Microsite Determines the Soil Nitrogen and Carbon Mineralization in Response to Nitrogen Addition in a Temperate Desert
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site Description
2.2. Experimental Design and Sample Collection
2.3. Soil Sampling and Measurements
2.4. Soil Properties Measurements
2.5. Net N-mineralization In Situ
2.6. Soil C Mineralization
2.7. Soil Microbial Biomass and Microbial Community Composition Measurements
2.8. Herbaceous Biomass Measurement
2.9. Statistical Analysis
3. Results
3.1. N Mineralization Rate and Nitrification, and C Mineralization Rate in Response to N Addition
3.2. Seasonal Dynamics of Soil Inorganic Nitrogen
3.3. Microbial Biomass and Community Composition in Response to N Addition
3.4. Factors Determining Soil N and C Mineralization
4. Discussion
4.1. Effects of N Addition on Soil Net N Mineralization and Nitrification Rate
4.2. Effects of N Addition on Soil C Mineralization
4.3. The Opposite Responses of Microbial Characteristics between Interplant and Beneath Shrubs
4.4. Implications for N Cycling in Deserts
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Cmic | Microbial biomass carbon |
| Nmic | Microbial biomass nitrogen |
| Cmic:Nmic | The ratio of microbial biomass carbon to nitrogen |
| RmN | Nitrogen mineralization rate |
| RNN | Nitrogen nitrification rate |
| RCM | Carbon mineralization rate |
| In-N | Inorganic nitrogen |
| SM | Soil moisture |
| F:B PLFAs | The ratio of fungal to bacterial PLFAs |
References
- de Vries, W. Preface. For. Ecol. Manag. 2009, 258, vii–x. [Google Scholar] [CrossRef]
- Liu, X.; Chen, C.R.; Wang, W.J.; Hughes, J.M.; Lewis, T.; Hou, E.Q.; Shen, J.P. Soil environmental factors rather than denitrification gene abundance control N2O fluxes in a wet sclerophyll forest with different burning frequency. Soil Biol. Biochem. 2013, 57, 292–300. [Google Scholar] [CrossRef]
- Grant, T.; Sethuraman, A.; Escobar, M.A.; Vourlitis, G.L. Chronic dry nitrogen inputs alter soil microbial community composition in Southern California semi-arid shrublands. Appl. Soil Ecol. 2022, 176, 104496. [Google Scholar] [CrossRef]
- Huang, G.; Cao, Y.F.; Wang, B.; Li, Y. Effects of nitrogen addition on soil microbes and their implications for soil C emission in the Gurbantunggut Desert, center of the Eurasian Continent. Sci. Total Environ. 2015, 515, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.F.; Wu, J.G.; Clark, C.M.; Naeem, S.; Pan, Q.M.; Huang, J.H.; Zhang, L.X.; Han, X.G. Tradeoffs and thresholds in the effects of nitrogen addition on biodiversity and ecosystem functioning: Evidence from inner Mongolia Grasslands. Glob. Chang. Biol. 2010, 16, 358–372. [Google Scholar] [CrossRef]
- Mo, J.; Zhang, W.; Zhu, W.; Gundersen, P.; Fang, Y.; Li, D.; Wang, H. Nitrogen addition reduces soil respiration in a mature tropical forest in southern China. Glob. Chang. Biol. 2008, 14, 403–412. [Google Scholar] [CrossRef]
