The Role of Forest Stands Characteristics on Formation of Exterior Migratory Outbreak Spots by the Siberian Silk Moth Dendrolimus sibiricus (Tschetv.) during Population Collapse
Abstract
1. Introduction
2. Materials and Methods
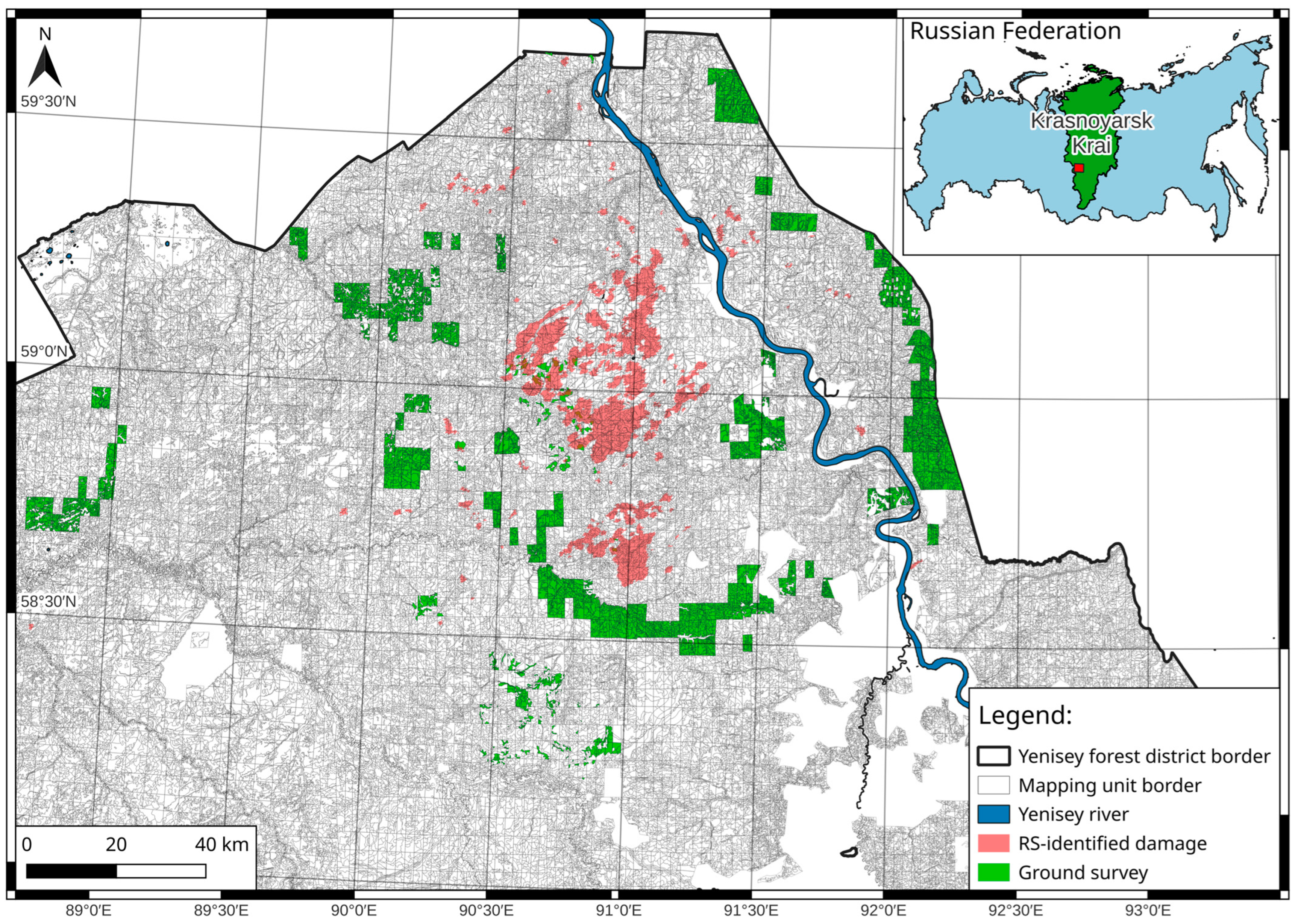
2.1. Study Area
2.2. The Siberian Silk Moth Outbreak in 2014–2017
2.3. Forest Stand Characteristics
2.4. Statistical Processing and Modeling
3. Results
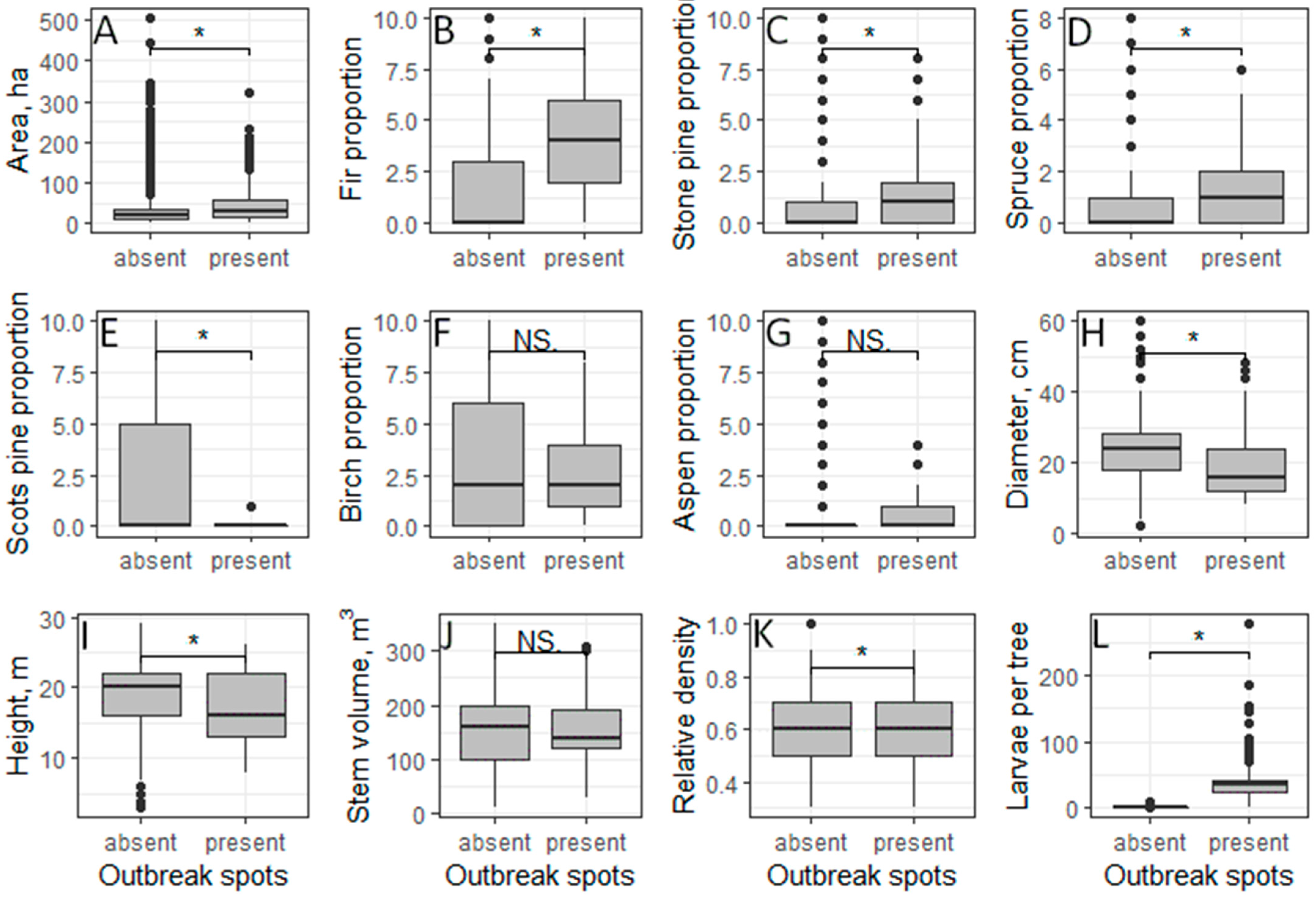
3.1. Characteristics of Forest Stands Where There Are Some or No Outbreak Spots
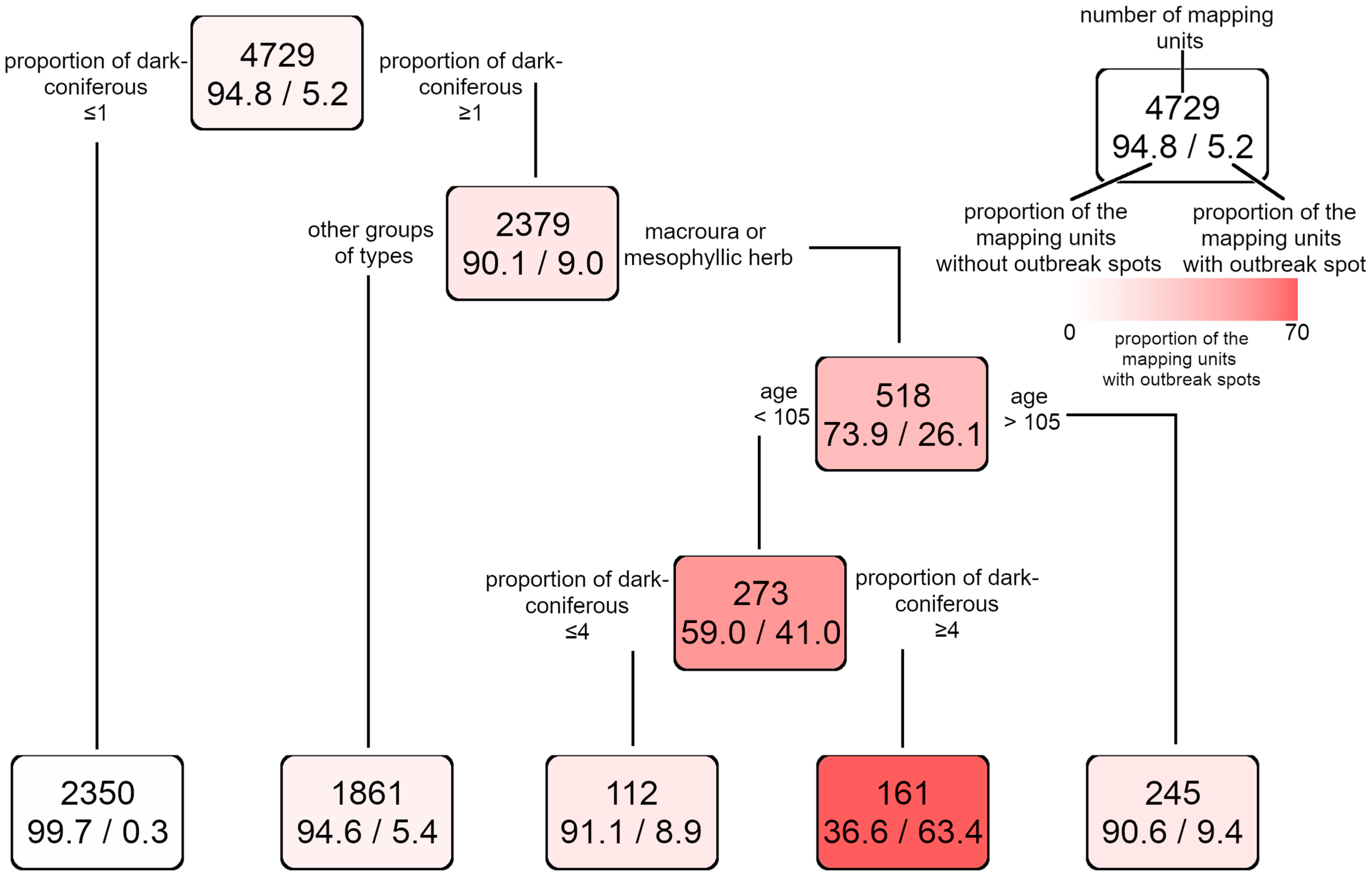
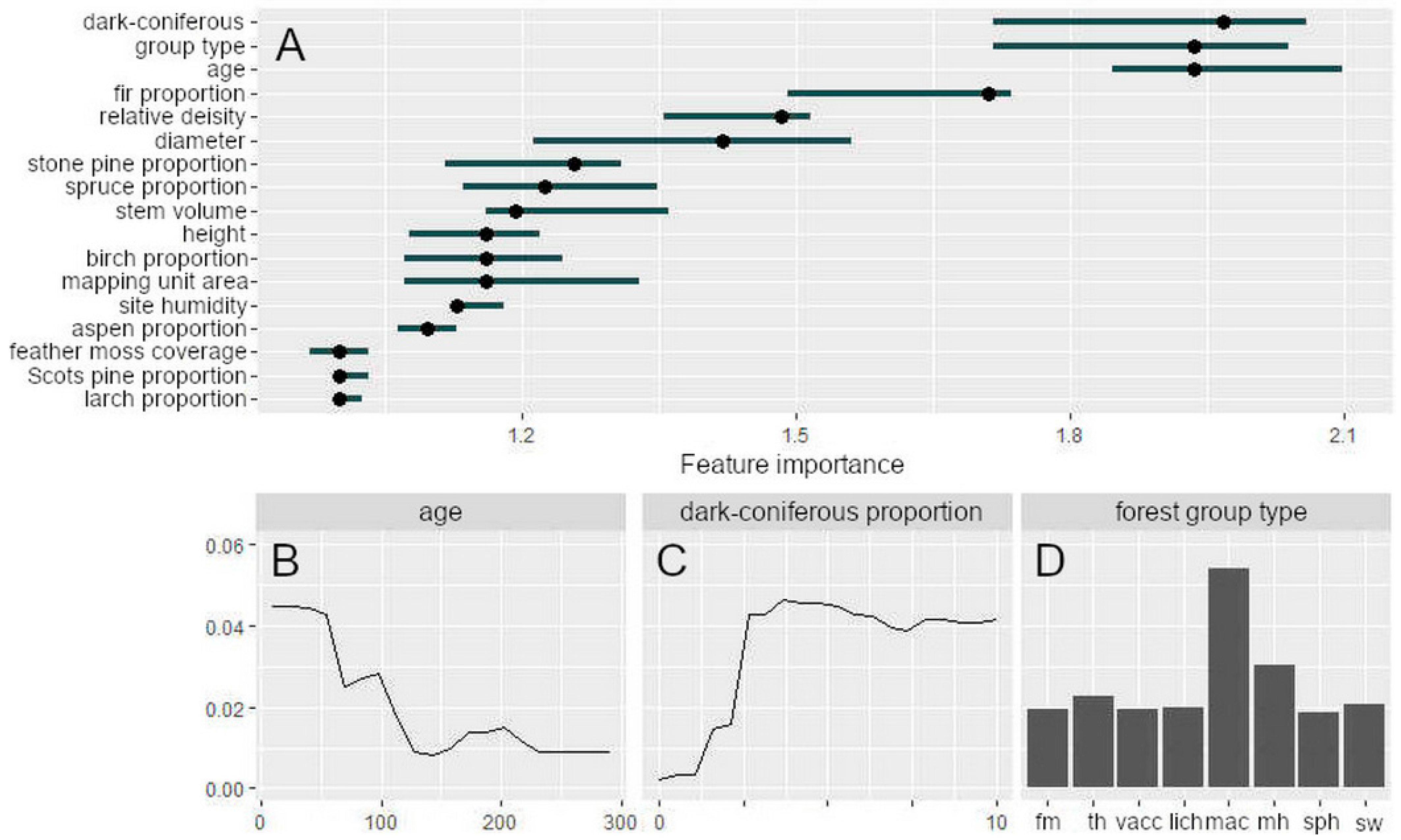
3.2. Modelling the Occurrence of Outbreak Spots
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olson, D.M.; Dinerstein, E.; Wikramanayake, E.D.; Burgess, N.D.; Powell, G.V.N.; Underwood, E.C.; D’amico, J.A.; Itoua, I.; Strand, H.E.; Morrison, J.C.; et al. Terrestrial Ecoregions of the World: A New Map of Life on Earth: A New Global Map of Terrestrial Ecoregions Provides an Innovative Tool for Conserving Biodiversity. BioScience 2001, 51, 933–938. [Google Scholar] [CrossRef]
- Santoro, M.; Cartus, O.; Carvalhais, N.; Rozendaal, D.M.A.; Avitabile, V.; Araza, A.; de Bruin, S.; Herold, M.; Quegan, S.; Rodríguez-Veiga, P.; et al. The Global Forest above-Ground Biomass Pool for 2010 Estimated from High-Resolution Satellite Observations. Earth Syst. Sci. Data 2021, 13, 3927–3950. [Google Scholar] [CrossRef]
