Abstract
A review of papers related to cambium activity and wood formation in tropical and subtropical trees and their response to climate in South America, tropical Africa, Southwest Asia, and Southeast Asia reveals a complex picture of the factors that influence tree growth and wood formation. One key finding is that while temperature and rainfall are essential drivers of tree growth in tropical regions, the specific effects of these factors can vary widely depending on local conditions. For example, in some areas, increased rainfall may lead to higher rates of wood formation, while in others, it may have little effect or even be detrimental to tree growth. Another key finding is that tree species can exhibit different cambium activity and wood formation patterns, even within the same region. These observations highlight the need for careful species-level studies to understand the factors influencing tree growth in tropical regions fully. Some studies have also found that extreme events, such as droughts and floods, can significantly impact tree growth and wood formation in tropical regions. These events can lead to cambium activity and wood density changes and may have long-term effects on forest structure and composition. Overall, this review suggests that much is still to be learned about the complex interactions between climate, soil, and other environmental factors that influence tree growth and wood formation in tropical and subtropical regions. Continued research and monitoring efforts will be essential for understanding these important ecosystems and developing effective conservation and management strategies.
1. Introduction
Wood is a natural material; it is not only a source of energy but also provides countless utilization services [1,2]. In addition, the wood formation absorbs a large amount of carbon dioxide from the air, thus helping to reduce global warming [3] The tropics refer to the region of the Earth located between the Tropic of Cancer (23°26′10.6″ N) and Tropic of Capricorn (23°26′10.6″ S) (Figure 1). This region is known for its warm climate, with high temperatures and humidity throughout the year, and is home to a diverse range of flora and fauna. The tropics are also important for global biodiversity and are home to many endemic species not found elsewhere on Earth [4]. They also play a crucial role in regulating the Earth’s climate, as they are a significant source of heat and moisture that circulates around the planet [5,6]. The global mapping of tree density demonstrated that worldwide, a total of approximately 1.39 trillion trees exist in tropical and subtropical forests [7].
Studying the growth of trees in natural forests is, therefore, very useful for determining the growth factors affected by climate or how trees grow to adapt to more severe climates. The formation of wood—xylogenesis—is a consequence of cell division in the cambium. Various factors causing that division depend on the species and environmental conditions (e.g., temperature, rainfall, and humidity). Studying the relationship between the anatomy of wood and changing environmental conditions can explain tree physiology and provide perspectives on climate adaptation and growth strategies [8,9,10].
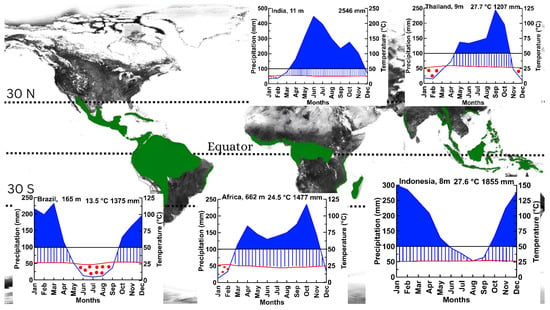
Figure 1.
Tropical areas and weather conditions in each area. Walter and Lieth climate diagram [11]. The blue line indicates the precipitation curve, the red line indicates the temperature, the red dots indicate the dry period, blue stripes indicate the humid period, and the blue area shows the super humid period. The average maximum temperature of the warmest month and the average minimum temperature of the coldest month are indicated on the right axis. The annual average temperature and annual precipitation are shown in the upper right corner of the diagram.
Several problems are affecting tropical rainforests worldwide. Some of the most pressing issues include deforestation, one of the biggest threats to tropical rainforests. Deforestation occurs when forests are cleared for agriculture, logging, mining, and other human activities. It destroys habitats, reduces biodiversity, and releases carbon dioxide into the atmosphere, contributing to climate change [12]. In turn, climate change has a significant impact on tropical rainforests. Rising temperatures, changing rainfall patterns, and more frequent extreme weather events are causing stress on forest ecosystems and can lead to changes in the distribution and abundance of plant and animal species [13,14]. Illegal logging is a major problem in many tropical rainforests, leading to deforestation and the loss of biodiversity and contributing to carbon emissions and climate change [15,16]. These problems threaten the health and stability of tropical rainforests and have far-reaching consequences for local and global communities. Addressing these issues requires a multifaceted approach that includes sustainable development practices, conservation efforts, and policies to reduce greenhouse gas emissions and limit climate change.
