Life History Traits of the Pentatomidae (Hemiptera) for the Development of Pest Management Tools
Abstract
1. Introduction
2. Vibratory and Pheromone Signals in Stink Bug Mating
3. Alarm Pheromones
4. Aggregation Behaviour and Pheromones in Pentatomidae
5. Symbiotic Relationships and Vertical Transmission of Bacteria in Pentatomidae
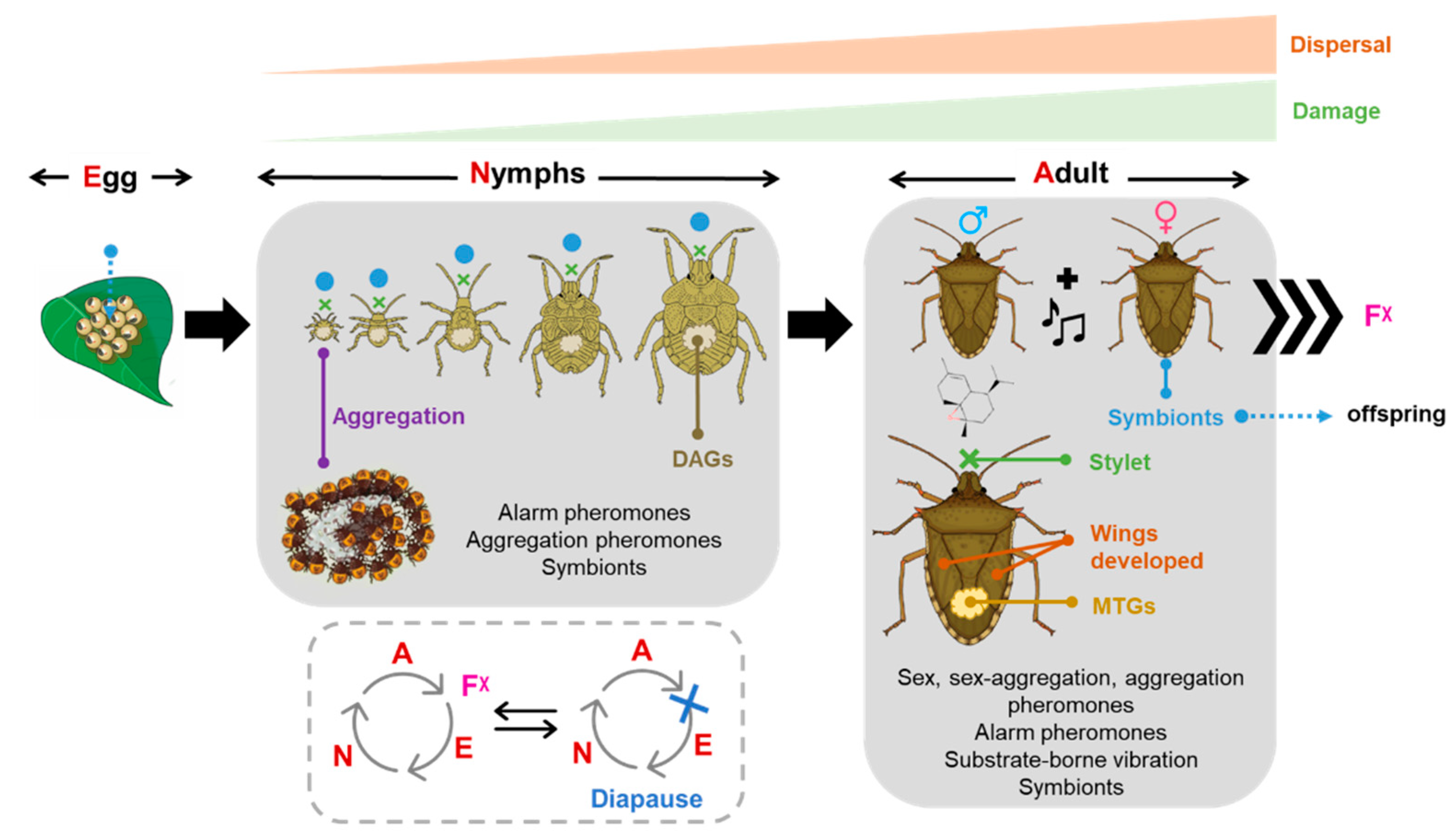
6. Other Important Traits to Consider for Pest Management
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McPherson, J.E.; Robert, M. General introduction to stink bugs. In Stink Bugs of Economic Importance in America North of Mexico, 1st ed.; CRC Press: Boca Raton, FL, USA, 2000; pp. 1–6. [Google Scholar]
- McPherson, J.E.; Bundy, C.S.; Wheeler, A.G. Overview of the superfamily Pentatomoidea. In Invasive Stink Bugs and Related Species (Pentatomoidea): Biology, Higher Systematics, Semiochemistry, and Management; CRC Press: Boca Raton, FL, USA, 2018; pp. 3–22. [Google Scholar]
- Panizzi, A.R.; McPherson, J.E.; James, D.G.; Javahery, M.; McPherson, R.M. Stink bugs (Pentatomidae). In Heteroptera of Economic Importance, 1st ed.; Schaefer, C.W., Panizzi, A.R., Eds.; CRC Press: Boca Raton, FL, USA, 2000; pp. 421–474. [Google Scholar]
- Schuh, R.T.; Slater, J.A. Chapter 73 Pentatomidae. In True Bugs of the World (Hemiptera: Heteroptera). Classification and Natural History; Schuh, R.T., Slater, J.A., Eds.; Siri Scientific Press: Rochdale, UK, 1995; Volume 8, pp. 229–233. [Google Scholar]
- Grazia, J.; Schwertner, C.F. Stink Bug Classification, Phylogeny, Biology and Reproductive Behavior, 1st ed.; Čokl, A., Borges, M., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 1–30. [Google Scholar]
- Sosa-Gómez, D.R.; Corrêa-Ferreira, B.S.; Kraemer, B.; Pasini, A.; Husch, P.E.; Delfino Vieira, C.E.; Reis Martinez, C.B.; Negrão Lopes, I.O. Prevalence, damage, management and insecticide resistance of stink bug populations (Hemiptera: Pentatomidae) in commodity crops. Agric. For. Entomol. 2020, 22, 99–118. [Google Scholar] [CrossRef]
- Greene, J.K.; Baum, J.A.; Benson, E.P.; Bundy, C.S.; Jones, W.A.; Kennedy, G.G.; McPherson, J.E.; Musser, F.R.; Reay-Jones, F.P.F.; Toews, M.D.; et al. General insect management. In Invasive Stink Bugs and Related Species (Pentatomoidea); McPherson, J.E., Ed.; CRC Press: Boca Raton, FL, USA, 2018; pp. 729–774. [Google Scholar]
- Horowitz, A.R.; Ellsworth, P.C.; Ishaaya, I. Biorational pest control—An Overview. In Biorational Control of Arthropod Pests: Application and Resistance Management; Springer: Dordrecht, The Netherlands, 2009; pp. 1–20. [Google Scholar]
- Abe, Y.; Mishiro, K.; Takanashi, M. Symbiont of brown-winged green bug, Plautia stali Scott. Jpn. J. Appl. Entomol. Zool. 1995, 39, 109–115. [Google Scholar] [CrossRef]
- Prado, S.S.; Almeida, R.P.P. Role of symbiotic gut bacteria in the development of Acrosternum hilare and Murgantia histrionica. Entomol. Exp. Appl. 2009, 132, 21–29. [Google Scholar] [CrossRef]
- Prado, S.S.; Rubinoff, D.; Almeida, R.P.P. Vertical transmission of a pentatomid caeca-associated symbiont. Entomol. Exp. Appl. 2006, 99, 577–585. [Google Scholar] [CrossRef]
- Bistolas, K.S.I.; Sakamoto, R.I.; Fernandes, J.A.M.; Goffredi, S.K. Symbiont polyphyly, co-evolution, and necessity in pentatomid stinkbugs from Costa Rica. Front. Microbiol. 2014, 5, 349. [Google Scholar] [CrossRef] [PubMed]
- Douglas, A.E. Microbial brokers of insect-plant interactions revisited. J. Chem. Ecol. 2013, 39, 952–961. [Google Scholar] [CrossRef]
- Prado, S.S.; Almeida, R.P.P. Phylogenetic placement of pentatomid stink bug gut symbionts. Curr. Microbiol. 2009, 58, 64–69. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Prado, S.S.; Jenkins, T.M. Symbiotic microorganisms associated with Pentatomoidea. In Invasive Stink Bugs and Related Species (Pentatomoidea): Biology, Higher Systematics, Semiochemistry, and Management; CRC Press: Boca Raton, FL, USA, 2018; pp. 643–674. [Google Scholar]
- Weber, D.C.; Khrimian, A.; Blassioli-Moraes, M.C.; Millar, J.G. Semiochemistry of Pentatomoidea. In Invasive Stink Bugs and Related Species (Pentatomoidea): Biology, Higher Systematics, Semiochemistry, and Management; CRC Press: Boca Raton, FL, USA, 2018; pp. 677–726. [Google Scholar]
- Borges, M.; Blassioli-Moraes, M.C. The semiochemistry of Pentatomidae. In Stink Bugs: Biorational Control Based on Communication Processes; Čokl, A., Borges, M., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 116–124. [Google Scholar]
- Cardé, R.T. Defining attraction and aggregation pheromones: Teleological versus functional perspectives. J. Chem. Ecol. 2014, 40, 519–520. [Google Scholar] [CrossRef] [PubMed]
- Gonella, E.; Orrù, B.; Marasco, R.; Daffonchio, D.; Alma, A. Disruption of host-symbiont associations for the symbiotic control and management of pentatomid agricultural pests—A Review. Front. Microbiol. 2020, 11, 547031. [Google Scholar] [CrossRef]
- Karamipour, N.; Fathipour, Y.; Mehrabadi, M. Gammaproteobacteria as essential primary symbionts in the striped shield bug, Graphosoma Lineatum (Hemiptera: Pentatomidae). Sci. Rep. 2016, 6, 33168. [Google Scholar] [CrossRef] [PubMed]
- Kashkouli, M.; Fathipour, Y.; Mehrabadi, M. Potential management tactics for pistachio stink bugs, Brachynema germari, Acrosternum heegeri and Acrosternum arabicum (Hemiptera: Pentatomidae): High temperature and chemical surface sterilants leading to symbiont suppression. J. Econ. Entomol. 2019, 112, 244–254. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Hosokawa, T.; Nikoh, N.; Fukatsu, T. Gut symbiotic bacteria in the cabbage bugs Eurydema rugosa and Eurydema dominulus (Heteroptera: Pentatomidae). Appl. Entomol. Zool. 2012, 47, 1–8. [Google Scholar] [CrossRef]
- Taylor, C.M.; Coffey, P.L.; Delay, B.D.; Dively, G.P. The importance of gut symbionts in the development of the brown marmorated stink bug, Halyomorpha halys (Stål). PLoS ONE 2014, 9, e90312. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.; Johnson, V.; Dively, G. Assessing the use of antimicrobials to sterilize brown marmorated stink bug egg masses and prevent symbiont acquisition. J. Pest Sci. 2017, 90, 1287–1294. [Google Scholar] [CrossRef]
- Laumann, R.A.; Maccagnan, D.H.B.; Čokl, A. Use of vibratory signals for stink bug monitoring and control. In Stink Bugs: Biorational Control Based on Communication Processes; CRC Press: Boca Raton, FL, USA, 2017; pp. 226–245. [Google Scholar]
- Cocroft, R.B.; Rodríguez, R.L. The behavioral ecology of insect vibrational communication. BioScience 2005, 55, 323–334. [Google Scholar] [CrossRef]
- Claridge, M. Insect sounds and communication—An introduction. In Insect Sounds and Communication: Physiology, Behaviour, Ecology and Evolution, 1st ed.; Drosopoulos, S., Claridge, M.F., Eds.; CRC Press: Boca Raton, FL, USA, 2005; pp. 3–9. [Google Scholar]
- Hill, P.; Lakes-Harlan, R.; Mazzoni, V.; Narins, P.M.; Virant-Doberlet, M.; Wessel, A. (Eds.) Biotremology: Studying Vibrational Behavior; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; Volume 8, pp. 3–49. [Google Scholar]
- Hill, P.S.M. Vibration and animal communication: A Review. Am. Zool. 2001, 41, 1135–1142. [Google Scholar] [CrossRef]
- Čokl, A.; Žunič-Kosi, A.; Virant-Doberlet, M. Stink bug communication network and environment. In Stink Bugs: Biorational Control Based on Communication Processes; Čokl, A., Borges, M., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 165–179. [Google Scholar]
- Čokl, A.; Žunič-Kosi, A.; Stritih-Peljhan, N.; Blassioli-Moraes, M.C.; Laumann, R.A.; Borges, M. Stink bug communication and signal detection in a plant environment. Insects 2021, 12, 1058. [Google Scholar] [CrossRef]
- Čokl, A.; Žunič-Kosi, A.; Laumann, R.A. Stink bug communication with multimodal signals transmitted through air and substrate. Emerg. Sci. J. 2019, 3, 407–424. [Google Scholar] [CrossRef]
- Borges, M.; Jepson, P.C.; Howse, P.E. Long-range mate location and close-range courtship behaviour of the Green stink bug, Nezara viridula and its mediation by sex pheromones. Entomol. Exp. Appl. 1987, 44, 205–212. [Google Scholar] [CrossRef]
- Čokl, A.; Virant-Doberlet, M. Communication with substrate-borne signals in small plant-dwelling insects. Annu. Rev. Entomol. 2003, 48, 29–50. [Google Scholar] [CrossRef]
- Čokl, A.; Virant-Doberlet, M.; McDowell, A. Vibrational directionality in the southern green stink bug, Nezara viridula (L.), is mediated by female song. Anim. Behav. 1999, 58, 1277–1283. [Google Scholar] [CrossRef]
- Ota, D.; Čokl, A. Mate location in the southern green stink bug, Nezara viridula (Heteroptera: Pentatomidae), mediated through substrate-borne signals on ivy. J. Insect Behav. 1991, 4, 441–447. [Google Scholar] [CrossRef]
- Čokl, A.; Virant-Doberlet, M.; Stritih, N. The structure and function of songs emitted by southern green stink bugs from Brazil, Florida, Italy and Slovenia. Physiol. Entomol. 2000, 25, 196–205. [Google Scholar] [CrossRef]
- Čokl, A.; Virant-Doberlet, M.; Zorovic, M. Sense organs involved in the vibratory communication of bugs. In Insect Sounds and Communication; Drosopoulos, S., Claridge, M.F., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2005; pp. 71–80. [Google Scholar]
- Čokl, A.; Zorović, M.; Millar, J.G. Vibrational communication along plants by the stink bugs Nezara viridula and Murgantia histrionica. Behav. Process. 2007, 75, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Čokl, A.A.; Millar, J.G. Manipulation of insect signaling for monitoring and control of pest insects. In Biorational Control of Arthropod Pests: Application and Resistance Management; Ishaaya, I., Horowitz, R.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 279–316. [Google Scholar]