- Schepaschenko, D.; Moltchanova, E.; Fedorov, S.; Karminov, V.; Ontikov, P.; Santoro, M.; See, L.; Kositsyn, V.; Shvidenko, A.; Romanovskaya, A.; et al. Russian Forest Sequesters Substantially More Carbon than Previously Reported. Sci. Rep. 2021, 11, 12825. [Google Scholar] [CrossRef]
- Alexeyev, V.A.; Svyazeva, O.A. Woody Plants of Russian Forests. A List of Species and the State Account of Biodiversity of Forest Resources; SB RAS, Sukachev Institute of Forest: Krasnoyarsk, Russia, 2009; ISBN 978-5-94668-063-9. [Google Scholar]
- Talucci, A.C.; Loranty, M.M.; Alexander, H.D. Siberian Taiga and Tundra Fire Regimes from 2001–2020. Environ. Res. Lett. 2022, 17, 25001. [Google Scholar] [CrossRef]
- Kononov, A.; Ustyantsev, K.; Wang, B.; Mastro, V.C.; Fet, V.; Blinov, A.; Baranchikov, Y. Genetic Diversity among Eight Dendrolimus Species in Eurasia (Lepidoptera: Lasiocampidae) Inferred from Mitochondrial COI and COII, and Nuclear ITS2 Markers. BMC Genet. 2016, 17, 157. [Google Scholar] [CrossRef]
- Kharuk, V.I.; Im, S.T.; Ranson, K.J.; Yagunov, M.N. Climate-Induced Northerly Expansion of Siberian Silkmoth Range. Forests 2017, 8, 301. [Google Scholar] [CrossRef] [PubMed]
- Kondakov, Y.P. The Patterns of Siberian Moth Outbreaks. In The Population Ecology of Forest Animals; Petrenko, E.S., Ed.; Nauka: Novosibirsk, Russia, 1974; pp. 206–265. [Google Scholar]
- Kharuk, V.I.; Demidko, D.A.; Fedotova, E.V.; Dvinskaya, M.L.; Budnik, U.A. Spatial and Temporal Dynamics of Siberian Silk Moth Large-Scale Outbreak in Dark-Needle Coniferous Tree Stands in Altai. Contemp. Probl. Ecol. 2016, 9, 711–720. [Google Scholar] [CrossRef]
