Response of Fine-Root Traits of Populus tomentosa to Drought in Shallow and Deep Soil
Abstract
1. Introduction
2. Materials and Methods
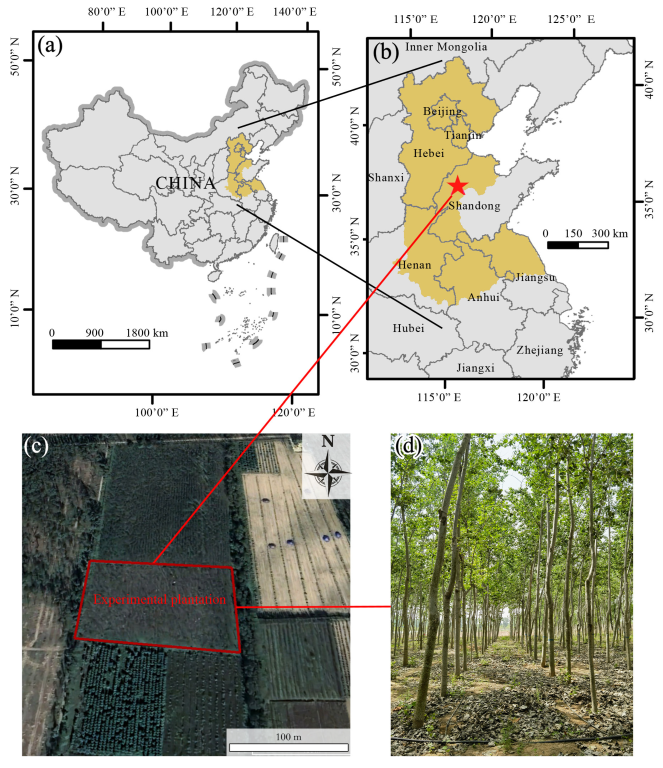
2.1. Experimental Site and Experimental Plantation
2.2. Experimental Design
2.3. Soil Water Content and Root Measurement
2.4. Data Analysis
3. Results
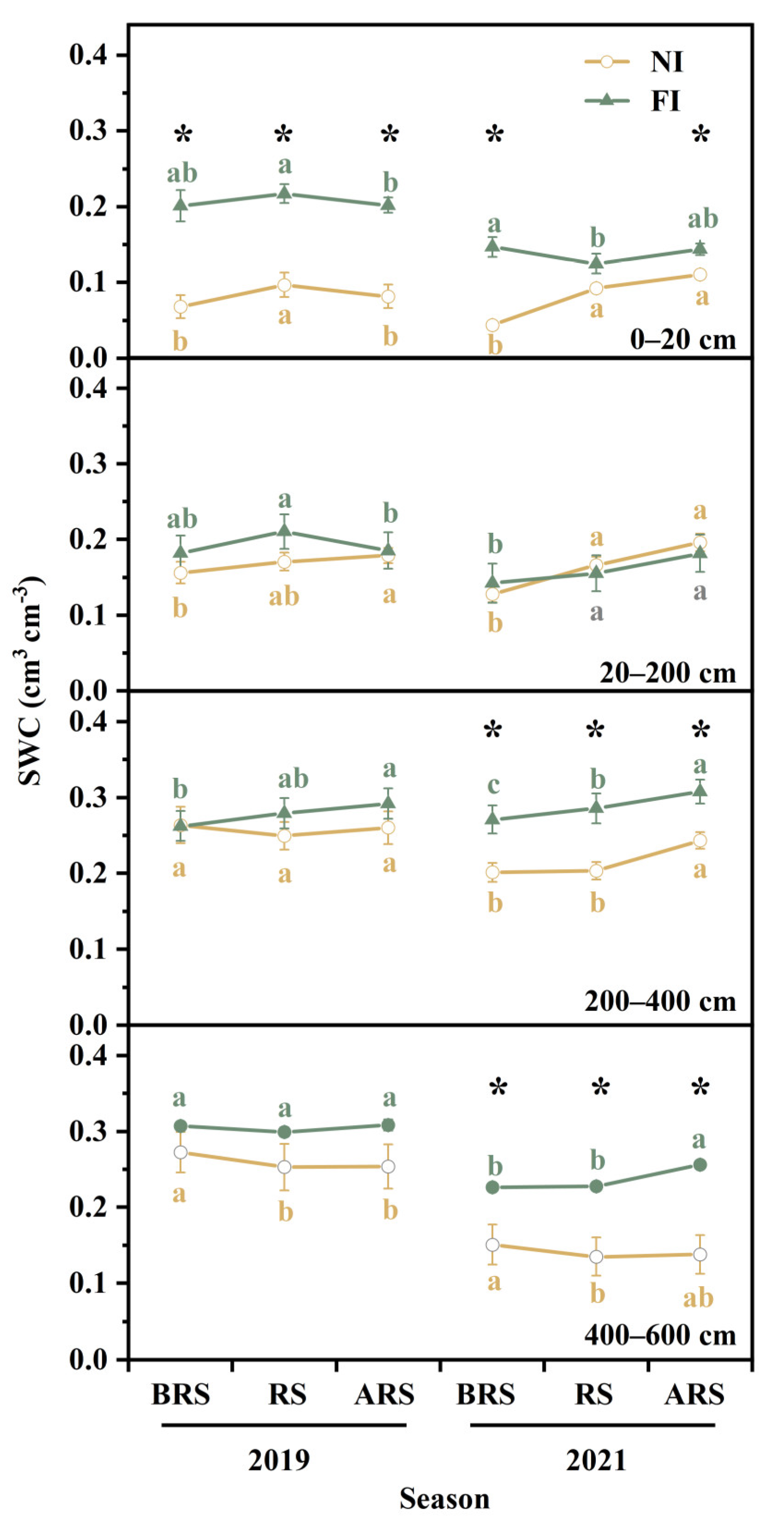
3.1. Soil Moisture Condition
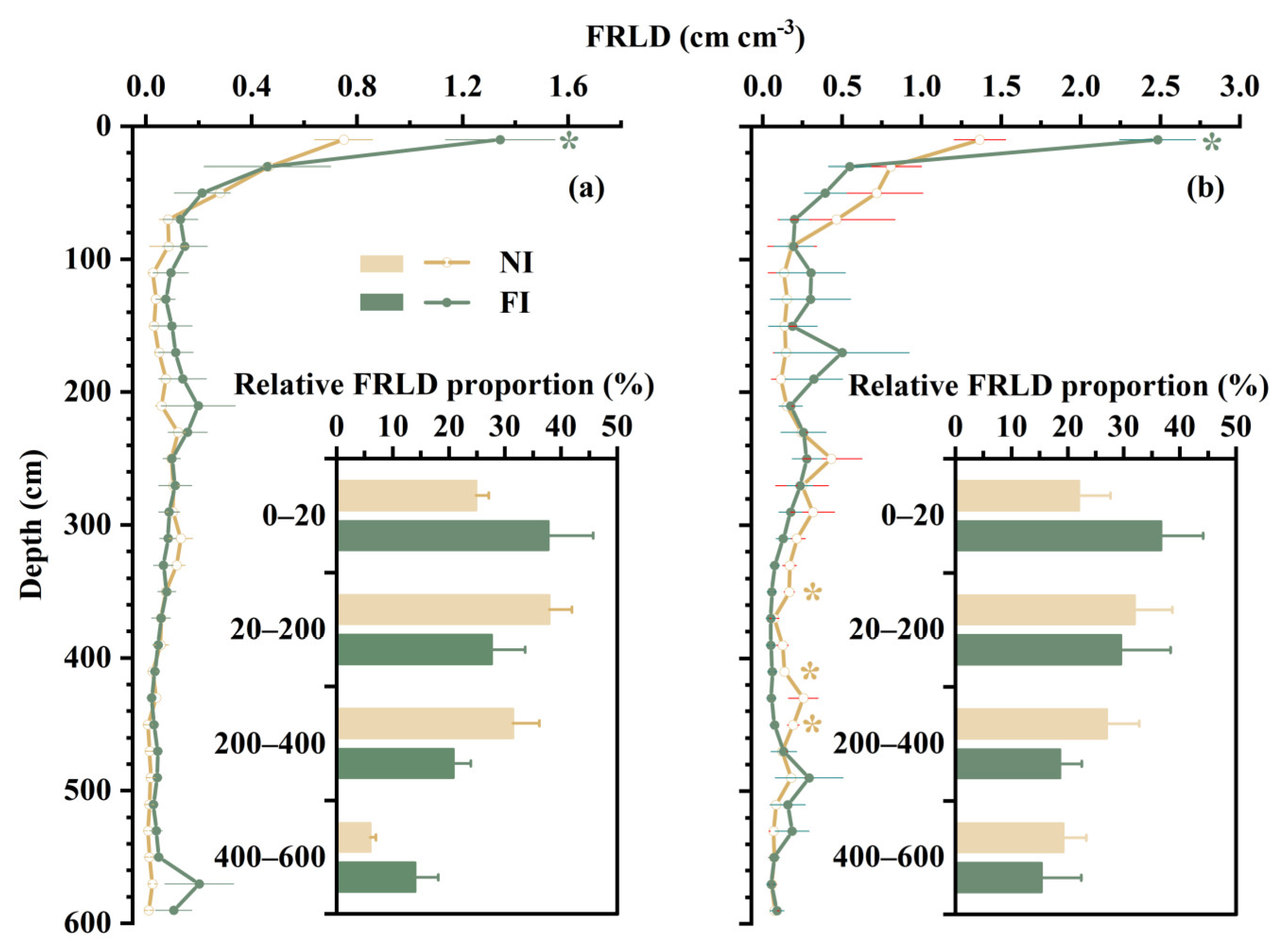
3.2. Fine-Root Vertical Distribution
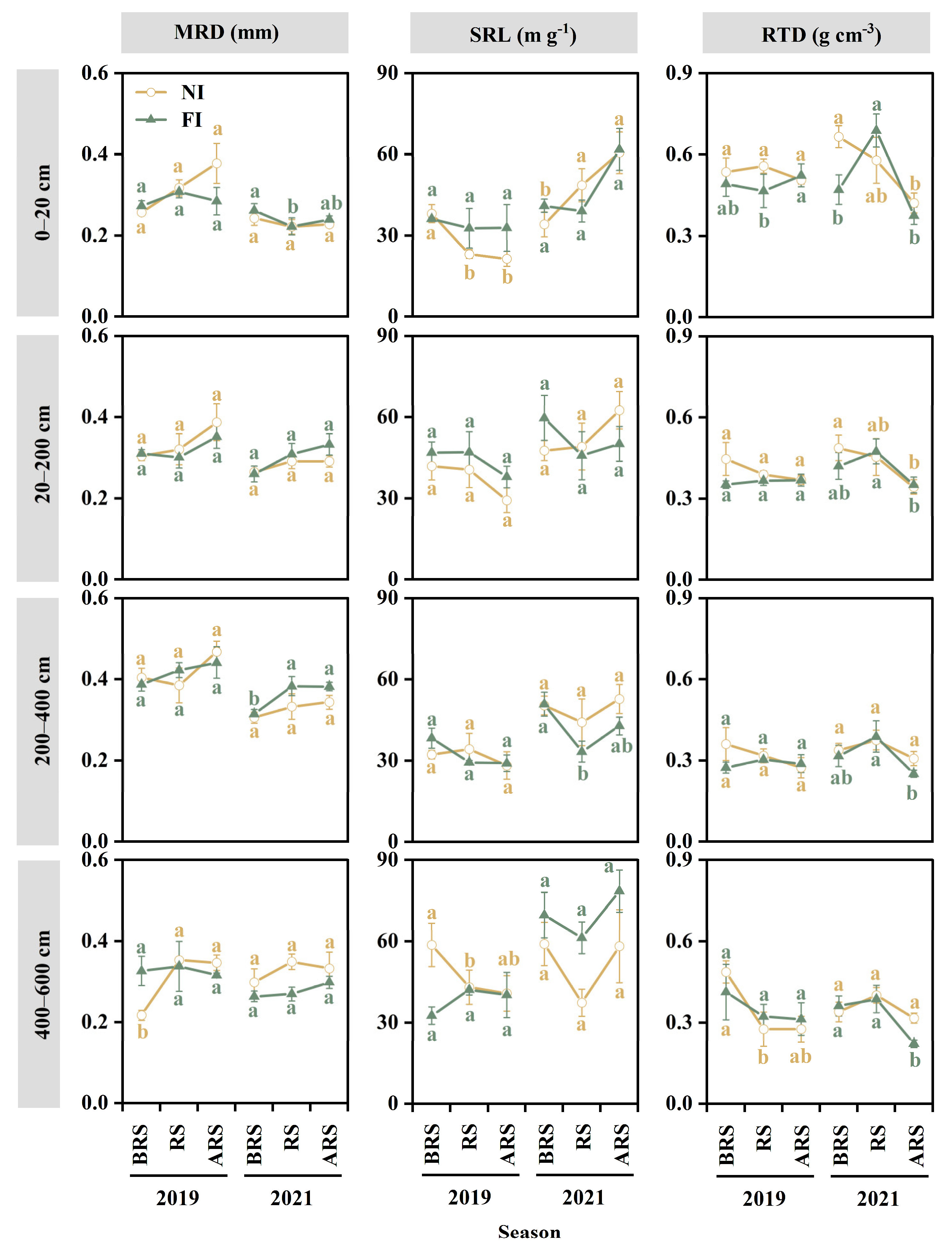
3.3. Fine-Root Morphological Traits
3.4. The Relationship between Fine-Root Morphological Traits
4. Discussion
4.1. Effect of Soil Drought on Vertical Distribution
4.2. Effect of Soil Drought on Fine-Root Morphological Traits
4.3. Effect of Soil Drought on the Relationship between Fine-Root Morphological Traits
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shefeld, J.; Wood, E.F.; Roderick, M.L. Little change in global drought over the past 60 years. Nature 2012, 491, 435–438. [Google Scholar] [CrossRef]
- Cavin, L.; Mountford, E.P.; Peterken, G.F.; Jump, A.S. Extreme drought alters competitive dominance within and between tree species in a mixed forest stand. Funct. Ecol. 2013, 27, 1424–1435. [Google Scholar] [CrossRef]
- Frank, D.; Reichstein, M.; Bahn, M.; Thonicke, K.; Frank, D.; Mahecha, M.D.; Smith, P.; Velde, M.V.D.; Vicca, S.; Babst, F.; et al. Effects of climate extremes on the terrestrial carbon cycle: Concepts, processes and potential future impacts. Glob. Chang. Biol. 2015, 21, 2861–2880. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachel, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Kane, J.M.; Anderegg, L.D.L. Consequences of widespread tree mortality triggered by drought and temperature stress. Nat. Clim. Chang. 2013, 3, 30–36. [Google Scholar] [CrossRef]
- Janssens, I.A.; Sampson, D.A.; Curiel-Yuste, J.; Carrara, A.; Ceulemans, R. The carbon cost of fine root turnover in a Scots pine forest. For. Ecol. Manag. 2002, 168, 231–240. [Google Scholar] [CrossRef]
