Evolutionary Processes Shaping Postglacial Gene Pools of High-Altitude Forests: Evidence from the Endemic Eucalypts of Tasmania
Abstract
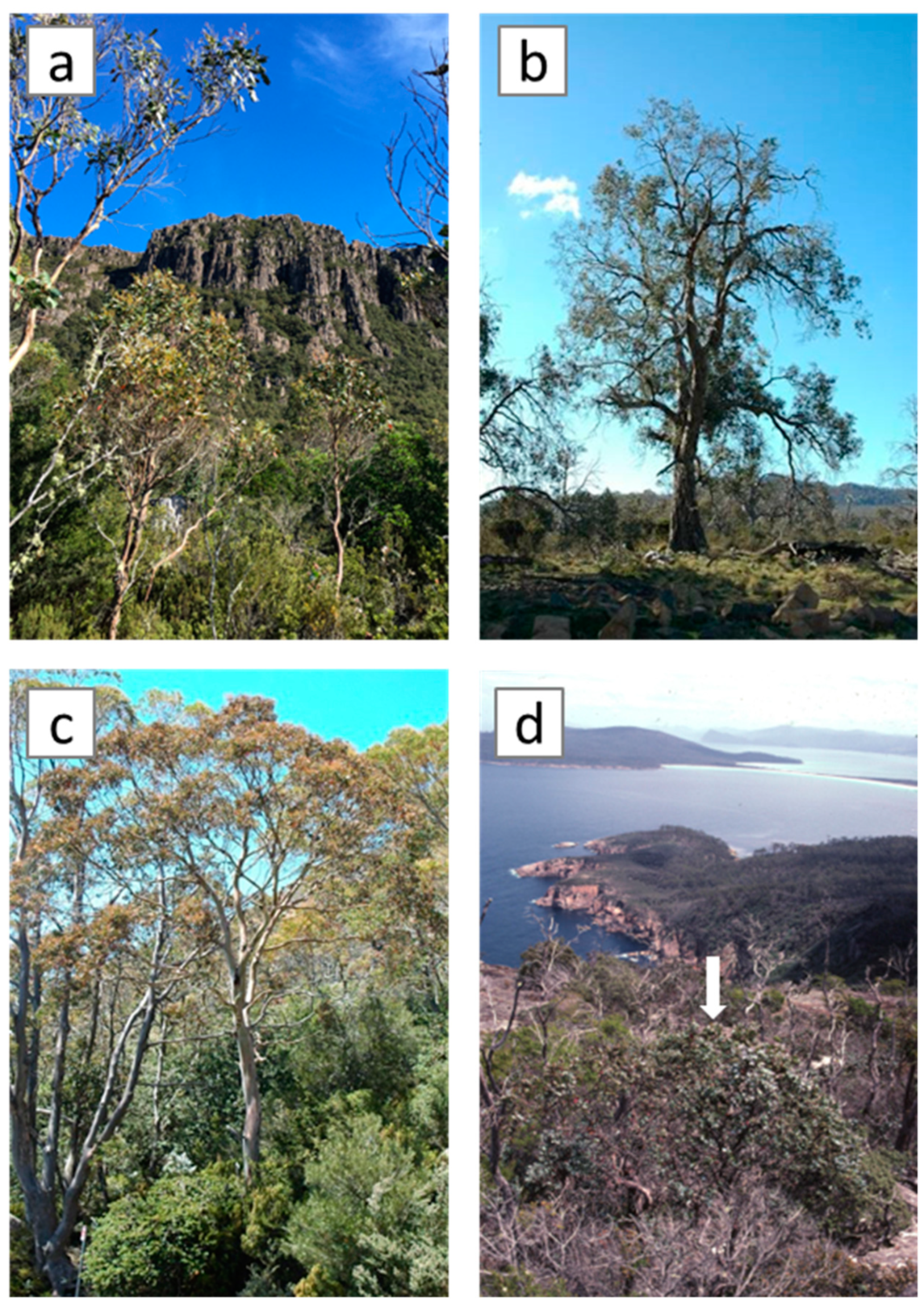
1. Introduction
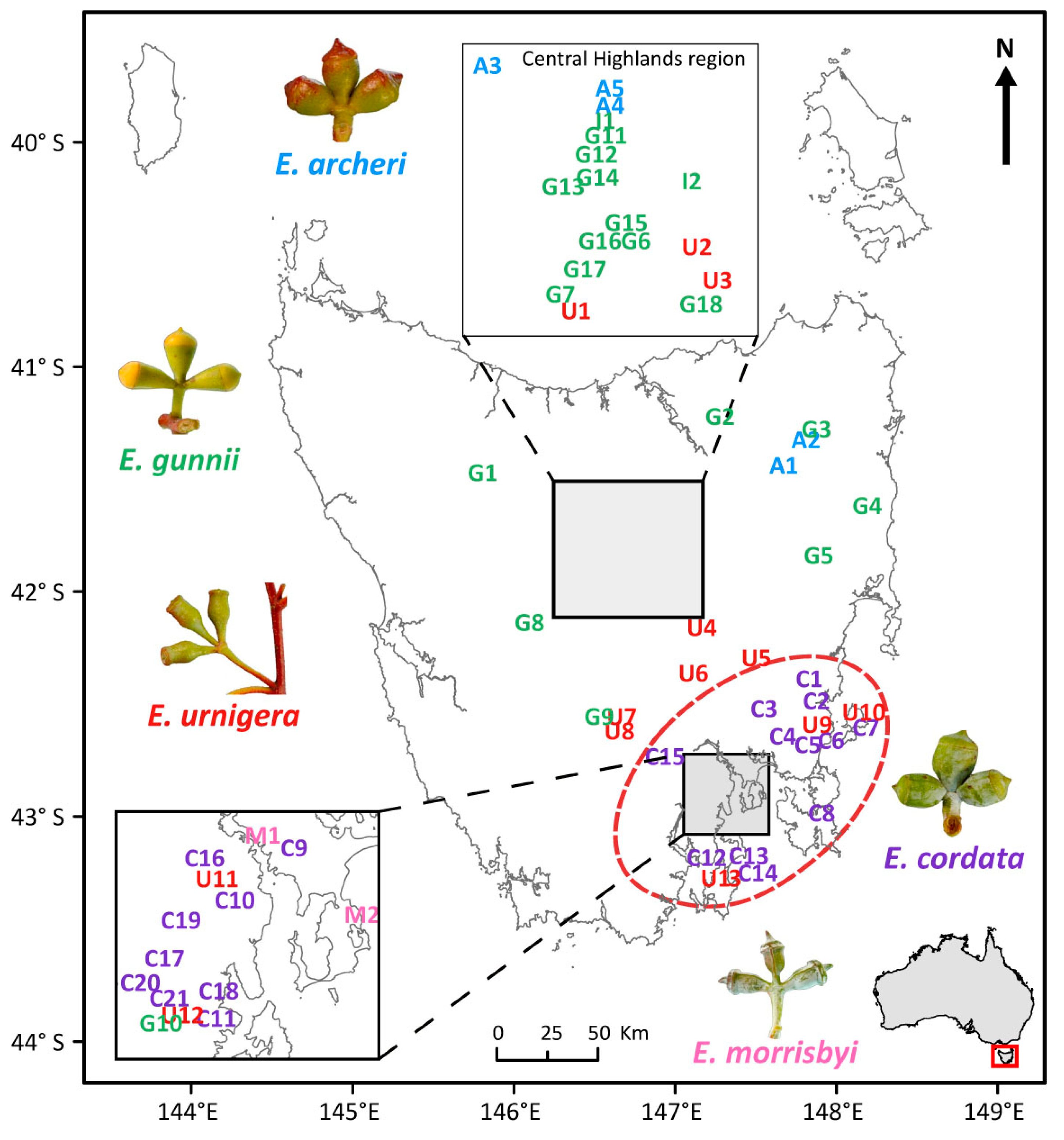
2. Materials and Methods
2.1. Molecular Markers
2.2. Chloroplast Sequencing
2.3. Nuclear Affinities and Diversity
2.4. Glasshouse Progeny Trial
2.5. Mantel Association Analyses
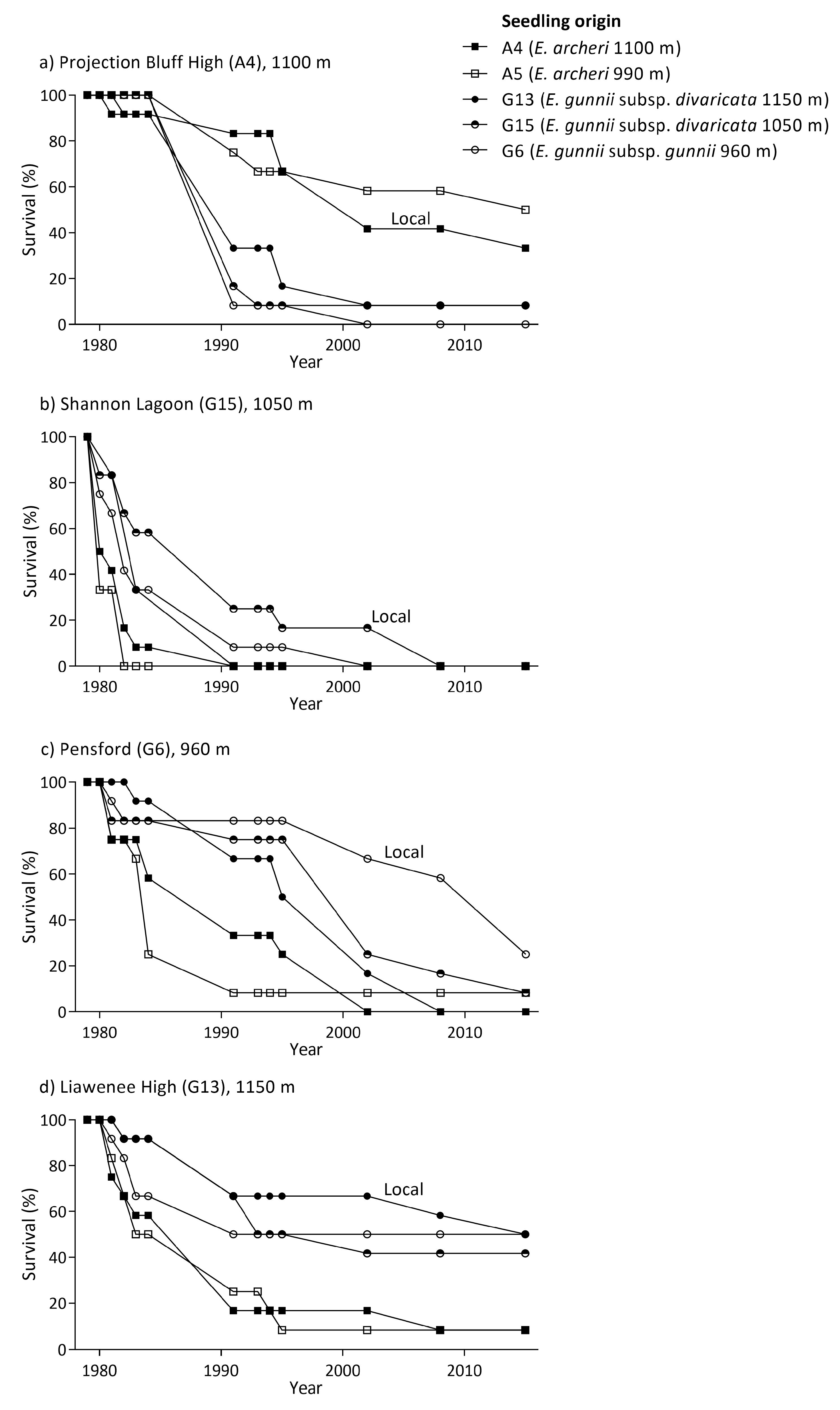
2.6. Reciprocal Common Garden Trials
3. Results
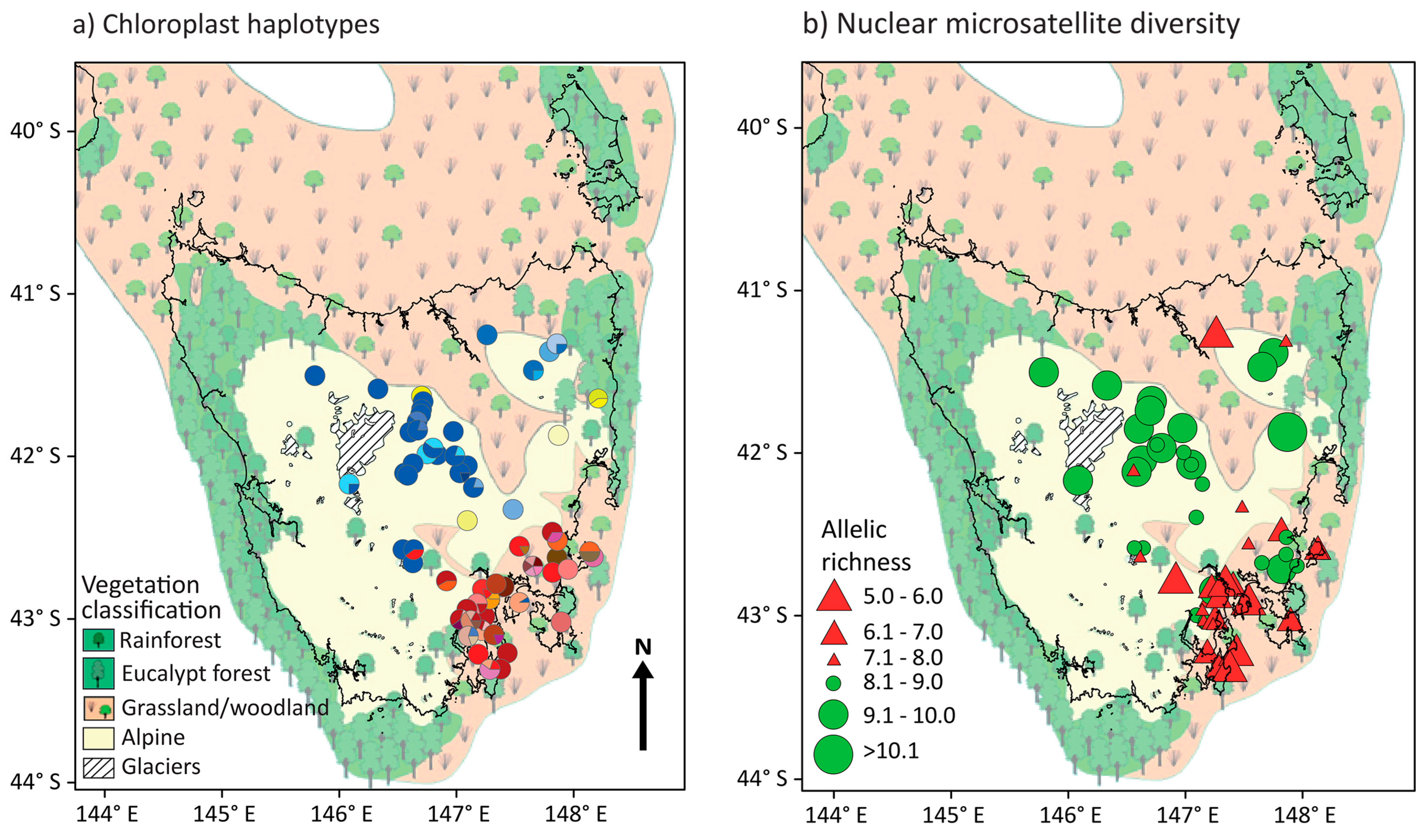
3.1. Chloroplast Haplotype Diversity
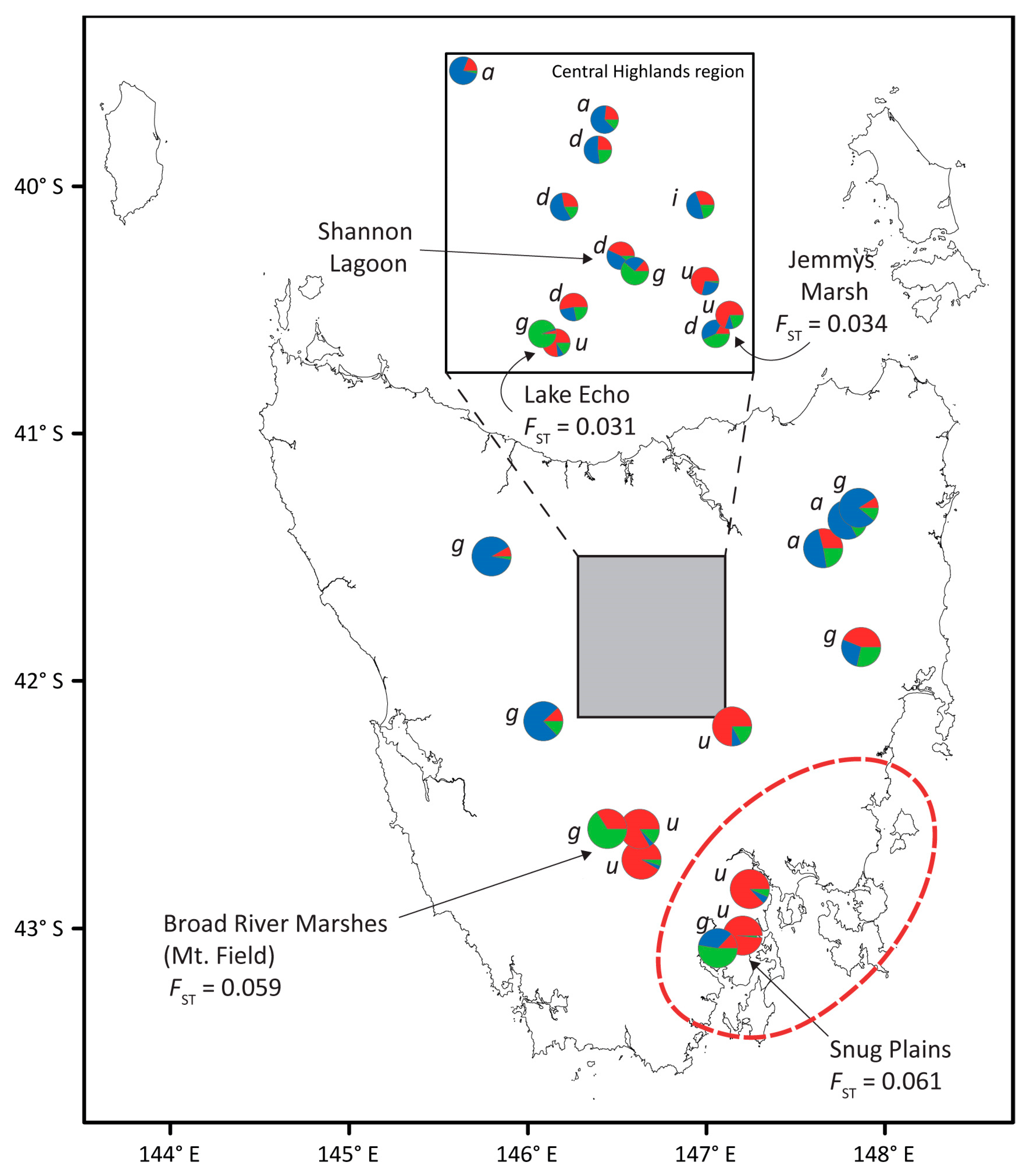
3.2. Nuclear Microsatellite Diversity
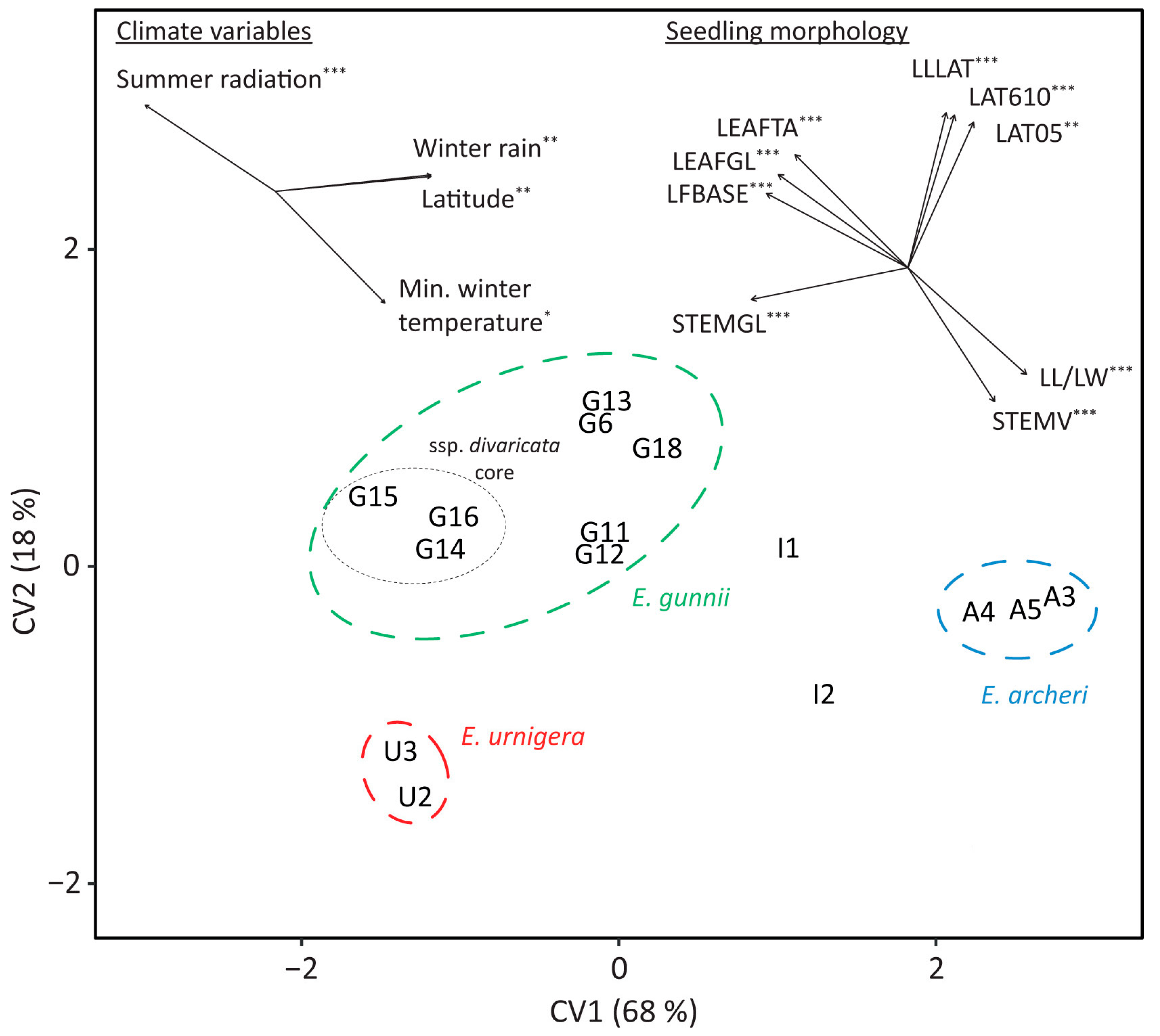
3.3. Morphological Differentiation between Populations (Glasshouse Trial)
| Between 10 Populations | Mantel Correlation | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Trait † | Brief Description | Units | Transformation | N | F9,89–90 | p | QST | SE | p | Trait vs. FST | Trait vs. Climate |
| Leaf traits (node 10 leaf) | |||||||||||
| LAML | Lamina length | mm | none | 483 | 4.52 | *** | 0.11 | 0.06 | ns | 0.05 ns | 0.81 * |
| LL/LW | Lamina length/width | ratio | log10 | 475 | 11.59 | *** | 0.28 | 0.12 | *** | −0.12 ns | 0.46 * |
| LL/LWP | LL/widest point | ratio | log10 | 475 | 3.88 | *** | 0.15 | 0.10 | ns | 0.21 ns | 0.40 ns |
| LEAFTA | Leaf tip angle | degrees | square | 475 | 10.07 | *** | 0.25 | 0.11 | *** | −0.24 ns | 0.52 * |
| LFBASE | Leaf base lobing | mm | log10 | 475 | 9.64 | *** | 0.24 | 0.11 | *** | −0.07 ns | 0.38 * |
| LEAFA | Leaf angle to stem | degrees | log10 | 483 | 2.83 | * | 0.06 | 0.05 | ns | 0.17 ns | 0.03 ns |
| LEAFP | Leaf plane orientation | degrees | sqrt | 483 | 1.92 | ns | 0.08 | 0.09 | ns | 0.04 ns | 0.02 ns |
| CRENM | Leaf crenulation | 0–1 | binary | 483 | 1.43 | ns | 0.29 | 0.46 | ns | 0.06 ns | 0.08 ns |
| LEAFGL | Leaf glaucousness | 0–7 | none | 483 | 25.56 | *** | 0.54 | 0.14 | *** | −0.13 ns | 0.23 ns |
| MRRED | Midrib redness | 0–1 | binary | 483 | 0.77 | ns | −0.09 | 0.21 | ns | −0.18 ns | −0.16 ns |
| Plant traits | |||||||||||
| HT10 | Height to node 10 | cm | none | 483 | 3.11 | ** | 0.07 | 0.05 | ns | 0.27 ns | 0.03 ns |
| LAT05 | Branching node 0–5 | count | log10 | 483 | 6.21 | *** | 0.20 | 0.10 | ** | 0.25 ns | −0.10 ns |
| LAT610 | Branching node 6–10 | count | log10 | 483 | 18.03 | *** | 0.38 | 0.13 | *** | 0.42 * | −0.15 ns |
| LLLAT | Length longest lateral | cm | none | 483 | 14.08 | *** | 0.31 | 0.12 | *** | 0.30 ns | −0.14 ns |
| COTCOL | Cotyledon colour | 0–1 | binary | 310 | 3.47 | *** | 0.59 | 0.36 | ns | −0.25 ns | 0.15 ns |
| NODERED | Leaf colour change | count | none | 310 | 2.98 | ** | 0.11 | 0.08 | ns | −0.19 ns | 0.17 ns |
| INTER10 | Internode 10 length | mm | none | 483 | 3.41 | *** | 0.08 | 0.05 | ns | −0.05 ns | −0.23 ns |
| Stem traits | |||||||||||
| STEMGL | Stem glaucousness | 0–2 | none | 483 | 53.32 | *** | 0.79 | 0.11 | *** | 0.32 ns | 0.46 * |
| STEMRED | Stem redness | 0–1 | binary | 483 | 1.14 | ns | −0.09 | 0.21 | ns | 0.04 ns | 0.35 ns |
| STEMV | Oil gland density | 0–2 | none | 483 | 6.56 | *** | 0.23 | 0.12 | ** | 0.21 ns | 0.44 * |
| STEMREC | Stem rectangularity | ratio | none | 483 | 4.54 | *** | 0.17 | 0.10 | * | 0.25 ns | −0.16 ns |
| STEMRO | Stem roundness | ratio | none | 483 | 4.51 | *** | 0.27 | 0.19 | * | 0.17 ns | 0.79 * |
3.4. Reciprocal Common Garden Trials
4. Discussion
4.1. Colonisation of the Central Highlands
4.2. Chloroplast Haplotype Sharing Implicates Hybridisation
4.3. Natural Selection Filters the Admixed Populations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gavin, D.G.; Fitzpatrick, M.C.; Gugger, P.F.; Heath, K.D.; Rodriguez-Sanchez, F.; Dobrowski, S.Z.; Hampe, A.; Hu, F.S.; Ashcroft, M.B.; Bartlein, P.J.; et al. Climate refugia: Joint inference from fossil records, species distribution models and phylogeography. New Phytol. 2014, 204, 37–54. [Google Scholar] [CrossRef] [PubMed]
