Abstract
Forest protection against eastern spruce budworm, Choristoneura fumiferana (Clemens), relies on aerial applications of Bacillus thuringiensis (Btk). However, Btk prescriptions have been developed for balsam fir (Abies balsamea (L.) Mill.) stands, generating uncertainty as to the relevance of this protection approach on white spruce (Picea glauca (Moench) Voss). The main goal of this study was to evaluate the efficacy of three Btk application treatments (early application, late application, and double application) for protecting white spruce and balsam fir in mixed stands. Our results show that all Btk treatments tested kept defoliation under the 50% threshold on balsam fir (27.7 to 38.1% less defoliation than in controls). In contrast, differences in defoliation among treatments were not significant for white spruce. Larval mortality was significantly lower on white spruce than on balsam fir. The low efficacy of Btk treatments on white spruce may be explained by its shoot phenology (most bud caps were still present during the early application), and its foliar chemistry, which could decrease Btk efficacy. Consequently, many larvae may survive simply because Btk spores do not reach them, or because they may consume sublethal doses. We recommend maintaining the current strategy in mixed stands, as it provides a good protection for balsam fir. Further research is needed to determine the causes of the low efficacy of Btk treatments on white spruce.
1. Introduction
White spruce (Picea glauca (Moench) Voss) is a major component of Canadian forest ecosystems and is intensively harvested for lumber production [1]. Given its economic value, it is one of the most widely used species in breeding and reforestation programs in Canada, e.g., [1,2]. In Quebec, white spruce abundance began to decline during the 19th century due to selective logging, e.g., [3]. This decline continued during the 20th century with the rise of the pulp and paper industry, which required large volumes of wood that were obtained by large-scale clearcutting, which favored balsam fir (Abies balsamea (L.) Mill.). Efforts to restore white spruce are currently underway in Quebec, where about 25 million seedlings have been planted each year in balsam fir ecosystems since 2009 [4].
Eastern spruce budworm, Choristoneura fumiferana (Clemens) (Lepidoptera: Tortricidae) (hereafter spruce budworm), feeds on several conifer hosts, with the damage being most important on balsam fir, followed by white spruce, red spruce (Picea rubens Sarg.), and black spruce (Picea mariana (Mill.) BSP). In the past, outbreaks of this native defoliator occurred periodically, at intervals of about 35 years [5,6], leading to a substantial decline in vigour and increased mortality of affected trees [7]. On white spruce, defoliation by the spruce budworm may produce growth suppression from the second year of severe defoliation [8,9] and tree mortality after 6 to 7 consecutive years of severe defoliation [7,10].
The approach currently used to protect Canadian forests against spruce budworm has been developed for balsam fir stands, and involves large-scale aerial spray operations with the biological insecticide Bacillus thuringiensis ssp. kurstaki (Btk), e.g., [11,12]. In Quebec, spraying operations aim to protect at least 50% of current-year foliage, to ensure tree survival during outbreaks. Biological insecticide application begins after one year of moderate to severe defoliation, and the first application is generally synchronized with the bud break of balsam fir, which usually occurs at the peak of the third larval instar [11,12]. A second Btk application may be needed if high larval density is expected, staminate flowers are produced, or if the photosynthetic capacity of trees is expected to be reduced to under 38% [12]. This protection approach has usually been effective in keeping defoliation under the 50% threshold in balsam fir stands [13,14,15]. However, the literature shows that the aerial spraying operations may be less effective to protect white spruce foliage, e.g., [16,17,18].
Previous studies have shown that white spruce characteristics may affect the effectiveness of Btk applications. Specifically, white spruce foliar chemistry and shoot phenological development have been identified as factors that may decrease Btk efficacy [13]. For example, Carisey et al. [13] reported that Btk produced lower larval mortality for spruce budworm feeding on white spruce compared to those feeding on balsam fir foliage. This lower Btk efficacy in white spruce trees has been related to the higher tannin concentrations in its foliage compared with that of balsam fir [13]. However, the antagonistic effect of tannins on Btk efficacy also seems to be affected by the concentrations of both tannins and Btk toxin [19]. In addition, white spruce shoots retain their bud cap for a few weeks after bud break, unlike balsam fir, whose needles break the bud cap. Consequently, larvae feeding on white spruce may be protected by the bud cap when the first Btk application is done against the spruce budworm, and this may lower Btk efficacy, e.g., [20] as shown in Carisey et al. [13]. Moreover, a feeding inhibition of 3–5 days has been reported on balsam fir after Btk spray [21], but how long it lasts on white spruce is unknown. It is therefore necessary to evaluate the efficacy of Btk treatment on white spruce, and to study the factors that may affect its efficacy, to determine the relevance of the current strategy, especially in terms of the timing of the treatment application, given the differences in shoot phenology and larval development between balsam fir and white spruce, to optimize the protection of white spruce in mixed forests and plantations. To do this, we developed a novel method which included all treatments in one experimental site, by using sleeve cages as barriers to protect branches from Btk deposit. In this way, we overcame problems such as high heterogeneity among sites and trees, and variation in environmental conditions during Btk spray application. The main goal of this study was to evaluate the efficacy of three Btk application treatments (early application, late application, and double application) on larval mortality and final defoliation on white spruce and balsam fir in mixed stands. We also evaluated the impact that host tree foliage characteristics may have on the acquisition of a lethal dose by assessing the proportion of larvae exhibiting feeding inhibition and the time to resume feeding, to properly synchronize the timing of the second application of Btk in white spruce-balsam fir mixed forests.