- Ma, Z.Q.; Guo, D.L.; Xu, X.L.; Lu, M.Z.; Bardgett, R.D.; Mccormack, M.L.; Hedin, L.O. Evolutionary history resolves global organization of root functional traits. Nature 2018, 556, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Iversen, C.M.; McCormack, M.L. Filling gaps in our understanding of belowground plant traits across the world: An introduction to a virtual issue. New Phytol. 2021, 231, 2097–2103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xing, Y.J.; Yan, G.Y.; Han, S.J.; Wang, Q.G. Effects of precipitation change on fine root morphology and dynamics at a global scale: A meta-analysis. Can. J. Soil Sci. 2019, 99, 1–11. [Google Scholar] [CrossRef]
- Nakahata, R. Time-varying response of fine root growth to soil temperature and soil moisture in cypress and deciduous oak forests. Plant-Environ. Interact. 2022, 3, 60–73. [Google Scholar] [CrossRef]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef] [PubMed]
- Parhizkar, M.; Shabanpour, M.; Lucas-Borja, M.E.; Zema, D.A.; Li, S.Y.; Tanaka, N.; Cerda, A. Effects of length and application rate of rice straw mulch on surface runoff and soil loss under laboratory simulated rainfall. Int. J. Sediment Res. 2021, 36, 468–478. [Google Scholar] [CrossRef]
- Jackson, R.B.; Mooney, H.A.; Schulze, E.D. A global budget for fine root biomass, surface area, and nutrient contents. Proc. Natl. Acad. Sci. USA 1997, 94, 7362–7366. [Google Scholar] [CrossRef] [PubMed]
- Pregitzer, K.S. Fine roots of trees—A new perspective. New Phytol. 2002, 154, 267–270. [Google Scholar] [CrossRef]
- Block, R.M.A.; Rees, K.C.J.; Knight, J.D. A review of fine root dynamics in Populus plantations. Agrofor. Syst. 2006, 67, 73–84. [Google Scholar] [CrossRef]
- Germon, A.; Jourdan, C.; Bordron, B.; Robin, A.; Nouvellon, Y.; Chapuis-Lardy, L.; de Moraes, J.L.; Pradier, C.; Guerrini, I.A.; Laclau, J.P. Consequences of clear-cutting and drought on fine root dynamics down to 17 m in coppice-managed eucalypt plantations. For. Ecol. Manag. 2019, 445, 48–59. [Google Scholar] [CrossRef]
- Zou, S.Y.; Li, D.D.; Di, N.; Liu, J.Q.; Li, L.Y.; Liu, Y.; Xi, B.Y.; Coleman, M. Stand development modifies effects of soil water availability and spatial location on poplar root traits: Evidence from a six-year experiment. Plant Soil 2022, 480, 165–184. [Google Scholar] [CrossRef]