- Polajnar, J.; Čokl, A. The effect of vibratory disturbance on sexual behaviour of the southern green stink bug Nezara viridula (Heteroptera, Pentatomidae). Open Life Sci. 2008, 3, 189–197. [Google Scholar] [CrossRef]
- Čokl, A.; Laumann, R.A.; Žunič Kosi, A.; Blassioli-Moraes, M.C.; Virant-Doberlet, M.; Borges, M. Interference of overlapping insect vibratory communication signals: An Eushistus heros model. PLoS ONE 2015, 10, e0130775. [Google Scholar] [CrossRef]
- De Groot, M.; Čokl, A.; Virant-Doberlet, M. Effects of heterospecific and conspecific vibrational signal overlap and signal-to-noise ratio on male responsiveness in Nezara viridula (L.). J. Exp. Biol. 2010, 213, 3213–3222. [Google Scholar] [CrossRef]
- Miklas, N.; Čokl, A.; Renou, M.; Virant-Doberlet, M. Variability of vibratory signals and mate choice selectivity in the southern green stink bug. Behav. Process. 2003, 61, 131–142. [Google Scholar] [CrossRef]
- Mazzoni, V.; Nieri, R.; Eriksson, A.; Virant-Doberlet, M.; Polajnar, J.; Anfora, G.; Lucchi, A. Mating disruption by vibrational signals: State of the field and perspectives. In Biotremology: Studying Vibrational Behavior; Animal Signals and Communication; Hill, P.S.M., Lakes-Harlan, R., Mazzoni, V., Narins, P.M., Virant-Doberlet, M., Wessel, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; Volume 6, p. 331. [Google Scholar]
- Laumann, R.A.; Maccagnan, D.H.B.; Čokl, A.; Blassioli-Moraes, M.C.; Borges, M. Substrate-borne vibrations disrupt the mating behaviors of the neotropical brown stink bug, Euschistus heros: Implications for pest management. J. Pest Sci. 2018, 91, 995–1004. [Google Scholar] [CrossRef]
- Dias, A.M.; Borges, M.; Blassioli Moraes, M.C.; Lorran Figueira Coelho, M.; Čokl, A.; Laumann, R.A. Inhibitory copulation effect of vibrational rival female signals of three stink bug species as a tool for mating disruption. Insects 2021, 12, 177. [Google Scholar] [CrossRef]
- Laumann, R.A.; Moraes, M.C.B.; Čokl, A.; Borges, M. Eavesdropping on sexual vibratory signals of stink bugs (Hemiptera: Pentatomidae) by the egg parasitoid Telenomus podisi. Anim. Behav. 2007, 73, 637–649. [Google Scholar] [CrossRef]
- Laumann, R.A.; Čokl, A.; Lopes, A.P.S.; Fereira, J.B.C.; Moraes, M.C.B.; Borges, M. Silent singers are not safe: Selective response of a parasitoid to substrate-borne vibratory signals of stink bugs. Anim. Behav. 2011, 82, 1175–1183. [Google Scholar] [CrossRef]
- Kaplan, I. Attracting carnivorous arthropods with plant volatiles: The future of biocontrol or playing with fire? Biol. Control 2012, 60, 77–89. [Google Scholar] [CrossRef]
- Mankin, R.W.; Hagstrum, D.W.; Smith, M.T.; Roda, A.L.; Kairo, M.T.K. Perspective and promise: A century of insect acoustic detection and monitoring. Am. Entomol. 2011, 57, 30–44. [Google Scholar] [CrossRef]
- Mankin, R.W. Applications of acoustics in insect pest management. CAB Rev. 2012, 7, 1–7. [Google Scholar] [CrossRef]
- Ganchev, T.; Potamitis, I. Automatic acoustic identification of singing insects. Bioacoustics 2007, 16, 281–328. [Google Scholar] [CrossRef]
- Pinhas, J.; Soroker, V.; Hetzroni, A.; Mizrach, A.; Teicher, M.; Goldberger, J. Automatic acoustic detection of the red palm weevil. Comput. Electron. Agric. 2008, 63, 131–139. [Google Scholar] [CrossRef]
- Korinšek, G.; Derlink, M.; Virant-Doberlet, M.; Tuma, T. An autonomous system of detecting and attracting leafhopper males using species- and sex-specific substrate borne vibrational signals. Comput. Electron. Agric. 2016, 123, 29–39. [Google Scholar] [CrossRef]
- Lampson, B.D.; Han, Y.J.; Khalilian, A.; Greene, J.; Mankin, R.W.; Foreman, E.G. Automatic detection and identification of brown stink bug, Euschistus servus, and southern green stink bug, Nezara viridula, (Heteroptera: Pentatomidae) using intraspecific substrate-borne vibrational signals. Comput. Electron. Agric. 2013, 91, 154–159. [Google Scholar] [CrossRef]
- Millar, J.G. Pheromones of True Bugs. In The Chemistry of Pheromones and Other Semiochemicals II; Schulz, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 37–84. [Google Scholar] [CrossRef]
- Moraes, M.C.B.; Pareja, M.; Laumann, R.A.; Borges, M. The chemical volatiles (semiochemicals) produced by neotropical stink bugs (Hemiptera: Pentatomidae). Neotrop. Entomol. 2008, 37, 489–505. [Google Scholar] [CrossRef]
- Leal, W.S.; Kuwahara, S.; Shi, X.; Higuchi, H.; Marino, C.E.B.; Ono, M.; Meinwald, J. Male-released sex pheromone of the stink bug Piezodorus hybneri. J. Chem. Ecol. 1998, 24, 1817–1829. [Google Scholar] [CrossRef]
- Endo, N.; Sasaki, R.; Muto, S. Pheromonal cross-attraction in true bugs (Heteroptera): Attraction of Piezodorus hybneri (Pentatomidae) to its pheromone versus the pheromone of Riptortus pedestris (Alydidae). Environ. Entomol. 2010, 39, 1973–1979. [Google Scholar] [CrossRef] [PubMed]
- McBrien, H.L.; Millar, J.G.; Rice, R.E.; McElfresh, J.S.; Cullen, E.; Zalom, F.G. Sex attractant pheromone of the red-shouldered stink bug Thyanta pallidovirens: A pheromone blend with multiple redundant components. J. Chem. Ecol. 2002, 28, 1797–1818. [Google Scholar] [CrossRef] [PubMed]
- Moraes, M.C.; Millar, J.G.; Laumann, R.A.; Sujii, E.R.; Pires, C.S.; Borges, M. Sex attractant pheromone from the neotropical red-shouldered stink bug, Thyanta perditor (F.). J. Chem. Ecol. 2005, 31, 1415–1427. [Google Scholar] [CrossRef] [PubMed]
- Aldrich, J.R.; Oliver, J.E.; Lusby, W.R.; Kochansky, J.P.; Borges, M. Identification of male-specific volatiles from nearctic and neotropical stink bugs (Heteroptera: Pentatomidae). J. Chem. Ecol. 1994, 20, 1103–1111. [Google Scholar] [CrossRef]
- Borges, M.; Aldrich, J.R. Attractant pheromone for nearctic stink bug, Euschistus obscurus (Heteroptera: Pentatomidae): Insight into a neotropical relative. J. Chem. Ecol. 1994, 20, 1095–1102. [Google Scholar] [CrossRef]
- Borges, M.; Mori, K.; Costa, M.L.M.; Sujii, E.R. Behavioural evidence of methyl-2,6,10-trimethyltridecanoate as a sex pheromone of Euschistus heros (Het., Pentatomidae). J. Appl. Entomol. 1998, 122, 335–338. [Google Scholar] [CrossRef]
- Fávaro, C.F.; Santos, T.B.; Zarbin, P.H.G. Defensive compounds and male-produced sex pheromone of the stink bug, Agroecus griseus. J. Chem. Ecol. 2012, 38, 1124–1132. [Google Scholar] [CrossRef]
- Zarbin, P.H.G.; Fávaro, C.F.; Vidal, D.M.; Rodrigues, M.A.C.M. Male-produced sex pheromone of the stink bug Edessa meditabunda. J. Chem. Ecol. 2012, 38, 825–835. [Google Scholar] [CrossRef]
- Borges, M.; Birkett, M.; Aldrich, J.R.; Oliver, J.E.; Chiba, M.; Murata, Y.; Laumann, R.A.; Barrigossi, J.A.; Pickett, J.A.; Moraes, M.C.B. Sex attractant pheromone from the rice stalk stink bug, Tibraca limbativentris Stal. J. Chem. Ecol. 2006, 32, 2749–2761. [Google Scholar] [CrossRef]
- Blassioli-Moraes, M.C.; Khrimian, A.; Michereff, M.F.F.; Magalhães, D.M.; Hickel, E.; De Freitas, T.F.S.; Barrigossi, J.A.F.; Laumann, R.A.; Silva, A.T.; Guggilapu, S.D.; et al. Male-produced sex pheromone of Tibraca limbativentris revisited: Absolute configurations of zingiberenol stereoisomers and their influence on chemotaxis behavior of conspecific females. J. Chem. Ecol. 2020, 46, 1–9. [Google Scholar] [CrossRef]
- De Freitas, T.F.S.; Hickel, E.R.; Khrimian, A.; Borges, M.; Michereff, M.F.F.; Barrigossi, J.A.; Laumann, R.A.; Guggilapu, S.D.; Sant’Ana, J.; Blassioli-Moraes, M.C. Field responses of rice stalk stink bug, Tibraca limbativentris, to synthetic sex pheromone and isomers of 1,10-Bisaboladien-3-ol. Neotrop. Entomol. 2021, 50, 282–288. [Google Scholar] [CrossRef]
- De Oliveira, M.W.M.; Borges, M.; Andrade, C.K.Z.; Laumann, R.A.; Barrigossi, J.A.F.; Blassioli-Moraes, M.C. Zingiberenol, (1S,4R,1′S)-4-(1′,5-Dimethylhex-4′-enyl)-1 methylcyclohex-2-en-1-ol, identified as the sex pheromone produced by males of the rice stink bug Oebalus poecilus (Heteroptera: Pentatomidae). J. Agric. Food Chem. 2013, 61, 7777–7785. [Google Scholar] [CrossRef] [PubMed]
- Prestwich, G.D. Composition of the scents of eight east african hemipterans. nymph-adult chemical polymorphism in Coreids. Ann. Entomol. Soc. Am. 1976, 69, 812–814. [Google Scholar] [CrossRef]
- Gunawardena, N.E.; Herath, H.M.W.K.B. Significance of medium chainn-alkanes as accompanying compounds in hemipteran defensive secretions: An investigation based on the defensive secretion of Coridius janus. J. Chem. Ecol. 1991, 17, 2449–2458. [Google Scholar] [CrossRef]
- Sagar, C.V.; Janaiah, C.; Narender, E.R. Volatile constituents of metathoracic scent secretions of adult Cyclopelta siccifolia Westwood (Hemiptera: Pentatomidae). Entomologia 2000, 25, 39–46. [Google Scholar]
- Meneses-Arias, M.G.; Cruz-López, L.; Huerta, G.; Rojas, J.C. Volatile compounds emitted by the stink bug Antiteuchus innocens (Hemiptera: Pentatomidae). Fla. Entomol. 2019, 102, 431. [Google Scholar] [CrossRef]
- Nagnan, P.; Cassier, P.; Andre, M.; Llosa, J.F.; Guillaumin, D. Fine structure and physicochemical analysis of the metathoracic scent glands of Lincus malevolus (Rolston) and L. spurcus (Rolston) (Heteroptera: Pentatomidae). Int. J. Insect Morphol. Embryol. 1994, 23, 355–370. [Google Scholar] [CrossRef]
- Cassier, P.; Nagnan, P.; Llosa, J.F.; Andre, M.; Guillaumin, D. Fine structure and physicochemical analysis of the nymphal and imaginal scent gland systems of Lincus spurcus (Rolston) (Heteroptera: Pentatomidae). Int. J. Insect Morphol. Embryol. 1994, 23, 371–382. [Google Scholar] [CrossRef]