- Zhou, X.B.; Zhang, Y.M.; Downing, A. Non-linear response of microbial activity across a gradient of nitrogen addition to a soil from the Gurbantunggut Desert. Soil Biol Biochem. Soil Biol. Biochem. 2012, 47, 67–77. [Google Scholar] [CrossRef]
- Niu, G.X.; Hasi, M.; Wang, R.Z.; Wang, Y.L.; Geng, Q.Q.; Hu, S.Y.; Xu, X.H.; Yang, J.J.; Wang, C.H.; Han, X.G.; et al. Soil microbial community responses to long-term nitrogen addition at different soil depths in a typical steppe. Appl. Soil Ecol. 2021, 167, 104054. [Google Scholar] [CrossRef]
- Song, B.; Li, Y.; Yang, L.Y.; Shi, H.Q.; Li, L.H.; Bai, W.M.; Zhao, Y. Soil Acidification Under Long-Term N Addition Decreases the Diversity of Soil Bacteria and Fungi and Changes Their Community Composition in a Semiarid Grassland. Microb. Ecol. 2023, 85, 221–231. [Google Scholar] [CrossRef]
- Wang, C.; Liu, D.W.; Bai, E. Decreasing soil microbial diversity is associated with decreasing microbial biomass under nitrogen addition. Soil Biol. Biochem. 2018, 120, 126–133. [Google Scholar] [CrossRef]
- Zhang, X.; Song, X.; Wang, T.T.; Huang, L.; Ma, H.Y.; Wang, M.; Tan, D.Y. The responses to long-term nitrogen addition of soil bacterial, fungal, and archaeal communities in a desert ecosystem. Front. Microbiol. 2022, 13, 1015588. [Google Scholar] [CrossRef]
- Zheng, P.; Zhao, R.A.; Jiang, L.C.; Yang, G.J.; Wang, Y.L.; Wang, R.Z.; Han, X.G.; Ning, Q.S. Increasing nitrogen addition rates suppressed long-term litter decomposition in a temperate meadow steppe. J. Plant Ecol. 2023, 16, rtac078. [Google Scholar] [CrossRef]
- Liu, W.X.; Jiang, L.; Yang, S.; Wang, Z.; Tian, R.; Peng, Z.Y.; Chen, Y.L.; Zhang, X.X.; Kuang, J.L.; Ling, N.; et al. Critical transition of soil bacterial diversity and composition triggered by nitrogen enrichment. Ecology 2020, 101, e03053. [Google Scholar] [CrossRef] [PubMed]
- Bowman, W.D.; Ayyad, A.; de Mesquita, C.P.B.; Fierer, N.; Potter, T.S.; Sternagel, S. Limited ecosystem recovery from simulated chronic nitrogen deposition. Ecol. Appl. 2018, 28, 1762–1772. [Google Scholar] [CrossRef]
- Parton, W.; Silver, W.L.; Burke, I.C.; Grassens, L.; Harmon, M.E.; Currie, W.S.; King, J.Y.; Adair, E.C.; Brandt, L.A.; Hart, S.C.; et al. Global-Scale Similarities in Nitrogen Release Patterns during Long-Term Decomposition. Science 2007, 315, 361–364. [Google Scholar] [CrossRef]
- Schimel, J.P.; Bennett, J. Nitrogen Mineralization: Challenges of a Changing Paradigm. Ecology 2004, 85, 591–602. [Google Scholar] [CrossRef]
- Zhong, X.L.; Li, J.T.; Li, X.J.; Ye, Y.C.; Liu, S.S.; Hallett, P.D.; Ogden, M.R.; Naveed, M. Physical protection by soil aggregates stabilizes soil organic carbon under simulated N deposition in a subtropical forest of China. Geoderma 2017, 285, 323–332. [Google Scholar] [CrossRef]