- Brewer, S.; Cheddadi, R.; de Beaulieu, J.L.; Reille, M. The spread of deciduous Quercus throughout Europe since the last glacial period. For. Ecol. Manag. 2002, 156, 27–48. [Google Scholar] [CrossRef]
- Davis, M.B.; Shaw, R.G. Range shifts and adaptive responses to Quaternary climate change. Science 2001, 292, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Kremer, A.; Hipp, A.L. Oaks: An evolutionary success story. New Phytol. 2020, 226, 987–1011. [Google Scholar] [CrossRef]
- Payette, S.; Couillard, P.-L.; Frégeau, M.; Laflamme, J.; Lavoie, M. The velocity of postglacial migration of fire-adapted boreal tree species in eastern North America. Proc. Natl. Acad. Sci. USA 2022, 119, e2210496119. [Google Scholar] [CrossRef]
- Petit, R.J.; Brewer, S.; Bordacs, S.; Burg, K.; Cheddadi, R.; Coart, E.; Cottrell, J.; Csaikl, U.M.; van Dam, B.; Deans, J.D.; et al. Identification of refugia and post-glacial colonisation routes of European white oaks based on chloroplast DNA and fossil pollen evidence. For. Ecol. Manag. 2002, 156, 49–74. [Google Scholar] [CrossRef]
- Jordan, G.J.; Harrison, P.A.; Worth, J.R.P.; Williamson, G.J.; Kirkpatrick, J.B. Palaeoendemic plants provide evidence for persistence of open, well-watered vegetation since the Cretaceous. Glob. Ecol. Biogeogr. 2016, 25, 127–140. [Google Scholar] [CrossRef]
- Alberto, F.J.; Aitken, S.N.; Alia, R.; Gonzalez-Martinez, S.C.; Hanninen, H.; Kremer, A.; Lefevre, F.; Lenormand, T.; Yeaman, S.; Whetten, R.; et al. Potential for evolutionary responses to climate change evidence from tree populations. Glob. Change Biol. 2013, 19, 1645–1661. [Google Scholar] [CrossRef]
- Kremer, A.; Potts, B.M.; Delzon, S. Genetic divergence in forest trees: Understanding the consequences of climate change. Funct. Ecol. 2014, 28, 22–36. [Google Scholar] [CrossRef]
- Kremer, A.; Ronce, O.; Robledo-Arnuncio, J.J.; Guillaume, F.; Bohrer, G.; Nathan, R.; Bridle, J.R.; Gomulkiewicz, R.; Klein, E.K.; Ritland, K.; et al. Long-distance gene flow and adaptation of forest trees to rapid climate change. Ecol. Lett. 2012, 15, 378–392. [Google Scholar] [CrossRef]
- Hampe, A.; Pemonge, M.-H.; Petit, R.J. Efficient mitigation of founder effects during the establishment of a leading-edge oak population. Proc. R. Soc. B-Biol. Sci. 2013, 280, 20131070. [Google Scholar] [CrossRef] [PubMed]
- Petit, R.J.; Pineau, E.; Demesure, B.; Bacilieri, R.; Ducousso, A.; Kremer, A. Chloroplast DNA footprints of postglacial recolonization by oaks. Proc. Natl. Acad. Sci. USA 1997, 94, 9996–10001. [Google Scholar] [CrossRef] [PubMed]
- Pfennig, K.S.; Kelly, A.L.; Pierce, A.A. Hybridization as a facilitator of species range expansion. Proc. R. Soc. B Biol. Sci. 2016, 283, 20161329. [Google Scholar] [CrossRef] [PubMed]
- Buck, R.; Flores-Rentería, L. The syngameon enigma. Plants 2022, 11, 895. [Google Scholar] [CrossRef] [PubMed]
- Cannon, C.H.; Petit, R.J. The oak syngameon: More than the sum of its parts. New Phytol. 2020, 226, 978–983. [Google Scholar] [CrossRef] [PubMed]
- Bai, W.N.; Liao, W.J.; Zhang, D.Y. Nuclear and chloroplast DNA phylogeography reveal two refuge areas with asymmetrical gene flow in a temperate walnut tree from East Asia. New Phytol. 2010, 188, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Callahan, C.M.; Rowe, C.A.; Ryel, R.J.; Shaw, J.D.; Madritch, M.D.; Mock, K.E. Continental-scale assessment of genetic diversity and population structure in quaking aspen (Populus tremuloides). J. Biogeogr. 2013, 40, 1780–1791. [Google Scholar] [CrossRef]
- Latta, R.G.; Mitton, J.B. Historical separation and present gene flow through a zone of secondary contact in ponderosa pine. Evolution 1999, 53, 769–776. [Google Scholar] [CrossRef]