The diversity of trees in tropical forests is vast, but our understanding of tropical tree growth is limited. For example, while we know that rainfall and temperature are essential factors in determining tree growth in the tropics, there is still much to be learned about how these factors interact with other environmental variables, such as soil type, nutrient availability, and disturbance regimes [17,18]. In addition, further studies are required about the complex ecological interactions occurring in tropical forests, such as how trees interact with other plants, animals, and microorganisms and how disturbances such as monsoons, fires, and logging impact forest dynamics. There is also a need for more long-term studies of tropical tree growth and forest dynamics, as many existing studies only cover short periods, making it difficult to fully understand the factors that control forest growth and structure over the long term [19,20]. Improving our understanding of tropical tree growth and forest dynamics is essential for many reasons, including conserving tropical forests, managing forest resources, and predicting future global changes in the carbon cycle and climate. Ongoing research and monitoring efforts are essential for improving our knowledge of these important ecosystems [21].
Studying tree age and xylogenesis in tropical trees can be challenging due to a variety of reasons, such as the following:
- A lack of distinct growth rings: In temperate regions, trees often produce annual growth rings that can be used to determine their age accurately. However, many tropical trees do not produce distinct annual rings, or they may produce rings that are difficult to distinguish, making it more challenging to accurately determine their age and track changes in growth over time [22].
- High species diversity: Tropical regions are known for their high species diversity, which can make it challenging to study specific tree species in depth. Researchers may study multiple species in order to obtain a comprehensive understanding of tree age and xylogenesis in a given region [23].
- Limited data availability: In many tropical regions, data on tree age and xylogenesis may be limited or unavailable, making it difficult for researchers to develop accurate models and identify patterns over time.
- Environmental factors: Tropical regions are subject to various environmental factors impacting tree growth, including temperature, rainfall, and soil nutrient availability. Understanding how these factors impact tree age and xylogenesis can be complex and may require long-term studies and advanced analytical tools [24].
Overall, studying tree age and xylogenesis in tropical trees requires careful consideration of these factors and others, as well as a deep understanding of the unique challenges presented by tropical ecosystems.
Waisel and Fahn (1965) explained that cambial activity is regulated by an endogenous activity rhythm and a complex of environmental factors [25]. They mentioned that plants of tropical origin usually exhibit active cambium throughout the year. Therefore, it is not easy to study trees through woody ring counting and the process of wood formation. Bass and Vetter (1989) wrote in the preface of a Special Issue of the IAWA Bulletin that it was time to find a method for studying trees in tropical forests to determine their age and growth rate [26]. Botanists and foresters recognize the importance of such studies in achieving wiser use of tropical timber. Subsequently, more extensive studies of tree age and stem growth rates in tropical climates have been conducted. Another important driver is climate change studies, as in some areas, the climate data collected by the meteorological department are insufficient or cover short periods of time. Therefore, studying the age of long-lived tropical forests would help determine past climates.
In the early twentieth century, the study of dendrochronology expanded to tropical zones. The rings that trees produce annually provide information about the environment in which the tree grows, and the benefits of studying dendrochronology can be applied to many other subjects (e.g., past climates [27], forest fires [28], insect outbreaks [29], and archeology [30]). Climate change is now evident and is the biggest challenge of this century, affecting both plants and animals [31]. Therefore, the study of annual rings, which can shed light on past climates, now raises questions about the impact of climate change on tree growth [32].
In temperate regions, the annual course of cambial activity (dormancy and activation) is regulated by temperature and/or photoperiod [33]. In temperate zones, earlywood has a shorter and lower density, resulting from thinned tracheids or fibers of large radial diameter. Latewood is formed in the late summer or autumn when cambial cell division and expansion decline. The growth of temperate trees is generally slower than that of tropical trees, resulting in denser wood with smaller cells. Boreal trees [34] typically have a short growing season, lasting only a few months during the summer when temperatures are above freezing. The growing season in boreal regions is shorter than in temperate regions. As the days shorten and temperatures begin to drop in the fall, cambial activity slows down and eventually stops during cold season. In the spring, when temperatures once again rise above freezing, cambial activity resumes, and new growth begins. The timing and duration of cambial activity in boreal trees can vary depending on factors such as temperature, heat sum, and the availability of nutrients [35,36].