- Asner, G.P.; Seastedt, T.R.; Townsend, A.R. The decoupling of terrestrial carbon and nitrogen cycles. Bioscience 1997, 47, 226–234. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, X.L.; Liu, L.X.; Li, T.; Dou, Y.X.; Qiao, J.B.; Wang, Y.Q.; An, S.S.; Chang, S.X. Nitrogen fertilization weakens the linkage between soil carbon and microbial diversity: A global meta-analysis. Glob. Chang. Biol. 2022, 28, 6446–6461. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.H.; Wu, S.M.; Nie, C.; Lorenz, N.; Lee, N.R.; Dick, R.P. Composition and carbon utilization of soil microbial communities subjected to long-term nitrogen fertilization in a temperate grassland in northern China. Appl. Soil Ecol. 2018, 124, 252–261. [Google Scholar] [CrossRef]
- Galloway, J.N.; Dentener, F.J.; Capone, D.G.; Boyer, E.W.; Howarth, R.W.; Seitzinger, S.P.; Asner, G.P.; Cleveland, C.C.; Green, P.A.; Holland, E.A.; et al. Nitrogen Cycles: Past, Present, and Future. Biogeochemistry 2004, 70, 153–226. [Google Scholar] [CrossRef]
- Wang, Y.X.; Wang, H.M.; Dai, X.Q.; Kou, L.; Meng, S.W.; Fu, X.L. Decoupled responses of leaf and root decomposition to nutrient deposition in a subtropical plantation. Soil Biol. Biochem. 2022, 168, 108643. [Google Scholar] [CrossRef]
- Ulm, F.; Gouveia, C.; Dias, T.; Cruz, C. N fertilization in a Mediterranean ecosystem alters N and P turnover in soil, roots and the ectomycorrhizal community. Soil Biol. Biochem. 2017, 113, 60–70. [Google Scholar] [CrossRef]
- Fernandez-Alonso, M.J.; Yuste, J.C.; Kitzler, B.; Ortiz, C.; Rubio, A. Changes. in litter chemistry associated with global change-driven forest succession resulted in time-decoupled responses of soil carbon and nitrogen cycles. Soil Biol. Biochem. 2018, 120, 200–211. [Google Scholar] [CrossRef]
- Mueller, R.C.; Belnap, J.; Kuske, C.R. Soil bacterial and fungal community responses to nitrogen addition across soil depth and microhabitat in an arid shrubland. Front. Microbiol. 2015, 6, 891. [Google Scholar] [CrossRef] [PubMed]
- Zhai, B.; Dang, X.H.; Liu, X.J.; Wang, J. Fertile island effect in the sedimentary process of Tetraena mongolica Maxim nebkhas in steppe-desert ecotones on the Inner Mongolia Plateau, China. J. Mt. Sci. 2022, 19, 2791–2805. [Google Scholar] [CrossRef]
- Li, S.Y.; Wang, C.; Yang, S.S.; Chen, W.M.; Li, G.Q.; Luo, W.; Wei, G.H.; Chen, C. Determining the contribution of microbiome complexity to the soil nutrient heterogeneity of fertile islands in a desert ecosystem. Sci. Total Environ. 2023, 857, Yang159355. [Google Scholar] [CrossRef]
- Schaeffer, S.M.; Evans, R.D. Pulse additions of soil carbon and nitrogen affect soil nitrogen dynamics in an arid Colorado Plateau shrubland. Oecologia 2005, 145, 425–433. [Google Scholar] [CrossRef]
