Abstract
Furfurylated wood has many advantages, such as decay resistance, dimensional stability, hardness, etc. However, furfurylation increases the brittleness and decreases the flexural resistance of wood, which greatly limits its application. Therefore, caprolactam (CPL) is incorporated with furfuryl alcohol (FA) to improve the performance of furfurylated wood. In this study, an FA and CPL combinational modifier was used to treat masson pine (Pinus massoniana Lamb.) earlywood and latewood. The synergistic interaction of both components with the wood cell walls was systematically evaluated via microstructural, chemical, and thermal analysis using scanning electron microscopy (SEM), infrared spectroscopy (ATR-FTIR), X-ray photoelectron spectrometry (XPS), and differential scanning calorimetry (DSC). The SEM images showed that polymerized modifiers were distributed in tracheids, ray cells, and pits, with a higher degree of distribution in latewood tissues. The FA-CPL co-treatment led to the highest degree of distribution in cell cavities as well as of cell wall swelling. The results of the weight percentage gain (WPG) of modified wood agreed with the SEM findings that the FA-CPL co-treatment could more effectively increase the WPG than individual modification. The results of FTIR and XPS revealed that FA and CPL might chemically bind with each other as well as react with lignin and hemicellulose in the cell walls during the curing process. In addition, the interactions between modifiers and cell walls were slightly different for earlywood and latewood. DSC analysis indicated that the wood hygroscopicity decreased and the thermal stability improved after modification.
1. Introduction
Wood modification and preservation have become a popular area of research in recent years as advancements in technology have provided us with new ways to improve the properties of wood and extend its service life. It is well known that wood preservatives containing heavy metals (such as CCA) have been banned by many countries because of their toxicity and pollution to the environment. The use of wood preservatives with environmental benignity and abundant resources [1] is an urgent task to be achieved. Furfurylation, a chemical process that involves impregnating wood with furfuryl alcohol (FA), is one such technique that has received considerable attention.
FA, as a substitute material for phenolic resin, has been studied extensively in resin impregnation and metal casting [2]. Scientists have conducted a series of studies on its impregnation performance. As early as in the 1950s, Stamm [3] investigated the use of phenolic and FA to modify wood and made certain progress. Subsequently, wood furfurylation has been a hot topic in the area of improving wood properties, yet the furfurylation reaction was not stable. Consequently, research on how to control the reaction and optimize the process parameters has been conducted. Peter [4] et al. studied the furfurylation catalyzed by maleic anhydride, which has been widely recognized since. The furfurylation process was further optimized by incorporating sodium tetraborate as the stabilizer. Furfurylated wood is a bio-sustainable material, an environmentally friendly wood product [5], which has the potential to replace traditional heavy metal preservative-treated counterparts.
Studies have shown that the main reaction mechanism of furfurylation is the self-polymerization of FA molecules and cross-linking reaction with wood cell wall components [6]. Furfurylation increases the surface hardness and stiffness of wood. In addition, FA molecules solidify to form a protective film, which effectively reduces the moisture absorption of wood, improves its dimension stability, and endows it with excellent biological resistance [7]. However, after furfurylation, the brittleness increases and the impact toughness weakens [8]. Therefore, how to improve the brittleness and toughness of furfurylated wood has become a new research direction.
Caprolactam (CPL) is the raw material commonly used to fabricate polyamide. CPL has high toughness and is used to prepare flexible materials such as nylon fibers. CPL can also be used as a wood preservative [9,10] and filler for adhesives in wood-based panels [11]. Mechanistically, CPL can be cross-linked with FA via a ring-opening reaction, and the merits of both modifiers can be exerted when they are introduced into the cellular structure of wood simultaneously to enhance the wood properties. In this study, FA and CPL were introduced into masson pine (Pinus massoniana Lamb.) through vacuum impregnation. The interaction mechanism between the composite modifiers and wood as well as the evolution of microstructure and chemical properties were systematically evaluated based on the characterization of ATR-FTIR, SEM, XPS, and DSC. The outcomes of this study are valuable in that they provide theoretical grounds for the composite modification of wood.
2. Materials and Methods
2.1. Materials
In this experiment, sapwood of Pinus massoniana Lamb., taken from Longli Forest Farm in Guizhou, was processed into quarter-sawn wood samples with the dimensions of 20 × 20 × 300 mm (width × thickness × length). Samples with rapid changes from earlywood to latewood were selected to separate earlywood from latewood. They were processed into wood chips with thicknesses depending on that of earlywood and latewood, and the width of 20 mm and length of 30 mm.
FA chemical (Beijing Innochem Science & Technology Co., Ltd., Beijing, China, molecular weight 98.10, 98%, analytical purity), CPL (Shanghai McLean Biochemical Technology Co., Ltd., Shanghai, China, molecular weight 113.16, analytical purity), maleic anhydride (Aladdin Reagent (Shanghai) Co., LTD., Shanghai, China, molecular weight 98.06, analytical purity), and sodium tetraborate (Nanjing Chemical Reagent Co., LTD., Nanjing, China, molecular weight 381.37, analytical purity) were used as received without further purification. Deionized water was produced in the lab at Nanjing Forestry University.