- Sultson, S.M.; Goroshko, A.A.; Verkhovets, S.V.; Mikhaylov, P.V.; Ivanov, V.A.; Demidko, D.A.; Kulakov, S.S. Orographic Factors as a Predictor of the Spread of the Siberian Silk Moth Outbreak in the Mountainous Southern Taiga Forests of Siberia. Land 2021, 10, 115. [Google Scholar] [CrossRef]
- Kirichenko, N.I.; Baranchikov, Y.N. Withdrawn Food Rate for Larvae of Siberian Moth on Conifers of Siberia. Contemp. Probl. Ecol. 2008, 1, 543–548. [Google Scholar] [CrossRef]
- Grodnitskiy, D.L.; Raznobarskiy, V.G.; Soldatov, V.V.; Remarchuk, N.P. The Tree Dieback in Taiga Forests Defoliated by Siberian Silk Moth. Contemporary Problem of Ecology. 2002, 1, 3–12. [Google Scholar]
- Gorbatenko, V.P.; Ippolitov, I.I.; Kabanov, M.V.; Loginov, S.V.; Podnebesnych, N.V.; Kharyutkina, E.V. Effect of atmospheric circulation on temperature variations in Siberia. Atmos. Ocean. Opt. 2011, 24, 15–21. [Google Scholar]
- Tchebakova, N.M.; Parfenova, E.; Soja, A.J. The Effects of Climate, Permafrost and Fire on Vegetation Change in Siberia in a Changing Climate. Environ. Res. Lett. 2009, 4, 45013. [Google Scholar] [CrossRef]
- Seidl, R.; Thom, D.; Kautz, M.; Martin-Benito, D.; Peltoniemi, M.; Vacchiano, G.; Wild, J.; Ascoli, D.; Petr, M.; Honkaniemi, J.; et al. Forest Disturbances under Climate Change. Nat. Clim. Chang. 2017, 7, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Niinemets, Ü. Responses of Forest Trees to Single and Multiple Environmental Stresses from Seedlings to Mature Plants: Past Stress History, Stress Interactions, Tolerance and Acclimation. For. Ecol. Manag. 2010, 260, 1623–1639. [Google Scholar] [CrossRef]