- Szczerbowski, D.; Schulz, S.; Zarbin, P.H.G. Total synthesis of four stereoisomers of methyl 4,8,12-trimethylpentadecanoate, a major component of the sex pheromone of the stink bug Edessa meditabunda. Org. Biomol. Chem. 2020, 18, 5034–5044. [Google Scholar] [CrossRef] [PubMed]
- Borges, M.; Aldrich, J.R. Instar-specific defensive secretions of stink bugs (Heteroptera: Pentatomidae). Experientia 1992, 48, 893–896. [Google Scholar] [CrossRef]
- Howard, J.J.; Wiemer, D.F. The defensive secretion of Edessa rufomarginata. Naturwissenschaften 1983, 70, 202–203. [Google Scholar] [CrossRef]
- Tsuyuki, T.; Ogata, Y.; Yamamoto, I.; Shimi, K. Stink bug aldehydes. Agric. Biol. Chem. 1965, 29, 419–427. [Google Scholar] [CrossRef]
- Sugie, H.; Yoshida, M.; Kawasaki, K.; Noguchi, H.; Moriya, S.; Takagi, K.; Fukuda, H.; Fujiie, A.; Yamanaka, M.; Ohira, Y.; et al. Identification of the Aggregation Pheromone of the brown-winged green bug, Plautia stali Scott (Heteroptera: Pentatomidae). Appl. Entomol. Zool. 1996, 31, 427–431. [Google Scholar] [CrossRef]
- Millar, J.G. Methyl (2E,4Z,6Z)-deca-2,4,6-trienoate, a thermally unstable, sex-specific compound from the stink bug Thyanta pallidovirens. Tetrahedron Lett. 1997, 38, 7971–7972. [Google Scholar] [CrossRef]
- Fucarino, A.; Millar, J.G.; McElfresh, J.S.; Colazza, S. Chemical and physical signals mediating conspecific and heterospecific aggregation behavior of first instar stink bugs. J. Chem. Ecol. 2004, 30, 1257–1269. [Google Scholar] [CrossRef] [PubMed]
- Pal, E.; Allison, J.; Guignard, Q.; Hurley, B.P.; Slippers, B.; Fourie, G. Characterisation of the alarm pheromone of Bathycoelia distincta (Pentatomidae). J. Chem. Ecol. 2022, 48, 791–801. [Google Scholar] [CrossRef]
- Harris, C.; Abubeker, S.; Yu, M.; Leskey, T.; Zhang, A. Semiochemical production and laboratory behavior response of the brown marmorated stink bug, Halyomorpha halys. PLoS ONE 2015, 10, e0140876. [Google Scholar] [CrossRef]
- Khrimian, A.; Zhang, A.; Weber, D.C.; Ho, H.-Y.; Aldrich, J.R.; Vermillion, K.E.; Siegler, M.A.; Shirali, S.; Guzman, F.; Leskey, T.C. Discovery of the aggregation pheromone of the brown marmorated stink bug (Halyomorpha halys) through the creation of stereoisomeric libraries of 1-bisabolen-3-ols. J. Nat. Prod. 2014, 77, 1708–1717. [Google Scholar] [CrossRef]
- Fraga, D.F.; Parker, J.; Busoli, A.C.; Hamilton, G.C.; Nielsen, A.L.; Rodriguez-Saona, C. Behavioral responses of predaceous minute pirate bugs to tridecane, a volatile emitted by the brown marmorated stink bug. J. Pest Sci. 2017, 90, 1107–1118. [Google Scholar] [CrossRef]
- Nixon, L.J.; Morrison, W.R.; Rice, K.B.; Brockerhoff, E.G.; Leskey, T.C.; Guzman, F.; Khrimian, A.; Goldson, S.; Rostás, M. Identification of volatiles released by diapausing brown marmorated stink bug, Halyomorpha halys (Hemiptera: Pentatomidae). PLoS ONE 2018, 13, e0191223. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.Z.; Zhang, J.P.; Ren, L.L.; Tang, R.; Zhan, H.X.; Chen, G.H.; Zhang, F. Behavioral responses of the egg parasitoid Trissolcus japonicus to volatiles from adults of its stink bug host, Halyomorpha halys. J. Pest Sci. 2017, 90, 1097–1105. [Google Scholar] [CrossRef]
- Solomon, D.; Dutcher, D.; Raymond, T. Characterization of Halyomorpha halys (brown marmorated stink bug) biogenic volatile organic compound emissions and their role in secondary organic aerosol formation. J. Air Waste Manag. Assoc. 2013, 63, 1264–1269. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.-Z.; Tang, R.; Zhang, J.-P.; Yang, S.-Y.; Chen, G.-H.; He, K.-L.; Wang, Z.-Y.; Zhang, F. Behavioral Evidence and olfactory reception of a single alarm pheromone component in Halyomorpha halys. Front. Physiol. 2018, 9, 1610. [Google Scholar] [CrossRef]
- Fávaro, C.F.; Zarbin, P.H.G. Identification of (Z)-4- and 1-tridecene in the metathoracic gland secretions of stink bugs employing the GC/FT-IR technique. J. Chem. Ecol. 2013, 39, 1182–1185. [Google Scholar] [CrossRef]
- Durak, D.; Kalender, Y. Structure and chemical analysis of the metathoracic scent glands of Carpocoris fuscispinus (Boheman, 1851) (Heteroptera: Pentatomidae) from Turkey. Turk. J. Zool. 2012, 36, 526–533. [Google Scholar] [CrossRef]
- Krall, B.S.; Bartelt, R.J.; Lewis, C.J.; Whitman, D.W. Chemical defense in the stink bug Cosmopepla bimaculata. J. Chem. Ecol. 1999, 25, 2477–2494. [Google Scholar] [CrossRef]
- Noge, K.; Prudic, K.L.; Becerra, J.X. Defensive roles of (E)-2-alkenals and related compounds in heteroptera. J. Chem. Ecol. 2012, 38, 1050–1056. [Google Scholar] [CrossRef]
- Aldrich, J.R.; Hoffmann, M.P.; Kochansky, J.P.; Lusby, W.R.; Eger, J.E.; Payne, J.A. Identification and attractiveness of a major pheromone component for nearctic Euschistus spp. stink bugs (Heteroptera: Pentatomidae). Environ. Entomol. 1991, 20, 477–483. [Google Scholar] [CrossRef]
- Borges, M.; Schmidt, F.G.V.; Sujii, E.R.; Medeiros, M.A.; Mori, K.; Zarbin, P.H.G.; Ferreira, J.T.B. Field responses of stink bugs to the natural and synthetic pheromone of the Neotropical brown stink bug, Euschistus heros (Heteroptera: Pentatomidae). Physiol. Entomol. 1998, 23, 202–207. [Google Scholar] [CrossRef]
- Pareja, M.; Borges, M.; Laumann, R.A.; Moraes, M.C.B. Inter- and intraspecific variation in defensive compounds produced by five neotropical stink bug species (Hemiptera: Pentatomidae). J. Insect Physiol. 2007, 53, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Lopes, R.B.; Laumann, R.A.; Blassioli-Moraes, M.C.; Borges, M.; Faria, M. The fungistatic and fungicidal effects of volatiles from metathoracic glands of soybean-attacking stink bugs (Heteroptera: Pentatomidae) on the entomopathogen Beauveria bassiana. J. Invertebr. Pathol. 2015, 132, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Lima, B.S.A.; Martínez, L.C.; Plata-Rueda, A.; Dos Santos, M.H.; De Oliveira, E.E.; Zanuncio, J.C.; Serrão, J.E. Interaction between predatory and phytophagous stink bugs (Heteroptera: Pentatomidae) promoted by secretion of scent glands. Chemoecology 2021, 31, 209–219. [Google Scholar] [CrossRef]
- Aldrich, J.R.; Rosi, M.C.; Bin, F. Behavioral correlates for minor volatile compounds from stink bugs (Heteroptera: Pentatomidae). J. Chem. Ecol. 1995, 21, 1907–1920. [Google Scholar] [CrossRef] [PubMed]
- Marques, F.A.; Wendler, E.P.; Maia, B.H.L.N.S.; Ventura, M.U.; Arruda-Gatti, I.C. Identification of defensive compounds in metathoracic glands of adults of the stink bug Dichelops melacanthus (Hemiptera: Pentatomidae). J. Braz. Chem. Soc. 2007, 18, 1242–1246. [Google Scholar] [CrossRef]
- Yang, C.Y.; Seo, M.H.; Lee, S.C. Male-produced aggregation pheromone of the sloe bug, Dolycoris baccarum L. (Hemiptera: Heteroptera: Pentatomidae). J. Chem. Ecol. 2019, 45, 818–822. [Google Scholar] [CrossRef]
- Durak, D. Morphology and chemical composition of metathoracic scent glands in Dolycoris baccarum (Linnaeus, 1758) (Heteroptera: Pentatomidae). Acta Zool. 2008, 89, 193–199. [Google Scholar] [CrossRef]
- Schildknecht, H.; Holoubek, K.; Weis, K.H.; Krämer, H. Defensive substances of the arthropods, their isolation and identification. Angew. Chem. Int. Ed. 1964, 3, 73–82. [Google Scholar] [CrossRef]
- Moliterno, A.A.C.; De Melo, D.J.; Zarbin, P.H.G. Identification of zingiberenol and murgantiol as components of the aggregation-sex pheromone of the rice stink bug, Mormidea v-luteum (Heteroptera: Pentatomidae). J. Chem. Ecol. 2020, 47, 1–9. [Google Scholar] [CrossRef]
- Blum, M.S.; Traynham, J.G.; Chidester, J.B.; Boggus, J.D. n-Tridecane and trans-2-heptenal in scent gland of the rice stink bug Oebalus pugnax (F.). Science 1960, 132, 1480–1481. [Google Scholar] [CrossRef]
- Mori, K.; Tashiro, T.; Yoshimura, T.; Takita, M.; Tabata, J.; Hiradate, S.; Sugie, H. Determination of the absolute configuration of the male aggregation pheromone, 2-methyl-6-(4′-methylenebicyclo[3.1.0]hexyl)hept-2-en-1-ol, of the stink bug Erysarcoris lewisi (Distant) as 2Z,6R,1′S,5′S by its synthesis. Tetrahedron Lett. 2008, 49, 354–357. [Google Scholar] [CrossRef]
- Takita, M.; Sugie, H.; Tabata, J.; Ishii, S.; Hiradate, S. Isolation and estimation of the aggregation pheromone from Eysarcoris lewisi (Distant) (Heteroptera: Pentatomidae). Appl. Entomol. Zool. 2008, 43, 11–17. [Google Scholar] [CrossRef]
- Alizadeh, B.H.; Kuwahara, S.; Leal, W.S.; Men, H.-C. Synthesis of the racemate of (Z)-exo-α-bergamotenal, a pheromone component of the white-spotted spined bug, Eysarcoris parvus Uhler. Biosci. Biotechnol. Biochem. 2002, 66, 1415–1418. [Google Scholar] [CrossRef]
- Everton, I.J.; Games, D.E.; Staddon, B.W. Composition of scents from Apodiphus amygdali. Ann. Entomol. Soc. Am. 1974, 67, 815–816. [Google Scholar] [CrossRef]
- Blum, M.S. The presence of 2-hexenal in the scent gland of the pentatomid Brochymena quadripustulata. Ann. Entomol. Soc. Am. 1961, 54, 410–412. [Google Scholar] [CrossRef]
- Kou, R.; Tang, D.S.; Chow, Y.S. Alarm pheromone of pentatomid bug, Erthesina fullo Thunberg (Hemiptera: Pentatomidae). J. Chem. Ecol. 1989, 15, 2695–2702. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, D.F.; Forss, D.A.; Hackman, R.H. Characteristic odour components of the scent of stink bugs. J. Insect Physiol. 1961, 6, 113–121. [Google Scholar] [CrossRef]
- Aldrich, J.R.; Numata, H.; Borges, M.; Bin, F.; Waite, G.K.; Lusby, W.R. Artifacts and pheromone blends from Nezara spp. and other stink bugs (Heteroptera: Pentatomidae). Z. Natforsch. C 1993, 48, 73–79. [Google Scholar] [CrossRef]