2. Materials and Methods
2.1. Site Description
Results from the 2017 fall L2 survey, carried out in preparation of the 2018 protection program, were used to select potential sites that met the double-Btk application criteria (≥40 L2 per 45 cm branch tip) in white spruce. This survey involved cutting branches at the mid-crown level of six randomly selected trees, and extracting overwintering larvae using the method described by Miller et al. [22]. The experimental site retained was easily accessible and located in a 35-year-old mixed balsam fir-white spruce stand that covered 43 ha within the limits of Causapscal in the Bas-Saint-Laurent region, Quebec, Canada (48°35′30″ N 66°56′30″ W) (Figure 1). A second L2 survey was carried out in the experimental site in spring 2018, in order to make sure that the experimental site met the criteria for a double application of Btk (≥40 L2 per 45 cm branch tip) in white spruce. When L2 emergence was forced using Sanders’ technique [23], population estimates were around 50% (i.e., 20 L2 per branch) of the threshold required for double application of Btk. To ensure populations met or exceeded the double application criteria, 40 third instar larvae (L3) were collected from surrounding trees one day prior to placement on each experimental branch. Larval species were confirmed as spruce budworm prior to placement.
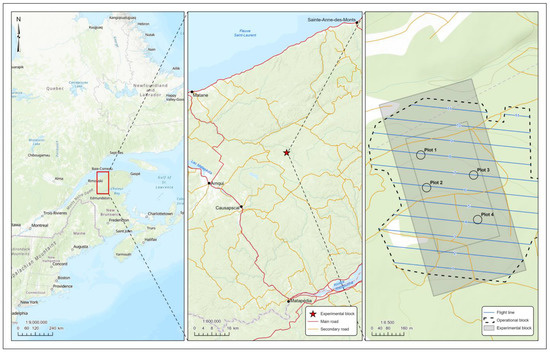
Figure 1.
Location of the experimental site, and diagram of the experimental design. The plots containing the selected trees are located under the flight lines.
2.2. Experimental Design
Two factors were tested: (i) Btk treatments (unsprayed, early application (16 June 2018), late application (22 June 2018), and double application (16 and 22 June 2018)), and (ii) host tree species (balsam fir and white spruce). Twenty balsam fir and twenty white spruce codominant trees were randomly selected along transects perpendicular to each of four flight lines of the aircraft applying Btk formulations in the selected site (Figure 1), yielding five trees of each species per flight line. To avoid spatial autocorrelation, trees selected along a given transect were at least 40 m apart [24]. Four branches were selected in the mid-crown of each tree. Branches were then covered with two-layer sleeve cages or barriers to prevent Btk deposition on needles. Then, branches of each tree covered with sleeve cages were randomly assigned to one of the four Btk treatments (unsprayed, early application, late application, and double application). Prior to the first Btk application, sleeve cages were removed from branches assigned to the early and double application treatments, and reinstalled a few hours after spraying. Then, prior to the second Btk application, sleeve cages were removed from branches assigned to the late and double application scenarios, and reinstalled a few hours after spraying. For branches assigned to the unsprayed treatment, sleeve cages remained in place for the entire duration of the experiment.
2.3. Btk Formulation and Aerial Spraying
Foray 76B, a Btk strain HD-1 commercial formulation at nominal potency of 20.0 billion international units per litre (BIU/L) (Abbott Laboratories, Chicago, IL, USA; on behalf of Valent BioSciences Corporation, Libertyville, IL, USA) was applied to the experimental area. The aircraft C-GMTG Air tractor 504TM was equipped with six MicronairTM atomizers (Micronair Sprayers Ltd., Bromyard, UK). MicronairTM atomizers, spinning at 8000 rpm, were located within 75% of the total wingspan. The aircraft was flying at a speed varying between 227 and 231 km/h, with 80 m spray widths. Aerial sprays were done in the early morning under good weather conditions (no rain and maximum wind speed of 16 km/h). The flow rate through the nozzles was calibrated to deliver 1.5 L/ha or 30 BIU/ha. The timing of Btk applications was based on balsam fir bud development, following the scheme of Auger [25]. The first application of Btk targets balsam fir bud break, when all needles are visible but not flaring. In our field experiment, the first Btk application was done on 16 June 2018, after the peak of the third instar (L3) larvae of the spruce budworm (development index of 3.5 on balsam fir and 3.7 on white spruce), whereas the second application was done 6 days later, when budworm larvae were near the peak of the fourth instar (L4) (development index of 3.9 on balsam fir and 4.2 on white spruce).