2.2. Test Methods and Data Analysis
2.2.1. Sample Preparation and Impregnation Process
The wood samples of Pinus massoniana Lamb. were divided into five groups. Samples were labeled, dried, weighed, and then placed in a drying dish for subsequent use. In order to determine the optimal ratio of FA, different mass fractions (30 wt%, 40 wt%, 50 wt%) of FA were used. Liu XY et al. [12] concluded that FA in the range of 30 wt%–50 wt% is preferable. P. Pereira et al. [13] used 5 wt% caprolactam as a filler for resin modification to make a resin with high toughness. The mass fraction of each component in the impregnation solution is shown in Table 1.

Table 1.
Composition of the impregnation solution.
Wood samples were treated via vacuum impregnation with the above five groups of solutions, respectively. The brief impregnation steps are as follows. We placed the samples at the bottom of a custom-made impregnation tank. We set the vacuum pressure to −95 kPa for 1.5 h. Then, the preset impregnation solution was slowly sucked into the tank driven by the vacuum. The volume of the impregnation solution was predetermined, and metal weights were placed on top of the samples to ensure they were fully immersed in the solution. The vacuum pressure (−95 kPa) was maintained for another 2 h. Finally, the vacuum was released and the samples were kept soaking for another 3 h under atmospheric pressure before they were collected. Samples modified with FA solutions (i.e., groups 1–3) were wrapped in aluminum foil for 12 h, followed by curing in an oven for 2 h at 103 °C. Then, we removed the aluminum foil, wiped away the unsolidified FA on the surface, and dried the sample stepwise at 60, 80, and 103 °C for 2 h, respectively.
For samples treated with CPL solutions (group 4), the curing process was conducted in a tube furnace with nitrogen gas as the protection environment. The gas flow rate was set at 600 mL/min. Samples were heated up to 240 °C at a ramp up rate of 10 °C/min and maintained for 2 h, then cooled naturally before being taken out of the furnace. It is worth noting that for samples treated with a combination of FA and CPL (group 5), the CPL curing process came after the FA curing.
The difference in the weight percentage gain (WPG) of samples treated with the five groups of solutions was compared. Subsequently, the effect of FA/CPL (groups 1, 4, and 5) on the microstructural and thermo-chemical properties of pine wood was evaluated with scanning electron microscopy (SEM), infrared spectrum analysis (ATR-FTIR), X-ray photoelectron spectroscopy (XPS), and differential scanning calorimetry (DSC) analysis.
2.2.2. Weight Percentage Gain (WPG)
The WPGs of both impregnated wood blocks and the earlywood as well as the latewood samples were calculated according to the formula below. Each group had 10 replicates.
2.2.3. Scanning Electron Microscopy (SEM)
A total of six groups of samples with untreated, CPL-treated and FA-CPL-co-treated earlywood and latewood were processed into 5 × 1 × 10 mm (width × thickness × length) slices. In order to ease the SEM sample preparation, two 5 × 1 × 5 mm (width × thickness × length) wood chips were used to clamp the slices through an adhesive, as illustrated in Figure 1.
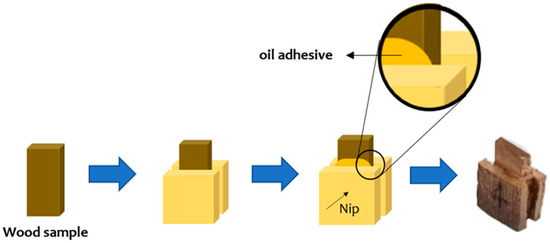
Figure 1.
Schematic diagram for SEM sample preparation.
Then, the cross section of the sample was trimmed with a microtome and gold-sputtered for SEM Phenom XL (Thermo scientific, Waltham, MA, USA) observation. The acceleration voltage was set at 10 kV. SEM images of the earlywood were used to analyze the cell wall thickness via ImageJ software (2.9.0, Bethesda, MD, USA). The latewood cell wall thickness was difficult to measure, since the cell cavities were filled with polymerized modifiers. Fifty complete cells were randomly selected for the measurement, and the average was used for comparison.
2.2.4. Infrared Spectrum Analysis (ATR FT-IR)
A total of six groups of untreated, CPL-treated and FA-CPL-co-treated earlywood and latewood were processed into 5 × 5 mm slices with a thickness less than 1 mm. A total reflection infrared spectrometer VERTEX 80 V (BRUKER, Karlsruhe, Baden-Wurttemberg, Germany) was used to scan and analyze the samples with the wavenumber ranging from 500 cm−1 to 4000 cm−1. The resolution is 4 cm−1 and the number of scans is 64.
2.2.5. X-ray Photoelectron Spectroscopy (XPS)
Specimens with a dimension of 5 × 1 × 5 mm (width × thickness × length) were obtained from the surface layer of the above six groups of samples using a blade to minimize the time necessary to achieve vacuum in the XPS chamber. We made sure the samples were not contaminated during the preparation, and the tests were performed immediately after plasma treatment. XPS was carried out using an AXIS Ultra DLD Spectrometer (Kratos, Manchester, United Kingdom) with a hemispherical energy analyzer and a monochromatic Al Kα X-ray source (600 W) at a vacuum pressure of 2 × 10−8 torr. Monochromatic Al Kα was used as the target material (1486.6 eV). An X-ray beam with 150 W power and 300 × 700 μm spot size were applied. The survey scans acquired with a pass energy of 50 eV at 1 eV steps were used to record the low-resolution spectra (0–1200 eV).