Tropical trees grow in regions near the equator, where the climate is warm and humid year-round. The wood formation in tropical trees is characterized by the absence of distinct growth rings. Instead, tropical trees may have a ring-porous, diffuse-porous, or semi-ring porous structure, with smaller and more uniformly sized cells throughout the wood. This results in a more uniform color and texture of the wood. The growth rate of tropical trees is generally faster than that of temperate or boreal trees, resulting in less dense wood with larger cells [37,38,39,40].
While observing a tree’s phenology (the study of cyclic and seasonal natural phenomena, especially in relation to climate and plant life) can provide valuable insights into tree growth and development, it is not enough to fully understand tree growth in tropical regions. Worbes (1995) proposed various methods, including non-destructive methods, such as phenological investigation. Plant phenology observations can provide valuable information about the timing of key developmental stages, such as budburst and leaf senescence, but they may not necessarily provide a clear indication of the process of wood formation in trees [41]. Dendrometer band measurements were used to track tree growth, but the enlargement and contraction of the tree may not reflect the actual growth of the tree. Destructive methods consist of wood anatomy (cambium wounding, pinning technique, micro cores), ring-width analysis, radiocarbon dating, and stable isotope signatures, which can provide accurate information on the temporal and spatial features of cell wall traits.
The combination of wood anatomy and stable carbon isotopes can be very useful in studying tree growth in tropical forests [42]. The anatomy of wood can provide information on the structure and composition of the cell wall, including the size, density, and arrangement of the cells. This information can be used to infer environmental conditions and how they may have affected the growth of the tree. Stable carbon isotopes can be used to determine the photosynthetic pathway of the tree, as well as its water-use efficiency. Trees that use the C3 pathway, which is more common in tropical forests, have a different stable carbon isotope signature than those that use the C4 pathway, which is more common in drier environments. By measuring the stable carbon isotope ratios in the wood of a tree, it is possible to infer the environmental conditions in which it grew, such as temperature, rainfall, and humidity, and to reconstruct changes in these conditions over time [43]. By combining these two approaches, researchers can gain a better understanding of how trees grow and respond to environmental conditions in tropical forests. This information can be used to develop more accurate models of forest dynamics and to inform conservation and management strategies [42,44]. Van der Sleen et al. (2015) pointed out that intrinsic water-use efficiency in both understory and canopy trees of tropical trees increased by 30–35% over the past 150 years as atmospheric CO2 concentrations increased [45]. They found no evidence for the recommended simultaneous acceleration of individual tree growth. They concluded that no growth of tropical trees was stimulated during the increase in the amount of CO2. This study is consistent with the study of tropical trees in Thailand [46].
In the case of oxygen and hydrogen isotopes, globally, the pattern of δ18O and δ 2H values in precipitation are correlated broadly with temperature, with low values characteristic of high latitudes and altitudes [47]. Tropical δ18O rainfall and paleo-records are often interpreted using the “amount effect” [48]. Current research shows that other factors affecting δ18O such as groundwater can also have a variable residence time, and its isotope composition may reflect current environmental conditions [49].
Searching for articles related to tropical and subtropical trees, cambium, wood formation, xylogenesis, and phenology is a common approach used in scientific research. Search engines provide access to a vast array of scientific literature and can help researchers stay up to date with the latest research in their field. However, a review summarizing all available knowledge related to cambium activity, wood formation in tropical and subtropical trees, and the response of wood formation to the climate composed of tropical trees in South America, tropical Africa, Southwest Asia, and Southeast Asia was not available yet. Therefore, we decided to undertake the necessary research and assemble all available state-of-the-art studies in this paper. Our study does not include any investigation on mangrove forests, since a mangrove forest is a unique type of forest that grows in the intertidal zones of tropical and subtropical coastlines. Mangrove forests are composed of a group of salt-tolerant trees and shrubs that are adapted to living in the harsh and dynamic environment of the tidal zone and were thus excluded.
