Impact of Fire on Secondary Forest Succession in a Sub-Tropical Landscape
Abstract
1. Introduction
2. Materials and Methods
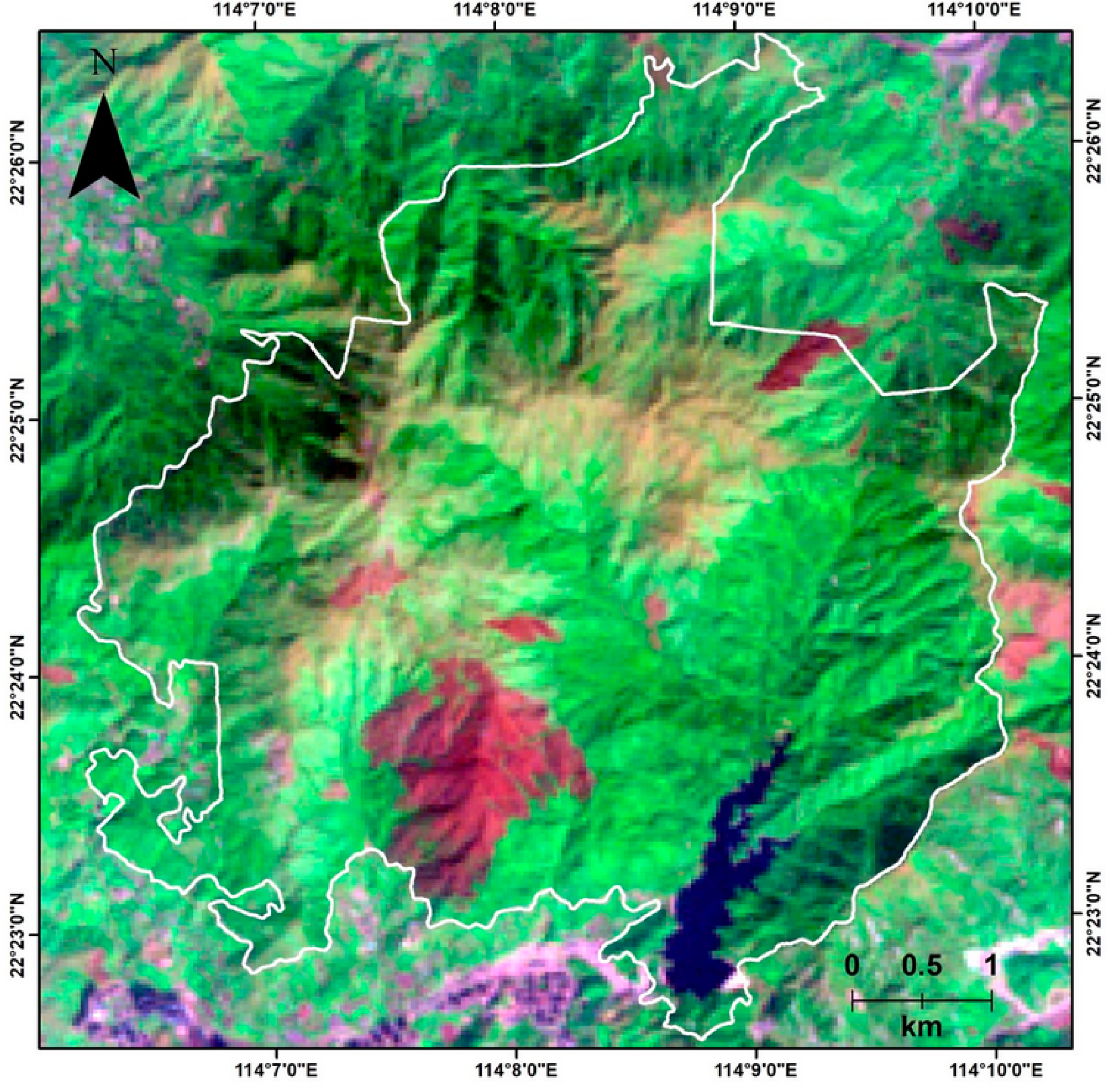
2.1. Study Area
2.2. Data Used
2.3. Data Analysis
3. Results
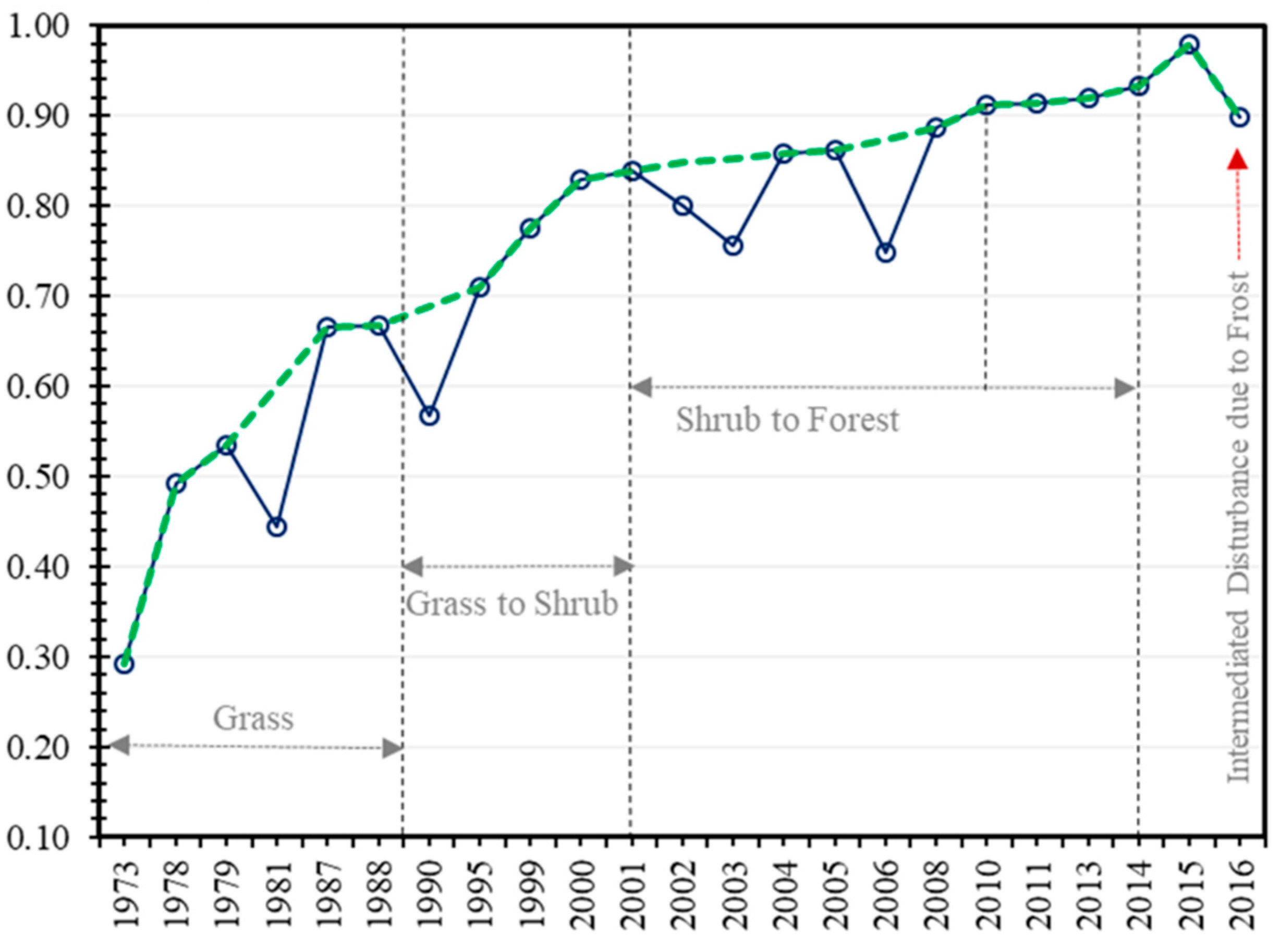
3.1. Impacts of Fire on Vegetation Growth
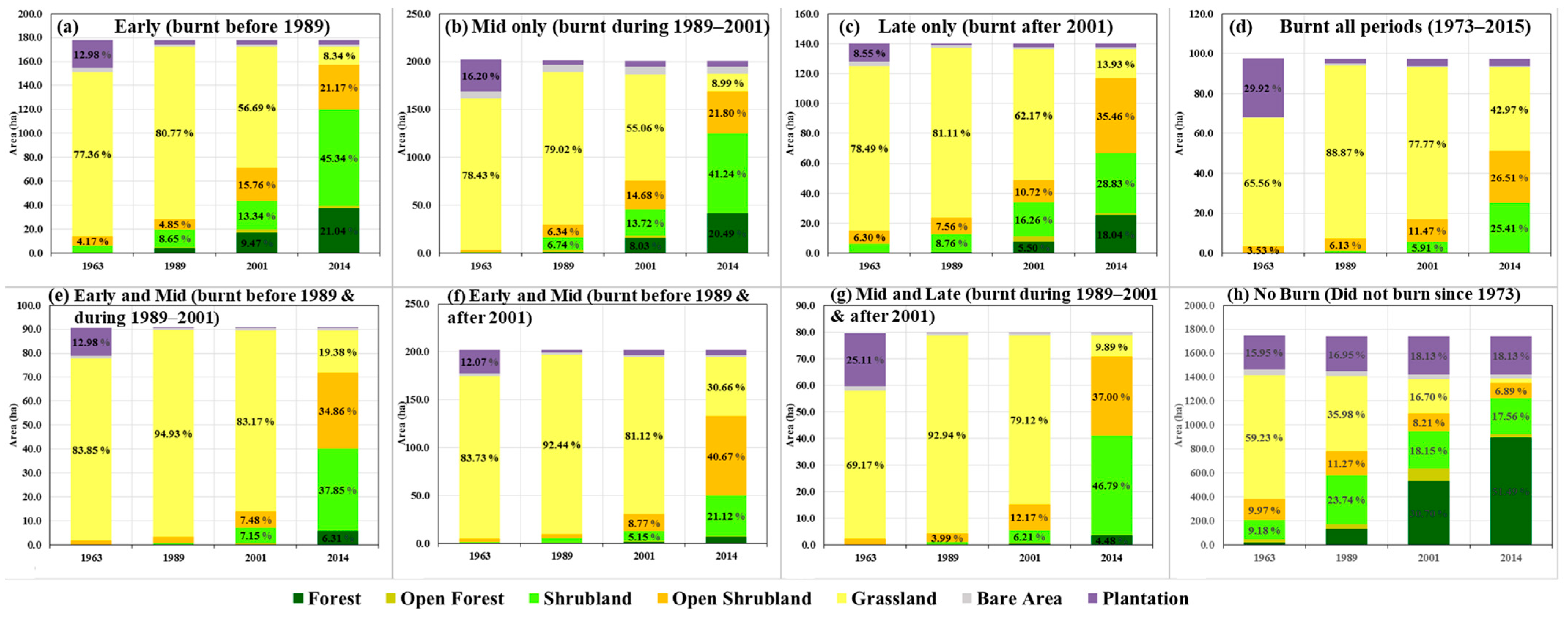
3.2. Impacts of Fire on Vegetation Successional Classes
3.3. Plot Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arroyo-Rodríguez, V.; Melo, F.P.L.; Martínez-Ramos, M.; Bongers, F.; Chazdon, R.L.; Meave, J.A.; Norden, N.; Santos, B.A.; Leal, I.R.; Tabarelli, M. Multiple successional pathways in human-modified tropical landscapes: New insights from forest succession, forest fragmentation and landscape ecology research. Biol. Rev. 2017, 92, 326–340. [Google Scholar] [CrossRef] [PubMed]
- Corlett, R.T.; Hau, B.C.H. Seed dispersal and forest restoration. In Forest Restoration for Wildlife Conservation; Elliott, S., Kerby, J., Blakesley, D., Hardwick, K., Woods, K., Anusarnsunthorn, V., Eds.; International Tropical Timber Organization and The Forest Restoration Research: Chiang Mai, Thailand, 2000; p. 31. [Google Scholar]
- Dent, D.H.; Estrada-Villegas, S. Uniting niche differentiation and dispersal limitation predicts tropical forest succession. Trends Ecol. Evol. 2021, 36, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Rozendaal, D.M.A.; Bongers, F.; Aide, T.M.; Alvarez-Dávila, E.; Ascarrunz, N.; Balvanera, P.; Becknell, J.M.; Bentos, T.V.; Brancalion, P.H.S.; Cabral, G.A.L.; et al. Biodiversity recovery of Neotropical secondary forests. Sci. Adv. 2019, 5, eaau3114. [Google Scholar] [CrossRef]
- Steidinger, B.S.; Crowther, T.W.; Liang, J.; Van Nuland, M.E.; Werner, G.D.A.; Reich, P.B.; Nabuurs, G.J.; De-Miguel, S.; Zhou, M.; Picard, N.; et al. Climatic controls of decomposition drive the global biogeography of forest-tree symbioses. Nature 2019, 569, 404–408. [Google Scholar] [CrossRef]
- Szefer, P.; Molem, K.; Sau, A.; Novotny, V. Impact of pathogenic fungi, herbivores and predators on secondary succession of tropical rainforest vegetation. J. Ecol. 2020, 108, 1978–1988. [Google Scholar] [CrossRef]
- Pérez-Cárdenas, N.; Mora, F.; Arreola-Villa, F.; Arroyo-Rodríguez, V.; Balvanera, P.; Flores-Casas, R.; Navarrete-Pacheco, A.; Ortega-Huerta, M.A. Effects of landscape composition and site land-use intensity on secondary succession in a tropical dry forest. For. Ecol. Manag. 2021, 482, 118818. [Google Scholar] [CrossRef]