- Ostonen, I.; Püttsepp, U.; Biel, C.; Alberton, O.; Bakker, M.R.; Lõhmus, K.; Majdi, H.; Metcalfe, D.; Olsthoorn, A.F.M.; Pronk, A.; et al. Specific root length as indicator of environmental change. Plan Biosyst. 2007, 141, 426–442. [Google Scholar] [CrossRef]
- Chapman, N.; Miller, A.J.; Lindsey, K.; Whalley, W.R. Roots, water, and nutrient acquisition: Let’s get physical. Trends Plant Sci. 2012, 17, 701–710. [Google Scholar] [CrossRef]
- Fuentealbal, M.P.; Zhang, J.; Kenworthy, K.E.; Erickson, J.E.; Kruse, J.; Trenholm, L.E. Root development and profile characteristics of bermudagrass and zoysiagrass. HortScience 2015, 50, 1429–1434. [Google Scholar] [CrossRef]
- Zhu, H.; Zhao, J.; Gong, L. The morphological and chemical properties of fine roots respond to nitrogen addition in a temperate Schrenk’s spruce (Picea schrenkiana) forest. Sci. Rep. 2021, 11, 3839. [Google Scholar] [CrossRef] [PubMed]
- Finer, L.; Ohashi, M.; Noguchi, K.; Hirano, Y. Factors causing variation in fine-root biomass in forest ecosystems. For. Ecol. Manag. 2011, 262, 701. [Google Scholar] [CrossRef]
- Hertel, D.; Strecker, T.; Muller-Haubold, H.; Leuschner, C. Fine-root biomass and dynamics in beech forests across a precipitation gradient-is optimal resource partitioning theory applicable to water-limited mature trees? J. Ecol. 2013, 101, 1183–1200. [Google Scholar] [CrossRef]
- Kunz, J.; Löfer, G.; Bauhus, J. Minor European broadleaved tree species are more drought-tolerant than Fagus sylvatica but not more tolerant than Quercus petraea. For. Ecol. Manag. 2018, 414, 15–27. [Google Scholar] [CrossRef]
- Knutzen, F.; Meier, I.C.; Leuschner, C. Does reduced precipitation trigger physiological and morphological drought adaptations in European beech (Fagus sylvatica L.)? Comparing provenances across a precipitation gradient. Tree Physiol. 2015, 35, 949–963. [Google Scholar] [CrossRef]
- Metcalfe, D.B.; Meir, P.; Aragao, L.E.O.C.; da Costa, A.C.L.; Braga, A.P.; Goncalves, P.H.L.; de Athaydes, J.; de Almeida, S.S.; Dawson, L.A.; Malhi, Y.; et al. The effects of water availability on root growth and morphology in an Amazon rainforest. Plant Soil 2008, 311, 189–199. [Google Scholar] [CrossRef]