After drawing the map, the relative atomic concentration of carbon and oxygen was measured according to the formula below:
Origin software (b9.5.1.195, Northampton, MA, USA) was used to deconvolute the XPS peaks and calculate the area of each peak, and the percentage of carbon in different chemical states was obtained according to the area of each peak. The O/C ratio can be measured by the peak area.
2.2.6. Differential Scanning Calorimetry Analysis (DSC)
Conventional DSC measurements were performed with a DSC 204 F1 (NETZSCH, Selb, Free State of Bavaria, Germany) heat-flux instrument, and NETZSCH software (8.0.2 (10.07.2020)) 20192.201, Selb, Free State of Bavaria, Germany) was used for data analysis. Wood flour passed through a 60-mesh screen (3–5 mg) was placed in an aluminum crucible sealed with a hermetic lid. The wood flour was dried at 60 °C for 12 h before use. Two consecutive runs were conducted. The first run started from 25 °C, ramped up to 100 °C at a rate of 10 °C min−1 to clear the thermal history, then ramped down to 25 °C at the same rate. The second run started from 25 °C to 320 °C at the same rate, and remained at 320 °C for 2 min, followed by ramping down to 25 °C. Only the second DSC curve was used for thermal performance analysis.
3. Results and Discussions
3.1. Weight Percentage Gain (WPG)
WPG is a direct indicator of the effect of FA/CPL modification on wood properties. The results showed that the extent of modification led to different WPGs. As shown in Figure 2a, with the increase in the FA concentration from 30 wt% to 40 wt%, the WPG only increased by 2.24%, whereas further increasing the concentration to 50 wt%, caused the WPG to increase by 15.39%. The reason might be that upon impregnation with 50 wt% FA, a significantly higher volume of FAmonomers penetrated cell walls as well as attached to them and polymerized on sites, which was evident in the SEM images (shown in Section 3.2). Therefore, the impregnation solution concentration of 50% was selected for subsequent modification involving FA.
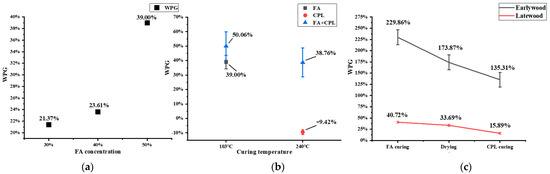
Figure 2.
(a) Comparison of WPG of FA-modified wood with different concentrations, (b) Comparison of WPG of wood blocks under different processes. (c) Comparison of WPG of earlywood and latewood under the same process.
The WPG of samples impregnated with the FA-CPL combination was 38.76% and that of samples impregnated with only FA was 39.00%, while the CPL-treated ones actually had a weight loss of 9.42%. The reason for the weight loss was that the curing temperature of CPL reached 240 °C, where the hemicellulose degraded greatly, leading to the decrease in WPG. Hemicellulose is reported to undergo pyrolysis starting at 150 °C due to its abundancy of branched chains [14]. The WPG of the FA-CPL-co-modified sample reached 50.06% at 103 °C. Studies have shown [15] that CPL can be used as a resin stabilizer to condense resin into a flexible substance, so its WPG is 11.06% higher than that of the sample modified with FA alone given that only 5 wt% of CPL was introduced into the solution. The WPG further lowered by 11.30% after CPL solidification at 240 °C. The reason for the higher weight loss by 1.88 percent(11.3% vs. 9.42%) might be that part of the free FA evaporated at higher temperatures.
Comparing the modification of the wood blocks, Figure 2c shows significant differences between earlywood and latewood under the same modification conditions. The WPG of earlywood was 135.31%, whereas that of latewood was only 15.89%. The reason for this was speculated to be that latewood has a higher cell wall/cavity ratio than earlywood, which constrained its ability to accommodate exotic modifiers. Moreover, the cell wall pits and the perforation between tracheids are more inclined to be aspirated than earlywood, resulting in a significantly lower WPG in latewood [16]. Combined with the ImageJ results, the cell wall of earlywood swelled from 2.68 to 5.41 μm after modification, resulting in the 119.42% higher WPG than that of latewood under the same treatment conditions.
3.2. Scanning Electron Microscopy (SEM)
The microstructure of earlywood and latewood before and after modification is shown in Figure 3. The degree of the distribution of polymers in the cell cavity of earlywood is lower than that of latewood. The majority of the self-polymerization and nucleation occurred in the tracheid cavity of latewood, as evidenced in Figure 3f–h, resulting in insufficient space for latewood to be infiltrated with more modifiers, and the cell wall reaction with the modifiers was insufficient. The phase differences of pits in the radial section shows that a large number of pits were aspirated after furfurylation and FA-CPL co-treatment, especially for the latewood (Figure 3i–p). This is also why the WPG values of earlywood are larger than those of latewood. According to ImageJ, the average thickness of untreated, furfurylated, CPL-treated, and FA/CPL-co-treated earlywood cell walls was 2.68 μm (standard deviation (SD: 0.61), 4.55 μm (SD: 0.75), 4.06 μm (SD: 0.57), and 5.41 μm (SD: 0.49), respectively, indicating that the degree of cell wall swelling of the earlywood was greatly improved after modification. The reason for this was that since the cell cavities of the earlywood were large and the structure was soft. The modified solution could easily penetrate the cell wall, and then self-polymerization or cross-linking with cell wall components occurred, making the cell walls permanently swollen [17]. The furfurylated sample swelled more than the CPL-treated one, which meant that a higher amount of FA was retained in the cell walls than CPL, or the curing condition for CPL was harsher than FA (240 vs. 103°C), causing more damage to the cell walls. We will use a lower curing temperature with CPL in future experiments in order to reduce the difference between FA and CPL. Another reason was related to the aspirated pits after furfurylation, whereas the CPL treatment may did not make the pits aspirated (Figure 3k,o), indicating that it was difficult for CPL to penetrate through the pits to the cell walls, and cure on-site. In contrast, when FA was introduced, either individually or in combination with CPL, the modifiers easily penetrated the cell walls via pits, making the cell walls permanently swollen [18].