- Jakovac, C.C.; Junqueira, A.B.; Crouzeilles, R.; Peña-Claros, M.; Mesquita, R.C.G.; Bongers, F. The role of land-use history in driving successional pathways and its implications for the restoration of tropical forests. Biol. Rev. 2021, 96, 1114–1134. [Google Scholar] [CrossRef]
- Corlett, R.T. Environmental forestry in Hong Kong: 1871–1997. For. Ecol. Manag. 1999, 116, 93–105. [Google Scholar] [CrossRef]
- Abbas, S.; Nichol, J.E.; Fischer, G.A. A 70-year perspective on tropical forest regeneration. Sci. Total Environ. 2016, 544, 544–552. [Google Scholar] [CrossRef]
- Abbas, S.; Nichol, J.E.; Fischer, G.A.; Wong, M.S.; Irteza, S.M. Impact assessment of a super-typhoon on Hong Kong’s secondary vegetation and recommendations for restoration of resilience in the forest succession. Agric. For. Meteorol. 2020, 280, 107784. [Google Scholar] [CrossRef]
- Abbas, S.; Nichol, J.E.; Fischer, G.A. Mapping and assessment of impacts of cold and frost on secondary forest in the marginally tropical landscape of Hong Kong. Agric. For. Meteorol. 2017, 232, 543–549. [Google Scholar] [CrossRef]
- Abbas, S.; Nichol, J.E.; Wong, M.S. Object-based, multi-sensor habitat mapping of successional age classes for effective management of a 70-year secondary forest succession. Land Use Policy 2020, 99, 103360. [Google Scholar] [CrossRef]
- Abbas, S.; Nichol, J.E.; Zhang, J.; Fischer, G.A. The accumulation of species and recovery of species composition along a 70 year succession in a tropical secondary forest. Ecol. Indic. 2019, 106, 105524. [Google Scholar] [CrossRef]
- Abbas, S.; Nichol, J.E.; Zhang, J.; Fischer, G.A.; Wong, M.S.; Irteza, S.M. Spatial and environmental constraints on natural forest regeneration in the degraded landscape of Hong Kong. Sci. Total Environ. 2021, 752, 141760. [Google Scholar] [CrossRef]
- USGS USGS:EROS Science Processing Architecture On Demand Interface. Available online: https://espa.cr.usgs.gov/ (accessed on 17 January 2021).
- Tucker, C.J. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef]
- Blaschke, T. Object based image analysis for remote sensing. ISPRS J. Photogramm. Remote Sens. 2010, 65, 2–16. [Google Scholar] [CrossRef]