- McBrien, H.L.; Millar, J.G.; Gottlieb, L.; Chen, X.; Rice, R.E. Male-produced sex attractant pheromone of the green stink bug, Acrosternum hilare (Say). J. Chem. Ecol. 2001, 27, 1821–1839. [Google Scholar] [CrossRef]
- Blassioli-Moraes, M.C.; Laumann, R.A.; Oliveira, M.W.M.; Woodcock, C.M.; Mayon, P.; Hooper, A.; Pickett, J.A.; Birkett, M.A.; Borges, M. Sex pheromone communication in two sympatric neotropical stink bug species Chinavia ubica and Chinavia impicticornis. J. Chem. Ecol. 2012, 38, 836–845. [Google Scholar] [CrossRef]
- Aldrich, J.R.; Lusby, W.R.; Marron, B.E.; Nicolaou, K.C.; Hoffmann, M.P.; Wilson, L.T. Pheromone blends of green stink bugs and possible parasitoid selection. Naturwissenschaften 1989, 76, 173–175. [Google Scholar] [CrossRef]
- Millar, J.G.; McBrien, H.M.; McElfresh, J.S. Field trials of aggregation pheromones for the stink bugs Chlorochroa uhleri and Chlorochroa sayi (Hemiptera: Pentatomidae). J. Econ. Entomol. 2010, 103, 1603–1612. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.-Y.; Millar, J.G. Identification and synthesis of male-produced sex pheromone components of the stink bugs Chlorochroa ligata and Chlorochroa uhleri. J. Chem. Ecol. 2001, 27, 2067–2095. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.Y.; Millar, J.G. Compounds in metathoracic glands of adults and dorsal abdominal glands of nymphs of the stink bugs, Chlorochroa uhleri, C. sayi, and C. ligata (Hemiptera: Pentatomidae). Zool. Stud. 2001, 40, 193–198. [Google Scholar]
- Ho, H.Y.; Millar, J.G. Identification and synthesis of a male-produced sex pheromone from the stink bug Chlorochroa sayi. J. Chem. Ecol. 2001, 27, 1177–1201. [Google Scholar] [CrossRef]
- Aldrich, J.R.; Oliver, J.E.; Lusby, W.R.; Kochansky, J.P.; Lockwood, J.A. Pheromone strains of the Cosmopolitan pest, Nezara viridula (Heteroptera: Pentatomidae). J. Exp. Zool. 1987, 244, 171–175. [Google Scholar] [CrossRef]
- Baker, R.; Borges, M.; Cooke, N.G.; Herbert, R.H. Identification and synthesis of (Z)-(1′S,3′R,4′S)(-)-2-(3′,4′-epoxy-4′-methylcyclohexyl)-6-methylhepta-2,5-diene, the sex pheromone of the southern green stinkbug, Nezara viridula (L.). J. Chem. Soc. Chem. Commun. 1987, 414–416. [Google Scholar] [CrossRef]
- Brézot, P.; Malosse, C.; Mori, K.; Renou, M. Bisabolene epoxides in sex pheromone in Nezara viridula (L.) (Heteroptera: Pentatomidae): Role of cis isomer and relation to specificity of pheromone. J. Chem. Ecol. 1994, 20, 3133–3147. [Google Scholar] [CrossRef]
- Borges, M. Attractant compounds of the southern green stink bug, Nezara viridula (L.) (Heteroptera: Pentatomidae). An. Soc. Entomol. Bras. 1995, 24, 215–225. [Google Scholar] [CrossRef]
- Lancaster, J.; Lehner, B.; Khrimian, A.; Muchlinski, A.; Luck, K.; Köllner, T.G.; Weber, D.C.; Gundersen-Rindal, D.E.; Tholl, D. An IDS-Type sesquiterpene synthase produces the pheromone precursor (Z)-α-bisabolene in Nezara viridula. J. Chem. Ecol. 2019, 45, 187–197. [Google Scholar] [CrossRef]
- Aldrich, J.R.; Blum, M.S.; Lloyd, H.A.; Fales, H.M. Pentatomid natural products—Chemistry and morphology of the III-IV dorsal abdominal glands of adults. J. Chem. Ecol. 1978, 4, 161–172. [Google Scholar] [CrossRef]
- Lockwood, J.A.; Story, R.N. Defensive secretion of the southern green stink bug (Hemiptera: Pentatomidae) as an alarm Pheromone. An. Soc. Entomol. Soc. Am. 1987, 80, 686–691. [Google Scholar] [CrossRef]
- Gilby, A.R.; Waterhouse, D.F. The composition of the scent of the green vegetable bug, Nezara viridula. Proc. R. Soc. B 1965, 162, 105–120. [Google Scholar] [CrossRef]
- Pavis, C.; Malosse, C.; Ducrot, P.H.; Descoins, C. Dorsal abdominal glands in nymphs of southern green stink bug, Nezara viridula (L.) (heteroptera: Pentatomidae): Chemistry of secretions of five instars and role of (E)-4-oxo-2-decenal, compound specific to first instars. J. Chem. Ecol. 1994, 20, 2213–2227. [Google Scholar] [CrossRef]
- Ishiwatari, T. Studies on the scent of stink bugs (Hemiptera: Pentatomidae): I. Alarm pheromone activity. Appl. Entomol. Zool. 1974, 9, 153–158. [Google Scholar] [CrossRef]
- Fávaro, C.F.; Soldi, R.A.; Ando, T.; Aldrich, J.R.; Zarbin, P.H. (6R,10S)-Pallantione: The first ketone identified as sex pheromone in stink bugs. Org. Lett. 2013, 15, 1822–1825. [Google Scholar] [CrossRef] [PubMed]
- Fávaro, C.F.; Rodrigues, M.A.C.D.M.; Aldrich, J.R.; Zarbin, P.H.G. Identification of semiochemicals in adults and nymphs of the stink bug Pallantia macunaima Grazia (Hemiptera: Pentatomidae). J. Braz. Chem. Soc. 2011, 22, 58–64. [Google Scholar] [CrossRef]
- Fávaro, C.F.; Millar, J.G.; Zarbin, P.H.G. Identification and synthesis of the male-produced sex pheromone of the stink bug, Pellaea stictica. J. Chem. Ecol. 2015, 41, 859–868. [Google Scholar] [CrossRef]
- Gomes, C.M.B.; Souza, J.P.A.; Millar, J.G.; Zarbin, P.H.G. Determination of the absolute configuration of the male-produced sex pheromone of the stink bug Pellaea stictica, (2R,4R,8R)-2,4,8,13-tetramethyltetradecan-1-ol by stereoselective synthesis coupled with enantiomeric resolution. J. Chem. Ecol. 2022, 48, 502–517. [Google Scholar] [CrossRef]
- Durak, D.; Kalender, Y. Scanning electron microscopic and chemical study of the metathoracic scent glands system of Rhaphigaster nebulosa (Poda, 1761) (Heteroptera: Pentatomidae). J. Entomol. Res. 2009, 11, 21–29. [Google Scholar]
- Borges, M.; Millar, J.G.; Laumann, R.A.; Moraes, M.C.B. A male-produced sex pheromone from the neotropical redbanded stink bug, Piezodorus guildinii (W.). J. Chem. Ecol. 2007, 33, 1235–1248. [Google Scholar] [CrossRef]
- Zarbin, P.H.G.; Borges, M.; Dos Santos, A.A.; De Oliveira, A.R.M.; Simonelli, F.; Marques, F.D.A. Alarm pheromone system of stink bug Piezodorus guildinii (Heteroptera: Pentatomidae). J. Braz. Chem. Soc. 2000, 11, 424–428. [Google Scholar] [CrossRef]
- Gilchrist, T.L.; Stansfield, F.; Cloudsley-Thompson, J.L. The odoriferous principle of Piezodorus teretipes (Stål) (Hemiptera: Pentatomoidea). Proc. R. Entomol. Soc. Lond. 1966, 41, 55–56. [Google Scholar] [CrossRef]
- Oliver, J.E. A male-produced pheromone of the spined citrus bug. Tetrahedron Lett. 1992, 33, 891–894. [Google Scholar] [CrossRef]
- James, D.G.; Heffer, R.; Amaike, M. Field attraction of Biprorulus bibax Breddin (Hemiptera: Pentatomidae) to synthetic aggregation pheromone and (E)-2-hexenal, a pentatomid defense chemical. J. Chem. Ecol. 1996, 22, 1697–1708. [Google Scholar] [CrossRef]
- James, D.G.; Mori, K.; Aldrich, J.R.; Oliver, J.E. Flight-mediated attraction of Biprorulus bibax breddin (Hemiptera: Pentatomidae) to natural and synthetic aggregation pheromone. J. Chem. Ecol. 1994, 20, 71–80. [Google Scholar] [CrossRef]
- Mori, K.; Amaike, M.; Oliver, J.E. Synthesis and absolute configuration of the hemiacetal pheromone of the spined citrus bug Biprorulus bibax. Liebigs Ann. Chem. 1992, 1992, 1185–1190. [Google Scholar] [CrossRef]
- Mori, K.; Amaike, M.; Watanabe, H. New synthesis and revision of the absolute configuration of the hemiacetal pheromone of the spined citrus bug Biprorulus bibax. Liebigs Ann. Chem. 1993, 1993, 1287–1294. [Google Scholar] [CrossRef]
- Macleod, J.K.; Howe, I.; Cable, J.; Blake, J.D.; Baker, J.T.; Smith, D. Volatile scent gland components of some tropical Hemiptera. J. Insect Physiol. 1975, 21, 1219–1224. [Google Scholar] [CrossRef]
- Smith, R.M. The defensive secretion of Vitellus insularis (Heteroptera: Pentatomidae). N. Z. J. Zool. 1974, 1, 375–376. [Google Scholar] [CrossRef]
- Arif, M.A.; Guarino, S.; Colazza, S.; Peri, E. The role of (E)-2-octenyl acetate as a pheromone of Bagrada hilaris (Burmeister): Laboratory and field evaluation. Insects 2020, 11, 109. [Google Scholar] [CrossRef] [PubMed]
- Guarino, S.; De Pasquale, C.; Peri, E.; Alonzo, G.; Colazza, S. Role of volatile and contact pheromones in the mating behaviour of Bagrada hilaris (Heteroptera: Pentatomidae). Eur. J. Entomol. 2008, 105, 613–617. [Google Scholar] [CrossRef]
- Aldrich, J.R.; Avery, J.W.; Lee, C.-J.; Graf, J.C.; Harrison, D.J.; Bin, F. Semiochemistry of cabbage bugs (Heteroptera: Pentatomidae: Eurydema and Murgantia). J. Entomol. Sci. 1996, 31, 172–182. [Google Scholar] [CrossRef]
- Ishiwatari, T. Studies on the scent of stink bugs (Hemiptera: Pentatomidae): II. Aggregation heromone activity. Appl. Entomol. Zool. 1976, 11, 38–44. [Google Scholar] [CrossRef]
- Khrimian, A.; Shirali, S.; Vermillion, K.E.; Siegler, M.A.; Guzman, F.; Chauhan, K.; Aldrich, J.R.; Weber, D.C. Determination of the stereochemistry of the aggregation pheromone of harlequin bug, Murgantia histrionica. J. Chem. Ecol. 2014, 40, 1260–1268. [Google Scholar] [CrossRef]
- Zahn, D.K.; Moreira, J.A.; Millar, J.G. Identification, synthesis, and bioassay of a male-specific aggregation pheromone from the harlequin bug, Murgantia histrionica. J. Chem. Ecol. 2008, 34, 238–251. [Google Scholar] [CrossRef]
- Lancaster, J.; Khrimian, A.; Young, S.; Lehner, B.; Luck, K.; Wallingford, A.; Ghosh, S.K.B.; Zerbe, P.; Muchlinski, A.; Marek, P.E.; et al. De novo formation of an aggregation pheromone precursor by an isoprenyl diphosphate synthase-related terpene synthase in the harlequin bug. Proc. Natl. Acad. Sci. USA 2018, 115, E8634–E8641. [Google Scholar] [CrossRef]
- Durak, D.; Kalender, Y. Fine structure and chemical analysis of the metathoracic scent gland secretion in Graphosoma lineatum (Linnaeus, 1758) (Heteroptera, Pentatomidae). C. R. Biol. 2009, 332, 34–42. [Google Scholar] [CrossRef]