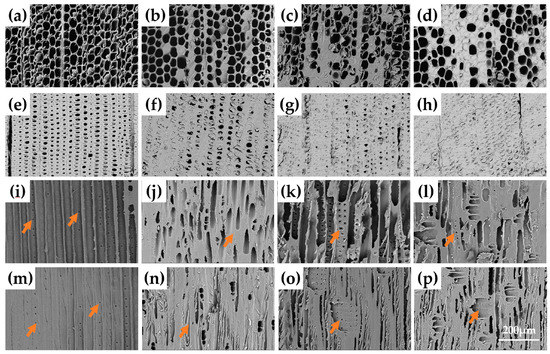
Figure 3.
SEM images of the cross sections of earlywood (a) untreated; (b) treated with FA; (c) treated with CPL; (d) treated with FA and CPL and latewood (e) untreated; (f) treated with FA; (g) treated with CPL; (h) treated with FA and CPL. Radial sections of earlywood (i) untreated; (j) treated with FA; (k) treated with CPL; (l) treated with FA and CPL as well as latewood (m) untreated; (n) treated with FA; (o) treated with CPL; (p) treated with FA and CPL.
Compared with earlywood, when FA/CPL was added, a larger number of polymerized modifiers were distributed in the cell cavities of the latewood (Figure 3f–h), and the structure was denser. Correlated with the result of lower WPG and pit aspiration tendency in latewood, it was concluded that the cell cavities and the pits of the latewood were more inclined to be blocked by the impregnation solution than those of the earlywood, making it difficult for the solution to penetrate the wood cell walls. Moreover, no obvious swelling of the latewood cell walls supported our speculation that with a high accumulation of polymerized modifiers in the cell cavities, the penetration of modifiers was severely impeded, hence the WPG in latewood was smaller than that of earlywood.
3.3. Infrared Spectral Analysis (ATR FT-IR)
The infrared spectra of earlywood before and after modification are shown in Figure 4. In this study, only the infrared spectra of the earlywood were analyzed because there was no significant difference between the earlywood and latewood. The characteristic infrared peaks as well as their attributions are summarized in Table 2. There is a broad peak at 3340 cm−1, which is ascribed to free hydroxyl or N-H stretching vibration, and its intensity decreases gradually with the increase in modification degree (from mono-component to combinational components). It can be concluded that the peak is mainly attributed to the free hydroxyl rather than N-H stretching vibration, since the peak intensity actually decreases after CPL or FA-CPL co-treatment. The decrease in the free hydroxyl group in the modified wood overwhelmed the impact of the increase in N-H stretching vibration caused by the increase in the amount of CPL added. The absorption peaks of 2923 cm−1 and 2852 cm−1 are ascribed to methylene and methyne, respectively. The intensity reduction of these two peaks was due to the partial degradation of hemicellulose by heat during the curing process [19]. At 1706 cm−1, with the increase in modification degree, the characteristic peak of the carboxyl group gradually decreased, which might be due to the reaction between the amino group and carboxyl group upon CPL ring opening [20]. Due to the cross-linking reaction with FA/CPL, the characteristic peaks of lignin at 1510 cm−1, 1423 cm−1, and 1267 cm−1 decreased [21]. Compared with unmodified samples, the peak intensities attributed to C-O and C-N of CPL-modified wood samples increased at 1029 cm−1, indicating that CPL penetrated the wood cells, because the peak intensity was still the largest despite the significant degradation of hemicellulose at the CPL curing temperature (i.e., 240 °C). Nevertheless, the peak intensity at 1029 cm−1 for FA-CPL-co-treated samples was the lowest. The reason for this was that the mass fraction of CPL in the impregnation solution was low, the carboxyl group produced by FA curing was substituted with CPL during the curing process, and the hemicellulose was significantly degraded at the CPL curing temperature, leading to a decrease in peak intensity.
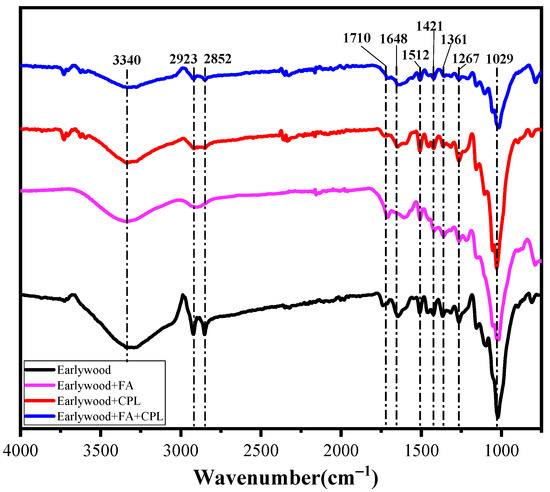
Figure 4.
Infrared spectrum comparison of earlywood with different modifications.

Table 2.
Characteristic peaks and attribution of tested pine samples (Data from: [22]).