- Yang, X.J.; Xiang, G.H.; Sun, W.C.; Chi, Y.G.; Li, W.H.; Bai, Y.F.; Zhou, Z.C.; Zheng, S.X. Shrub encroachment drives different responses of soil respiration to increased precipitation and N enrichment. Agr. For. Meteorol. 2022, 325, 109155. [Google Scholar] [CrossRef]
- Hu, X.; Li, X.Y.; Zhao, Y.D.; Gao, Z.; Zhao, S.J. Changes in soil microbial community during shrub encroachment process in the Inner Mongolia grassland of northern China. Catena 2021, 202, 105230. [Google Scholar] [CrossRef]
- Austin, A.T. Has water limited our imagination for aridland biogeochemistry? Trends Ecol. Evol. 2011, 26, 229–235. [Google Scholar] [CrossRef]
- Austin, A.T.; Yahdjian, L.; Stark, J.M.; Belnap, J.; Porporato, A.; Norton, U.; Ravetta, D.A.; Schaeffer, S.M. Water pulses and biogeochemical cycles in arid and semiarid ecosystems. Oecologia 2004, 141, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Li, Y.; Su, Y.G. Divergent responses of soil microbial communities to water and nitrogen addition in a temperate desert. Geoderma 2015, 251, 55–64. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Dell, E.; Yao, H.Y.; Rufty, T.; Shi, W. Microbial and soil properties in bentgrass putting greens: Impacts of nitrogen fertilization rates. Geoderma 2011, 162, 215–221. [Google Scholar] [CrossRef]
- Sponseller, R.A. Precipitation pulses and soil CO2flux in a Sonoran Desert ecosystem. Glob. Chang. Biol. 2007, 13, 426–436. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, L.; Zhao, J.N.; Wang, S.C.; Zhou, Y.J.; Xiao, C.W. The Diversity of Soil Bacteria and Fungi Under Altered Nitrogen and Rainfall Patterns in a Temperate Steppe. Front. Microbiol. 2022, 13, 906818. [Google Scholar] [CrossRef]
- Di Cesare, A.; Eckert, E.M.; Rogora, M.; Corno, G. Rainfall increases the abundance of antibiotic resistance genes within a riverine microbial community. Environ. Pollut. 2017, 226, 473–478. [Google Scholar] [CrossRef]
- Raison, R.; Connell, M.; Khanna, M. Methodology for studying fluxes of soil mineral-N in situ. Soil Biol. Biochem. 1987, 19, 521–530. [Google Scholar] [CrossRef]
- Lang, M.; Cai, Z.; Chang, S.X. Effects of land use type and incubation temperature on greenhouse gas emissions from Chinese and Canadian soils. J. Soils Sediments 2010, 11, 15–24. [Google Scholar] [CrossRef]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- White, D.C.; Davis, W.M.; Nickels, J.S. Determination of the sedimentary microbial biomass by extractible lipid phosphate. Oecologia 1979, 40, 51–62. [Google Scholar] [CrossRef]
- Frostegard, A.; Tunlid, A.; Baath, E. Phospholipid fatty acid composition, biomass, and activity of microbial communities from two soil types experimentally exposed to different heavy metals. Appl. Environ. Microb. 1993, 59, 3605–3617. [Google Scholar] [CrossRef]