- Escuin, S.; Navarro, R.; Fernández, P. Fire severity assessment by using NBR (Normalized Burn Ratio) and NDVI (Normalized Difference Vegetation Index) derived from LANDSAT TM/ETM images. Int. J. Remote Sens. 2008, 29, 1053–1073. [Google Scholar] [CrossRef]
- Song, W.; Mu, X.; Ruan, G.; Gao, Z.; Li, L.; Yan, G. Estimating fractional vegetation cover and the vegetation index of bare soil and highly dense vegetation with a physically based method. Int. J. Appl. Earth Obs. Geoinf. 2017, 58, 168–176. [Google Scholar] [CrossRef]
- Marafa, L.M.; Chau, K. Effect of hill fire on upland soil in Hong Kong. For. Ecol. Manag. 1999, 120, 97–104. [Google Scholar] [CrossRef]
- Svensson, J.R.; Lindegarth, M.; Jonsson, P.R.; Pavia, H. Disturbance–diversity models: What do they really predict and how are they tested? Proc. R. Soc. B Biol. Sci. 2012, 279, 2163–2170. [Google Scholar] [CrossRef]
- Chazdon, R.L.; Guariguata, M.R. Natural regeneration as a tool for large-scale forest restoration in the tropics: Prospects and challenges. Biotropica 2016, 48, 716–730. [Google Scholar] [CrossRef]
- Murphy, B.P.; Bowman, D.M.J.S. What controls the distribution of tropical forest and savanna? Ecol. Lett. 2012, 15, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Staal, A.; Nes, E.H.; Hantson, S.; Holmgren, M.; Dekker, S.C.; Pueyo, S.; Xu, C.; Scheffer, M. Resilience of tropical tree cover: The roles of climate, fire, and herbivory. Glob. Chang. Biol. 2018, 24, 5096–5109. [Google Scholar] [CrossRef] [PubMed]
- McLauchlan, K.K.; Higuera, P.E.; Miesel, J.; Rogers, B.M.; Schweitzer, J.; Shuman, J.K.; Tepley, A.J.; Varner, J.M.; Veblen, T.T.; Adalsteinsson, S.A.; et al. Fire as a fundamental ecological process: Research advances and frontiers. J. Ecol. 2020, 108, 2047–2069. [Google Scholar] [CrossRef]
- Lamont, B.B.; He, T.; Yan, Z. Evolutionary history of fire-stimulated resprouting, flowering, seed release and germination. Biol. Rev. 2019, 94, 903–928. [Google Scholar] [CrossRef]
- Guariguata, M.R.; Chazdon, R.L.; Denslow, J.S.; Dupuy, J.M.; Anderson, L. Structure and floristics of secondary and old-growth forest stands in lowland Costa Rica. Plant Ecol. 1997, 132, 107–120. [Google Scholar] [CrossRef]
- Kumar, S.; Getirana, A.; Libonati, R.; Hain, C.; Mahanama, S.; Andela, N. Changes in land use enhance the sensitivity of tropical ecosystems to fire-climate extremes. Sci. Rep. 2022, 12, 964. [Google Scholar] [CrossRef]