2.4. Spray Deposit
Spray deposit on host foliage was assessed by collecting two current-year shoots from unprotected branches of each tree after the second Btk application, as well as from branches previously covered by one- or two-layer sleeve cages placed on additional branches of each tree used in the field experiment. Quantitative assessment of the Btk deposit on foliage was determined using a microplate redox assay technique. This technique was described by van Frankenhuyzen et al. [21] and Seligy and Rancourt [26] and consists of spiking microplate wells with known-volume aliquots, derived from washes of each individual shoot samples. Quantification relies on a differential bioreduction in XTT dye, which is directly correlated with Btk levels (enzyme activity of vegetative cells germinating from spores under controlled incubation conditions of the assay), in reference to calibration curves derived from serial dilution of the formulation applied in each trial. The assay technique is highly sensitive, capable of detecting a single Btk spore, and is considered a significant improvement in quantification of Btk [21]. Btk deposition was expressed as the average number of spores deposited per gram of dry weight foliage.
2.5. Evaluation of Treatment Efficacy
The 45 cm tip of each branch used in the field experiment was collected from each of the 20 balsam firs and 20 white spruces when 85% of the larvae had reached the pupal stage. Spruce budworm pupae and remaining larvae were counted in the laboratory, and defoliation was estimated on current-year shoots using the Fettes grid [27,28]. Budworm density on branches always covered by two-layer sleeve cages (unsprayed scenario) was used as the control to estimate mortality from Btk spray scenarios on each tree, using this equation:
Mortality (%) = (Densityunsprayed scenario − DensityBtk spray scenario)/Densityunsprayed scenario × 100
Densityunsprayed scenario = Density on branches that did not receive Btk (always covered with two-layer sleeve cages; unsprayed scenario).
DensityBtk spray scenario = Density on branches that received Btk, according to one of the three scenarios.
2.6. Feeding Inhibition
The proportion of larvae that exhibited feeding inhibition resulting from the ingestion of sub-lethal doses of Btk, and the time to resume feeding, was assessed for 135 larvae collected on each host tree one day before the first Btk application, and the same number one day after the first Btk application, yielding 540 larvae. Larvae were brought back to the laboratory and placed individually in 1 oz Solo cups half-filled with artificial diet [29] in a rearing room (23 °C, 60% RH, 16L:8D photoperiod). Feeding behaviour of individual larvae was monitored daily for six days, in order to detect signs of feeding resumption (i.e., frass production). The day larvae resumed feeding was recorded.
2.7. Statistical Analysis
All analysis were performed using R version 4.0.2 (R Core Team 2020). All models were fitted using the “lme4” package [30]. We first evaluated the significance of the random effect using a likelihood ratio test [31]. If the random effect was not significant, a linear model was fitted instead, using the functions lm or glm. We compared spray deposit among barrier (sleeve cage) treatments using linear mixed models (LMM). Prior to model fitting, spray deposition data were normalized using the “bestNormalize” package [32], which automatically evaluates, chooses, and applies the best procedure to normalize data. The lmer procedure was used to fit a model with a barrier treatment (no sleeve cage vs. one sleeve cage vs. two sleeve cages per branch) and host species (balsam fir vs. white spruce) as fixed effects, and trees nested within flight line as random effect to consider spatial heterogeneity and unmeasured sources of variation at the tree level. Tukey–Kramer tests from the multcomp package [33] were used to contrast barrier treatments and host trees.
To test for an effect of Btk spray scenario (control vs. early application vs. late application vs. double application) and host species on defoliation, we fitted a linear mixed model (LMM). Host species, Btk treatments, and their interactions were considered fixed effects, and trees nested within flight line were included as random effects.
We compared larval mortality among treatments using generalized linear models (GLM) that accounted for binomial distributions. The glm procedure was used to fit a model with Btk treatment and host species and their interaction as fixed effects, assuming binomial distribution. Differences between the levels of significant fixed effects were verified using pairwise comparisons, with Tukey’s correction on the marginal means estimated by the models using the “emmeans” package [34].