- Zhao, L.H.; He, N.N.; Wang, J.P.; Siddique, K.H.M.; Gao, X.D.; Zhao, X.N. Plasticity of root traits in a seedling apple intercropping system driven by drought stress on the Loess Plateau of China. Plant Soil 2022, 480, 541–560. [Google Scholar] [CrossRef]
- Xi, B.Y.; Di, N.; Cao, Z.G.; Liu, J.Q.; Li, D.D.; Wang, Y.; Li, G.D.; Duan, J.; Jia, L.M.; Zhang, R.N. Characteristics and underlying mechanisms of plant deep soil water uptake and utilization: Implication for the cultivation of plant trees. Chin. J. Plant Ecol. 2018, 42, 885–905, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Liu, J.; Li, D.; Fernández, J.E.; Coleman, M.; Hu, W.; Di, N.; Zou, S.; Liu, Y.; Xi, B. Variations in water-balance components and carbon stocks in poplar plantations with differing water inputs over a whole rotation: Implications for sustainable forest management under climate change. Agric. For. Meteorol. 2022, 320, 108958. [Google Scholar] [CrossRef]
- Ma, L.H.; Liu, X.L.; Wang, Y.K.; Wu, P.T. Effects of drip irrigation on deep root distribution, rooting depth, and soil water profile of jujube in a semiarid region. Plant Soil 2013, 373, 995–1006. [Google Scholar] [CrossRef]
- Adriano, E.; Laclau, J.P.; Rodrigues, J.D. Deep rooting of rainfed and irrigated orange trees in Brazil. Trees 2017, 31, 285–297. [Google Scholar] [CrossRef]
- Phillips, R.P.; Ibáñez, I.; D’Orangeville, L.; Hanson, P.J.; Ryan, M.G.; McDowell, N.G. A belowground perspective on the drought sensitivity of forests: Towards improved understanding and simulation. For. Ecol. Manag. 2016, 380, 309–320. [Google Scholar] [CrossRef]
- Germon, A.; Laclaum, J.P.; Robin, A.; Jourdan, C. Tamm Review: Deep fine roots in forest ecosystems: Why dig deeper? For. Ecol. Manag. 2020, 466, 118135. [Google Scholar] [CrossRef]
- Gewin, V. Food an underground revolution. Nature 2010, 466, 552–553. [Google Scholar] [CrossRef]
- Pierret, A.; Maeght, J.L.; Clément, C.; Montoroi, J.P.; Hartmann, C.; Gonkhamdee, S. Understanding deep roots and their functions in ecosystems: An advocacy for more unconventional research. Ann. Bot. 2016, 118, 621–635. [Google Scholar] [CrossRef]