- Li, L.L.; Milesi, P.; Tiret, M.; Chen, J.; Sendrowski, J.; Baison, J.; Chen, Z.Q.; Zhou, L.H.; Karlsson, B.; Berlin, M.; et al. Teasing apart the joint effect of demography and natural selection in the birth of a contact zone. New Phytol. 2022, 236, 1976–1987. [Google Scholar] [CrossRef]
- Fussi, B.; Lexer, C.; Heinze, B. Phylogeography of Populus alba (L.) and Populus tremula (L.) in Central Europe: Secondary contact and hybridisation during recolonisation from disconnected refugia. Tree Genet. Genomes 2010, 6, 439–450. [Google Scholar] [CrossRef]
- Remington, C.L. Suture-zones of hybrid interaction between recently joined biotas. In Evolutionary Biology; Dobzhansky, T., Hecht, M.K., Steere, W.C., Eds.; Plenum: New York, NY, USA, 1968. [Google Scholar]
- Hunter, E.A.; Matocq, M.D.; Murphy, P.J.; Shoemaker, K.T. Differential effects of climate on survival rates drive hybrid zone movement. Curr. Biol. 2017, 27, 3898–3903.e3894. [Google Scholar] [CrossRef] [PubMed]
- Pfeilsticker, T.R.; Jones, R.C.; Steane, D.A.; Harrison, P.A.; Vaillancourt, R.E.; Potts, B.M. Expansion of the rare Eucalyptus risdonii under climate change through hybridization with a closely related species despite hybrid inferiority. Ann. Bot. 2022, 129, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wielstra, B. Historical hybrid zone movement: More pervasive than appreciated. J. Biogeogr. 2019, 46, 1300–1305. [Google Scholar] [CrossRef]
- Chunco, A.J. Hybridization in a warmer world. Ecol. Evol. 2014, 4, 2019–2031. [Google Scholar] [CrossRef] [PubMed]
- Leroy, T.; Louvet, J.M.; Lalanne, C.; Le Provost, G.; Labadie, K.; Aury, J.M.; Delzon, S.; Plomion, C.; Kremer, A. Adaptive introgression as a driver of local adaptation to climate in European white oaks. New Phytol. 2019, 226, 1171–1182. [Google Scholar] [CrossRef]
- Petit, R.J.; Bodenes, C.; Ducousso, A.; Roussel, G.; Kremer, A. Hybridization as a mechanism of invasion in oaks. New Phytol. 2004, 161, 151–164. [Google Scholar] [CrossRef]
- Todesco, M.; Pascual, M.A.; Owens, G.L.; Ostevik, K.L.; Moyers, B.T.; Hubner, S.; Heredia, S.M.; Hahn, M.A.; Caseys, C.; Bock, D.G.; et al. Hybridization and extinction. Evol. Appl. 2016, 9, 892–908. [Google Scholar] [CrossRef]
- Potts, B.M.; Reid, J.B. Hybridization as a dispersal mechanism. Evolution 1988, 42, 1245–1255. [Google Scholar] [CrossRef]
- Petit, R.J.; Aguinagalde, I.; de Beaulieu, J.L.; Bittkau, C.; Brewer, S.; Cheddadi, R.; Ennos, R.; Fineschi, S.; Grivet, D.; Lascoux, M.; et al. Glacial refugia: Hotspots but not melting pots of genetic diversity. Science 2003, 300, 1563–1565. [Google Scholar] [CrossRef]
- Saeki, I.; Dick, C.W.; Barnes, B.V.; Murakami, N. Comparative phylogeography of red maple (Acer rubrum L.) and silver maple (Acer saccharinum L.): Impacts of habitat specialization, hybridization and glacial history. J. Biogeogr. 2011, 38, 992–1005. [Google Scholar] [CrossRef]
- Kelleher, C.T.; Hodkinson, T.R.; Douglas, G.C.; Kelly, D.L. Species distinction in Irish populations of Quercus petraea and Q. robur: Morphological versus molecular analyses. Ann. Bot. 2005, 96, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Manos, P.S.; Doyle, J.J.; Nixon, K.C. Phylogeny, biogeography, and processes of molecular differentiation in Quercus subgenus Quercus (Fagaceae). Mol. Phylogenet. Evol. 1999, 12, 333–349. [Google Scholar] [CrossRef] [PubMed]
- Soliani, C.; Gallo, L.; Marchelli, P. Phylogeography of two hybridizing southern beeches (Nothofagus spp.) with different adaptive abilities. Tree Genet. Genomes 2012, 8, 659–673. [Google Scholar] [CrossRef]
- Worth, J.R.P.; Jordan, G.J.; McKinnon, G.E.; Vaillancourt, R.E. The major Australian cool temperate rainforest tree Nothofagus cunninghamii withstood Pleistocene glacial aridity within multiple regions: Evidence from the chloroplast. New Phytol. 2009, 182, 519–532. [Google Scholar] [CrossRef]