To analyse the effect of Btk treatment (control vs. early application) and host species on larval feeding resumption in the feeding inhibition experiment, we produced Kaplan–Meier curves for each treatment combination, and compared them using a log-rank test (p < 0.05) in the “survival” package [35].
3. Results
Our results showed that sleeve cages were efficient in preventing the deposition of Btk spores on needles, a significant difference existing when a barrier treatment is used (Table 1). However, adding a second layer to the sleeve cages does not significantly reduce the deposition of Btk spores. Nevertheless, compared to the control (no sleeve cage), the two- and one-layer sleeve cage treatments reduced the amount of Btk spores per gram of foliage dry weight by 96.1% and 84.9% on balsam fir, and by 98.3% and 83.3% on white spruce (Table 2). Host species, and interaction between host species and barrier treatment, were not statistically different (Table 1).

Table 1.
Influence of host tree species (balsam fir and white spruce) and barrier treatments (0 vs. 1 vs. 2 sleeve cages on branches) on Btk spray deposition.

Table 2.
Spray deposition (spores/g dry weight) on current-year shoots of balsam fir and white spruce protected by 0, 1 or 2 sleeve cages (LSMEANS ± SEM).
A significant interaction between host species and Btk spray treatments was detected for defoliation (Table 3), indicating that the response to the Btk spray treatments differed between the two coniferous species (Figure 2). The double-application treatment of Btk reduced defoliation significantly on balsam fir, but not on white spruce (Figure 2). Similarly, early and late applications significantly reduced the defoliation of balsam fir (27.7% and 29.1%, respectively) compared to control, but no significant difference was noticed on white spruce (Figure 2).

Table 3.
Influence of host tree species (balsam fir and white spruce) and Btk spray treatments (unsprayed, early application, late application, and double application) on tree defoliation.
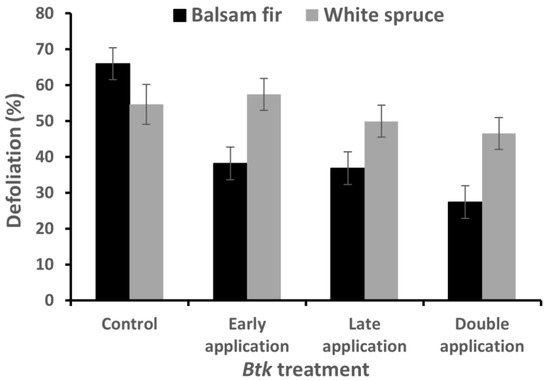
Figure 2.
Final defoliation tabulated by Btk treatment and host species (lsmeans ± SEM).
Larval mortality was significantly lower on white spruce than on balsam fir, and on branches that received the early application treatment, compared to the late and double application treatments (Table 4; Figure 3). There was no interaction between the two factors (Table 4).

Table 4.
Influence of host tree species (balsam fir and white spruce) and Btk spray scenarios (early application, late application, and double application) on spruce budworm larval mortality *.
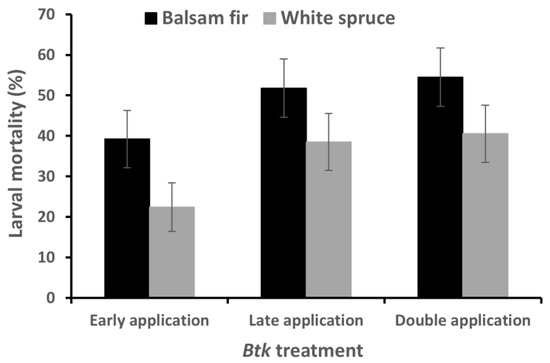
Figure 3.
Mortality of spruce budworm larvae (LSMEANS ± SEM) exposed to three different field applied Btk treatments.
Kaplan–Meier curves comparing the cumulative feeding resumption rates on each tree species and Btk treatment (control vs. one application) were significantly different (log-rank = 135, df = 3, p < 0.0001). They showed that over 50% of larvae from untreated trees of both species resumed feeding by the second day, and at least 80% of larvae from both hosts had resumed feeding by the end of the experiment (day 6). In fact, the feeding resumption rate on control trees was always slower on white spruce than on balsam fir, but it followed the same trend on both tree species (Figure 4). In contrast, the feeding resumption rate was similar for larvae collected on sprayed balsam fir and white spruce for the first three days (Figure 4). Then, the feeding resumption rate increased slower for larvae collected on sprayed balsam fir than on sprayed white spruce, reaching 53% by the fifth day and 58% by the end of the experiment on balsam fir, compared to 68% and 72% on white spruce (Figure 4).