- Nardini, A.; Casolo, V.; Dal Borgo, A.; Savi, T.; Stenni, B.; Bertoncin, P.; Zini, L.; McDowell, N.G. Rooting depth, water relations and nonstructural carbohydrate dynamics in three woody angiosperms differentially affected by an extreme summer drought. Plant Cell Environ. 2016, 39, 618–627. [Google Scholar] [CrossRef]
- Prakash, M.; Sunikumar, B.; Bharathi, J.K.; Viswanathan, C. Root dynamics and drought stress management in plants—An overview. Indian J. Exp. Biol. 2022, 60, 449–455. Available online: http://op.niscpr.res.in/index.php/IJEB/article/view/64063 (accessed on 1 April 2023).
- Roupsard, O.; Ferhi, A.; Granier, A.; Pallo, F.; Depommier, D.; Mallet, B.; Joly, H.I.; Dreyer, E. Reverse phenology and dry-season water uptake by Faidherbia albida (Del.) A. Chev. in an agroforestry parkland of Sudanese west Africa. Funct. Ecol. 1999, 13, 460–472. [Google Scholar] [CrossRef]
- Maeght, J.L.; Rewald, B.; Pierret, A. How to study deep roots-and why it matters. Front. Plant Sci. 2013, 4, 299. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.Y.; Li, D.D.; Wang, J.S.; Di, N.; Liu, J.Q.; Wang, Y.; Li, G.D.; Duan, J.; Jia, L.M.; Xi, B.Y. Response of fine roots to soil moisture of different gradients in young Populus tomentosa plantation. Sci. Silvae Sin. 2019, 55, 124–137, (In Chinese with English abstract). [Google Scholar]
- Zhou, Z.X.; Wang, Y.Q.; An, Z.S.; Li, R.J.; Xu, Y.T.; Zhang, P.P.; Yang, Y.; Wang, T. Deep root information “hidden in the dark”: A case study on the 21-m soil profile of Robinia pseudoacacia in the critical zone of the Chinese loess Plateau. Catena 2022, 213, 106121. [Google Scholar] [CrossRef]
- Kang, X.Y.; Zhu, Z.T. Status and role of triploid Populus tomentosa in pulp production in China. J. Beijing For. Univ. 2002, 24, 51–56. (In Chinese) [Google Scholar]
- Li, D.D.; Liu, J.Q.; Verhoef, A.; Xi, B.Y.; Hernandez-Santana, V. Understanding the relationship between biomass production and water use of Populus tomentosa trees throughout an entire short-rotation. Agric. Water Manag. 2021, 246, 106710. [Google Scholar] [CrossRef]