- Bossio, D.A.; Scow, K.M. Impacts of Carbon and Flooding on Soil Microbial Communities: Phospholipid Fatty Acid Profiles and Substrate Utilization Patterns. Microb. Ecol. 1998, 35, 265–278. [Google Scholar] [CrossRef]
- Frostegard, A.; Baath, E. The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol. Fertil. Soils 1996, 22, 59–65. [Google Scholar] [CrossRef]
- Zelles, L.; Rai, Q.Y.; Reck, T. Signature fatty acids in phospholipids and lipopolysaccharides as indicators of microbial biomass and community structure in agricultural soils. Soil Biol. Biochem. 1992, 24, 317–323. [Google Scholar] [CrossRef]
- Madan, R.; Pankhurst, C.; Hawke, B. Use of fatty acids for identification of AM fungi and estimation of the biomass of AM spores in soil. Soil Biol. Biochem. 2002, 34, 125–128. [Google Scholar] [CrossRef]
- Zelles, L. Phospholipid fatty acid profiles in selected members of soil microbial communities. Chemosphere 1997, 35, 275–294. [Google Scholar] [CrossRef] [PubMed]
- Zogg, G.P.; Zak, D.R.; Ringelberg, D.B. Compositional and functional shifts in microbial communities due to soil warming. Soil Sci. Soc. Am. J. 1997, 61, 475–481. [Google Scholar] [CrossRef]
- Fierer, N.; Schimel, J.P.; Holden, P.A. Variation in microbial community composition through two soil depth profiles. Soil Biol. Biochem. 2002, 35, 167–176. [Google Scholar] [CrossRef]
- Grace, J.B. Structural Equation Modeling and Natural Systems; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Duan, P.P.; Xiao, K.C.; Jiang, Y.L.; Li, D.J. Mechanisms underlying the responses of soil N2O production by ammonia oxidizers to nitrogen addition are mediated by topography in a subtropical forest. Geoderma 2022, 425, 116036. [Google Scholar] [CrossRef]
- Shaw, M.R.; Harte, J. Response of nitrogen cycling to simulated climate change differential responses along a subalpine ecotone. Glob. Chang. Biol. 2001, 7, 193–210. [Google Scholar] [CrossRef]
- Ehlers, K.; Bakken, L.R.; Frostegard, A.; Frossard, E.; Bunemann, E.K. Phosphorus limitation in a Ferralsol: Impact on microbial activity and cell internal P pools. Soil Biol. Biochem. 2010, 42, 558–566. [Google Scholar] [CrossRef]
- Corre, M.D.; Veldkamp, E.; Arnold, J.; Wright, S.J. Impact of elevated N input on soil N cycling and losses in old-growth lowland and montane forests in Panama. Ecology 2010, 91, 1715–1729. [Google Scholar] [CrossRef]
- Wang, L.; Wen, Y.; Tong, R.; Zhang, H.; Chen, H.; Hu, T.; Liu, G.; Wang, J.; Zhu, L.; Wu, T. Understanding Responses of Soil Microbiome to the Nitrogen and Phosphorus Addition in Metasequoia glyptostroboides Plantations of Different Ages. Microb. Ecol. 2021, 84, 565–579. [Google Scholar] [CrossRef]