2. South America
South America is home to more than 10,000 plant species. The Amazon Rainforest is a tropical forest that is older and covers a larger area than other tropical forests worldwide [50,51]. Due to the abundance of species diversity in tropical and subtropical forests and the absence of a clear seasonality in tropical climates, the study of tree age or tree growth patterns should be based on an integrated approach.
Since these regions are home to a diverse array of forest types, it can be useful to focus on each forest type separately to better understand the unique factors that affect tree growth in each ecosystem. Borchert (1999) stated that in a dry forest in Costa Rica, deciduous hardwood species grow in dry sites, and the stem water potential declines before the leaves fall [52]. In contrast, in trees in wet sites, the stem water potential increases simultaneously with the leaf fall and thereafter with new leaves and flowering. He concluded that many trees in tropical dry forests could avoid the impact of seasonal droughts. Water stored in the soil or in stem tissues can be used after leaf fall, favoring the production of new leaves and flowers. In dry forests of Costa Rica, simultaneously observing rainfall, soil moisture, evaporation, growth, and phenology, Borchert concluded that heavy rain caused tree trunk expansion, leading to leaf production and cambial activity. At the end of the rainy season, soil moisture decreases, causing stem shrinkage, cambium dormancy, and water stress in leaves. Borchert et al. (2005) noticed that in many seasonal dry tropical forests, the growing season is not significantly reduced by prolonged periods with low rainfall because soil water storage buffers trees against seasonal drought [53]. For example, in eastern Amazonian evergreen forests with an annual rainfall of 1500–2000 mm, there is a distinct dry season of 4–5 months, but the extraction of >500 mm soil water from up to an 8 m soil depth enables trees to retain full leaf cover during seasonal droughts [54].
Trees from the Central Amazonian inundation forest and the Gran Sabana in Venezuela were investigated using a combination of techniques, such as wood anatomy, radiocarbon dating, and ring width [37]. The author concluded that the trees in tropical regions with severe annual dry seasons or inundation phases form annual rings, and he recommended three characteristics of wood/tree to choose for determining their growth rhythm, i.e., the trees show regular growth around the entire stem, the trees show lobate growth and cannot be used, and the surface of the stem disks should be sanded to be examined under a microscope. Nevertheless, Worbes (1995) proposed that an annual dry season of 2–3 months and less than 60 mm of monthly precipitation induce the production of annual rings in tropical trees [41]. In the central Amazonian floodplain, the growth rhythm of trees is triggered by the annual long-term flooding, which results in a cambial dormancy and the formation of annual rings. This allows the determination of tree ages and growth rates [55]. In addition, Worbes (2002) summarized that the long-lasting and high-rising flooding in the floodplains in Amazonia induces cambial dormancy and the consequent formation of annual rings in the wood [39]. During periods of flooding, the availability of oxygen to the roots may be limited, which can slow down or even halt cambium activity. However, non-flooded tropical trees shed their leaves in short dry periods.
In addition, Dünish et al. (2002) studied three species, Swietenia macrophylla (semi-deciduous), Carapa guianensis (evergreen), and Cedrela odorata (deciduous), which belong to the Meliaceae family. These trees are from forest plantations, and their ages are known. These authors used stem discs and the pining method to explain the relationship between the external factors and the intra-annual cambial growth dynamic of these species. They found that the juvenile wood of C. odorata and S. macrophylla formed annual rings, but C. guianensis had more bands than its age [56]. They observed, before a cambial dormancy, that terminal parenchyma bands were formed in S. macrophylla and C. guianensis, whereas a band of fibers with reduced lumina was formed in C. odorata. Dünish et al. (2003) reported that the cambiums of C. odorata and S. macrophylla were active once a year but had different beginnings and endings. They concluded that the water supply determined the cambium growth of C. odorata and S. macrophylla [57].