- McKinnon, G.E.; Jordan, G.J.; Vaillancourt, R.E.; Steane, D.A.; Potts, B.M. Glacial refugia and reticulate evolution: The case of the Tasmanian eucalypts. Philos. Trans. R. Soc. Lond. Ser. B-Biol. Sci. 2004, 359, 275–284. [Google Scholar] [CrossRef]
- Nevill, P.G.; Bossinger, G.; Ades, P.K. Phylogeography of the world’s tallest angiosperm, Eucalyptus regnans: Evidence for multiple isolated Quaternary refugia. J. Biogeogr. 2010, 37, 179–192. [Google Scholar] [CrossRef]
- Nevill, P.G.; Despres, T.; Bayly, M.J.; Bossinger, G.; Ades, P.K. Shared phylogeographic patterns and widespread chloroplast haplotype sharing in Eucalyptus species with different ecological tolerances. Tree Genet. Genomes 2014, 10, 1079–1092. [Google Scholar] [CrossRef]
- Capblancq, T.; Lachmuth, S.; Fitzpatrick, M.C.; Keller, S.R. From common gardens to candidate genes: Exploring local adaptation to climate in red spruce. New Phytol. 2023, 237, 1590–1605. [Google Scholar] [CrossRef]
- Sork, V.L. Genomic studies of local adaptation in natural plant populations. J. Hered. 2018, 109, 3–15. [Google Scholar] [CrossRef]
- Leinonen, T.; McCairns, R.J.S.; O’Hara, R.B.; Merila, J. QST-FST comparisons: Evolutionary and ecological insights from genomic heterogeneity. Nat. Rev. Genet. 2013, 14, 179–190. [Google Scholar] [CrossRef]
- Dutkowski, G.W.; Potts, B.M. Genetic variation in the susceptibility of Eucalyptus globulus to drought damage. Tree Genet. Genomes 2012, 8, 757–773. [Google Scholar] [CrossRef]
- Prober, S.M.; Potts, B.M.; Harrison, P.A.; Wiehl, G.; Bailey, T.G.; Silva, J.C.E.; Price, M.R.; Speijers, J.; Steane, D.A.; Vaillancourt, R.E. Leaf economic and hydraulic traits signal disparate climate adaptation patterns in two co-occurring woodland eucalypts. Plants 2022, 11, 1846. [Google Scholar] [CrossRef] [PubMed]
- Boshier, D.; Buggs, R.J.A. The potential for field studies and genomic technologies to enhance resistance and resilience of British tree populations to pests and pathogens. Forestry 2015, 88, 27–40. [Google Scholar] [CrossRef]
- Saenz-Romero, C.; O’Neill, G.; Aitken, S.N.; Lindig-Cisneros, R. Assisted migration field tests in Canada and Mexico: Lessons, limitations, and challenges. Forests 2021, 12, 9. [Google Scholar] [CrossRef]
- Colhoun, E.A. Vegetation and climate change during the Last Interglacial-Glacial cycle in western Tasmania, Australia. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2000, 155, 195–209. [Google Scholar] [CrossRef]
- Jackson, W.D. Vegetation types. In Vegetation of Tasmania; Reid, J.B., Hill, R.S., Brown, M.J., Hovenden, M.J., Eds.; Australian Biological Resource Study: Melbourne, Australia, 1999; pp. 89–124. [Google Scholar]
- Mokany, K.; Jordan, G.J.; Harwood, T.D.; Harrison, P.A.; Keppel, G.; Gilfedder, L.; Carter, O.; Ferrier, S. Past, present and future refugia for Tasmania’s palaeoendemic flora. J. Biogeogr. 2017, 44, 1537–1546. [Google Scholar] [CrossRef]
- Jordan, G.J. Evidence of Pleistocene plant extinction and diversity from Regatta Point, western Tasmania, Australia. Bot. J. Linn. Soc. 1997, 123, 45–71. [Google Scholar] [CrossRef]
- Colhoun, E.A.; Hannan, D.; Kiernan, K. Late Wisconsin glaciation of Tasmania. Pap. Proc. R. Soc. Tasman. 1996, 130, 33–45. [Google Scholar] [CrossRef]
- Kirkpatrick, J.B.; Fowler, M. Locating likely glacial forest refugia in Tasmania using palynological and ecological information to test alternative climatic models. Biol. Conserv. 1998, 85, 171–182. [Google Scholar] [CrossRef]
- Potts, B.M.; Jackson, W.D. Evolutionary processes in the Tasmanian high altitude eucalypts. In Flora and Fauna of Alpine Australasia. Ages and Origins; Barlow, B.A., Ed.; CSIRO: Melbourne, Australia, 1986; pp. 511–527. [Google Scholar]
- Nicolle, D.; Jones, R.C. A revised classification for the predominantly eastern Australian Eucalyptus subgenus Symphyomyrtus sections Maidenaria, Exsertaria, Latoangulatae and related smaller sections (Myrtaceae). Telopea 2018, 21, 129–145. [Google Scholar] [CrossRef]