Figure 4 reveals the influence of modification on the chemical properties of latewood. Similar to earlywood, samples co-treated with FA-CPL had the lowest absorption peak intensities. In addition, the peak intensity at 1029 cm−1 of latewood modified with CPL was even lower than that of the untreated one. The reason for this was that the internal channels of latewood were smaller than those of earlywood, and the penetration difficulty for the CPL solution increased in latewood. Therefore, the C-N stretching vibration peak from CPL was not obvious. Moreover, the degradation of hemicellulose during CPL curing further reduced the C-O stretching vibration peak, rendering an overall decrease at 1029 cm−1.
3.4. X-ray Photoelectron Spectroscopy (XPS)
The elemental composition and chemical bonding determination of the sample surface were analyzed through X-ray photoelectron spectroscopy. It is worth noting that the spectra of the furfurylated group were not evaluated. The survey spectra of the earlywood and latewood with different treatments are shown in Figure 5. There are two intense peaks, C 1s and O 1s, representing the carbon and oxygen elements in the sample, respectively. The main elements of wood are carbon, hydrogen, and oxygen, while the modifier CPL contains a small amount of nitrogen. Since XPS cannot characterize hydrogen, this experiment mainly characterizes carbon, nitrogen, and oxygen.
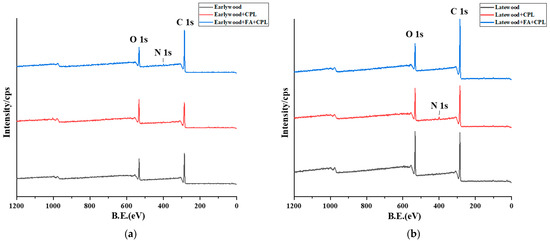
Figure 5.
(a) Comparison of XPS full spectrum of earlywood after different impregnation processes. (b) Comparison of XPS full spectrum of latewood after different impregnation processes.
Figure 6 shows the narrow-region high-energy resolution scans of the C 1s peaks of the earlywood and latewood after different treatments. The analysis of its satellite peaks referring to the binding energies in Table 3 shows that the intensity of the C 1s narrow spectrum of the earlywood was lower than that of the latewood, which might be caused by the differences in cell wall thickness between earlywood and latewood. In addition, the intensities ascribed to C-C/C-H and C-O-C at 284.8 eV and 286.5 eV decreased after curing at 240 °C due to hemicellulose pyrolysis, as shown in Figure 6b,e. In contrast, Figure 6c,f shows that the intensity of C-C at 284.8 eV increased upon the introduction of FA, which was due to FA self-polymerization as well as its reaction with lignin. Moreover, the furan ring opening during the curing process produced a carboxyl group; thus, the intensity of carboxyl groups at 289.5 eV increased, indicating that FA entered the wood cell, reacted with lignin, and self-polymerized. Figure 6c also shows the lower peak intensity of carboxyl groups with the incorporation of CPL with FA, since CPL reacted with carboxylic acid during the curing process, resulting in the reduction in carboxyl groups. The reaction of CPL with FA was verified by the results of infrared spectroscopy and scanning electron microscopy.
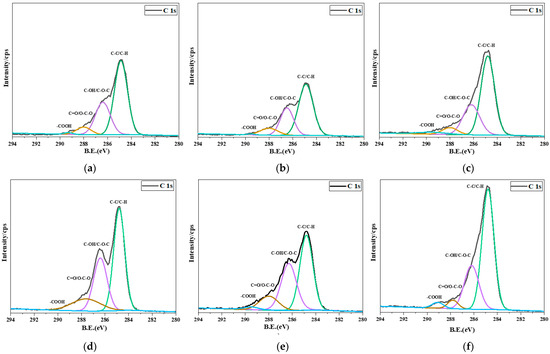
Figure 6.
C 1s narrow spectrum of earlywood treated with different processes. (a) Untreated earlywood; (b) CPL-modified earlywood; (c) FA + CPL-modified earlywood. C 1s narrow spectrum of latewood treated with different processes. (d) Untreated latewood; (e) CPL-modified latewood; (f) FA + CPL modified-latewood.

Table 3.
Binding energy and binding form of carbon in wood (Data from: [23,24]).
The peak intensity of amide can be measured in the N 1s map (Figure 7a). However, it is relatively weak, which might be due to the low concentration of CPL in the modifier solution. In the O 1s map (Figure 7b,c), the chemical components representing the peak C=O were lignin and wood extractives [25], while the peak C-O was associated with hemicellulose and cellulose [26,27]. The relative content of C=O in earlywood was greater than that in latewood. Therefore, the lignin content of earlywood was greater than that of latewood in this experiment. Furthermore, the relative content of C-O in earlywood was lower than that in latewood, indicating that the cellulose and hemicellulose content of earlywood was lower than that of latewood in this experiment [28]. The contents of cellulose, hemicellulose, and lignin in earlywood and latewood will be calculated to further test the hypothesis.
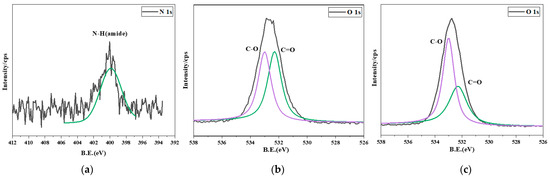
Figure 7.
(a) N 1s map of latewood + CPL pine; (b) O 1s map of earlywood; (c) O 1s map of latewood.