- Ma, W.; Feng, Z.; Cheng, Z.; Chen, S.; Wang, F. Identifying forest fire driving factors and related impacts in china using random forest algorithm. Forests 2020, 11, 507. [Google Scholar] [CrossRef]
- Denslow, J. Patterns of structure and diversity across a tropical moist forest chronosequence. Proc. IAVS Symp. 2000, 237–241. [Google Scholar]
- Connell, J.H. Diversity in Tropical Rain Forests and Coral Reefs. Science 1978, 199, 1302–1310. [Google Scholar] [CrossRef]
- Datta, R. To extinguish or not to extinguish: The role of forest fire in nature and soil resilience. J. King Saud Univ.-Sci. 2021, 33, 101539. [Google Scholar] [CrossRef]
- Herawati, H.; Santoso, H. Tropical forest susceptibility to and risk of fire under changing climate: A review of fire nature, policy and institutions in Indonesia. For. Policy Econ. 2011, 13, 227–233. [Google Scholar] [CrossRef]
- Stevens-Rumann, C.S.; Kemp, K.B.; Higuera, P.E.; Harvey, B.J.; Rother, M.T.; Donato, D.C.; Morgan, P.; Veblen, T.T. Evidence for declining forest resilience to wildfires under climate change. Ecol. Lett. 2018, 21, 243–252. [Google Scholar] [CrossRef]
- Sun, Q.; Burrell, A.; Barrett, K.; Kukavskaya, E.; Buryak, L.; Kaduk, J.; Baxter, R. Climate variability may delay post-fire recovery of boreal forest in southern siberia, russia. Remote Sens. 2021, 13, 2247. [Google Scholar] [CrossRef]
- Sutomo; van Etten, E.J.B. Fire Impacts and Dynamics of Seasonally Dry Tropical Forest of East Java, Indonesia. Forests 2023, 14, 106. [Google Scholar] [CrossRef]
- Mata, S.; Braga, J.M.A.; Moser, P.; Sartori, R.A.; Sánchez-Tapia, A.; Sansevero, J.B.B. Forever young: Arrested succession in communities subjected to recurrent fires in a lowland tropical forest. Plant Ecol. 2022, 223, 659–670. [Google Scholar] [CrossRef]
- Claverie, M.; Ju, J.; Masek, J.G.; Dungan, J.L.; Vermote, E.F.; Roger, J.-C.; Skakun, S.V.; Justice, C. The Harmonized Landsat and Sentinel-2 surface reflectance data set. Remote Sens. Environ. 2018, 219, 145–161. [Google Scholar] [CrossRef]
- Lacouture, D.L.; Broadbent, E.N.; Crandall, R.M. Detecting Vegetation Recovery after Fire in A Fire-Frequented Habitat Using Normalized Difference Vegetation Index (NDVI). Forests 2020, 11, 749. [Google Scholar] [CrossRef]
- Chau, K.L. The Ecology of Fire in Hong Kong. Ph.D. Thesis, University of Hong Kong, Hong Kong, China, 1994. [Google Scholar]