- Šanda, M.; Žáček, P.; Streinz, L.; Dračínský, M.; Koutek, B. Profiling and characterization of volatile secretions from the European stink bug Graphosoma lineatum (Heteroptera: Pentatomidae) by two-dimensional gas chromatography/time-of-flight mass spectrometry. J. Chromatogr. B 2012, 881–882, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Durak, D.; Kalender, Y. Fine structure and chemical analysis of the metathoracic scent glands Graphosoma semipunctatum (Fabricius, 1775) (Heteroptera, Pentatomidae). J. Appl. Biol. Sci. 2007, 1, 43–50. [Google Scholar]
- Endo, N.; Yasuda, T.; Wada, T.; Muto, S.E.; Sasaki, R. Age-related and individual variation in male Piezodorus hybneri (Heteroptera: Pentatomidae) pheromones. Psyche 2012, 2012, 608572. [Google Scholar] [CrossRef]
- Miklas, N.; Renou, M.; Malosse, I.; Malosse, C. Repeatability of pheromone blend composition in individual males of the Southern green stink bug, Nezara viridula. J. Chem. Ecol. 2000, 26, 2473–2485. [Google Scholar] [CrossRef]
- Moraes, M.C.B.; Borges, M.; Pareja, M.; Vieira, H.G.; De Souza Sereno, F.T.P.; Laumann, R.A. Food and humidity affect sex pheromone ratios in the stink bug, Euschistus heros. Physiol. Entomol. 2008, 33, 43–50. [Google Scholar] [CrossRef]
- Ryan, M.A.; Moore, C.J.; Walter, G.H. Individual variation in pheromone composition in Nezara viridula (Heteroptera: Pentatomidae): How valid is the basis for designating “pheromone strains”? Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1995, 111, 189–193. [Google Scholar] [CrossRef]
- Cullen, E.M.; Zalom, F.G. Relationship between Euschistus conspersus (Hem., Pentatomidae) pheromone trap catch and canopy samples in processing tomatoes. J. Appl. Entomol. 2005, 129, 505–514. [Google Scholar] [CrossRef]
- Zapponi, L.; Nieri, R.; Zaffaroni-Caorsi, V.; Pugno, N.M.; Mazzoni, V. Vibrational calling signals improve the efficacy of pheromone traps to capture the brown marmorated stink bug. J. Pest Sci. 2023, 96, 587–597. [Google Scholar] [CrossRef]
- Mazzoni, V.; Polajnar, J.; Baldini, M.; Rossi Stacconi, M.V.; Anfora, G.; Guidetti, R.; Maistrello, L. Use of substrate-borne vibrational signals to attract the brown marmorated stink bug, Halyomorpha halys. J. Pest Sci. 2017, 90, 1219–1229. [Google Scholar] [CrossRef]
- Aldrich, J. Chemical ecology of the Heteroptera. Ann. Rev. Entomol. 1988, 33, 211–238. [Google Scholar] [CrossRef]
- Ho, H.Y.; Kou, R.; Tseng, H.K. Semiochemicals from the predatory stink bug Eocanthecona furcellata (Wolff): Components of metathoracic gland, dorsal abdominal gland, and sternal gland secretions. J. Chem. Ecol. 2003, 29, 2101–2114. [Google Scholar] [CrossRef] [PubMed]
- Pavis, C. Les sécrétions exocrines des hétéroptères point bibliographique. Agronomie 1987, 7, 547–561. [Google Scholar] [CrossRef]
- Staddon, B.W. The Scent Glands of Heteroptera. In Advances in Insect Physiology; Treherne, J.E., Berridge, M.J., Wigglesworth, V.B., Eds.; Academic Press: Cambridge, MA, USA, 1989; Volume 14, pp. 351–418. [Google Scholar]
- Kment, P.; Vilímová, J. Thoracic scent efferent system of Pentatomoidea (Hemiptera: Heteroptera): A review of terminology. Zootaxa 2010, 2706, 1–77. [Google Scholar] [CrossRef]
- Lewis, W.J.; Martin, W.R. Semiochemicals for use with parasitoids: Status and future. J. Chem. Ecol. 1990, 16, 3067–3089. [Google Scholar] [CrossRef] [PubMed]
- Powell, W.; Pickett, J.A. Manipulation of parasitoids for aphid pest management: Progress and prospects. Pest Manag. Sci. 2003, 59, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Colazza, S.; Salerno, G.; Wajnberg, E. Volatile and contact chemicals released by Nezara viridula (Heteroptera: Pentatomidae) have a kairomonal effect on the egg parasitoid Trissolcus basalis (Hymenoptera: Scelionidae). Biol. Control 1999, 16, 310–317. [Google Scholar] [CrossRef]
- Laumann, R.A.; Aquino, M.F.S.; Moraes, M.C.B.; Pareja, M.; Borges, M. Response of the egg parasitoids Trissolcus basalis and Telenomus podisi to compounds from defensive secretions of stink bugs. J. Chem. Ecol. 2009, 35, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Vieira, C.R.; Blassioli -Moraes, M.C.; Borges, M.; Pires, C.S.S.; Sujii, E.R.; Laumann, R.A. Field evaluation of (E)-2-hexenal efficacy for behavioral manipulation of egg parasitoids in soybean. BioControl 2014, 59, 525–537. [Google Scholar] [CrossRef]
- Mattiacci, L.; Vinson, S.B.; Williams, H.J.; Aldrich, J.R.; Bin, F. A long-range attractant kairomone for egg parasitoid Trissolcus basalis, isolated from defensive secretion of its host, Nezara viridula. J. Chem. Ecol. 1993, 19, 1167–1181. [Google Scholar] [CrossRef]
- Aldrich, J.R.; Khrimian, A.; Camp, M.J. Methyl 2,4,6-decatrienoates attract stink bugs and tachinid parasitoids. J. Chem. Ecol. 2007, 33, 801–815. [Google Scholar] [CrossRef]
- Aldrich, J.R.; Barros, T.M. Chemical attraction of male crab spiders (Araneae, Thomisidae) and kleptoparasitic flies (Diptera, Milichiidae and Chloropidae). J. Arachnol. 1995, 23, 212–214. [Google Scholar]
- Eisner, T.; Eisner, M.; Deyrup, M. Chemical attraction of kleptoparasitic flies to heteropteran insects caught by orb-weaving spiders. Proc. Natl. Acad. Sci. USA 1991, 88, 8194–8197. [Google Scholar] [CrossRef]
- Colazza, S.; Cusumano, A.; Lo Giudice, D.; Peri, E. Chemo-orientation responses in hymenopteran parasitoids induced by substrate-borne semiochemicals. BioControl 2014, 59, 1–17. [Google Scholar] [CrossRef]
- Moraes, M.C.B.; Pareja, M.; Laumann, R.A.; Hoffmann-Campo, C.B.; Borges, M. Response of the parasitoid Telenomus podisi to induced volatiles from soybean damaged by stink bug herbivory and oviposition. J. Plant Interact. 2008, 3, 111–118. [Google Scholar] [CrossRef]
- Conti, E.; Salerno, G.; Bin, F.; Williams, H.J.; Vinson, S.B. Chemical cues from Murgantia histrionica eliciting host location and recognition in the egg parasitoid Trissolcus brochymenae. J. Chem. Ecol. 2003, 29, 115–130. [Google Scholar] [CrossRef]
- Silva, C.C.; Moraes, M.C.B.; Laumann, R.A.; Borges, M. Sensory response of the egg parasitoid Telenomus podisi to stimuli from the bug Euschistus heros. Pesqui. Agropecu. Bras. 2006, 41, 1093–1098. [Google Scholar] [CrossRef]
- Borges, M.; Colazza, S.; Ramirez-Lucas, P.; Chauhan, K.R.; Moraes, M.C.B.; Richard Aldrich, J. Kairomonal effect of walking traces from Euschistus heros (Heteroptera: Pentatomidae) on two strains of Telenomus podisi (Hymenoptera: Scelionidae). Physiol. Entomol. 2003, 28, 349–355. [Google Scholar] [CrossRef]
- Boyle, S.M.; Weber, D.C.; Hough-Goldstein, J.; Hoelmer, K.A. Host kairomones influence searching behavior of Trissolcus japonicus (Hymenoptera: Scelionidae), a parasitoid of Halyomorpha halys (Heteroptera: Pentatomidae). Environ. Entomol. 2020, 49, 15–20. [Google Scholar] [CrossRef] [PubMed]
- de Aquino, M.F.S.; Dias, A.M.; Borges, M.; Moraes, M.C.B.; Laumann, R.A. Influence of visual cues on host-searching and learning behaviour of the egg parasitoids Telenomus podisi and Trissolcus basalis. Entomol. Exp. Appl. 2012, 145, 162–174. [Google Scholar] [CrossRef]
- Pålsson, J.; Porcel, M.; Dekker, T.; Tasin, M. Attract, reward and disrupt: Responses of pests and natural enemies to combinations of habitat manipulation and semiochemicals in organic apple. J. Pest Sci. 2022, 95, 619–631. [Google Scholar] [CrossRef]
- Simpson, M.; Gurr, G.M.; Simmons, A.T.; Wratten, S.D.; James, D.G.; Leeson, G.; Nicol, H.I.; Orre-Gordon, G.U.S. Attract and reward: Combining chemical ecology and habitat manipulation to enhance biological control in field crops. J. Appl. Ecol. 2011, 48, 580–590. [Google Scholar] [CrossRef]
- Fountain, M.T.; Deakin, G.; Farman, D.; Hall, D.; Jay, C.; Shaw, B.; Walker, A. An effective ‘push–pull’ control strategy for European tarnished plant bug, Lygus rugulipennis (Heteroptera: Miridae), in strawberry using synthetic semiochemicals. Pest Manag. Sci. 2021, 77, 2747–2755. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Han, S.; Wu, Z.; Pan, C.; Wang, M.; Tang, Y.; Zhang, Q.-H.; Tan, G.; Han, B. A push–pull strategy for controlling the tea green leafhopper (Empoasca flavescens F.) using semiochemicals from Tagetes erecta and Flemingia macrophylla. Pest Manag. Sci. 2022, 78, 2161–2172. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, X.; Luo, Z.; Gao, Y.; Chen, Z. The manipulation mechanism of “push–pull” habitat management strategy and advances in its application. Acta Ecol. Sin. 2013, 33, 94–101. [Google Scholar] [CrossRef]
- Wertheim, B.; Van Baalen, E.J.A.; Dicke, M.; Vet, L.E.M. Pheromone-mediated aggregation in nonsocial arthropods: An evolutionary ecological perspective. Ann. Rev. Entomol. 2005, 50, 321–346. [Google Scholar] [CrossRef]
- Toyama, M.; Ihara, F.; Yaginuma, K. Formation of aggregations in adults of the brown marmorated stink bug, Halyomorpha halys (Stal) (Heteroptera: Pentatomidae): The role of antennae in short-range locations. Appl. Entomol. Zool. 2006, 41, 309–315. [Google Scholar] [CrossRef]
- Bedoya, C.L.; Brockerhoff, E.G.; Hayes, M.; Leskey, T.C.; Morrison, W.R.; Rice, K.B.; Nelson, X.J. Brown marmorated stink bug overwintering aggregations are not regulated through vibrational signals during autumn dispersal. R. Soc. Open Sci. 2020, 7, 201371. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.M. The Pherobase: Database of Pheromones and Semiochemicals. 2019. Available online: https://www.pherobase.com/database/family/family-Pentatomidae.php (accessed on 5 January 2023).