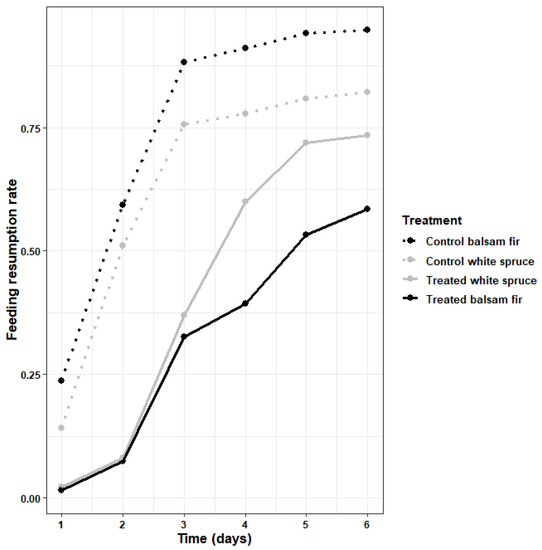
Figure 4.
Cumulative feeding resumption rate tabulated by Btk treatment (control vs. one application) and host species.
4. Discussion
This study showed that sleeve cages protect balsam fir and white spruce branches from Btk deposit, allowing comparison of the effect of various Btk treatments at the tree level within a single stand. In the past, such study required numerous replicated stands, e.g., [11,13,36], which added several sources of variation related to stand location and characteristics (e.g., topography, tree density, stand composition). This affects local climatic conditions and, thus, insect larval development. Aerial application of Btk needs favourable meteorological conditions (low wind, no rain, low UV), which only occur for a few hours a day, usually early in the morning and in the evening [12]. It is almost impossible to standardise conditions for an aerial application of Btk in numerous stands. When a double application is tested, it increases this variability. Even if differences were not significant, we recommend using double-layer sleeve cages, as it reduces Btk spore density by 96%–98%, compared to 83%–85% for single-layer sleeve cages.
This field study showed that aerial application of Btk was more effective in reducing balsam fir defoliation than white spruce. Indeed, all Btk treatments tested kept defoliation under the 50% threshold on balsam fir and produced reductions ranging between 27.7 and 38.1% of defoliation compared with control. These results are in agreement with the literature, e.g., [11,12,13,14,15] and confirm that the approach currently used in the province of Quebec provides good protection to balsam fir stands. In contrast, only the late and double application treatments achieved the foliage protection objective on white spruce, but their effectiveness in reducing defoliation (4.7 and 8.1% less defoliation than in controls, respectively) was either nonsignificant or lower than reported in previous studies, which ranged from 14 to 65% [13,14,15,16,17,18]. In fact, the literature indicates that the efficacy of Btk aerial applications against the spruce budworm is much more variable on white spruce than on balsam fir. We observed that most bud caps were still present on white spruce current-year shoots during the early application, and this could explain, at least in part, this inefficacy of the early application of Btk on white spruce. As a result, many larvae may survive simply because Btk spores do not reach them, or because they may consume sublethal doses. This hypothesis is supported by results of the feeding inhibition experiment in which larvae resumed feeding more rapidly on white spruce than on balsam fir. Moreover, larvae feeding on Btk-treated foliage not only resumed feeding faster on white spruce than they did on balsam fir (50% resumed feeding on white spruce after 4 days vs. 5 days on balsam fir), but more larvae were feeding normally at the end of the experiment (around 15% more larvae) than larvae collected on Btk-treated balsam fir foliage. These results suggest that larvae feeding on white spruce were more likely to consume a sublethal dose of Btk than those feeding on balsam fir. A higher survival means that residual larval populations may eat greater foliage biomass on white spruce than on balsam fir. Spray deposition on white spruce should be further investigated to determine if it can influence the effectiveness of Btk against spruce budworm.
Foliar chemistry may also play an important role in the efficacy of Btk formulations in white spruce. In fact, white spruce phenology and foliar chemistry could explain, at least in part, differences in foliage protection between balsam fir and white spruce. Tannins may reduce Btk efficacy against lepidopterous insects by decreasing the toxicity of Btk [37,38,39,40]. For example, tannins have been reported to have a negative impact on Btk toxicity in the case of Lymantria dispar (L.) [37,38], Pieris brassicae (L.) [39], and Heliothis virescens [40]. As for spruce budworm, Bauce et al. [19] found that at high Btk concentrations, tannin antagonized Btk potency against spruce budworms, and lowered Btk-related spruce budworm larval mortality from 83 to 43%. This effect may be related to the protein precipitating ability of tannins [41], which may have led to inactivation of the Btk proteinaceous toxin. The lower Btk efficacy in white spruce trees observed in our study may therefore be related to the higher tannin concentrations in foliage compared with that of balsam fir, e.g., [13].