Carbon exhibits various binding energies in different chemical states. The O/C ratio and the percentage of carbon in different chemical states are shown in Table 4. The O/C ratios of natural wood cellulose, hemicellulose, and lignin are reported as 0.83, 0.80, and 0.33, respectively, whereas those of FA and CPL are 0.53 and 0.22, respectively [29]. The results showed that the O/C ratio of earlywood increased from 0.25 to 0.41 after impregnation with CPL. It was speculated that the O/C ratio increased as a result of hemicellulose pyrolysis after heating [30]. A large number of oxygenated side branches mainly consisting of O-acetyl and uronic acid were retained in the hemicellulose structure after CPL curing at 240 °C, which might even have reacted with CPL. This speculation was further supported by the results from FTIR that polycaprolactam can react with oxygenated side branches such as carboxyl. In contrast, the O/C ratio of earlywood decreased to 0.23 after the addition of FA, since FA self-polymerization resulted in the formation and evaporation of formaldehyde. Regarding latewood, the O/C ratio decreased from 0.35 to 0.31 after impregnation with CPL. The reason for this might be that charcoal produced through hemicellulose pyrolysis inhibited further pyrolysis of hemicellulose [31], leading to few oxygenated side branches reacting with CPL. It is worth noting that the O/C ratio of latewood was greater than that of earlywood [32], due to the fact that the lignin content of earlywood was greater than that of latewood, whereas the hemicellulose and cellulose contents of earlywood were lower than those of latewood (Figure 7b,c).

Table 4.
O/C values of earlywood and latewood after different treatments and percentage of carbon in different chemical states (%).
The percentage of carbon in different chemical states can be determined according to the peak area at the respective binding energies, which is shown in Table 4. In terms of earlywood, the relative content of C1 and C2 had no significant change. After the addition of CPL, the percentage of C3 increased by 38.20%, indicating that CPL had cured in wood cells. After the addition of FA, the percentage of C4 increased by 182.22%, because the furfurylation reaction produced carboxyl groups. In terms of latewood, the relative content of C3 varied differently compared with earlywood. The relative content of C3 decreased (from 13.22% to 12.44%) after the addition of CPL, because the impregnation quantity of CPL was lower than that in earlywood (Figure 2b), which was mainly attributed to the size and ease of the internal penetration channels. The relative content of C3 decreased by 70.27% after the addition of FA. Hemicellulose pyrolysis and the reaction between FA and lignin led to the reduction in the relative content of C3, which was further supported by the results from FTIR (Figure 4).
3.5. Differential Scanning Calorimetry (DSC)
The difference in the thermodynamical properties of earlywood and latewood after different modifications was evaluated with DSC. There was an endothermic peak at around 110 °C for the untreated and CPL-treated samples, which was attributed to water evaporation (Figure 8a,b), while those involving FA did not have this peak or at least the peak was weak, demonstrating that both furfurylated and FA-CPL-co-treated wood had a low equilibrium moisture content. There was an exothermic peak at around 160 °C for the untreated and CPL-treated samples, which was ascribed to hemicellulose degradation. The exothermic peak was absent in the furfurylated and FA-CPL-co-treated ones, which means that reactions between hemicellulose and FA/CPL might occur, improving the thermal stability of hemicellulose. In general, the DSC curve of FA-CPL-co-modified wood was the flattest compared with untreated, furfurylated, and CPL-modified earlywood in the range of room temperature to 250 °C, indicating that FA-CPL co-modification improved the thermal stability of earlywood the most. In addition, after CPL modification, the sample showed a weak endothermic peak at 246 °C, which was attributed to the melting of polyamide. However, no endothermic peak was found in FA-CPL-co-modified wood, illustrating that cross-linking reactions between CPL and FA occurred and more stable bonds formed.
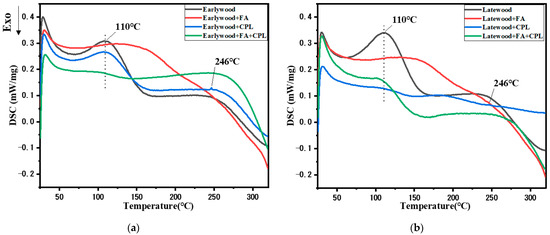
Figure 8.
(a) DSC comparison of earlywood after different impregnation processes. (b) DSC comparison of latewood after different impregnation processes.
The thermal dynamic properties of latewood are slightly different from those of earlywood, as shown in Figure 8b. The wood samples modified with CPL had the lowest endothermic peak at 110 °C, indicating that the hygroscopicity of modified specimens decreased under CPL treatment. Similarly, after CPL modification, the sample also had a weak melting absorption peak at 246 °C. It is worth noting that the DSC curve of the CPL modified latewood was the flattest, which was different from that of earlywood. In contrast, for the FA-CPL-co-treated latewood, an endothermic and an exothermic peak were located at 110 °C and around 160 °C, respectively, further proving that FA-CPL had difficulties penetrating the cell walls due to the size limit of the internal channels.
