci. 2017, 8, 1527. [Google Scholar] [CrossRef] [PubMed]
- Cagnola, J.I.; Ploschuk, E.; Benech-Arnold, T.; Finlayson, S.A.; Casal, J.J. Stem transcriptome reveals mechanisms to reduce the energetic cost of shade-avoidance responses in tomato. Plant Physiol. 2012, 160, 1110–1119. [Google Scholar] [CrossRef] [PubMed]
- Klem, K.; Gargallo-Garriga, A.; Rattanapichai, W.; Oravec, M.; Holub, P.; Vesela, B.; Sardans, J.; Penuelas, J.; Urban, O. Distinct morphological, physiological, and biochemical responses to light quality in barley leaves and roots. Front. Plant Sci. 2019, 10, 1026. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Dai, J.; Ge, Q. Responses of autumn phenology to climate change and the correlations of plant hormone regulation. Sci. Rep. 2020, 10, 9039. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2009, 15, 89–97. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).