- Teshome, D.T.; Zharare, G.E.; Naidoo, S. The Threat of the Combined Effect of Biotic and Abiotic Stress Factors in Forestry Under a Changing Climate. Front. Plant Sci. 2020, 11, 601009. [Google Scholar] [CrossRef] [PubMed]
- Kharuk, V.I.; Ranson, K.J.; Kozuhovskaya, A.G.; Kondakov, Y.P.; Pestunov, I.A. NOAA/AVHRR Satellite Detection of Siberian Silkmoth Outbreaks in Eastern Siberia. Int. J. Remote Sens. 2004, 25, 5543–5556. [Google Scholar] [CrossRef]
- Isaev, A.S.; Ryapolov, V.Y. The analysis of landscape-ecological confinedness of Siberian silk moth outbreak spots by aerospace survey data. In The Remote Sensing of Taiga Landscapes; Nauka: Novosibirsk, Russia, 1979; pp. 152–167. [Google Scholar]
- Kondakov, Y.P. The outbreaks of Siberian silk moth in Krasnoyarsk krai forests. In Entomological Researches in Siberia; KF RES: Krasnoyarsk, Russia, 2002; Volume 2, pp. 25–74. [Google Scholar]
- Kochenkov, E.A.; Frolov, A.A.; Zvonka, D.V. The History of Siberian Silk Moth Outbreaks Onsets and Their Control in Altai Republic. Nat. Resourses Altai Repub. 2004, 2, 98–103. [Google Scholar]
- Mikhailov, J.Z.; Sumina, N.Y. Siberian Moth Dendrolimus Superans (Butler, 1877) and Control of It in Irkutsk Region. Baikal Zool. J. 2012, 11, 25–29. [Google Scholar]
- Isaev, A.S.; Khlebopros, R.G.; Nedorezov, L.V.; Kondakov, Y.P.; Soukhovolsky, V.G. Population Dynamics of Forest Insects; Nauka: Moskow, Russia, 2001; ISBN 5-02-004270-6. [Google Scholar]