4. Conclusions
The present study evaluated the feasibility and mechanism of FA/CPL-impregnated masson pine. The WPG of earlywood was higher than latewood because the size of the internal channels was larger and this made the transportation of modifiers into cell walls easier. The high temperature of CPL curing severely degraded the cell wall components, thus lowering the WPG of CPL-treated samples. The SEM results agreed well with the WPG outcomes. A large number of polymerized modifiers were distributed in cell cavities, cell walls, and ray cells, and filled in the pits, especially in latewood. In addition, the FA-CPL co-treatment swelled the cell walls the most, since FA and CPL self-polymerized on-site in the cell walls and even cross-linked with the cell wall components (e.g., lignin, hemicellulose). The FTIR and XPS data both illustrated the successful incorporation of modifiers into wood cells and reduced accessible hydroxyl groups, corresponding to improved hygroscopic resistance. DSC curves revealed increased thermal stability after modification. In future work, the cellulose, hemicellulose, and lignin contents of earlywood and latewood before and after modification will be investigated to further evaluate the interaction of modifiers with cell wall components. Mechanical properties will also be tested, especially for stiffness and impact toughness, to check if the incorporation of CPL indeed reduces the stiffness and improves the impact toughness of furfurylated wood. Finally, catalysts will be introduced to significantly decrease the curing temperature of CPL-modified wood, since 240 °C is far beyond the commencement point of degradation for normal wood.
Author Contributions
Conceptualization, W.L., S.H. and X.Z.; methodology, Z.W; validation, J.S.; formal analysis, Z.W.; investigation, Z.W.; resources, W.L.; data curation, W.L.; writing—original draft preparation, Z.W.; writing—review and editing, W.L., S.H., J.S. and X.Z.; supervision, W.L.; project administration, W.L.; funding acquisition, W.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number: 32001259, the Natural Science Foundation of Jiangsu Province, grant number: BK20200796, and Key Research & Development Program of Zhejiang Province, grant number: 2021C02012.
Data Availability Statement
Data are contained within this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lande, S.; Eikenes, M.; Westin, M. Chemistry and Ecotoxicology of Furfurylated Wood. Scand. J. For. Res. 2004, 19, 14–21. [Google Scholar] [CrossRef]
- Leng, W.; Barnes, H. Properties of pine scrim lumber made from modified scrim. Wood Fiber Sci. 2017, 49, 158–167. [Google Scholar]
- Lande, S.; Westin, M.; Schneider, M. Development of Modified Wood Products Based on Furan Chemistry. Mol. Cryst. Liq. Cryst. 2008, 484, 367–378. [Google Scholar] [CrossRef]
- Peter, A.; Singaram, B. Reactions of Furfuryl Alcohols with Maleic Anhydride. Tetrahedron Lett. 1982, 23, 245–248. [Google Scholar] [CrossRef]
- Lande, S.; Westin, M.; Schneider, M. Eco-Efficient Wood Protection: Furfurylated Wood as Alternative to Traditional Wood Preservation. Abstr. Pap. Am. Chem. Soc. 2005, 229, U304. [Google Scholar] [CrossRef]
- Leng, W.; He, S.; Zhang, X.; Zhai, S.; Wang, X.; Pan, B.; Shi, J. Research Progress and Consideration on the Mechanism of Furfuryl Alcohol Resin Modified Wood. J. For. Eng. 2021, 6, 35–43. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Z.; Wang, X.; Xu, W.; Yao, L. Effect of Furfuryl Alcohol Modification on the Aging Resistance of Outdoor Wood. China For. Prod. Ind. 2020, 57, 23–25, 34. [Google Scholar] [CrossRef]
- Moazzen, K.; Zohuriaan-Mehr, M.; Jahanmardi, R.; Kabiri, K. Toward Poly(Furfuryl Alcohol) Applications Diversification: Novel Self-Healing Network and Toughening Epoxy-Novolac Resin. J. Appl. Polym. Sci. 2018, 135, 45921. [Google Scholar] [CrossRef]
- Lan, P.; Yang, R.; Mao, H.; Cui, J.; Brosse, N. Production of Melamine Formaldehyde Resins Used in Impregnation by Incorporation of Ethylene Glycol and Caprolactam with High Flexibility, Storage Stability, and Low Formaldehyde Content. Bioresources 2019, 14, 9916–9927. [Google Scholar] [CrossRef]
- Tondi, G.; Hu, J.; Rizzo, F.; Buh, J.; Medved, S.; Petutschnigg, A.; Thevenon, M.-F. Tannin-Caprolactam and Tannin-PEG Formulations as Outdoor Wood Preservatives: Weathering Properties. Ann. For. Sci. 2017, 74, 19. [Google Scholar] [CrossRef]
- Wang, S.; Ru, B.; Lin, H.; Sun, W. Pyrolysis Behaviors of Four O-Acetyl-Preserved Hemicelluloses Isolated from Hardwoods and Softwoods. Fuel 2015, 150, 243–251. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Dong, Y.; Hughes, M.; Wu, M.; Li, J. Evaluation of Natural Weathering and Thermal Degradation Behavior of Furfurylated Bamboo Strips at Different Weight Percent Gain. Eur. J. Wood Wood Prod. 2022, 80, 289–299. [Google Scholar] [CrossRef]