- Au, A.Y.Y.; Corlett, R.T.; Hau, B.C.H. Seed rain into upland plant communities in Hong Kong, China. Plant Ecol. 2006, 186, 13–22. [Google Scholar] [CrossRef]
- Hang Hau, C. Tree seed predation on degraded hillsides in Hong Kong. For. Ecol. Manage. 1997, 99, 215–221. [Google Scholar] [CrossRef]
- Guariguata, M.R.; Ostertag, R. Neotropical secondary forest succession: Changes in structural and functional characteristics. For. Ecol. Manag. 2001, 148, 185–206. [Google Scholar] [CrossRef]
- Zhuang, X.Y.; Gorlett, R.T. Forest and forest succession in Hong Kong, China. J. Trop. Ecol. 1997, 13, 857–866. [Google Scholar] [CrossRef]
- Weir, J.E.S.; Corlett, R.T. How far do birds disperse seeds in the degraded tropical landscape of Hong Kong, China? Landsc. Ecol. 2007, 22, 131–140. [Google Scholar] [CrossRef]
- Kaewsong, K.; Chang-Yang, C.-H.; Bunyavejchewin, S.; Kraichak, E.; Yang, J.; Sun, Z.; Zhang, C.; Li, W.; Lin, L.; Sun, I.-F. Effects of fire disturbance on species and functional compositions vary with tree sizes in a tropical dry forest. PeerJ 2022, 10, e13270. [Google Scholar] [CrossRef] [PubMed]
- Slik, J.W.F.; Bernard, C.S.; Van Beek, M.; Breman, F.C.; Eichhorn, K.A.O. Tree diversity, composition, forest structure and aboveground biomass dynamics after single and repeated fire in a Bornean rain forest. Oecologia 2008, 158, 579–588. [Google Scholar] [CrossRef]
- Fischer, R. The long-term consequences of forest fires on the carbon fluxes of a tropical forest in Africa. Appl. Sci. 2021, 11, 4696. [Google Scholar] [CrossRef]
- Reilly, M.; Outcalt, K.; O’Brien, J.; Wade, D. Effects of Repeated Growing Season Prescribed Fire on the Structure and Composition of Pine–Hardwood Forests in the Southeastern Piedmont, USA. Forests 2016, 8, 8. [Google Scholar] [CrossRef]
- Uriarte, M.; Pinedo-Vasquez, M.; DeFries, R.S.; Fernandes, K.; Gutierrez-Velez, V.; Baethgen, W.E.; Padoch, C. Depopulation of rural landscapes exacerbates fire activity in the western Amazon. Proc. Natl. Acad. Sci. USA 2012, 109, 21546–21550. [Google Scholar] [CrossRef]
- Pereira, P.; Cerdà, A.; Lopez, A.J.; Zavala, L.M.; Mataix-Solera, J.; Arcenegui, V.; Misiune, I.; Keesstra, S.; Novara, A. Short-Term Vegetation Recovery after a Grassland Fire in Lithuania: The Effects of Fire Severity, Slope Position and Aspect. L. Degrad. Dev. 2016, 27, 1523–1534. [Google Scholar] [CrossRef]