- Nemergut, D.R.; Townsend, A.R.; Sattin, S.R.; Freeman, K.R.; Fierer, N.; Neff, J.C.; Bowman, W.D.; Schadt, C.W.; Weintraub, M.N.; Schmidt, S.K. The effects of chronic nitrogen fertilization on alpine tundra soil microbial communities: Implications for carbon and nitrogen cycling. Environ. Microbiol. 2008, 10, 3093–3105. [Google Scholar] [CrossRef]
- Borken, W.; Matzner, E. Reappraisal of drying and wetting effects on C and N mineralization and fluxes in soils. Glob. Chang. Biol. 2009, 15, 808–824. [Google Scholar] [CrossRef]
- Orwin, K.H.; Dickie, I.A.; Wood, J.R.; Bonner, K.I.; Holdaway, R.J.; Jones, H. Soil microbial community structure explains the resistance of respiration to a dry–rewet cycle, but not soil functioning under static conditions. Funct. Ecol. 2015, 30, 1430–1439. [Google Scholar] [CrossRef]
- Treseder, K.K. Nitrogen additions and microbial biomass: A meta-analysis of ecosystem studies. Ecol. Lett. 2008, 11, 1111–1120. [Google Scholar] [CrossRef]
- Zhong, Y.Q.; Yan, W.M.; Zhouping, S.G. Impact of long-term N additions upon coupling between soil microbial community structure and activity, and nutrient-use efficiencies. Soil Biol. Biochem. 2015, 91, 151–159. [Google Scholar] [CrossRef]
- He, D.; Guo, Z.M.; Shen, W.J.; Ren, L.J.; Sun, D.; Yao, Q.; Zhu, H.H. Fungal Communities Are More Sensitive to the Simulated Environmental Changes than Bacterial Communities in a Sub-tropical Forest: The Single and Interactive Effects of Nitrogen Addition and Precipitation Seasonality Change. Microb. Ecol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Schimel, J.; Balser, T.C.; Wallenstein, M. Microbial stress-response physiology and its implications for ecosystem function. Ecology 2007, 88, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Blagodatskaya, E.; Kuzyakov, Y. Mechanisms of real and apparent priming effects and their dependence on soil microbial biomass and community structure: Critical review. Biol. Fertil. Soils 2008, 45, 115–131. [Google Scholar] [CrossRef]
- Luo, R.Y.; Luo, J.F.; Fan, J.L.; Liu, D.Y.; He, J.S.; Perveen, N.; Ding, W.X. Responses of soil microbial communities and functions associated with organic carbon mineralization to nitrogen addition in a Tibetan grassland. Pedosphere 2020, 30, 214–225. [Google Scholar] [CrossRef]
- Li, S.L.; Wang, S.; Fan, M.C.; Wu, Y.; Shangguan, Z.P. Interactions between biochar and nitrogen impact soil carbon mineralization and the microbial community. Soil Till Res. 2020, 196, 104437. [Google Scholar] [CrossRef]
- Kopacek, J.; Cosby, B.J.; Evans, C.D.; Hruska, J.; Moldan, F.; Oulehle, F.; Santruckova, H.; Tahovska, K.; Wright, R.F. Nitrogen, organic carbon and sulphur cycling in terrestrial ecosystems: Linking nitrogen saturation to carbon limitation of soil microbial processes. Biogeochemistry 2013, 115, 33–51. [Google Scholar] [CrossRef]