- McKinnon, G.E.; Vaillancourt, R.E.; Steane, D.A.; Potts, B.M. An AFLP marker approach to lower-level systematics in Eucalyptus (Myrtaceae). Am. J. Bot. 2008, 95, 368–380. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.A.; Jones, R.C.; Vaillancourt, R.E.; Wiltshire, R.J.E.; Potts, B.M. Unravelling the evolutionary history of Eucalyptus cordata (Myrtaceae) using molecular markers. Aust. J. Bot. 2014, 62, 114–131. [Google Scholar] [CrossRef]
- Jones, R.C.; Nicolle, D.; Steane, D.A.; Vaillancourt, R.E.; Potts, B.M. High density, genome-wide markers and intra-specific replication yield an unprecedented phylogenetic reconstruction of a globally significant, speciose lineage of Eucalyptus. Mol. Phylogenet. Evol. 2016, 105, 63–85. [Google Scholar] [CrossRef] [PubMed]
- Potts, B.M.; Reid, J.B. Variation in the Eucalyptus gunnii-archeri complex in Tasmania. I. Variation in the adult phenotype. Aust. J. Bot. 1985, 33, 337–359. [Google Scholar] [CrossRef]
- Davidson, N.J. Ecophysiological studies of factors determining the distribution of sub-alpine eucalypts at Snug Plains, southern Tasmania. Aust. J. Ecol. 1987, 12, 197–199. [Google Scholar]
- Doyle, J.J.; Doyle, J.L. Extraction of plant DNA from fresh tissue. Focus 1990, 12, 13–15. [Google Scholar]
- McKinnon, G.E.; Vaillancourt, R.E.; Steane, D.A.; Potts, B.M. The rare silver gum, Eucalyptus cordata, is leaving its trace in the organellar gene pool of Eucalyptus globulus. Mol. Ecol. 2004, 13, 3751–3762. [Google Scholar] [CrossRef]
- Freeman, J.S.; Jackson, H.D.; Steane, D.A.; McKinnon, G.E.; Dutkowski, G.W.; Potts, B.M.; Vaillancourt, R.E. Chloroplast DNA phylogeography of Eucalyptus globulus. Aust. J. Bot. 2001, 49, 585–596. [Google Scholar] [CrossRef]
- McKinnon, G.E.; Vaillancourt, R.E.; Tilyard, P.A.; Potts, B.M. Maternal inheritance of the chloroplast genome in Eucalyptus globulus and interspecific hybrids. Genome 2001, 44, 831–835. [Google Scholar] [CrossRef]
- Swofford, D.L. PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods). Version 4; Sinauer Associates: Sunderland, MA, USA, 2002. [Google Scholar]
- Templeton, A.R.; Crandall, K.A.; Sing, C.F. A cladistic analysis of phenotypic associations with haplotypes inferred from restriction endonuclease mapping and DNA sequence data. III. Cladogram estimation. Genetics 1992, 132, 619–633. [Google Scholar] [CrossRef]
- Brondani, R.P.V.; Brondani, C.; Grattapaglia, D. Towards a genus-wide reference linkage map for Eucalyptus based exclusively on highly informative microsatellite markers. Mol. Genet. Genom. 2002, 267, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Brondani, R.P.V.; Williams, E.R.; Brondani, C.; Grattapaglia, D. A microsatellite-based consensus linkage map for species of Eucalyptus and a novel set of 230 microsatellite markers for the genus. BMC Plant Biol. 2006, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Brondani, R.P.V.; Brondani, C.; Tarchini, R.; Grattapaglia, D. Development, characterization and mapping of microsatellite markers in Eucalyptus grandis and E. urophylla. Theor. Appl. Genet. 1998, 97, 816–827. [Google Scholar] [CrossRef]
- Steane, D.A.; Vaillancourt, R.E.; Russell, J.; Powell, W.; Marshall, D.; Potts, B.M. Development and characterisation of microsatellite loci in Eucalyptus globulus (Myrtaceae). Silvae Genet. 2001, 50, 89–91. [Google Scholar]
- Peakall, R.; Smouse, P.E. GENALEX 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research—An update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef] [PubMed]
- Goudet, J. FSTAT, a Program to Estimate and Test Gene Diversities and Fixation Indices. Version 2.9.3.2. Available online: http://www2.unil.ch/popgen/softwares/fstat.htm (accessed on 31 March 2023).