- Zhong, C.; Guo, M.; Zhou, F.; Li, J.; Yu, F.; Guo, F.; Li, W. Forest succession trajectories after fires in valleys and on slopes in the Greater Khingan Mountains, China. J. For. Res. 2023, 1–18. [Google Scholar] [CrossRef]
- Nichol, J.E.; Abbas, S.; Fischer, G.A. Spatial patterns of degraded tropical forest and biodiversity restoration over 70-years of succession. Glob. Ecol. Conserv. 2017, 11, 134–145. [Google Scholar] [CrossRef]
- Costa, F.R.C.; Magnusson, W.E.; Luizao, R.C. Mesoscale distribution patterns of Amazonian understorey herbs in relation to topography, soil and watersheds. J. Ecol. 2005, 93, 863–878. [Google Scholar] [CrossRef]
- Bohlman, S.A.; Laurance, W.F.; Laurance, S.G.; Nascimento, H.E.M.; Fearnside, P.M.; Andrade, A. Importance of soils, topography and geographic distance in structuring central Amazonian tree communities. J. Veg. Sci. 2008, 19, 863–874. [Google Scholar] [CrossRef]
- Punchi-Manage, R.; Getzin, S.; Wiegand, T.; Kanagaraj, R.; Savitri Gunatilleke, C.V.; Nimal Gunatilleke, I.A.U.; Wiegand, K.; Huth, A. Effects of topography on structuring local species assemblages in a Sri Lankan mixed dipterocarp forest. J. Ecol. 2013, 101, 149–160. [Google Scholar] [CrossRef]
- Segura, G.; Balvanera, P.; Durán, E.; Pérez, A. Tree community structure and stem mortality along a water availability gradient in a Mexican tropical dry forest. Plant Ecol. 2003, 169, 259–271. [Google Scholar] [CrossRef]
- Lovett, J.C.; Clarke, G.P.; Moore, R.; Morrey, G.H. Elevational distribution of restricted range forest tree taxa in eastern Tanzania. Biodivers. Conserv. 2001, 10, 541–550. [Google Scholar] [CrossRef]
- Vasquez, J.A.; Givnish, T.J. Altitudinal gradients in tropical forest composition, structure, and diversity in the Sierra de Manantlan. J. Ecol. 1998, 86, 999–1020. [Google Scholar]
- Gibbons, J.M.; Newbery, D.M. Drought avoidance and the effect of local topography on trees in the understorey of Bornean lowland rain forest. Plant Ecol. 2003, 164, 1–18. [Google Scholar] [CrossRef]
- Balvanera, P.; Quijas, S.; Perez-Jimenez, A. Distribution Patterns of Tropical Dry Forest Trees Along a Mesoscale Water Availability Gradient. Biotropica 2011, 43, 414–422. [Google Scholar] [CrossRef]
- Arekhi, S.; Heydari, M.; Pourbabaei, H. Vegetation-Environmental Relationships and Ecological Species Groups of the Ilam Oak Forest Landscape, Iran. Casp. J. Environ. Sci. 2010, 8, 115–125. [Google Scholar]