- Galkin, G.I. Some issues of Siberian silk moth refugiums and primary outbreak spots formation in the forests of Krasnoyarsk krai. In The Problem of Siberian Silk Moth (Workshop Reports); USSR Academy of Science Publishing House: Novosibirsk, Russia, 1960; pp. 21–33. [Google Scholar]
- Kondakov, Y.P. On the issue of Siberian silk moth (Dendrolimus sibiricus Tshtv) bioecology in the fir-dominated forests of Krasnoyarsk krai. In Scientific Notes of Krasnoyarsk State Pedagogical Institute; Golosov, V.F., Konikov, A.S., Cherepnin, L.M., Eds.; Krasnoyarsk State Pedagogical Institute: Krasnoyarsk, Russia, 1957; Volume X, pp. 144–153. [Google Scholar]
- Forestry Regulation of Eniseiskoye Lesnichestvo 2018. Available online: http://www.krskstate.ru/docs/0/doc/51516 (accessed on 23 May 2023).
- Forest Plan of Krasnoyarsk Krai for the Period 2019–2028 2018. Available online: http://mlx.krskstate.ru/lesplan?eyes=no (accessed on 23 May 2023).
- Gerasimov, I.P. Middle Siberia; Geographical Description and Natural Resourses of USSR; Nauka: Moskow, Russia, 1964. [Google Scholar]
- RIHMI-WDC. Available online: http://aisori.meteo.ru/ClspR (accessed on 15 October 2020).
- Okunev, P.P. Geographical Distribution and Damage Areas of Siberian Silk Moth. In The Geographical Compendium. V. Geographical Issues of Forestry; USSR Academy of Science Publishing House: Moskow-Leningrad, Russia, 1955; pp. 210–222. [Google Scholar]
- Vlăduţ, A.; Nikolova, N.; Licurici, M. Aridity Assessment within Southern Romania and Northern Bulgaria. Hrvat. Geogr. Glas. 2017, 79, 5–26. [Google Scholar] [CrossRef]