- Weir, B.S.; Cockerham, C.C. Estimating F-statistics for the analysis of population structure. Evolution 1984, 38, 1358–1370. [Google Scholar]
- Falush, D.; Stephens, M.; Pritchard, J.K. Inference of population structure using multilocus genotype data: Linked loci and correlated allele frequencies. Genetics 2003, 164, 1567–1587. [Google Scholar] [CrossRef]
- Falush, D.; Stephens, M.; Pritchard, J.K. Inference of population structure using multilocus genotype data: Dominant markers and null alleles. Mol. Ecol. Notes 2007, 7, 574–578. [Google Scholar] [CrossRef]
- Hubisz, M.J.; Falush, D.; Stephens, M.; Pritchard, J.K. Inferring weak population structure with the assistance of sample group information. Mol. Ecol. Resour. 2009, 9, 1322–1332. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Earl, D.; vonHoldt, B. Structure Harvester: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 2011, 4, 359–361. [Google Scholar] [CrossRef]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software Structure: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef]
- Jakobsson, M.; Rosenberg, N.A. Clumpp: A cluster matching and permutation program for dealing with label switching and multimodality in analysis of population structure. Bioinformatics 2007, 23, 1801–1806. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, N.A. Distruct: A program for the graphical display of population structure. Mol. Ecol. Notes 2004, 4, 137–138. [Google Scholar] [CrossRef]
- Kopelman, N.M.; Mayzel, J.; Jakobsson, M.; Rosenberg, N.A.; Mayrose, I. CLUMPAK: A program for identifying clustering modes and packaging population structure inferences across K. Mol. Ecol. Resour. 2015, 15, 1179–1191. [Google Scholar] [CrossRef] [PubMed]
- Gilmour, A.R.; Gogel, B.J.; Cullis, B.R.; Welham, S.J.; Thompson, R. ASReml User Guide Release 4.1 Structural Specification; VSN International Ltd.: Hemel Hempstead, UK, 2015. [Google Scholar]
- Gauli, A.; Vaillancourt, R.E.; Bailey, T.G.; Steane, D.A.; Potts, B.M. Evidence for local climate adaptation in early-life traits of Tasmanian populations of Eucalyptus pauciflora. Tree Genet. Genomes 2015, 11, 104. [Google Scholar] [CrossRef]
- Jordan, G.J.; Potts, B.M.; Wiltshire, R.J.E. Strong, independent, quantitative genetic control of the timing of vegetative phase change and first flowering in Eucalyptus globulus ssp. globulus (Tasmanian Blue Gum). Heredity 1999, 83, 179–187. [Google Scholar]
- Bush, D.; Kain, D.; Kanowski, P.; Matheson, C. Genetic parameter estimates informed by a marker-based pedigree: A case study with Eucalyptus cladocalyx in southern Australia. Tree Genet. Genomes 2015, 11, 16. [Google Scholar] [CrossRef]
- Latta, R.G. Differentiation of allelic frequencies at quantitative trait loci affecting locally adaptive traits. Am. Nat. 1998, 151, 283–292. [Google Scholar] [CrossRef]
- Yang, R.C.; Yeh, F.C.; Yanchuk, A.D. A comparison of isozyme and quantitative genetic variation in Pinus contorta ssp latifolia by FST. Genetics 1996, 142, 1045–1052. [Google Scholar] [CrossRef]
- Zar, J.H. Statistical significance of mutation frequencies, and the power of statistical testing, using the Poisson-distribution. Biom. J. 1984, 26, 83–88. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.; O’Hara, R.; Simpson, G.; Solymos, P.; et al. Vegan: Community Ecology Package in R. Version 2.4-2. Available online: https://CRAN.R-project.org/package=vegan (accessed on 31 March 2023).
- Goslee, S.C.; Urban, D.L. The ecodist package for dissimilarity-based analysis of ecological data. J. Stat. Softw. 2007, 22, 1–19. [Google Scholar] [CrossRef]
- Xu, T.B.; Hutchinson, M.F. New developments and applications in the ANUCLIM spatial climatic and bioclimatic modelling package. Environ. Model. Softw. 2013, 40, 267–279. [Google Scholar] [CrossRef]
- Nychka, D.; Furrer, R.; Paige, J.; Sain, S. Fields: Tools for Spatial Data. Available online: https://github.com/NCAR/Fields (accessed on 31 March 2023).