- Pereira, P.; Almeida, M.; Pereira, J.; Paiva, N.; Ferra, J.; Martins, J.; Carvalho, L.; Magalhaes, F. Improvement of Storage Stability of UF Resins by Adding Caprolactam. Int. J. Adhes. Adhes. 2019, 92, 105–110. [Google Scholar] [CrossRef]
- Hu, S. Study on Pyrolysis and Product Characteristics of Lacquer Tree; Northwest A&F University: Xianyang, China, 2021. [Google Scholar]
- Ding, T.; Peng, W.; Li, T. Mechanism of Color Change of Heat-Treated Ash Wood Based on FT-IR and XPS. J. For. Eng. 2017, 2, 25–30. [Google Scholar] [CrossRef]
- Shen, X.; Jiang, P.; Guo, D.; Li, G.; Chu, F.; Yang, S. Effect of Furfurylation on Hierarchical Porous Structure of Poplar Wood. Polymers 2021, 13, 32. [Google Scholar] [CrossRef]
- Singh, A.; Singh, T.; Rickard, C. Visualising Impregnated Chitosan in Pinus Radiata Early Wood Cells Using Light and Scanning Electron Microscopy. Micron 2010, 41, 263–267. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, J.; Wang, Z.; Hua, F.; He, S.; Lu, B.; Wang, X.; Zhang, X.; Leng, W. Microstructural and Thermo-Mechanical Characterization of Furfurylated Douglas Fir. Polymers 2022, 14, 4641. [Google Scholar] [CrossRef]
- Gupta, N.K.; Prakash, P.; Kalaichelvi, P.; Sheeba, K.N. The Effect of Temperature and Hemicellulose-Lignin, Cellulose-Lignin, and Cellulose-Hemicellulose on Char Yield from the Slow Pyrolysis of Rice Husk. Energy Source Part A Recovery Util. Environ. Eff. 2016, 38, 1428–1434. [Google Scholar] [CrossRef]
- Zhang, L.; Xiong, Y.; Ou, E.; Chen, Z.; Xiong, Y.; Xu, W. Preparation and Properties of Nylon 6/Carboxylic Silica Nanocomposites via in Situ Polymerization. J. Appl. Polym. Sci. 2011, 122, 1316–1324. [Google Scholar] [CrossRef]
- Zhang, J.; Xi, X.; Zhou, X.; Liang, J.; Du, G.; Cai, J. Synthesis and characterization of lignin-based adhesive cross-linked with furfuryl alcohol-formaldehyde and epoxy resins. Cellul. Chem. Technol. 2019, 53, 449–458. [Google Scholar] [CrossRef]
- Dong, S.; Wang, C.; Xiang, J.; Zhang, G. Study on Chemical Composition and Structural Changes of Hu County Gongshu Shrine Wood Based on FTIR-ATR. Infrared 2020, 41, 30–37. [Google Scholar] [CrossRef]
- Zhao, H.; Zheng, Y.; Wang, Z.; Xie, W.; Zhou, J.; Zhong, C. Preparation of a Bacterial Flocculant by Using Caprolactam as a Sole Substrate and Its Application in Amoxicillin Removal. J. Environ. Manag. 2021, 294, 113026. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Kocaefe, D.; Kocaefe, Y.; Boluk, Y.; Pichette, A. Study of the Degradation Behavior of Heat-Treated Jack Pine (Pinus Banksiana) under Artificial Sunlight Irradiation. Polym. Degrad. Stab. 2012, 97, 1197–1214. [Google Scholar] [CrossRef]
- Gerardin, P.; Petric, M.; Petrissans, M.; Lambert, J.; Ehrhrardt, J. Evolution of Wood Surface Free Energy after Heat Treatment. Polym. Degrad. Stab. 2007, 92, 653–657. [Google Scholar] [CrossRef]
- Kamdem, D.P.; Riedl, B.; Adnot, A.; Kaliaguine, S. ESCA Spectroscopy of Poly(Methyl Methacrylate) Grafted onto Wood Fibers. J. Appl. Polym. Sci. 1991, 43, 1901–1912. [Google Scholar] [CrossRef]
- Hua, X.; Kaliaguine, S.; Kokta, B.V.; Adnot, A. Surface Analysis of Explosion Pulps by ESCA Part 1. Carbon (1s) Spectra and Oxygen-to-Carbon Ratios. Wood Sci. Technol. 1993, 27, 449–459. [Google Scholar] [CrossRef]
- Orton, C.R.; Parkinson, D.Y.; Evans, P.D.; Owen, N.L. Fourier Transform Infrared Studies of Heterogeneity, Photodegradation, and Lignin/Hemicellulose Ratios within Hardwoods and Softwoods. Appl. Spectrosc. 2004, 58, 1265–1271. [Google Scholar] [CrossRef]
- Hu, J.; Thevenon, M.; Palanti, S.; Tondi, G. Tannin-Caprolactam and Tannin-PEG Formulations as Outdoor Wood Preservatives: Biological Properties. Ann. For. Sci. 2017, 74, 18. [Google Scholar] [CrossRef]
- Yu, H.; Gui, C.; Ji, Y.; Li, X.; Rao, F.; Huan, W.; Li, L. Changes in Chemical and Thermal Properties of Bamboo after Delignification Treatment. Polymers 2022, 14, 2573. [Google Scholar] [CrossRef]
- Sun, C.; Tan, H.; Zhang, Y. Simulating the Pyrolysis Interactions among Hemicellulose, Cellulose and Lignin in Wood Waste under Real Conditions to Find the Proper Way to Prepare Bio-Oil. Renew. Energy 2023, 205, 851–863. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, J.; Zhan, X.; Mei, C.; Li, W.; Deng, Y.; Wang, X. Effect of Plasma Treatment on the Surface Characteristics and Adhesive Penetration Performance of Heat-Treated Wood. Holzforschung 2022, 76, 941–953. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).