- Leśniak, A. Climatic and Meteorological Conditions of the Pine Moth (Dendrolimus pini L.) Outbreaks. Ekol. Pol. 1976, 24, 515–547. [Google Scholar]
- Ray, D.; Peace, A.; Moore, R.; Petr, M.; Grieve, Y.; Convery, C.; Ziesche, T. Improved Prediction of the Climate-Driven Outbreaks of Dendrolimus Pini in Pinus Sylvestris Forests. Forestry 2016, 89, 230–244. [Google Scholar] [CrossRef]
- Kharuk, V.I.; Im, S.T.; Yagunov, M.N. Migration of the Northern Boundary of the Siberian Silk Moth. Contemp. Probl. Ecol. 2018, 11, 26–34. [Google Scholar] [CrossRef]
- Pavlov, I.N.; Litovka, Y.A.; Golubev, D.V.; Astapenko, S.A.; Chromogin, P.V. New Outbreak of Dendrolimus Sibiricus Tschetv. in Siberia (2012–2017): Monitoring, Modeling and Biological Control. Contemp. Probl. Ecol. 2018, 11, 406–419. [Google Scholar] [CrossRef]
- Baranchikov, Y.N.; Bobrinskiy, A.N.; Golubev, A.V.; Gordienko, P.V.; Denisov, B.S.; Zhirin, V.M.; Kondakov, Y.P.; Lyamtsev, N.I.; Malysheva, N.V.; Maslov, A.D.; et al. Methods of the Forest Pests and Disiases Monitoring; Tuzov, V.K., Ed.; Forest pests and disiases of Russia; ARRISMF: Moskow, Russia, 2004; Volume III, ISBN 5-94219-112-3. [Google Scholar]
- Kozlovskiy, V.B.; Pavlov, V.M. The Growth of the Main Forest Tree Species of USSR; Lesnaya Promyshlennost: Moskow, Russia, 1967. [Google Scholar]
- Rysin, L.P. Siberian Stone Pine Forests of Russia; KMK Publishing: Moskow, Russia, 2011; ISBN 978-5-87317-771-4. [Google Scholar]
- Anonimous R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 2 October 2020).