- Yang, T.; Li, D.D.; Clothier, B.; Wang, Y.; Duan, J.; Di, N.; Li, G.D.; Li, X.; Jia, L.M.; Xi, B.Y.; et al. Where to monitor the soil-water potential for scheduling drip irrigation in Populus tomentosa plantations located on the North China Plain? For. Ecol. Manag. 2019, 437, 99–112. [Google Scholar] [CrossRef]
- Liu, Y.; Nadezhdina, N.; Di, N.; Ma, X.; Liu, J.Q.; Zou, S.Y.; Xi, B.Y.; Clothier, B. An undiscovered facet of hydraulic redistribution driven by evaporation—A study from a Populus tomentosa plantation. Plant Physiol. 2021, 186, 361–372. [Google Scholar] [CrossRef] [PubMed]
- McCormack, M.L.; Guo, D.L. Impacts of environmental factors on fine root lifespan. Front. Plant Sci. 2014, 5, 205. [Google Scholar] [CrossRef]
- Coleman, M.D.; Aubrey, D.P. Stand development and other intrinsic factors largely control fine-root dynamics with only subtle modifications from resource availability. Tree Physiol. 2018, 38, 1805–1819. [Google Scholar] [CrossRef]
- Green, S.; Clothier, B. The root zone dynamics of water uptake by a mature apple tree. Plant. Soil. 1999, 206, 61–77. [Google Scholar] [CrossRef]
- Xi, B.Y.; Wang, Y.; Jia, L.M.; Bloomberg, M.; Li, G.D.; Di, N. Characteristics of fine root system and water uptake in a triploid Populus tomentosa plantation in the North China Plain: Implications for irrigation water management. Agric. Water Manag. 2013, 117, 83–92. [Google Scholar] [CrossRef]
- Schenk, H.J. The shallowest possible water extraction profile: A null model for global root distribution. Vadose Zone. J. 2008, 7, 1119–1124. [Google Scholar] [CrossRef]
- Maeght, J.L.; Gonkhamdee, S.; Clément, C.; Na Ayutthaya, S.i; Stokes, A.; Pierret, A. Seasonal patterns of fine root production and turnover in a mature rubber tree (Hevea brasiliensis Müll. Arg.) stand-differentiation with soil depth and implications for soil carbon stocks. Front. Plant. Sci. 2015, 6, 1022. [Google Scholar] [CrossRef]
- Germon, A.; Cardinael, R.; Prieto, I.; Mao, Z.; Kim, J.; Stokes, A.; Dupraz, C.; Laclau, J.P.; Jourdan, C. Unexpected phenology and lifespan of shallow and deep fine roots of walnut trees grown in a silvoarable Mediterranean agroforestry system. Plant Soil 2016, 401, 409–426. [Google Scholar] [CrossRef]