- Rebholz, Z.; Lancaster, J.; Larose, H.; Khrimian, A.; Luck, K.; Sparks, M.E.; Gendreau, K.L.; Shewade, L.; Köllner, T.G.; Weber, D.C.; et al. Ancient origin and conserved gene function in terpene pheromone and defense evolution of stink bugs and hemipteran insects. Insect Biochem. Mol. Biol. 2023, 152, 103879. [Google Scholar] [CrossRef]
- Weber, D.C.; Leskey, T.C.; Walsh, G.C.; Khrimian, A. Synergy of aggregation pheromone with methyl (E,E,Z)-2,4,6-decatrienoate in attraction of Halyomorpha halys (Hemiptera: Pentatomidae). J. Econ. Entomol. 2014, 107, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Morrison, W.R.; Milonas, P.; Kapantaidaki, D.E.; Cesari, M.; Di Bella, E.; Guidetti, R.; Haye, T.; Maistrello, L.; Moraglio, S.T.; Piemontese, L.; et al. Attraction of Halyomorpha halys (Hemiptera: Pentatomidae) haplotypes in North America and Europe to baited traps. Sci. Rep. 2017, 7, 16941. [Google Scholar] [CrossRef]
- Leskey, T.C.; Agnello, A.; Bergh, J.C.; Dively, G.P.; Hamilton, G.C.; Jentsch, P.; Khrimian, A.; Krawczyk, G.; Kuhar, T.P.; Lee, D.H.; et al. Attraction of the invasive Halyomorpha halys (Hemiptera: Pentatomidae) to traps baited with semiochemical stimuli across the United States. J. Econ. Entomol. 2015, 44, 746–756. [Google Scholar] [CrossRef]
- Hogmire, H.W.; Leskey, T.C. An Improved trap for monitoring stink bugs (Heteroptera: Pentatomidae) in apple and peach orchards. J. Entomol. Sci. 2006, 41, 9–21. [Google Scholar] [CrossRef]
- Leskey, T.C.; Hogmire, H.W. Monitoring stink bugs (Hemiptera: Pentatomidae) in Mid-Atlantic apple and peach orchards. J. Econ. Entomol. 2005, 98, 143–153. [Google Scholar] [CrossRef]
- Cullen, E.M.; Zalom, F.G. Phenology-based field monitoring for consperse stink bug (Hemiptera: Pentatomidae) in processing tomatoes. Environ. Entomol. 2000, 29, 560–567. [Google Scholar] [CrossRef]
- Krupke, C.H.; Brunner, J.F.; Doerr, M.D.; Kahn, A.D. Field attraction of the stink bug Euschistus conspersus (Hemiptera: Pentatomidae) to synthetic pheromone-baited host plants. J. Econ. Entomol. 2001, 94, 1500–1505. [Google Scholar] [CrossRef]
- Yamanaka, T.; Teshiba, M.; Tuda, M.; Tsutsumi, T. Possible use of synthetic aggregation pheromones to control stinkbug Plautia stali in kaki persimmon orchards. Agric. For. Entomol. 2011, 13, 321–331. [Google Scholar] [CrossRef]
- Morrison, W.R.; Cullum, J.P.; Leskey, T.C. Evaluation of trap designs and deployment strategies for capturing Halyomorpha halys (Hemiptera: Pentatomidae). J. Econ. Entomol. 2015, 108, 1683–1692. [Google Scholar] [CrossRef]
- Morrison, W.R.; Lee, D.-H.; Short, B.D.; Khrimian, A.; Leskey, T.C. Establishing the behavioral basis for an attract-and-kill strategy to manage the invasive Halyomorpha halys in apple orchards. J. Pest Sci. 2016, 89, 81–96. [Google Scholar] [CrossRef]
- Morrison, W.R.; Blaauw, B.R.; Short, B.D.; Nielsen, A.L.; Bergh, J.C.; Krawczyk, G.; Park, Y.-L.; Butler, B.; Khrimian, A.; Leskey, T.C. Successful management of Halyomorpha halys (Hemiptera: Pentatomidae) in commercial apple orchards with an attract-and-kill strategy. Pest Manag. Sci. 2019, 75, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, Y. Endosymbiotic bacteria in insects: Their diversity and culturability. Microbes Environ. 2009, 24, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H. Insect symbiosis: An introduction. In Insect Symbiosis; Bourtzis, K., Miller, T.A., Eds.; CRC Press: Boca Raton, FL, USA, 2003; pp. 1–16. [Google Scholar]
- Engel, P.; Moran, N.A. The gut microbiota of insects—Diversity in structure and function. FEMS Microbiol. Rev. 2013, 37, 699–735. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Zhou, X.; Zhang, Y. Symbiont-mediated functions in insect hosts. Commun. Integr. Biol. 2013, 6, e23804. [Google Scholar] [CrossRef] [PubMed]
- Douglas, A.E. Phloem-sap feeding by animals: Problems and solutions. J. Exp. Bot. 2006, 57, 747–754. [Google Scholar] [CrossRef]
- Sudakaran, S.; Kost, C.; Kaltenpoth, M. Symbiont acquisition and replacement as a source of ecological innovation. Trends Microbiol. 2017, 25, 375–390. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R.; Michel, A.P.; Sabree, Z.L. The crypt-dwelling primary bacterial symbiont of the polyphagous pentatomid pest Halyomorpha halys (Hemiptera: Pentatomidae). Environ. Entomol. 2014, 43, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Kashkouli, M.; Fathipour, Y.; Mehrabadi, M. Habitat visualization, acquisition features and necessity of the gammaproteobacterial symbiont of pistachio stink bug, Acrosternum heegeri (Hem.: Pentatomidae). Bull. Entomol. Res. 2020, 110, 22–33. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Fukatsu, T. Diversity of Wolbachia endosymbionts in Heteropteran bugs. Appl. Environ. Microbiol. 2003, 69, 6082–6090. [Google Scholar] [CrossRef]
- Duron, O.; Noël, V. A wide diversity of Pantoea lineages are engaged in mutualistic symbiosis and cospeciation processes with stink bugs. Environ. Microbiol. Rep. 2016, 8, 715–727. [Google Scholar] [CrossRef]
- Kenyon, L.J.; Meulia, T.; Sabree, Z.L. Habitat visualization and genomic analysis of “Candidatus Pantoea carbekii” the primary symbiont of the brown marmorated stink bug. Genome Biol. Evol. 2015, 7, 620–635. [Google Scholar] [CrossRef]
- Otero-Bravo, A.; Goffredi, S.; Sabree, Z.L. Cladogenesis and genomic streamlining in extracellular endosymbionts of tropical stink bugs. Genome Biol. Evol. 2018, 10, 680–693. [Google Scholar] [CrossRef]
- Medina, V.; Sardoy, P.M.; Soria, M.; Vay, C.A.; Gutkind, G.O.; Zavala, J.A. Characterized non-transient microbiota from stinkbug (Nezara viridula) midgut deactivates soybean chemical defenses. PLoS ONE 2018, 13, e0200161. [Google Scholar] [CrossRef]
- Hosokawa, T.; Kikuchi, Y.; Nikoh, N.; Shimada, M.; Fukatsu, T. Strict host-symbiont cospeciation and reductive genome evolution in insect gut bacteria. PLoS Biol. 2006, 4, e337. [Google Scholar] [CrossRef]
- Nikoh, N.; Hosokawa, T.; Oshima, K.; Hattori, M.; Fukatsu, T. Reductive evolution of bacterial genome in insect gut environment. Genome Biol. Evol. 2011, 3, 702–714. [Google Scholar] [CrossRef] [PubMed]
- Geerinck, M.W.J.; Van Hee, S.; Gloder, G.; Crauwels, S.; Colazza, S.; Jacquemyn, H.; Cusumano, A.; Lievens, B. Diversity and composition of the microbiome associated with eggs of the Southern green stinkbug, Nezara viridula (Hemiptera: Pentatomidae). Microbiologyopen 2022, 11, e1337. [Google Scholar] [CrossRef] [PubMed]
- Oishi, S.; Moriyama, M.; Koga, R.; Fukatsu, T. Morphogenesis and development of midgut symbiotic organ of the stinkbug Plautia stali (Hemiptera: Pentatomidae). Zool. Lett. 2019, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, T.; Kikuchi, Y.; Shimada, M.; Fukatsu, T. Symbiont acquisition alters behaviour of stinkbug nymphs. Biol. Lett. 2008, 4, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Glasgow, H. The gastric caeca and the caecal bacteria of the Heteroptera. Biol. Bull. 1914, 16, 101–171. [Google Scholar] [CrossRef]
- Hayashi, T.; Hosokawa, T.; Meng, X.Y.; Koga, R.; Fukatsu, T. Female-specific specialization of a posterior end region of the midgut symbiotic organ in Plautia splendens and allied stinkbugs. Appl. Environ. Microbiol. 2015, 81, 2603–2611. [Google Scholar] [CrossRef]
- Hosokawa, T.; Ishii, Y.; Nikoh, N.; Fujie, M.; Satoh, N.; Fukatsu, T. Obligate bacterial mutualists evolving from environmental bacteria in natural insect populations. Nat. Microbiol. 2016, 1, 15011. [Google Scholar] [CrossRef]
- Tada, A.; Kikuchi, Y.; Hosokawa, T.; Musolin, D.L.; Fujisaki, K.; Fukatsu, T. Obligate association with gut bacterial symbiont in Japanese populations of the southern green stinkbug Nezara viridula (Heteroptera: Pentatomidae). Appl. Entomol. Zool. 2011, 46, 483–488. [Google Scholar] [CrossRef]
- Gonella, E.; Orrù, B.; Alma, A. Egg masses treatment with micronutrient fertilizers has a suppressive effect on newly-emerged nymphs of the brown marmorated stink bug Halyomorpha halys. Entomol. Gen. 2019, 39, 231–238. [Google Scholar] [CrossRef]
- Mathews, C.R.; Barry, S. Compost tea reduces egg hatch and early stage nymphal development of Halyomorpha halys (Hemiptera: Pentatomidae). Fla. Entomol. 2014, 97, 1726–1732. [Google Scholar] [CrossRef]
- Prokopy, R.J.; Owens, E.D. Visual detection of plants by herbivorous insects. Ann. Rev. Entomol. 1983, 28, 337–364. [Google Scholar] [CrossRef]
- Tillman, P.G.; Cottrell, T.E. Use of pheromones for monitoring phytophagous stink bugs (Hemiptera: Pentatomidae). In Stink Bugs: Biorational Control Based on Communication Processes; Čokl, A., Borges, M., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 210–225. [Google Scholar]
- Leskey, T.C.; Wright, S.E.; Short, B.D.; Khrimian, A. Development of behaviorally-based monitoring tools for the brown marmorated stink bug (Heteroptera: Pentatomidae) in commercial tree fruit orchards. J. Entomol. Sci. 2012, 47, 76–85. [Google Scholar] [CrossRef]
- Joseph, S.V. Effect of trap color on captures of bagrada bug, Bagrada hilaris (Hemiptera: Pentatomidae). J. Entomol. Sci. 2014, 49, 318–321. [Google Scholar] [CrossRef]
- DiMeglio, A.S.; Kuhar, T.P.; Weber, D.C. Color preference of harlequin bug (Heteroptera: Pentatomidae. J. Econ. Entomol. 2017, 110, 2275–2277. [Google Scholar] [CrossRef] [PubMed]
- Duehl, A.J.; Cohnstaedt, L.W.; Arbogast, R.T.; Teal, P.E.A. Evaluating light attraction to increase trap efficiency for Tribolium castaneum (Coleoptera: Tenebrionidae). J. Econ. Entomol. 2011, 104, 1430–1435. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-G.; Yang, J.-Y.; Lee, H.-S. Phototactic behavior: Repellent effects of cigarette beetle, Lasioderma serricorne (Coleoptera: Anobiidae), to light-emitting diodes. J. Korean Soc. Appl. Biol. Chem. 2013, 56, 331–333. [Google Scholar] [CrossRef]
- Kim, K.N.; Huang, Q.Y.; Lei, C.L. Advances in insect phototaxis and application to pest management: A review. Pest Manag. Sci. 2019, 75, 3135–3143. [Google Scholar] [CrossRef]
- Shimoda, M.; Honda, K.-I. Insect reactions to light and its applications to pest management. Appl. Entomol. Zool. 2013, 48, 413–421. [Google Scholar] [CrossRef]
- Nielsen, A.L.; Holmstrom, K.; Hamilton, G.C.; Cambridge, J.; Ingerson-Mahar, J. Use of black light traps to monitor the abundance, spread, and flight behavior of Halyomorpha halys (Hemiptera: Pentatomidae). J. Econ. Entomol. 2013, 106, 1495–1502. [Google Scholar] [CrossRef]
- Antignus, Y. Manipulation of wavelength-dependent behaviour of insects: An IPM tool to impede insects and restrict epidemics of insect-borne viruses. Virus Res. 2000, 71, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Coombe, P.E. Wavelength specific behaviour of the whitefly Trialewodes vaporariorum (Homoptera: Aleyrodidae). J. Comp. Physiol. 1981, 144, 83–90. [Google Scholar] [CrossRef]
- Hardie, J. Spectral specificity for targeted flight in the black bean aphid, Aphis fabae. J. Insect Physiol. 1989, 35, 619–626. [Google Scholar] [CrossRef]