- Ripley, B. Tree: Classification and Regression Trees. R Package. Available online: https://cran.r-project.org/package=tree (accessed on 26 October 2020).
- Liaw, A.; Wiener, M. Classification and Regression by RandomForest. R News 2002, 2, 18–22. [Google Scholar]
- Molnar, C.; Bischl, B.; Casalicchio, G. Iml: An R Package for Interpretable Machine Learning. J. Open Source Softw. 2018, 3, 786. [Google Scholar] [CrossRef]
- Kuhn, M. Caret: Classification and Regression Training. R Package. Available online: https://cran.r-project.org/package=caret (accessed on 26 October 2020).
- Klapwijk, M.J.; Björkman, C. Mixed Forests to Mitigate Risk of Insect Outbreaks. Scand. J. For. Res. 2018, 33, 772–780. [Google Scholar] [CrossRef]
- Rozhkov, A.S. Siberian Silk Moth Outbreaks and Their Control; Nauka: Moskow, Russia, 1965. [Google Scholar]
- Sukachev, V.N. Basics of Forest Typology and Biogeocenology: Selected Works; Nauka: Leningrad, Russia, 1972. [Google Scholar]
- Rozhkov, A.S. Siberian Silk Moth; Nauka: Moskow, Russia, 1963. [Google Scholar]
- Konikov, A.S.; Platonova-Chernysheva, L.; Kondakov, Y.P.; Zaitseva, A.I. Siberian Silk Moth Adaptation to Environmental Conditions. In Scientific Notes of Krasnoyarsk State Pedagogical Institute; Konikov, A.S., Pshonik, A.T., Cherepnin, L.M., Eds.; Krasnoyarsk State Pedagogical University: Krasnoyarsk, Russia, 1959; Volume XV, pp. 145–175. [Google Scholar]
- Kostin, I.A. Siberian silk moth outbreak in mountain forests of Eastern Kazakhstan. In Issues of Zoological Institute of Kazakh SSR Academy of Sciences; Academy of Kazakh SSR Publishing House: Alma-Ata, Kazakhstan, 1958; pp. 122–126. [Google Scholar]
- Pologova, N.N.; Chernova, N.A.; Klimova, N.W.; Dyukarev, A.G. Diversity of Siberian Pine Forests Related to Their Habitat. Russ. J. For. Sci. 2013, 4, 32–42. [Google Scholar]
- Stoszek, K.J.; Mika, P.G.; Moore, J.A.; Osborne, H.L. Relationships of Douglas-Fir Tussock Moth Defoliation to Site and Stand Characteristics in Northern Idaho. For. Sci. 1981, 27, 431–442. [Google Scholar]
- Williams, C.B.; Wenz, J.M.; Dahlsten, D.L.; Norick, N.X. Relation of Forest Site and Stand Characteristics to Douglas-Fir Tussock Moth (Lep. Lymantriidae) Outbreaks in California. Mitt. Schweiz. Entomol. Ges. 1979, 52, 297–307. [Google Scholar]
- Krylov, G.V.; Maradudin, I.I.; Mikheev, N.I.; Kozakova, N.F. Fir; Agropromizdat: Moskow, Russia, 1986. [Google Scholar]
- Royama, T. Population Dynamics of the Spruce Budworm Choristoneura fumiferana. Ecol. Monogr. 1984, 54, 429–462. [Google Scholar] [CrossRef]
- Larroque, J.; Johns, R.; Canape, J.; Morin, B.; James, P.M.A. Spatial Genetic Structure at the Leading Edge of a Spruce Budworm Outbreak: The Role of Dispersal in Outbreak Spread. For. Ecol. Manag. 2020, 461, 117965. [Google Scholar] [CrossRef]
- Furyaev, V.V. Forests that Died after Siberian Silk Moth Outbreaks and Thier Prescribed Burning; Nauka: Moskow, Russia, 1966. [Google Scholar]
- Sedykh, V.N. Formation of Siberian Stone Pine Forests in Priob’e; Nauka: Novosibirsk, Russia, 1979. [Google Scholar]
- Buryak, L.V.; Ivanov, V.A.; Zlenko, L.V. Forest Formation Processes in Violation Fire Light Coniferous Plantations Lower Angara Region. Fundam. Res. 2015, 2, 1709–1714. [Google Scholar]