- Konôpka, B.; Lukac, M. Moderate drought alters biomass and depth distribution of fine roots in Norway spruce. For Pathol. 2013, 43, 115–123. [Google Scholar] [CrossRef]
- Brunner, I.; Herzog, C.; Dawes, M.A.; Arend, M.A.; Sperisen, C. How tree roots respond to drought. Front. Plant Sci. 2015, 6, 547. [Google Scholar] [CrossRef] [PubMed]
- Bristiel, P.; Roumet, C.; Violle, C.; Volaire, F. Coping with drought: Root trait variability within the perennial grass Dactylis glomerata captures a trade-off between dehydration avoidance and dehydration tolerance. Plant Soil 2019, 434, 327–342. [Google Scholar] [CrossRef]
- Meier, I.C.; Leuschner, C. Genotypic variation and phenotypic plasticity in the drought response of fine roots of European beech. Tree Physiol. 2007, 28, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Olmo, M.; Lopez-Iglesias, B.; Villar, R. Drought changes the structure and elemental composition of very fine roots in seedlings of ten woody tree species: Implications for a drier climate. Plant Soil 2014, 384, 113–129. [Google Scholar] [CrossRef]
- Kou, X.Y.; Han, W.H.; Kang, J. Responses of root system architecture to water stress at multiple levels: A meta-analysis of trials under controlled conditions. Front. Plant. Sci. 2022, 13, 1085409. [Google Scholar] [CrossRef]
- Arend, M.; Kuster, T.; Günthardt-Goerg, M.; Dobbertin, M. Provenance-specific growth responses to drought and air warming in three European oak species (Quercus robur, Q. petraea and Q. pubescens). Tree Physiol. 2011, 31, 287–297. [Google Scholar] [CrossRef]
- Herzog, C.; Peter, M.; Pritsch, K.; Günthardt-Goerg, M.S.; Egli, S. Drought and air warming affects abundance and exoenzyme profiles of Cenococcum geophilum associated with Quercus robur, Q. petraea and Q. pubescens. Plant Biol. 2013, 15 (Suppl. 1), 230–237. [Google Scholar] [CrossRef]
- Brunner, I.; Herzog, C.; Galiano, L.; Gessler, A. Plasticity of Fine-Root Traits Under Long-Term Irrigation of a Water-Limited Scots Pine Forest. Front. Plant. Sci. 2019, 10, 701. [Google Scholar] [CrossRef]