- Cambridge, J.E.; Francoeur, L.; Hamilton, G.C. Brown marmorated stink bug (Hemiptera: Pentatomidae) attraction to various light stimuli. Fla. Entomol. 2017, 100, 583–588. [Google Scholar] [CrossRef]
- Leskey, T.C.; Lee, D.-H.; Glenn, D.M.; Morrison, W.R. Behavioral responses of the invasive Halyomorpha halys (Stål) (Hemiptera: Pentatomidae) to light-based stimuli in the laboratory and field. J. Insect Behav. 2015, 28, 674–692. [Google Scholar] [CrossRef]
- Bergmann, P.; Richter, S.; Glöckner, N.; Betz, O. Morphology of hindwing veins in the shield bug Graphosoma italicum (Heteroptera: Pentatomidae). Arthropod Struct. Dev. 2018, 47, 375–390. [Google Scholar] [CrossRef]
- Lee, D.H.; Leskey, T.C. Flight behavior of foraging and overwintering brown marmorated stink bug, Halyomorpha halys (Hemiptera: Pentatomidae). Bull. Entomol. Res. 2015, 105, 566–573. [Google Scholar] [CrossRef]
- Wiman, N.G.; Walton, V.M.; Shearer, P.W.; Rondon, S.I.; Lee, J.C. Factors affecting flight capacity of brown marmorated stink bug, Halyomorpha halys (Hemiptera: Pentatomidae). J. Pest Sci. 2015, 88, 37–47. [Google Scholar] [CrossRef]
- Minter, M.; Pearson, A.; Lim, K.S.; Wilson, K.; Chapman, J.W.; Jones, C.M. The tethered flight technique as a tool for studying life-history strategies associated with migration in insects. Ecol. Entomol. 2018, 43, 397–411. [Google Scholar] [CrossRef]
- Naranjo, S.E. Assessing insect flight behavior in the laboratory: A primer on flight mill methodology and what can be learned. Ann. Entomol. Soc. Am. 2019, 112, 182–199. [Google Scholar] [CrossRef]
- Aita, R.C.; Kees, A.M.; Aukema, B.H.; Hutchison, W.D.; Koch, R.L. Effects of starvation, age, and mating status on flight capacity of laboratory-reared brown marmorated stink bug (Hemiptera: Pentatomidae). Environ. Entomol. 2021, 50, 532–540. [Google Scholar] [CrossRef]
- Babu, A.; Del Pozo-Valdivia, A.I.; Reisig, D.D. Baseline flight potential of Euschistus servus (Hemiptera: Pentatomidae) and its implications on local dispersal. Environ. Entomol. 2020, 49, 699–708. [Google Scholar] [CrossRef]
- Ali, S.; Ullah, M.I.; Sajjad, A.; Shakeel, Q.; Hussain, A. Environmental and health effects of pesticide residues. In Sustainable Agriculture Reviews; Springer: Cham, Switzerland, 2021; Volume 48, pp. 311–336. [Google Scholar]
- Le Goff, G.; Giraudo, M. Effects of pesticides on the environment and insecticide resistance. In Olfactory Concepts of Insect Control—Alternative to Insecticides; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 51–78. [Google Scholar]
- Lowenstein, D.M.; Andrews, H.; Mugica, A.; Wiman, N.G. Sensitivity of the egg parasitoid Trissolcus japonicus (Hymenoptera: Scelionidae) to field and laboratory-applied insecticide residue. J. Econ. Entomol. 2019, 112, 2077–2084. [Google Scholar] [CrossRef]
- Guedes, R.N.C.; Smagghe, G.; Stark, J.D.; Desneux, N. Pesticide-induced stress in arthropod pests for optimized integrated pest management programs. Annu. Rev. Entomol. 2016, 61, 43–62. [Google Scholar] [CrossRef]
- Hill, M.P.; Macfadyen, S.; Nash, M.A. Broad spectrum pesticide application alters natural enemy communities and may facilitate secondary pest outbreaks. PeerJ 2017, 5, e4179. [Google Scholar] [CrossRef]
- Brzozowski, L.; Mazourek, M. A sustainable agricultural future relies on the transition to organic agroecological pest management. Sustainability 2018, 10, 2023. [Google Scholar] [CrossRef]
- Sparks, M.E.; Shelby, K.S.; Kuhar, D.; Gundersen-Rindal, D.E. Transcriptome of the invasive brown marmorated stink bug, Halyomorpha halys (Stål) (Heteroptera: Pentatomidae). PLoS ONE 2014, 9, e111646. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Huang, Y.; Qin, Z.; Zhan, H.; Zhang, J.; Liu, Y.; Yang, S. Identification of candidate olfactory genes in the antennal transcriptome of the stink bug Halyomorpha halys. Front. Physiol. 2020, 11, 876. [Google Scholar] [CrossRef] [PubMed]
- Cagliari, D.; Dias, N.P.; Dos Santos, E.Á.; Rickes, L.N.; Kremer, F.S.; Farias, J.R.; Lenz, G.; Galdeano, D.M.; Garcia, F.R.M.; Smagghe, G.; et al. First transcriptome of the Neotropical pest Euschistus heros (Hemiptera: Pentatomidae) with dissection of its siRNA machinery. Sci. Rep. 2020, 10, 4856. [Google Scholar] [CrossRef]
- King, R.; Buer, B.; Davies, T.G.E.; Ganko, E.; Guest, M.; Hassani-Pak, K.; Hughes, D.; Raming, K.; Rawlings, C.; Williamson, M.; et al. The complete genome assemblies of 19 insect pests of worldwide importance to agriculture. Pestic. Biochem. Phys. 2023, 191, 105339. [Google Scholar] [CrossRef] [PubMed]
- Leal, W.S. Reverse chemical ecology at the service of conservation biology. Proc. Nat. Acad. Sci. USA 2017, 114, 12094–12096. [Google Scholar] [CrossRef] [PubMed]
- Pickett, J.A. Chemical ecology in the post genomics era. J. Chem. Ecol. 2014, 40, 319. [Google Scholar] [CrossRef] [PubMed]
- Cagliari, D.; Smagghe, G.; Zotti, M.; Taning, C.N.T. RNAi and CRISPR/Cas9 as functional genomics tools in the neotropical stink bug, Euschistus heros. Insects 2020, 11, 838. [Google Scholar] [CrossRef]
- Singh, S.; Rahangdale, S.; Pandita, S.; Saxena, G.; Upadhyay, S.K.; Mishra, G.; Verma, P.C. CRISPR/Cas9 for insect pests management: A comprehensive review of advances and applications. Agriculture 2022, 12, 1896. [Google Scholar] [CrossRef]
- Henderson, W.G.; Khalilian, A.; Han, Y.J.; Greene, J.K.; Degenhardt, D.C. Detecting stink bugs/damage in cotton utilizing a portable electronic nose. Comput. Electron. Agric. 2010, 70, 157–162. [Google Scholar] [CrossRef]
- Lampson, B.D.; Khalilian, A.; Greene, J.K.; Han, Y.J.; Degenhardt, D.C. Development of a portable electronic nose for detection of cotton damaged by Nezara viridula (Hemiptera: Pentatomidae). J. Insects 2014, 2014, 297219. [Google Scholar] [CrossRef]
- Martinazzo, J.; Moraes, M.C.B.; Steffens, J.; Steffens, C. Application of gas nanosensor for detection pheromone and its interferents compounds in vivo Euschistus heros (F.) stink bugs insects. Sens. Actuat. A Phys. 2022, 345, 113804. [Google Scholar] [CrossRef]
- Reddy, G.V.P.; Guerrero, A. Interactions of insect pheromones and plant semiochemicals. Trends Plant Sci. 2004, 9, 253–261. [Google Scholar] [CrossRef]
- Thrift, E.M.; Herlihy, M.V.; Wallingford, A.K.; Weber, D.C. Fooling the Harlequin Bug (Hemiptera: Pentatomidae) using synthetic volatiles to alter host plant choice. Environ. Entomol. 2018, 47, 432–439. [Google Scholar] [CrossRef]
- Guignard, Q.; Allison, J.D.; Slippers, B. The evolution of insect visual opsin genes with specific consideration of the influence of ocelli and life history traits. BMC Ecol. Evol. 2022, 22, 2. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Lu, B.; Chao, J.; Holdbrook, R.; Liang, G.; Lu, Y. The evolution of opsin genes in five species of mirid bugs: Duplication of long-wavelength opsins and loss of blue-sensitive opsins. BMC Ecol. Evol. 2021, 21, 66. [Google Scholar] [CrossRef] [PubMed]
 and
and  indicate adults and nymphs, respectively, whereas the sex, sex–aggregation and aggregation pheromones are emitted by males (“M”). Information regarding the life stage and the sex (males (M); females (F); nymphs (n)) of responder(s) to the alarm, sex, aggregation, or sex–aggregation pheromones are also listed if behavioural studies have been conducted, with red, green, and grey indicating the degree of response (red = does not respond to the pheromone, green = responds to the pheromone, grey = response has not been tested). In terms of compounds listed for each pheromone, only the most abundant compound is listed for the alarm pheromones, except for species in which bioassays have been conducted. For these species, all the compounds (as well as the instar and sex emitter) are listed, and the most abundant compound is underlined. For the sex pheromones, aggregation pheromones and sex–aggregation pheromones all compounds assayed are listed.
indicate adults and nymphs, respectively, whereas the sex, sex–aggregation and aggregation pheromones are emitted by males (“M”). Information regarding the life stage and the sex (males (M); females (F); nymphs (n)) of responder(s) to the alarm, sex, aggregation, or sex–aggregation pheromones are also listed if behavioural studies have been conducted, with red, green, and grey indicating the degree of response (red = does not respond to the pheromone, green = responds to the pheromone, grey = response has not been tested). In terms of compounds listed for each pheromone, only the most abundant compound is listed for the alarm pheromones, except for species in which bioassays have been conducted. For these species, all the compounds (as well as the instar and sex emitter) are listed, and the most abundant compound is underlined. For the sex pheromones, aggregation pheromones and sex–aggregation pheromones all compounds assayed are listed.
 and
and  indicate adults and nymphs, respectively, whereas the sex, sex–aggregation and aggregation pheromones are emitted by males (“M”). Information regarding the life stage and the sex (males (M); females (F); nymphs (n)) of responder(s) to the alarm, sex, aggregation, or sex–aggregation pheromones are also listed if behavioural studies have been conducted, with red, green, and grey indicating the degree of response (red = does not respond to the pheromone, green = responds to the pheromone, grey = response has not been tested). In terms of compounds listed for each pheromone, only the most abundant compound is listed for the alarm pheromones, except for species in which bioassays have been conducted. For these species, all the compounds (as well as the instar and sex emitter) are listed, and the most abundant compound is underlined. For the sex pheromones, aggregation pheromones and sex–aggregation pheromones all compounds assayed are listed.
indicate adults and nymphs, respectively, whereas the sex, sex–aggregation and aggregation pheromones are emitted by males (“M”). Information regarding the life stage and the sex (males (M); females (F); nymphs (n)) of responder(s) to the alarm, sex, aggregation, or sex–aggregation pheromones are also listed if behavioural studies have been conducted, with red, green, and grey indicating the degree of response (red = does not respond to the pheromone, green = responds to the pheromone, grey = response has not been tested). In terms of compounds listed for each pheromone, only the most abundant compound is listed for the alarm pheromones, except for species in which bioassays have been conducted. For these species, all the compounds (as well as the instar and sex emitter) are listed, and the most abundant compound is underlined. For the sex pheromones, aggregation pheromones and sex–aggregation pheromones all compounds assayed are listed.| Pheromone 1 | Emitter | Responder | Compounds 2 | References |
|---|---|---|---|---|
| Dinidorinae: Dinidorini | ||||
| Aspongopus sp. | ||||
| D |  | (E)-2-Hexenal | [72] | |
| Coridus janus | ||||
| D |  | (E)-2-Hexenal | [73] | |
| Cyclopelta siccifolia | ||||
| D |  | (E)-2-Hexenal | [74] | |
| Discocephalinae: Discocephalini | ||||