- Wu, H.; Yang, J.; Fu, W.; Rillig, M.C.; Cao, Z.; Zhao, A.; Hao, Z.; Zhang, X.; Chen, B.; Han, X. Identifying thresholds of nitrogen enrichment for substantial shifts in arbuscular mycorrhizal fungal community metrics in a temperate grassland of northern China. New Phytol. 2022, 237, 279–294. [Google Scholar] [CrossRef]
- Yuan, X.B.; Niu, D.C.; Gherardi, L.A.; Liu, Y.B.; Wang, Y.; Elser, J.J.; Fu, H. Linkages of stoichiometric imbalances to soil microbial respiration with increasing nitrogen addition: Evidence from a long-term grassland experiment. Soil Biol. Biochem. 2019, 138, 107580. [Google Scholar] [CrossRef]
- Zhang, M.N.; Li, S.P.; Wu, X.P.; Zheng, F.J.; Song, X.J.; Lu, J.J.; Liu, X.T.; Wang, B.S.; Abdelrhmana, A.A.; Degre, A. Nitrogen addition mediates the effect of soil microbial diversity on microbial carbon use efficiency under long-term tillage practices. Land Degrad. Dev. 2022, 33, 2258–2275. [Google Scholar] [CrossRef]
- Niu, Y.Y.; Duan, Y.L.; Li, Y.Q.; Wang, X.Y.; Yun, C.; Wang, L.L. Soil microbial community responses to short-term nitrogen addition in China’s Horqin Sandy Land. PLoS ONE 2021, 16, e0242643. [Google Scholar] [CrossRef]
- Kaiser, C.; Fuchslueger, L.; Koranda, M.; Gorfer, M.; Stange, C.F.; Kitzler, B.; Rasche, F.; Strauss, J.; Sessitsch, A.; Zechmeister-Boltenstern, S.; et al. Plants control the seasonal dynamics of microbial N cycling in a beech forest soil by belowground C allocation. Ecology 2011, 92, 1036–1051. [Google Scholar] [CrossRef]
- Iwaoka, C.; Imada, S.; Taniguchi, T.; Du, S.; Yamanaka, N.; Tateno, R. The Impacts of Soil Fertility and Salinity on Soil Nitrogen Dynamics Mediated by the Soil Microbial Community Beneath the Halophytic Shrub Tamarisk. Microb. Ecol. 2018, 75, 985–996. [Google Scholar] [CrossRef]
- Boberg, J.B.; Finlay, R.D.; Stenlid, J.; Lindahl, B.D. Fungal C translocation restricts N-mineralization in heterogeneous environments. Funct. Ecol. 2010, 24, 454–459. [Google Scholar] [CrossRef]
- Gallardo, A.; Schlesinger, W.H. Factors determining soil microbial biomass and nutrient immobilization in desert soils. Biogeochemistry 1995, 28, 55–68. [Google Scholar] [CrossRef]
- Okiobe, S.T.; Pirhofer-Walzl, K.; Leifheit, E.F.; Rillig, M.C.; Veresoglou, S.D. Proximal and distal mechanisms through which arbuscular mycorrhizal associations alter terrestrial denitrification. Plant Soil 2022, 476, 315–336. [Google Scholar] [CrossRef]
- McCalley, C.K.; Sparks, J.P. Abiotic Gas Formation Drives Nitrogen Loss from a Desert Ecosystem. Science 2009, 326, 837–840. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, S.M.; Billings, S.A.; Evans, R.D. Responses of soil nitrogen dynamics in a Mojave Desert ecosystem to manipulations in soil carbon and nitrogen availability. Oecologia 2003, 134, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.L.; Halvorson, J.J.; Bolton, H.J. Spatial relationships of soil microbial biomass and C and N mineralization in a semiarid shrub-steppe ecosystem. Soil Biol. Biochem. 1994, 26, 1151–1159. [Google Scholar] [CrossRef]