- Clark, D.B.; Palmer, M.W.; Clark, D.A. Edaphic factors and the landscape-scale distributions of tropical rain forest trees. Ecology 1999, 80, 2662–2675. [Google Scholar] [CrossRef]
- Liu, J.; Yunhong, T.; Slik, J.W.F. Topography related habitat associations of tree species traits, composition and diversity in a Chinese tropical forest. For. Ecol. Manag. 2014, 330, 75–81. [Google Scholar] [CrossRef]
- Baldeck, C.A.; Tupayachi, R.; Sinca, F.; Jaramillo, N.; Asner, G.P. Environmental drivers of tree community turnover in western Amazonian forests. Ecography 2016, 39, 1089–1099. [Google Scholar] [CrossRef]
- Ivanova, S.; Prosekov, A.; Kaledin, A. A Survey on Monitoring of Wild Animals during Fires Using Drones. Fire 2022, 5, 60. [Google Scholar] [CrossRef]




| Year | No of Images | Day of Year | |
|---|---|---|---|
| 1973–1974 | 1 | 304 | |
| 1978–1979 | 1 | 306 | |
| 1979–1980 | 2 | 292, 310 | |
| 1987–1988 | 1 | 342 | |
| 1988–1989 | 5 | 329, 338, 354, 044, 196 | |
| 1989–1990 | 1 | 231 | |
| 1990–1991 | 5 | 343, 358, 113, 257, 266 | |
| 1991–1992 | 1 | 282 | |
| 1992–1993 | 1 | 285 | |
| 1993–1994 | 1 | 278 | |
| 1994–1995 | 1 | 297 | |
| 1995–1996 | 6 | 293, 316, 031, 040, 063, 136 | |
| 1998–1999 | 2 | 232, 255 | |
| 1999–2000 | 16 | 287, 319, 328, 360, 002, 003, 019, 042, 107, 179, 195, 211, 242, 243, 258, 259 | |
| 2000–2001 | 13 | 291, 306, 307, 315, 363, 005, 020, 053, 060, 133, 220, 260, 261 | |
| 2001–2002 | 13 | 293, 324, 325, 356, 364, 365, 007, 008, 031, 048, 064, 240, 247 | |
| 2002–2003 | 10 | 311, 312, 010, 018, 019, 027, 058, 131, 187, 235 | |
| 2003–2004 | 10 | 290, 299, 331, 347, 021, 046, 069, 110, 165, 206 | |
| 2004–2005 | 15 | 270, 286, 293, 309, 325, 334, 341, 350, 007, 016, 023, 064, 112, 192, 224 | |
| 2005–2006 | 6 | 288, 295, 327, 042, 243, 266 | |
| 2006–2007 | 10 | 314, 355, 362, 013, 029, 038, 102, 214, 230, 262 | |
| 2007–2008 | 7 | 064, 137, 185, 208, 217, 233, 240 | |
| 2008–2009 | 10 | 272, 297, 329, 336, 011, 018, 034, 123, 139, 251, | |
| 2009–2010 | 7 | 283, 299, 014, 030, 078, 085, 206 | |
| 2010–2011 | 7 | 302, 357, 008, 033, 072, 097, 152 | |
| 2011–2012 | 1 | 305 | |
| 2012–2013 | 1 | 221 | |
| 2013–2014 | 11 | 278, 294, 301, 333, 358, 365, 016, 153, 185, 249, 265 | |
| 2014–2015 | 3 | 281, 320, 329 | |
| Summary | |||
| Period | No. of Years | No of Years with Images during Dry Season | No. of Season with Fire Event |