- Sedykh, V.N. Forest Forming Process; Nauka: Novosibirsk, Russia, 2009. [Google Scholar]
- Lyubimova, E.L.; Simakova, L.A. Modern Paludification of Forests. In Scientific Preconditions of West Siberian Bogs Exploitation; Nauka: Moskow, Russia, 1977; pp. 158–171. [Google Scholar]
- Robert, L.; Sturtevant, B.R.; Cooke, B.J.; James, P.M.A.; Fortin, M.; Townsend, P.A.; Wolter, P.T.; Kneeshaw, D. Landscape Host Abundance and Configuration Regulate Periodic Outbreak Behavior in Spruce Budworm Choristoneura fumiferana. Ecography 2018, 41, 1556–1571. [Google Scholar] [CrossRef]
- Ellis, T.M.; Flower, A. A Multicentury Dendrochronological Reconstruction of Western Spruce Budworm Outbreaks in the Okanogan Highlands, Northeastern Washington. Can. J. For. Res. 2017, 47, 1266–1277. [Google Scholar] [CrossRef]
- Demidko, D.A.; Efremenko, A.A.; Baranchikov, Y.N. History of the Siberian Moth Outbreaks at the Eastern Foothills of Kuznetskiy Alatau Mountains: Dendrochronolohical Reconstruction. Sib. J. For. Sci. 2023, 1, 98–110. [Google Scholar] [CrossRef]
- Konikov, A.S.; Platonova-Chernysheva; Kondakov, Y.P. About two critical periods of Siberian silk moth larval stage and their role in population dynamics. In Scientific Notes of Krasnoyarsk State Pedagogical Institute. Iss. II; Krasnoyarsk Publishing House: Krasnoyarsk, Russia, 1961; Volume XX, pp. 3–16. [Google Scholar]

| Outbreak Spots | Soil Moisture | Feather Mosses Cover (%) | Total | ||
|---|---|---|---|---|---|
| 0–30 | 31–70 | 70–100 | |||
| Present | Moderately moist | 57.1 1 | 16.5 | 0.9 | 74.6 |
| Moist | 4.0 | 11.2 | 5.6 | 20.8 | |
| Wet | 0.9 | 0.9 | |||
| Extremely wet | 3.7 | 3.7 | |||
| Total | 65.8 | 27.7 | 6.5 | ||
| Absent | Extremely dry | 0.3 | 0.3 | ||
| Dry | 3.2 | 0.2 | 3.4 | ||
| Moderately moist | 26.7 | 24.6 | 10.1 | 61.4 | |
| Moist | 5.0 | 6.4 | 11.6 | 23.0 | |
| Wet | 4.7 | 0.2 | 0.2 | 5.1 | |
| Extremely wet | 6.5 | 6.5 | |||
| Total | 46.3 | 31.2 | 21.7 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demidko, D.A.; Goroshko, A.A.; Slinkina, O.A.; Mikhaylov, P.V.; Sultson, S.M. The Role of Forest Stands Characteristics on Formation of Exterior Migratory Outbreak Spots by the Siberian Silk Moth Dendrolimus sibiricus (Tschetv.) during Population Collapse. Forests 2023, 14, 1078. https://doi.org/10.3390/f14061078
Demidko DA, Goroshko AA, Slinkina OA, Mikhaylov PV, Sultson SM. The Role of Forest Stands Characteristics on Formation of Exterior Migratory Outbreak Spots by the Siberian Silk Moth Dendrolimus sibiricus (Tschetv.) during Population Collapse. Forests. 2023; 14(6):1078. https://doi.org/10.3390/f14061078
Chicago/Turabian StyleDemidko, Denis A., Andrey A. Goroshko, Olga A. Slinkina, Pavel V. Mikhaylov, and Svetlana M. Sultson. 2023. "The Role of Forest Stands Characteristics on Formation of Exterior Migratory Outbreak Spots by the Siberian Silk Moth Dendrolimus sibiricus (Tschetv.) during Population Collapse" Forests 14, no. 6: 1078. https://doi.org/10.3390/f14061078
APA StyleDemidko, D. A., Goroshko, A. A., Slinkina, O. A., Mikhaylov, P. V., & Sultson, S. M. (2023). The Role of Forest Stands Characteristics on Formation of Exterior Migratory Outbreak Spots by the Siberian Silk Moth Dendrolimus sibiricus (Tschetv.) during Population Collapse. Forests, 14(6), 1078. https://doi.org/10.3390/f14061078