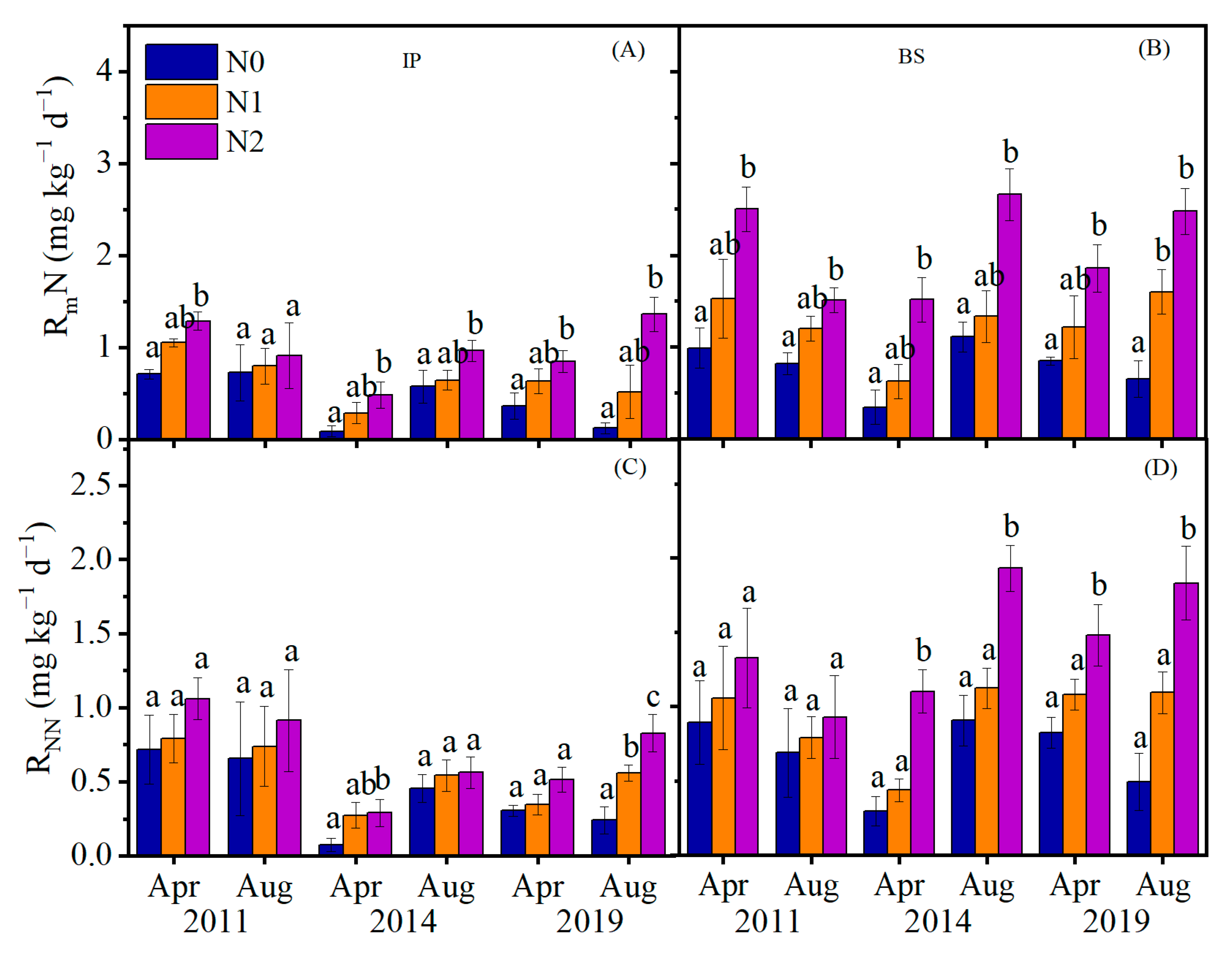

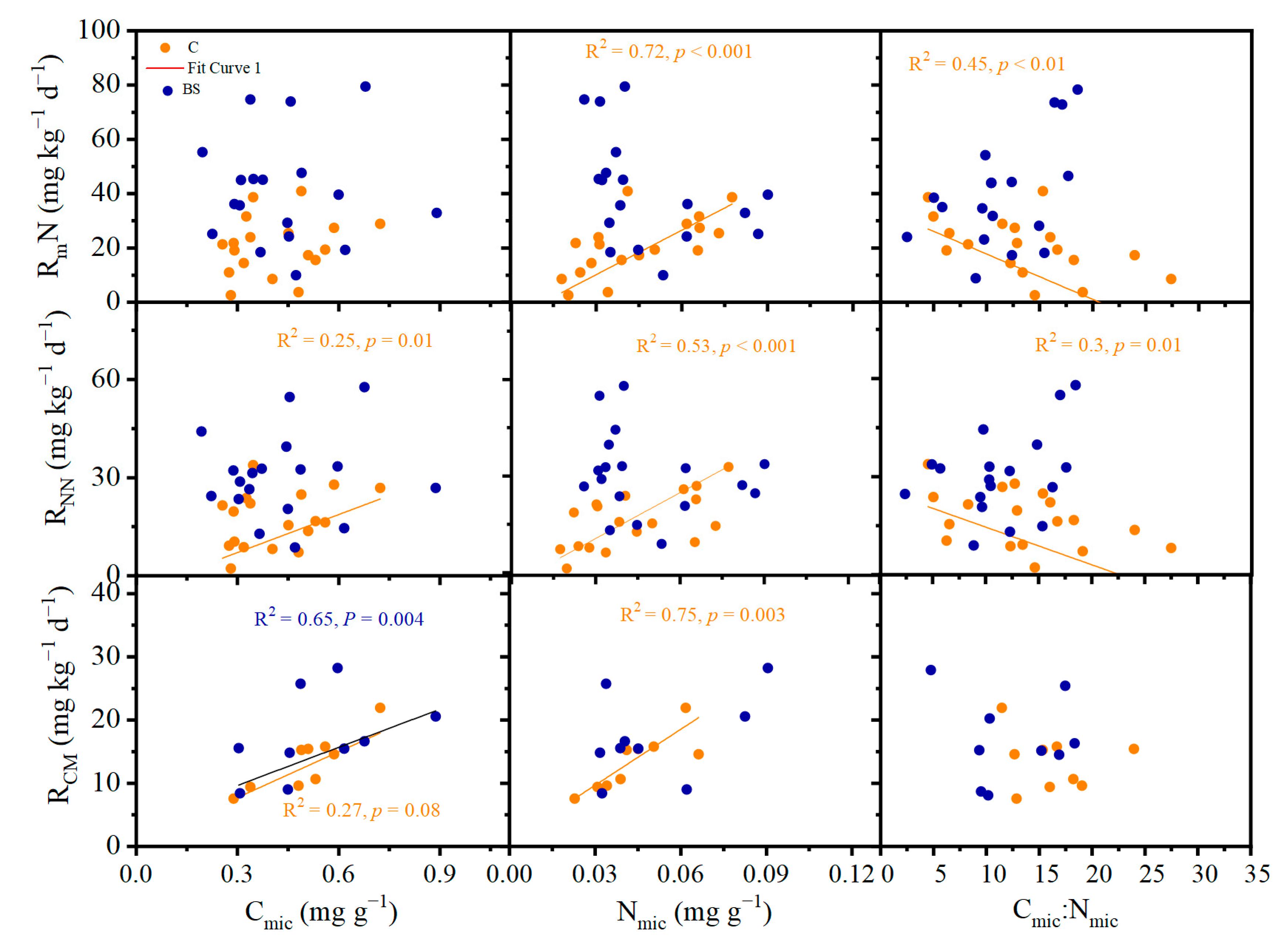


| Year | Factors | Samling Time | N | Site | N × Site | ||||
|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | ||
| 2011 | RmN | 16.57 | <0.001 | 26.25 | <0.001 | 35.18 | <0.001 | 6.34 | 0.004 |
| RNN | 4.43 | 0.04 | 5.4 | 0.008 | 2.03 | 0.16 | 0.03 | 0.96 | |
| Cmic | 0.99 | 0.32 | 1.07 | 0.35 | 0.04 | 0.84 | 6.35 | 0.004 | |
| Nmic | 0.14 | 0.71 | 1.4 | 0.25 | 3.4 | 0.07 | 8.37 | <0.001 | |
| Cmic:Nmic | 0.87 | 0.35 | 0.06 | 0.94 | 1.69 | 0.2 | 0.53 | 0.59 | |
| 2014 | RmN | 43.72 | <0.001 | 28.62 | <0.001 | 58.27 | <0.001 | 9.44 | <0.001 |
| RNN | 25.29 | <0.001 | 10.02 | <0.001 | 35.43 | <0.001 | 5.94 | 0.004 | |
| Cmic | 43.45 | <0.001 | 0.55 | 0.58 | 0.18 | 0.68 | 2.07 | 0.14 | |
| Nmic | 50.32 | <0.001 | 4.04 | 0.03 | 25.64 | <0.001 | 12.47 | <0.001 | |
| Cmic:Nmic | 0.001 | 0.98 | 0.38 | 0.68 | 11.00 | 0.002 | 5.49 | 0.008 | |
| 2019 | RmN | 0.78 | 0.38 | 13.05 | <0.001 | 19.28 | <0.001 | 0.77 | 0.46 |
| RNN | 0.93 | 0.34 | 22.73 | <0.001 | 62.16 | <0.001 | 4.2 | 0.02 | |
| Cmic | 49.97 | <0.001 | 0.28 | 0.75 | 4.55 | 0.03 | 4.26 | 0.02 | |
| Nmic | 12.29 | <0.001 | 0.51 | 0.6 | 0.4 | 0.52 | 8.33 | 0.001 | |
| Cmic:Nmic | 26.16 | <0.001 | 0.28 | 0.75 | 0.89 | 0.34 | 2.1 | 0.13 | |
| Factors | Year | N | Site | N × Site | ||||
|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | |
| RCM | 381.5 | <0.001 | 100.4 | <0.001 | 204.72 | <0.001 | 272.12 | <0.001 |
| Microbial PLFAs | 143.89 | <0.001 | 2.99 | 0.06 | 40.6 | <0.001 | 5.94 | 0.006 |
| Bacterial PLFAs | 409.73 | <0.001 | 4.83 | 0.01 | 58.36 | <0.001 | 37.18 | <0.001 |
| Fungal PLFAs | 335.58 | <0.001 | 1.55 | 0.23 | 0.3 | 0.58 | 24.24 | <0.001 |
| F:B | 11.66 | <0.001 | 0.18 | 0.83 | 60.46 | <0.001 | 4.68 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Li, H.; Sun, H.; Guo, Y. Microsite Determines the Soil Nitrogen and Carbon Mineralization in Response to Nitrogen Addition in a Temperate Desert. Forests 2023, 14, 1154. https://doi.org/10.3390/f14061154
Chen Y, Li H, Sun H, Guo Y. Microsite Determines the Soil Nitrogen and Carbon Mineralization in Response to Nitrogen Addition in a Temperate Desert. Forests. 2023; 14(6):1154. https://doi.org/10.3390/f14061154
Chicago/Turabian StyleChen, Yingwu, Haorui Li, Huilu Sun, and Yuxin Guo. 2023. "Microsite Determines the Soil Nitrogen and Carbon Mineralization in Response to Nitrogen Addition in a Temperate Desert" Forests 14, no. 6: 1154. https://doi.org/10.3390/f14061154
APA StyleChen, Y., Li, H., Sun, H., & Guo, Y. (2023). Microsite Determines the Soil Nitrogen and Carbon Mineralization in Response to Nitrogen Addition in a Temperate Desert. Forests, 14(6), 1154. https://doi.org/10.3390/f14061154