| 1973–1974 to 1988–1989 | 16 | 5 | 5 |
| 1989–1990 to 2000–2001 | 12 | 8 | 8 |
| 2001–2002 to 2014–2015 | 13 | 12 | 10 |
| 1973–1974 to 2014–2015 | 41 | 25 | 23 |
| Class | Burn Frequency | Class Designation |
|---|---|---|
| 1. | Area never burnt since the satellite record (since 1973) | No burn |
| 2. | Area that burnt in all periods, i.e., consistently burnt | All |
| 3. | Area that burnt before 1989 then did not burn after 1989 | Early |
| 4. | Area that burnt during 1989–2001 | Mid |
| 6. | Area that burnt during 2001–2014 | Late |
| 5. | Area that burnt before 1989 and during 1989–2001 | Early and Mid |
| 7. | Area that burnt before 1989 and during 2001–2014 | Early and Late |
| 8. | Area that burnt during 1989–2001 and after 2001 | Mid and Late |
| 1973/4–1988/9 | 1989/90–2000/1 | 2001/2–2013/4 | |
|---|---|---|---|
| The cumulative area burnt, including > 1 burn | 26,932 | 7593 | 9525 |
| Burnt at least once | 19,392 (24%) | 5780 (7%) | 7978 (10.2%) |
| Burnt at least twice | 2944 (3.61%) | 844 (3.6%) | 1586 (1.94%) |
| Burnt/year (av) | 1683 (2.1%) | 632 (0.78%) | 732 (0.91%) |
| Fire History | Mean Basal Area (m2) | Mean Species Richness |
|---|---|---|
| Old Growth Forest | 67.3 | 19.5 |
| Unburnt plots | 45.9 | 35.6 |
| Once burnt plots | 30.3 | 30.4 |
| Twice burnt plots | 11.4 | 21.3 |
| Period | Area Burnt (ha) | No of Trees Damaged |
|---|---|---|
| 1973–1988 | 30,423 | 19.5 |
| 1989–2000 | 45.9 | 35.6 |
| 2001–2014 | 30.3 | 30.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, S.; Nichol, J.E.; Muhammad Irteza, S.; Usman, M. Impact of Fire on Secondary Forest Succession in a Sub-Tropical Landscape. Forests 2023, 14, 865. https://doi.org/10.3390/f14050865
Abbas S, Nichol JE, Muhammad Irteza S, Usman M. Impact of Fire on Secondary Forest Succession in a Sub-Tropical Landscape. Forests. 2023; 14(5):865. https://doi.org/10.3390/f14050865
Chicago/Turabian StyleAbbas, Sawaid, Janet E. Nichol, Syed Muhammad Irteza, and Muhammad Usman. 2023. "Impact of Fire on Secondary Forest Succession in a Sub-Tropical Landscape" Forests 14, no. 5: 865. https://doi.org/10.3390/f14050865
APA StyleAbbas, S., Nichol, J. E., Muhammad Irteza, S., & Usman, M. (2023). Impact of Fire on Secondary Forest Succession in a Sub-Tropical Landscape. Forests, 14(5), 865. https://doi.org/10.3390/f14050865