5. Conclusions
Our results show that the current Btk aerial spray prescriptions used in the province of Quebec are efficient in terms of protecting foliage of balsam fir against spruce budworm. However, the efficacy of aerial spraying operations is reduced when applied to white spruce. This phenomenon could simply reflect the fact that these protection strategies were developed to protect balsam fir, e.g., [11]. As a result, the dosage or timing of insecticide applications might not have been optimal to protect white spruce trees. Nevertheless, results from this trial suggest that white spruce characteristics, such as foliar chemistry and phenology, may be more important to explain the low efficacy of the Btk treatments observed. As a result, we recommend maintaining the current strategy in mixed stands, as it provides a good control of budworm populations on balsam fir, a highly vulnerable tree species. In turn, the early and late applications provided similar results, which suggest that the time window for a single application of Btk at the stand level is greater than one week. However, if two applications are needed, it is recommended to target balsam fir bud burst for the first application. For white spruce, further research is needed under different field conditions to confirm the low efficacy of Btk in reducing defoliation observed in this study, to assess the importance of the bud cap as a protective shelter for spruce budworm larvae, and to determine when it falls off. Finally, the impact of spray droplet size and droplet density on efficacy of Btk in white spruce should be studied to determine if the operational spray deposit obtained under current application prescriptions delivers a lethal dose, and the proportion of needles that receive a lethal spray deposit in this host species. Finally, we think that the approach developed in this study may be very useful to design efficacy trials in the future, because it allows inclusion of several treatments in the same experimental site. This, in turn, may help to overcome several logistical, operational and experimental challenges that often affect this type of trial.
Author Contributions
Conceptualization, A.D., R.B., C.H. and É.B.; methodology, A.D., R.B., C.H. and É.B.; formal analysis, A.F.; investigation, É.P.-B.; resources, A.D. and É.B.; writing—original draft preparation, A.F.; writing—review and editing, A.D., R.B., C.H., A.F., É.P.-B. and É.B.; visualization, A.F.; supervision, A.D., R.B., C.H. and É.B.; project administration, A.D. and É.B.; funding acquisition, A.D. and É.B. All authors have read and agreed to the published version of the manuscript.
Funding
This project was funded by iFor Research Consortium through contributions from the Natural Sciences and Engineering Research Council of Canada (NSERC RDCPJ 479394-15), the Ministère des ressources naturelles et des forêts du Québec (MRNFQ), the Quebec Forest Industry Council (QFIC), the CFS, and SOPFIM.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank the technicians of SOPFIM and the staff of the laboratory of forest entomology at Université Laval (Martin Charest, Ioan Nicolae, Jonathan Guérard-Poirier et Sandrine Corriveau) for their time and efforts dedicated to this project. Special thanks to Sylvie Lemieux at SOPFIM for the analyses of Btk spray deposit and to le Service de Géomatique de la SOPFIM for producing the maps in Figure 1.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Farrar, J.L. Trees in Canada; Canadian Forest Service, Natural Resources Canada: Ottawa, ON, Canada, 1995; 502p. [Google Scholar]
- Beaulieu, J. Breeding Program and Strategy for White Spruce in Quebec; Canadian Forest Service, Natural Resources Canada: Ottawa, ON, Canada, 1996; Information Report LAU-X-117E; ISBN 978-0-662-24422-6. [Google Scholar]
- Boucher, Y.; Arseneault, D.; Sirois, L. Logging history (1820–2000) of a heavily exploited southern boreal forest landscape: Insights from sunken logs and forestry maps. For. Ecol. Manag. 2009, 258, 1359–1368. [Google Scholar] [CrossRef]
- Ministère des Forêts, de la Faune et des Parcs (MFFP). Ressources et Industries Forestières du Québec. Portrait Statistique; MFFP: Québec, QC, Canada, 2019. Available online: https://mffp.gouv.qc.ca/wp-content/uploads/PortraitStatistique_2019.pdf. (accessed on 19 October 2021).