- Potts, B.M. Variation in the Eucalyptus gunnii-archeri complex. III. Reciprocal transplant trials. Aust. J. Bot. 1985, 33, 687–704. [Google Scholar] [CrossRef]
- McKinnon, G.E.; Vaillancourt, R.E.; Jackson, H.D.; Potts, B.M. Chloroplast sharing in the Tasmanian eucalypts. Evolution 2001, 55, 703–711. [Google Scholar] [CrossRef]
- Potts, B.M.; Potts, W.C.; Kantvilas, G. The Miena cider gum, Eucalyptus gunnii subsp. divaricata (Myrtaceae): A taxon in rapid decline. Pap. Proc. R. Soc. Tasman. 2001, 135, 57–61. [Google Scholar]
- Potts, B.M.; Reid, J.B. Variation in the Eucalyptus gunnii-archeri complex in Tasmania. II. The origin of variation. Aust. J. Bot. 1985, 33, 519–541. [Google Scholar] [CrossRef]
- Tibbits, W.N. Germination and morphology of progeny from controlled pollinations of Eucalyptus nitens (Deane and Maiden) Maiden. Aust. J. Bot. 1988, 36, 677–691. [Google Scholar] [CrossRef]
- Armbruster, W.S.; Schwaegerle, K.E. Causes of covariation of phenotypic traits among populations. J. Evol. Biol. 1996, 9, 261–276. [Google Scholar] [CrossRef]
- Kremer, A.; Kleinschmit, J.; Cottrell, J.; Cundall, E.P.; Deans, J.D.; Ducousso, A.; Konig, A.O.; Lowe, A.J.; Munro, R.C.; Petit, R.J.; et al. Is there a correlation between chloroplastic and nuclear divergence, or what are the roles of history and selection on genetic diversity in European oaks? For. Ecol. Manag. 2002, 156, 75–87. [Google Scholar] [CrossRef]
- Hopf, F.V.L.; Colhoun, E.A.; Barton, C.E. Late-glacial and Holocene record of vegetation and climate from Cynthia Bay, Lake St Clair, Tasmania. J. Quat. Sci. 2000, 15, 725–732. [Google Scholar] [CrossRef]
- Macphail, M.K. Vegetation and climates in southern Tasmania since the last glaciation. Quat. Res. 1979, 11, 306–341. [Google Scholar] [CrossRef]
- Fletcher, M.S.; Bowman, D.M.J.S.; Whitlock, C.; Mariani, M.; Stahle, L. The changing role of fire in conifer-dominated temperate rainforest through the last 14,000 years. Quat. Sci. Rev. 2018, 182, 37–47. [Google Scholar] [CrossRef]
- Booth, T.H. Going nowhere fast: A review of seed dispersal in eucalypts. Aust. J. Bot. 2017, 65, 401–410. [Google Scholar] [CrossRef]
- Jones, T.H.; Vaillancourt, R.E.; Potts, B.M. Detection and visualization of spatial genetic structure in continuous Eucalyptus globulus forest. Mol. Ecol. 2007, 16, 697–707. [Google Scholar] [CrossRef]
- Potts, B.M.; Wiltshire, R.J.E. Eucalypt genetics and genecology. In Eucalypt Ecology: Individuals to Ecosystems; Williams, J., Woinarski, J., Eds.; Cambridge University Press: Cambridge, UK, 1997; pp. 56–91. [Google Scholar]
- Kahrood, H.; Rigault, P.; Spokevicius, A.; Bossinger, G.; Tibbits, J. RuBisCo evolution in eucalypts. In Proceedings of the IUFRO Tree Biotechnology 2015 Conference, Florence, Italy, 8–12 June 2015; pp. 228–229. [Google Scholar]
- Marthick, J.R. The Phylogeography of Two Eucalypts. Honours Thesis, University of Tasmania, Hobart, Australia, 2005. [Google Scholar]
- Hewitt, G.M. Some genetic consequences of ice ages, and their role in divergence and speciation. Biol. J. Linn. Soc. 1996, 58, 247–276. [Google Scholar] [CrossRef]
- Gauli, A.; Steane, D.A.; Vaillancourt, R.E.; Potts, B.M. Molecular genetic diversity and population structure in Eucalyptus pauciflora subsp. pauciflora (Myrtaceae) on the island of Tasmania. Aust. J. Bot. 2014, 62, 175–188. [Google Scholar] [CrossRef]
- Havrdova, A.; Douda, J.; Krak, K.; Vit, P.; Hadincova, V.; Zakravsky, P.; Mandak, B. Higher genetic diversity in recolonized areas than in refugia of Alnus glutinosa triggered by continent-wide lineage admixture. Mol. Ecol. 2015, 24, 4759–4777. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Takeuchi, Y.; Yamasaki, M.; Sakurai, S.; Isagi, Y. Lineage admixture during postglacial range expansion is responsible for the increased gene diversity of Kalopanax septemlobus in a recently colonised territory. Heredity 2011, 107, 338–348. [Google Scholar] [CrossRef]
- Petit, R.J.; Duminil, J.; Fineschi, S.; Hampe, A.; Salvini, D.; Vendramin, G.G. Comparative organization of chloroplast, mitochondrial and nuclear diversity in plant populations. Mol. Ecol. 2005, 14, 689–701. [Google Scholar] [CrossRef]
- Byrne, M. Phylogeny, diversity and evolution of eucalypts. In Plant Genome: Biodiversity and Evolution. Volume 1, Part E: Phanerogams-Angiosperm; Sharma, A.K., Sharma, A., Eds.; Science Publishers: London, UK, 2008; Volume 1, pp. 303–346. [Google Scholar]
- Lesser, M.R.; Jackson, S.T. Contributions of long-distance dispersal to population growth in colonising Pinus ponderosa populations. Ecol. Lett. 2013, 16, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Birky, C.W., Jr.; Maruyama, T.; Fuerst, P. An approach to population and evolutionary genetic theory for genes in mitochondria and chloroplasts, and some results. Genetics 1983, 103, 513–527. [Google Scholar] [CrossRef]
- Currat, M.; Ruedi, M.; Petit, R.J.; Excoffier, L. The hidden side of invasions: Massive introgression by local genes. Evolution 2008, 62, 1908–1920. [Google Scholar] [CrossRef] [PubMed]
- Thornhill, A.H.; Crisp, M.D.; Kulheim, C.; Lam, K.E.; Nelson, L.A.; Yeates, D.K.; Miller, J.T. A dated molecular perspective of eucalypt taxonomy, evolution and diversification. Aust. Syst. Bot. 2019, 32, 29–48. [Google Scholar] [CrossRef]
- Jordan, R.; Prober, S.M.; Andrew, R.L.; Freeman, J.S.; Kerr, R.; Steane, D.A.; Vaillancourt, R.E.; Potts, B.M. Population genomic research in eucalypts. In Population Genomics: Forest Trees; Rajora, O.P., Ed.; Springer Nauture: Cham, Switzerland, 2023. [Google Scholar]
- Mostert-O’Neill, M.M.; Reynolds, S.M.; Acosta, J.J.; Lee, D.J.; Borevitz, J.O.; Myburg, A.A. Genomic evidence of introgression and adaptation in a model subtropical tree species, Eucalyptus grandis. Mol. Ecol. 2021, 30, 625–638. [Google Scholar] [CrossRef]
- Costa e Silva, J.; Potts, B.M.; Tilyard, P. Epistasis causes outbreeding depression in eucalypt hybrids. Tree Genet. Genomes 2012, 8, 249–265. [Google Scholar] [CrossRef]
- Larcombe, M.J.; Silva, J.C.E.; Tilyard, P.; Gore, P.; Potts, B.M. On the persistence of reproductive barriers in Eucalyptus: The bridging of mechanical barriers to zygote formation by F1 hybrids is counteracted by intrinsic post-zygotic incompatibilities. Ann. Bot. 2016, 118, 431–444. [Google Scholar] [CrossRef]
- Larcombe, M.J.; Barbour, R.C.; Jones, R.C.; Vaillancourt, R.E.; Potts, B.M. Postmating barriers to hybridization between an island’s native eucalypts and an introduced congener. Tree Genet. Genomes 2016, 12, 26. [Google Scholar] [CrossRef]
- Larcombe, M.J.; Holland, B.; Steane, D.A.; Jones, R.C.; Nicolle, D.; Vaillancourt, R.E.; Potts, B.M. Patterns of reproductive isolation in Eucalyptus—A phylogenetic perspective. Mol. Biol. Evol. 2015, 32, 1833–1846. [Google Scholar] [CrossRef]
- Cauvin, B.; Potts, B.M.; Potts, W.C. Eucalyptus: Hybridation artificielle-barrieres et hérédité des caracteres. In Annales de Recherches Sylvicoles; Association Forêt-Cellulose: Paris, France, 1997; pp. 255–303. [Google Scholar]
- James, M.E.; Brodribb, T.; Wright, I.J.; Rieseberg, L.H.; Ortiz-Barrientos, D. Replicated Evolution in Plants. Annu. Rev. Plant Biol. 2023, 74, 1367–1380. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, G.E.; Smith, J.J.; Potts, B.M. Recurrent nuclear DNA introgression accompanies chloroplast DNA exchange between two eucalypt species. Mol. Ecol. 2010, 19, 1367–1380. [Google Scholar] [CrossRef] [PubMed]
- Yost, J.M.; Wise, S.L.; Love, N.L.R.; Steane, D.A.; Jones, R.C.; Ritter, M.K.; Potts, B.M. Origins, diversity and naturalization of Eucalyptus globulus (Myrtaceae) in California. Forests 2021, 12, 1129. [Google Scholar] [CrossRef]
- Gore, P.L.; Potts, B.M.; Volker, P.W.; Megalos, J. Unilateral cross-incompatibility in Eucalyptus-the case of hybridization between Eucalyptus globulus and Eucalyptus nitens. Aust. J. Bot. 1990, 38, 383–394. [Google Scholar] [CrossRef]
- Hewitt, G. The genetic legacy of the Quaternary ice ages. Nature 2000, 405, 907–913. [Google Scholar] [CrossRef]