- Li, D.D.; Fernández, J.E.; Li, X.; Xi, B.Y.; Jia, L.M.; Fernández, V. Tree growth patterns and diagnosis of water status based on trunk diameter fluctuations in fast-growing Populus tomentosa plantations. Agric. Water Manag. 2020, 241, 106348. [Google Scholar] [CrossRef]
- Weigelt, A.; Mommer, L.; Andraczek, K.; Iversen, C.M.; Bergmann, J.; Bruelheide, H.; Fan, Y.; Freschet, G.T.; Guerrero-Ramírez, N.R.; Kattge, J.; et al. An integrated framework of plant form and function: The belowground perspective. New Phytol. 2021, 232, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Carmona, C.P.; Bueno, C.G.; Toussaint, A.; Träger, S.; Díaz, S.; Moora, M.; Munson, A.D.; Pärtel, M.; Zobel, M.; Tamme, R. Fine-root traits in the global spectrum of plant form and function. Nature 2021, 597, 683–687. [Google Scholar] [CrossRef] [PubMed]



| Year | Month | Treatment | Block | Interval (cm) | Depth (cm) |
|---|---|---|---|---|---|
| 2019 | May, July, and September | NI, FI | 1, 2, 4 | 20 | 600 |
| June, August, and October | NI, FI | 1, 2, 3, 4 | 20 | 400 | |
| 2021 | June, August, and October | NI, FI | 1, 2, 3, 4, 5 | 20 | 600 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, J.; Yu, W.; Liu, Y.; Guo, Y.; Liu, N.; Fu, H.; Di, N.; Duan, J.; Li, X.; Xi, B. Response of Fine-Root Traits of Populus tomentosa to Drought in Shallow and Deep Soil. Forests 2023, 14, 951. https://doi.org/10.3390/f14050951
Tan J, Yu W, Liu Y, Guo Y, Liu N, Fu H, Di N, Duan J, Li X, Xi B. Response of Fine-Root Traits of Populus tomentosa to Drought in Shallow and Deep Soil. Forests. 2023; 14(5):951. https://doi.org/10.3390/f14050951
Chicago/Turabian StyleTan, Jianbiao, Weichen Yu, Yang Liu, Youzheng Guo, Nan Liu, Haiman Fu, Nan Di, Jie Duan, Ximeng Li, and Benye Xi. 2023. "Response of Fine-Root Traits of Populus tomentosa to Drought in Shallow and Deep Soil" Forests 14, no. 5: 951. https://doi.org/10.3390/f14050951
APA StyleTan, J., Yu, W., Liu, Y., Guo, Y., Liu, N., Fu, H., Di, N., Duan, J., Li, X., & Xi, B. (2023). Response of Fine-Root Traits of Populus tomentosa to Drought in Shallow and Deep Soil. Forests, 14(5), 951. https://doi.org/10.3390/f14050951