- Royama, T. Population dynamics of the spruce budworm Choristoneura fumiferana. Ecol. Monogr. 1984, 54, 429–462. [Google Scholar] [CrossRef]
- Jardon, Y.; Morin, H.; Dutilleul, P. Périodicité et synchronisme des épidémies de la tordeuse des bourgeons de l’épinette au Québec. Can. J. For. Res. 2003, 33, 1947–1961. [Google Scholar] [CrossRef]
- MacLean, D.A. Vulnerability of fir-spruce stands during uncontrolled spruce budworm outbreaks: A review and discussion. For. Chron. 1980, 56, 213–221. [Google Scholar] [CrossRef]
- Blais, J.R. Effects of defoliation by spruce budworm (Choristoneura fumiferana Clem.) on radial growth at breast height of balsam fir (Abies balsamea (L.) Mill.) and white spruce (Picea glauca (Moench) Voss). For. Chron. 1958, 34, 39–47. [Google Scholar] [CrossRef]
- Piene, H. The sensitivity of young white spruce to spruce budworm defoliation. N. J. Appl. For. 1991, 8, 168–171. [Google Scholar] [CrossRef]
- Blais, J.R. Mortality of balsam fir and white spruce following a spruce budworm outbreak in the Ottawa River watershed in Quebec. Can. J. For. Res. 1981, 11, 620–629. [Google Scholar] [CrossRef]
- Bauce, É.; Carisey, N.; van Frankenhuyzen, K.; Dupont, A. Bacillus thuringiensis subsp. kurstaki (Btk) aerial spray prescriptions for balsam fir protection against spruce budworm, Choristoneura fumiferana (Lepidoptera: Tortricidae). J. Econ. Entomol. 2004, 97, 624–634. [Google Scholar] [CrossRef]
- SOPFIM. Programmes de Pulvérisation Aérienne d’Insecticide Biologique (Btk) Contre la Tordeuse des Bourgeons de l’épinette; Rapport de Réalisation des Travaux; SOPFIM: Quebec, QC, Canada, 2020; 105p. [Google Scholar]
- Carisey, N.; Bauce, É.; Miron, S.; Dupont, A. Effects of bud phenology and foliage chemistry of balsam fir and white spruce trees on the efficacy of Bacillus thuringiensis against the spruce budworm, Choristoneura fumiferana. Agric. For. Entomol. 2004, 6, 55–69. [Google Scholar] [CrossRef]
- Fuentealba, A.; Bauce, É.; Dupont, A. Bacillus thuringiensis efficacy in reducing spruce budworm damage as affected by host tree species. J. Pest Sci. 2015, 88, 593–603. [Google Scholar] [CrossRef]
- Fuentealba, A.; Dupont, A.; Hébert, C.; Berthiaume, R.; Quezada-Garcia, R.; Bauce, É. Comparing the efficacy of various aerial spraying scenarios using Bacillus thuringiensis to protect trees from spruce budworm defoliation. For. Ecol. Manag. 2019, 432, 1013–1021. [Google Scholar] [CrossRef]
- Campbell, D.; Campbell, M.; Moore, R.; Thompson, J.; Meating, J.H.; Bolan, P.M.; Francis, M.W. The eastern spruce budworm in Saskatchewan—1999. In Forest Pest Management Forum Report; Natural Resources Canada: Ottawa, ON, Canada, 1999; 9p. [Google Scholar]
- Kettela, E.G. Evaluation of the Efficacy of Foray 96B (ABG-6470) against Spruce Budworm in Ontario in 1999; SERG-I Project 95/07 Comp. 5. Final Report; Great Lakes Forestry Centre: Sault Ste. Marie, ON, Canada, 2001; 27p. [Google Scholar]
- Magnussen, S.; Alfaro, R.I.; Boudewyn, P. Survival-time analysis of white spruce during spruce budworm defoliation. Silva Fenn. 2005, 39, 177–189. [Google Scholar] [CrossRef]
- Bauce, É.; Kumbaşlı, M.; van Frankenhuyzen, K.; Carisey, N. Interactions among white spruce tannins, Bacillus thuringiensis subsp. kurstaki, and spruce budworm (Lepidoptera: Tortricidae), on larval survival, growth, and development. J. Econ. Entomol. 2006, 99, 2038–2047. [Google Scholar] [CrossRef]
- Volney, W.J.A.; Cerezke, H.F. The phenology of white spruce and the spruce budworm in northern Alberta. Can. J. For. Res. 1992, 22, 198–205. [Google Scholar] [CrossRef]
- van Frankenhuyzen, K.; Nystrom, C.; Dedes, J.; Seligy, V. Mortality, feeding inhibition, and recovery of spruce budworm (Lepidoptera: Tortricidae) larvae following aerial application of a high-potency formulation of Bacillus thuringiensis subsp. kurstaki. Can. Entomol. 2000, 132, 505–518. [Google Scholar] [CrossRef]
- Miller, C.A.; Kettela, E.G.; McDougall, G.A. A Sampling Technique for Overwintering Spruce Budworm and Its Applicability to Population Surveys; Rep. M-X-25; Canadian Forest Service, Department of Fisheries and Forestry: Fredericton, NB, Canada, 1971; 12p. [Google Scholar]