- Byrne, M.; Yeates, D.K.; Joseph, L.; Kearney, M.; Bowler, J.; Williams, M.A.J.; Cooper, S.; Donnellan, S.C.; Keogh, J.S.; Leys, R.; et al. Birth of a biome: Insights into the assembly and maintenance of the Australian arid zone biota. Mol. Ecol. 2008, 17, 4398–4417. [Google Scholar] [CrossRef]
- Anderson, E.; Stebbins, G.L. Hybridization as an evolutionary stimulus. Evolution 1954, 8, 378–388. [Google Scholar] [CrossRef]
- Stebbins, G.L. The role of hybridization in evolution. Proc. Am. Philos. Soc. 1959, 103, 231–251. [Google Scholar]
- Levin, D.A. Flowering-time plasticity facilitates niche shifts in adjacent populations. New Phytol. 2009, 183, 661–666. [Google Scholar] [CrossRef]
- Buschbom, J.; Yanbaev, Y.; Degen, B. Efficient long-distance gene flow into an isolated relict oak stand. J. Hered. 2011, 102, 464–472. [Google Scholar] [CrossRef]
- Kramer, A.T.; Ison, J.L.; Ashley, M.V.; Howe, H.F. The paradox of forest fragmentation genetics. Conserv. Biol. 2008, 22, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Mimura, M.; Barbour, R.C.; Potts, B.M.; Vaillancourt, R.E.; Watanabe, K.N. Comparison of contemporary mating patterns in continuous and fragmented Eucalyptus globulus native forests. Mol. Ecol. 2009, 18, 4180–4192. [Google Scholar] [CrossRef] [PubMed]
- Barber, H.N. Adaptive gene substitutions in Tasmanian eucalypts: I. Genes controlling the development of glaucousness. Evolution 1955, 9, 1–15. [Google Scholar] [CrossRef]
- Martinsen, G.D.; Whitham, T.G.; Turek, R.J.; Keim, P. Hybrid populations selectively filter gene introgression between species. Evolution 2001, 55, 1325–1335. [Google Scholar] [PubMed]
- Gailing, O.; Hipp, A.L.; Plomion, C.; Carlson, J.E. Oak population genomics. In Population Genomics; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–37. [Google Scholar]
- Close, D.C.; Davidson, N.J.; Shields, C.B.; Wiltshire, R. Reflectance and phenolics of green and glaucous leaves of Eucalyptus urnigera. Aust. J. Bot. 2007, 55, 561–567. [Google Scholar] [CrossRef]
- Aitken, S.N.; Yeaman, S.; Holliday, J.A.; Wang, T.; Curtis-McLane, S. Adaptation, migration or extirpation: Climate change outcomes for tree populations. Evol. Appl. 2008, 1, 95–111. [Google Scholar] [CrossRef]
- Davidson, N.J.; Reid, J.B. Frost as a factor influencing the growth and distribution of subalpine eucalypts. Aust. J. Bot. 1985, 33, 657–667. [Google Scholar] [CrossRef]
- Harrison, P.A. Climate change and the suitability of local and non-local species for ecosystem restoration. Ecol. Manag. Restor. 2021, 22, 75–91. [Google Scholar] [CrossRef]
- Sanger, J.C.; Davidson, N.J.; O’Grady, A.P.; Close, D.C. Are the patterns of regeneration in the endangered Eucalyptus gunnii ssp divaricata shifting in response to climate? Austral. Ecol. 2011, 36, 612–620. [Google Scholar] [CrossRef]
- Calder, J.A.; Kirkpatrick, J.B. Climate change and other factors influencing the decline of the Tasmanian cider gum (Eucalyptus gunnii). Aust. J. Bot. 2008, 56, 684–692. [Google Scholar] [CrossRef]
- Griffin, A.R.; Burgess, I.P.; Wolf, L. Patterns of natural and manipulated hybridization in the genus Eucalyptus L’Herit—A review. Aust. J. Bot. 1988, 36, 41–66. [Google Scholar] [CrossRef]
- González-Orozco, C.E.; Pollock, L.J.; Thornhill, A.H.; Mishler, B.D.; Knerr, N.; Laffan, S.W.; Miller, J.T.; Rosauer, D.F.; Faith, D.P.; Nipperess, D.A.; et al. Phylogenetic approaches reveal biodiversity threats under climate change. Nat. Clim. Chang. 2016, 6, 1110. [Google Scholar] [CrossRef]
- Aguilee, R.; Raoul, G.; Rousset, F.; Ronce, O. Pollen dispersal slows geographical range shift and accelerates ecological niche shift under climate change. Proc. Natl. Acad. Sci. USA 2016, 113, E5741–E5748. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Liguori, J.A.; Ramírez-Barahona, S.; Gaut, B.S. The evolutionary genomics of species’ responses to climate change. Nat. Ecol. Evol. 2021, 5, 1350–1360. [Google Scholar] [CrossRef] [PubMed]
- Pfeilsticker, T.R.; Jones, R.C.; Steane, D.A.; Vaillancourt, R.E.; Potts, B.M. Molecular insights into the dynamics of species invasion by hybridisation in Tasmanian eucalypts. Mol. Ecol. 2023, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Brennan, A.C.; Woodward, G.; Seehausen, O.; Munoz-Fuentes, V.; Moritz, C.; Guelmami, A.; Abbott, R.J.; Edelaar, P. Hybridization due to changing species distributions: Adding problems or solutions to conservation of biodiversity during global change? Evol. Ecol. Res. 2014, 16, 475–491. [Google Scholar]
- Hamilton, J.A.; Miller, J.M. Adaptive introgression as a resource for management and genetic conservation in a changing climate. Conserv. Biol. 2016, 30, 33–41. [Google Scholar] [CrossRef]
- Janes, J.K.; Hamilton, J.A. Mixing it up: The role of hybridization in forest management and conservation under climate change. Forests 2017, 8, 237. [Google Scholar] [CrossRef]
- Baskett, M.L.; Gomulkiewicz, R. Introgressive hybridization as a mechanism for species rescue. Theor. Ecol. 2011, 4, 223–239. [Google Scholar] [CrossRef]
- DeMarche, M.L.; Doak, D.F.; Morris, W.F. Incorporating local adaptation into forecasts of species’ distribution and abundance under climate change. Glob. Change Biol. 2019, 25, 775–793. [Google Scholar] [CrossRef]
- Drake, J.E.; Aspinwall, M.J.; Pfautsch, S.; Rymer, P.D.; Reich, P.B.; Smith, R.A.; Crous, K.Y.; Tissue, D.T.; Ghannoum, O.; Tjoelker, M.G. The capacity to cope with climate warming declines from temperate to tropical latitudes in two widely distributed Eucalyptus species. Glob. Change Biol. 2015, 21, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Allendorf, F.W.; Hohenlohe, P.A.; Luikart, G. Genomics and the future of conservation genetics. Nat. Rev. Genet. 2010, 11, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Coates, D.J.; Byrne, M.; Moritz, C. Genetic diversity and conservation units: Dealing with the species-population continuum in the age of genomics. Front. Ecol. Evol. 2018, 6, 165. [Google Scholar] [CrossRef]
- Binks, R.M.; Steane, D.A.; Byrne, M. Genomic divergence in sympatry indicates strong reproductive barriers and cryptic species within Eucalyptus salubris. Ecol. Evol. 2021, 11, 5096–5110. [Google Scholar] [CrossRef]
- Steane, D.A.; Potts, B.M.; McLean, E.; Collins, L.; Prober, S.M.; Stock, W.D.; Vaillancourt, R.E.; Byrne, M. Genome-wide scans reveal cryptic population structure in a dry-adapted eucalypt. Tree Genet. Genomes 2015, 11, 33. [Google Scholar] [CrossRef]
- Hudson, C.J.; Kullan, A.R.K.; Freeman, J.S.; Faria, D.A.; Grattapaglia, D.; Kilian, A.; Myburg, A.A.; Potts, B.M.; Vaillancourt, R.E. High synteny and colinearity among Eucalyptus genomes revealed by high-density comparative genetic mapping. Tree Genet. Genomes 2012, 8, 339–352. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones, R.C.; Harrison, P.A.; Hudson, C.J.; Hirst, C.A.; Matthews, A.T.; Rouger, R.; Wise, S.L.; O’Reilly-Wapstra, J.M.; Wiltshire, R.J.E.; Jordan, G.J.; et al. Evolutionary Processes Shaping Postglacial Gene Pools of High-Altitude Forests: Evidence from the Endemic Eucalypts of Tasmania. Forests 2023, 14, 1072. https://doi.org/10.3390/f14061072
Jones RC, Harrison PA, Hudson CJ, Hirst CA, Matthews AT, Rouger R, Wise SL, O’Reilly-Wapstra JM, Wiltshire RJE, Jordan GJ, et al. Evolutionary Processes Shaping Postglacial Gene Pools of High-Altitude Forests: Evidence from the Endemic Eucalypts of Tasmania. Forests. 2023; 14(6):1072. https://doi.org/10.3390/f14061072
Chicago/Turabian StyleJones, Rebecca C., Peter A. Harrison, Corey J. Hudson, Cate A. Hirst, Alexander T. Matthews, Romuald Rouger, Sascha L. Wise, Julianne M. O’Reilly-Wapstra, Robert J. E. Wiltshire, Gregory J. Jordan, and et al. 2023. "Evolutionary Processes Shaping Postglacial Gene Pools of High-Altitude Forests: Evidence from the Endemic Eucalypts of Tasmania" Forests 14, no. 6: 1072. https://doi.org/10.3390/f14061072
APA StyleJones, R. C., Harrison, P. A., Hudson, C. J., Hirst, C. A., Matthews, A. T., Rouger, R., Wise, S. L., O’Reilly-Wapstra, J. M., Wiltshire, R. J. E., Jordan, G. J., Vaillancourt, R. E., & Potts, B. M. (2023). Evolutionary Processes Shaping Postglacial Gene Pools of High-Altitude Forests: Evidence from the Endemic Eucalypts of Tasmania. Forests, 14(6), 1072. https://doi.org/10.3390/f14061072