| Antiteuchus innocens | ||||
| D |  | Undecane (M, F) | [75] | |
| D |  | Undecane (1st–5th instar) | [75] | |
| Discocephalinae: Ochlerini | ||||
| Lincus malevolus | ||||
| D |  | Undecane (M, F); (E)-4-oxo-2-decenal (F) | [76] | |
| Lincus spurcus | ||||
| D |  | Undecane (M, F) | [76] | |
| D |  | Undecane (5th instar) | [77] | |
| Edessinae | ||||
| Edessa meditabunda | ||||
| S | M | M, F, n | Methyl 4,8,12-trimethylpentadecanoate | [67,78] |
| D |  | Undecane (1st–2nd instar) | [79] | |
| Edessa rufomarginata | ||||
| D |  | Undecane | [80] | |
| Pentatominae: Aeliini | ||||
| Aelia fieberi | ||||
| D |  | (E)-2-Decenal (M, F) | [81] | |
| Pentatominae: Antestiini | ||||
| Plautia stali | ||||
| Ag | M | M, F, n | Methyl (2E,4E,6Z)-2,4,6-decatrienoate | [82] |
| Thyanta custator accera | ||||
| ? | M | Methyl (2E,4Z,6Z)-2,4,6-decatrienoate; (+)-α-curcumene; (−)-zingiberene; (−)-β-sesquiphellandrene | [61] | |
| Thyanta pallidovirens | ||||
| S | M | M, F, n | Methyl (2E,4Z,6Z)-2,4,6-decatrienoate; (+)-α-curcumene; (−)-zingiberene; (−)-β-sesquiphellandrene | [61,83] |
| D |  | Tridecane (M, F) | [61] | |
| D |  | Tridecane (1st–2nd instar) | [84] | |
| Thyanta perditor | ||||
| S | M | M, F, n | Methyl (2E,4E,6Z)-2,4,6-decatrienoate | [62] |
| D |  | Tridecane (M, F) | [62] | |
| D |  | Tridecane (1st–2nd instar) | [79] | |
| Pentatominae: Bathycoliini | ||||
| Bathycoelia distincta | ||||
| D |  | M, F, n | (E)-2-Hexenal; 4-oxo-(E)-2-hexenal; (E)-2-decenal; (E)-2-decenyl acetate; tridecane; dodecane | [85] |
| Pentatominae: Cappaeini | ||||
| Caura rufiventris | ||||
| D |  | Tridecane | [72] | |
| Halyomorpha halys | ||||
| Ag | M | M, F, n | Isomers of murgantiol (10,11-Epoxy-1- bisabolen-3-ol) | [86,87] |
| D |  | Tridecane (M, F) | [86,88,89,90,91] | |
| D |  | Tridecane (3rd–5th instar) | [86,88] | |
| D | M, F, n | M, F, n | (E)-2-Decenal | [92] |
| Veterna patula | ||||
| D |  | Tridecane | [72] | |
| Pentatominae: Carpocorini | ||||
| Agroecus griseus | ||||
| S | M | M, F, n | Methyl 2,6,10 trimethyltridecanoate | [66] |
| D |  | Tridecane | [66,93] | |
| D |  | Tridecane (1st–5th instar) | [66] | |
| Carpocoris fuscispinus | ||||
| D |  | Tridecane (M, F) | [94] | |
| Cosmopepla bimaculate | ||||
| D |  | Tridecane (M, F) | [95] | |
| Euschistus biformis | ||||
| D |  | Tridecane | [96] | |
| Euschistus conspersus | ||||
| Ag | M | M, F, n | Methyl (2E,4Z)-2,4-decadienoate; methyl (2E,4E)-decadienoate; geranylacetone | [97] |
| D |  | Tridecane (1st–2nd instar) | [84] | |
| Euschistus heros | ||||
| S | M | M, F, n | Methy 2,6,10 trimethyltridecanoate; methyl (2E,4Z)-2,4-decadienoate; methyl 2,6,10-trimethyldodecanoate | [63,65,98] |
| D |  | M, F, n | (E)-2-Hexenal; 4-oxo-(E)-2-hexenal; hexenoic acid; (E)-2-hexenyl acetate; 2-octenal; (E)-2-octen-1-ol; nonanal; (E)-2-heptenyl acetate; (E)-2-decenal; (E)-2-octenyl acetate; tridecane; tetradecane; (E)-2-decenyl acetate; tridecanal; tetradecanal; pentadecane | [99,100,101] |
| D |  | Tridecane (1st–4th instar); tetradecanal (5th instar) | [79,96,99] | |
| Euschistus ictericus | ||||
| ? | M | Methyl (2E,4Z)-2,4-decadienoate; methyl (2E,4E)-decadienoate | [97] | |
| Euschistus obscurus | ||||
| S | M | M, F, n | Methyl (2E,4Z)-2,4-decadienoate; methy 2,6,10 trimethyltridecanoate; methyl 2,6,10-trimethyldodecanoate; methyl (2E,4E)-decadienoate | [63,64] |
| Euschistus politus | ||||
| Ag | M | M, F, n | Methyl (2E,4Z)-2,4-decadienoate; methyl (2E,4E)-decadienoate | [97] |
| Euschistus servus | ||||
| Ag | M | M, F, n | Methyl (2E,4Z)-2,4-decadienoate; methyl (2E,4E)-decadienoate; geranylacetone | [97] |
| D |  | (E)-2-Hexenal | [102] | |
| Euschistus tristigmus | ||||
| Ag | M | M, F, n | Methyl (2E,4Z)-2,4-decadienoate; geranylacetone; decanoic acid | [97] |
| D |  | Hexanal | [102] | |
| D |  | Tridecane (1st–5th instar) | [79] | |
| Dichelops melacanthus | ||||
| D |  | Tridecane (M, F); 4-oxo-(E)-2-hexenal (M, F) | [99,100,103] | |
| D |  | 4-Oxo-(E)-2-hexenal (5th instar) | [99] | |
| Dolycoris baccarum | ||||
| Ag | M | M, F, n | α-Bisabolol *; trans-α-bergamotene *; (S)-β-bisabolene * | [104] |
| D |  | Tridecane (M, F); hexenal | [105,106] | |
| Mormidea v-luteum | ||||
| Ag | M | M, F, n | Isomers of zingiberenol (cis-(1S,4R,1′S)-zingiberenol; trans-(1R,4R,1′S)-zingiberenol); murgantiol *; sesquipiperitol | [107] |
| Oebalus poecilus | ||||
| S | M | M, F, n | Isomer of zingiberenol ((1S,4R,1′S)-4-(1′,5′-dimethylhex-4′-enyl)-1-methylcyclohex-2-en-1-ol) | [71] |
| Oebalus pugnax | ||||
| D |  | Tridecane | [108] | |
| Tibraca limbativentris | ||||
| S | M | M, F, n | Isomers of zingiberenol ((3S,6S,7R)-1,10-bisaboladien-3-ol; (3R,6S,7R)-1,10-bisaboladien-3-ol); sesquipiperitol | [68,69] |
| Pentatominae: Eysarcorini | ||||
| Eysarcoris lewisi | ||||
| Ag | M | M (3), F, n | Sesquisabinen-1-ol | [109,110] |
| Eysarcoris parvus | ||||
| ? | M | (Z)-exo-α-Bergamotene | [111] | |
| Pentatominae: Halyini | ||||
| Apodiphus amygdali | ||||
| D |  | 4-Oxo-(E)-2-hexenal | [112] | |
| D |  | 4-Oxo-(E)-2-hexenal (1st–5th instar) | [112] | |
| Brochymena quadripustulata | ||||
| D |  | (E)-2-Hexenal | [113] | |
| Erthesina fullo | ||||
| D |  | Tridecane (M, F) | [114] | |
| Poecilometris strigatus | ||||
| D |  | (E)-2-Hexenal; (E)-2-octenal | [115] | |
| Pentatominae: Myrocheini | ||||
| Delegorguella lautus | ||||
| D |  | Tridecane | [72] | |
| Pentatominae: Nezarini | ||||
| Chinavia aseada | ||||
| ? | M | cis-(Z)-Bisabolene epoxide; trans-(Z)-bisabolene epoxide; (Z)-α-bisabolene | [116] | |
| D |  | (Z)-4-Nonenal | [102] | |
| D |  | Tridecane (1st–2nd instar) | [79] | |
| Chinavia hilaris | ||||
| S | M | M, F, n | cis-(Z)-Bisabolene epoxide; trans-(Z)-bisabolene epoxide | [117] |
| D |  | (E)-2-Hexenal | [102] | |
| D |  | Tridecane (1st–2nd instar) | [84] | |
| Chinavia impicticornis | ||||
| S | M | M, F, n | trans-(Z)-Bisabolene epoxide; cis-(Z)-bisabolene epoxide | [118] |
| D |  | Tridecane (M); 4-oxo-(E)-2-hexenal (F) | [99,118] | |
| D |  | 4-Oxo-(E)-2-hexenal (5th instar) | [99] | |
| Chinavia marginata | ||||
| ? | M | trans-(Z)-Bisabolene epoxide; cis-(Z)-bisabolene epoxide | [119] | |
| Chinavia ubica | ||||
| S | M | M, F, n | trans-(Z)-Bisabolene epoxide; cis-(Z)-bisabolene epoxide | [118] |
| D |  | Tridecane (M, F) | [99,100,118] | |
| D |  | 4-Oxo-(E)-2-hexenal (5th instar) | [99] | |
| Chlorochroa ligata | ||||
| S | M | M (3), F, n | Methyl (E)-6,2,3-dihydrofarnesoate; methyl farnesoate; methyl (E)-5-2,6,10-trimethyl-5,9-undecadienoate | [120,121] |
| D |  | Tridecane (M, F) | [122] | |
| D |  | Tridecane (5th instar) | [122] | |
| Chlorochroa sayi | ||||
| SAg | M | M, F, n | Methyl geranate; methyl citronellate; methyl farnesoate | [120,123] |
| D |  | Tridecane (M, F) | [122] | |
| D |  | 4-Oxo-(E)-2-hexenal (5th instar) | [122] | |
| Chlorochroa uhleri | ||||
| Ag | M | M, F, n | Methyl geranate; methyl citronellate; methyl farnesoate | [120,121] |
| D |  | Tridecane (M, F) | [122] | |
| Nezara antennata | ||||
| ? | M | (Z)-α-Bisabolene; trans-(Z)-bisabolene epoxide; cis-(Z)-bisabolene epoxide; (E)-nerolidol | [116] | |
| D |  | (E)-2-Decenal (M, F) | [81] | |
| Nezara viridula | ||||
| Ag | M | M, F, n | trans-(Z)-Bisabolene epoxide; cis-(Z)-bisabolene epoxide; (Z)-α-bisabolene; (E)-nerolidol | [98,116,124,125,126,127,128] |
| D |  | M, F, n | Tridecane; (E)-2-decenal; (E)-2-decenyl acetate; (E)-2-hexenal; (E)-2-hexenyl acetate; dodecane | [124,129,130,131] |
| D |  | n | 4-Oxo-(E)-2-decenal (1st instar); tetradecane (2nd instar); tridecane (2nd–5th instar); (E)-2-hexenal | [79,84,132,133] |
| Palomena viridissima | ||||
| D |  | Unknown carbonyl compound | [106] | |
| Pentatominae: Pentatomini | ||||
| Pallantia macunaima | ||||
| S | M | M, F, n | (6R,10S)-Pallantione | [134] |
| D |  | Tridecane (M, F) | [93,135] | |
| D |  | (E)-4-Oxo-2-hexenal (1st instar) Tridecane (2nd–5th instar) | [135] | |
| Pellaea stictica | ||||
| P | M | M, F, n | Isomers of 2,4,8,13-tetramethyltetradecan-1-ol | [136,137] |
| D |  | (Z)-4-tridecene | [93] | |
| Rhaphigaster nebulosa | ||||
| D |  | Tridecane (M, F) | [138] | |
| Pentatominae: Piezodorini | ||||
| Piezodorus guildinii | ||||
| S | M | M, F, n | (7R)-(+)-β-sesquiphellandrene | [139] |
| D |  | (E)-4-oxo-2-hexenal (M, F) | [99,140] | |
| D |  | Unknown (5th instar) | [99] | |
| Piezodorus hybneri | ||||
| SAg | M | M, F, n | β-Sesquiphellandrene *; (R)-15-hexadecanolide; methyl (Z)-8-hexadecenoate | [59,60] |
| Piezodorus teretipes | ||||
| D |  | (E)-2-Hexenal | [141] | |
| Pentatominae: Rhynchocorini | ||||
| Biprorulus bibax | ||||
| SAg | M | M, F, n | Biprolure ((3R8,4S′,1′E)-3,4-bis(1′-buteny1)tetrahydro-2-furano; linalool; isomers of farnesol; nerolidol | [142,143,144,145,146] |
| D |  | Tridecane | [147] | |
| Vitellus insularis | ||||
| D |  | Tridecane | [148] | |
| Pentatominae: Strachiini | ||||
| Bagrada hilaris | ||||
| Ag | M | M, F, n | (E)-2-octenyl acetate | [149,150] |
| Eurydema oleraceum | ||||
| D |  | (E)-2-Octenal; (E)-2-hexenal | [102,151] | |
| Eurydema pulchrum | ||||
| D |  | n | (E)-2-Hexenal (1st–3rd instar); tridecane | [133] |
| Eurydema rugosa | ||||
| Ag | M | M, F, n | (E)-2-Hexenal | [152] |
| D |  | n | (E)-2-Hexenal (2nd–3rd instar); tridecane | [133] |
| Eurydema ventrale | ||||
| D |  | (2E,6E)-Octadienedial; (E)-2-octenal | [102,151] | |
| Murgantia histrionica | ||||
| Ag | M | M, F, n | Isomers of murgantiol ((3S,6S,7R,10S)-10,11-epoxy-1-bisabolen-3-ol; (3S,6S,7R,10R)-10,11-epoxy-1-bisabolen-3-ol) | [153,154,155] |
| D |  | (2E,6E)-Octadienedial | [151] | |
| Podopinae: Graphosomatini | ||||
| Graphosoma lineatum | ||||
| D |  | 1-phenanthrenecarboxylic acid (M); tridecane (F); (E)-2-decenal (F) | [156,157] | |
| Graphosoma rubrolineatum | ||||
| D |  | (E)-2-Decenal (M, F) | [81] | |
| Graphosoma semipunctatum | ||||
| D |  | Tridecane (M, F) | [158] | |
| Podopinae: Podopini | ||||
| Scotinophara lurida | ||||
| D |  | (E)-2-Decenal (M, F) | [81] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pal, E.; Allison, J.D.; Hurley, B.P.; Slippers, B.; Fourie, G. Life History Traits of the Pentatomidae (Hemiptera) for the Development of Pest Management Tools. Forests 2023, 14, 861. https://doi.org/10.3390/f14050861
Pal E, Allison JD, Hurley BP, Slippers B, Fourie G. Life History Traits of the Pentatomidae (Hemiptera) for the Development of Pest Management Tools. Forests. 2023; 14(5):861. https://doi.org/10.3390/f14050861
Chicago/Turabian StylePal, Elisa, Jeremy D. Allison, Brett P. Hurley, Bernard Slippers, and Gerda Fourie. 2023. "Life History Traits of the Pentatomidae (Hemiptera) for the Development of Pest Management Tools" Forests 14, no. 5: 861. https://doi.org/10.3390/f14050861
APA StylePal, E., Allison, J. D., Hurley, B. P., Slippers, B., & Fourie, G. (2023). Life History Traits of the Pentatomidae (Hemiptera) for the Development of Pest Management Tools. Forests, 14(5), 861. https://doi.org/10.3390/f14050861