- Sanders, C.J. A Summary of Current Techniques Used for Sampling Spruce Budworm Populations and Estimating Defoliation in Eastern Canada; Rep. O-X-306; Canadian Forest Service, Great Lakes Forestry Centre: Sault Ste. Marie, ON, Canada, 1980; 34p. [Google Scholar]
- van Frankenhuyzen, K.; Reardon, R.C.; Dubois, N.R. Forest defoliators. In Field Manual of Techniques in Invertebrate Pathology: Application and Evaluation of Pathogens for Control of Insects and Other Invertebrate Pests, 2nd ed.; Lacey, L.A., Kaya, H.K., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 481–504. [Google Scholar]
- Dorais, L.G.; Kettela, E. Revue, Par Région, des Techniques d’inventaire Entomologique et d’évaluation des Programmes de Pulvérisation à Grande Échelle Contre la Tordeuse des Bourgeons de l’épinette Choristoneura fumiferana (Clem.); Conseil de l’Est de la Tordeuse des Bourgeons de l’épinette. Rapport du Comité Pour la Standardisation des Techniques Entomologiques; Ministère de l’Énergie et des Ressources du Québec: Québec, QC, Canada, 1982; p. 51. [Google Scholar]
- Seligy, V.L.; Rancourt, J.M. Antibiotic MIC/MBC analysis of Bacillus-based commercial insecticides: Use of bioreduction and DNA-based assays. J. Ind. Microbiol. Biotechnol. 1999, 22, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Fettes, J.J. Investigations of Sampling Techniques for Population Studies of the Spruce Budworm on Balsam Fir in Ontario; Annual Technical Report; Forest Insect Laboratory: Sault Ste. Marie, ON, Canada, 1950; pp. 163–401. [Google Scholar]
- Dorais, L.G.; Hardy, Y.J. Méthode d’évaluation de la protection accordée au sapin baumier par les pulvérisations aériennes contre la tordeuse des bourgeons de l’épinette. Can. J. For. Res. 1976, 6, 86–92. [Google Scholar] [CrossRef]
- McMoran, A. A synthetic diet for the spruce budworm. Choristoneura fumiferana (Clem.) (Lepidoptera: Tortricidae). Can. Entomol. 1965, 97, 58–62. [Google Scholar] [CrossRef]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Bolker, B.M.; Brooks, M.E.; Clark, C.J.; Geange, S.W.; Poulsen, J.R.; Stevens, M.H.H.; White, J.-S.S. Generalized linear mixed models: A practical guide for ecology and evolution. Trends Ecol. Evol. 2009, 24, 127–135. [Google Scholar] [CrossRef]
- Peterson, R.A.; Cavanaugh, J.E. Ordered quantile normalization: A semiparametric transformation built for the cross-validation era. J. Appl. Stat. 2020, 47, 2312–2327. [Google Scholar] [CrossRef] [PubMed]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed]
- Length, R. Mmeans: Estimated Marginal Means, aka Least-Squares Means. R Package Version 1.5.2-1. 2020. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 25 October 2022).
- Therneau, T.M. A Package for Survival Analysis in R. R Package Version 3.1-12. 2020. Available online: https://CRAN.R-project.org/package=survival (accessed on 25 October 2022).
- Sundaram, K.M.S.; Sundaram, A.; Hammock, B.D. Persistence of Bacillus thuringiensis deposits in a hardwood forest, after aerial application of a commercial formulation at two dosage rates. J. Environ. Sci. Health Part B 1994, 29, 999–1052. [Google Scholar] [CrossRef]
- Appel, H.M.; Schultz, J.C. Oak tannins reduce effectiveness of Thuricide (Bacillus thuringiensis) in the gypsy moth (Lepidoptera: Tortricidae). J. Econ. Entomol. 1994, 87, 1736–1742. [Google Scholar] [CrossRef]
- Hwang, S.Y.; Lindroth, R.L.; Montgomery, M.E.; Shields, K.S. Aspen leaf quality affects gypsy moth (Lepidoptera: Lymantriidae) susceptibility to Bacillus thuringiensis. J. Econ. Entomol. 1995, 88, 278–282. [Google Scholar] [CrossRef]
- Luthy, P.; Hofman, C.; Jaquet, F. Inactivation of delta-endotoxin of Bacillus thuringiensis by tannins. FEMS Microbiol. Lett. 1985, 28, 31–33. [Google Scholar] [CrossRef]
- Navon, A.; Hare, J.D.; Federici, B.A. Interactions among Heliothis virescens larvae, cotton condensed tannin and the CryIA(c) δ-endotoxin of Bacillus thuringiensis. J. Chem. Ecol. 1993, 19, 2485–2499. [Google Scholar] [CrossRef]
- Zucker, W.V. Tannins: Does structure determine function? An ecological perspective. Am. Nat. 1983, 121, 335–365. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).