Marcati et al. (2006) studied the wood formation and phenology (budding, mature leaves, leaf fall, flowering, and fruiting) of Cedrela fissilis (Meliaceae) from a semi-deciduous forest in Botucatu (São Paulo State, Brazil) [58]. C. fissilis forms annual rings, and cambium activity occurs during the rainy season when leaves emerge. Cambium activity stops during the dry season when these trees shed their leaves. The cambium began to actively form new layers of axial parenchyma, which appeared close to and around the large earlywood vessels, and when the cambial zone was inactive, it exhibited one or two layers of immature fibers and axial parenchyma cells. Bräuning et al. (2009) studied Cedrela montana, a deciduous broad-leaved tree species that grows in a humid mountain rainforest in southern Ecuador. They applied a high-resolution dendrometer and wood anatomy to investigate cambial activity and growth dynamics to uncover what factors affect the width of the ring. They found that C. montana showed a regular seasonal growth rhythm, with cambial activity from January to April. During the humid period, daily stem diameter variations appeared to be smaller than during the drier period [59]. The authors emphasized that cambial activity is limited by available moisture, even in such a humid mountain environment. The beginning of cambial growth showed the formation of a marginal parenchyma band. In addition, these authors constructed the C. montana tree-ring index and found that temperature influenced tree-ring width. Later, Marcati et al. (2008) investigated the relationship between seasonal cambial activity, xylem and phloem development, and phenology in Schizolobium parahyba, a fast-growing semi-deciduous forest tree. Wood samples were collected, and phenology was observed monthly from 2002 to 2003. S. parahyba produces annual growth in wood. These growth rings are a result of the seasonal changes in environmental conditions that affect the growth of the tree. The beginning of the cambial activity coincided with the water replacement in the soil, before the trees entered the budding, in September; the cambium was beginning its activity, when there was still a deficit of water in the soil. The highest cambial activity was observed in January and March, coinciding with excess water in the soil, when the trees had mature leaves. The reduction in cambial activity to a minimum in May and June coincided with the dry season, when the trees lost some their leaves.
Rodriguez-Ramirez et al. (2022) investigated the effect of climate on tree ring width and vessel traits (diameter, vessel density, vulnerability index and hydraulic diameter) of three Cedrela species (Cedrela fissilis, C. nebulosi and C. angustifolia) in Peruvian tropical Andean cloud forests [60]. They found that all Cedrela species showed a significant reduction in radial growth, and adjusted vessel traits correlated with temperature, precipitation, and evapotranspiration. They concluded that the diffuse and semi-ring porous Cedrela species developed smaller vessels in markedly dry periods to acclimate to actual climate variations. This adaptive response is known as “drought-induced plasticity”. In southeast Brazil, Lisi et al. (2008) examined 24 semi-deciduous tree species in forests using plant phenology observations, cambium marking, and permanent dendrometer bands for 7 years [61]. They found that trees lost their leaves during the dry season and grew new leaves at the end of the same season; stems expanded during the rainy season and shrunk during the dry season. The number of tree rings formed after cambium marking coincided with the years since the wood sample was extracted. They concluded that marginal parenchyma was the most common and most identifiable characteristic that defined the growth ring boundary [61]. The wood characteristics of 37 tree species in the subtropical Yungas and Chaco forests from northwestern Argentina were studied. The results showed that 27 out of the 37 wood species had distinct growth rings. In the drier area, the Chaco plain, the growth ring boundary was indicated by marginal parenchyma, in contrast to mountain forest trees, which showed thicker fibers at the end of the ring [62].
Grogan and Schulze (2012) studied inter-annual and seasonal in-stem diameters on three species—Swietenia macrophylla (Meliaceae), Hymenaea courbaril (Fabaceae), and Parkia pendula (Fabaceae)—in a dry tropical forest in southern Parà, Brazil [63]. Only the diameter growth rates of S. macrophylla displayed a strong positive correlation with the total annual precipitation. This finding confirms results found in Mexico, Belize, and southern Amazonia, indicating that the diameter growth rate of S. macrophylla increases with total annual rainfall [56]. The results from Vernier stem growth measurements showed that all three tree species simultaneously expanded during the rainy season and were almost unchanged or contracted during the dry season. The researchers concluded that external measurements of tree growth might not reflect true growth related to wood formation, as trunk expansion or contraction may depend on the moisture content in the bark [63].
Totti de Lara et al. (2016) investigated the growth dynamics of an evergreen shrubby species, Cordiera concolor, from a semi-deciduous tropical forest with distinct wet and dry seasons [64]. They found that the cambium is dormant during the rainy season, and dormancy lasts up to 9 months. Cambium activity was positively related to day length, and the periods of its onset and termination were not concurrent with the beginning and end of the rainy season. C. concolor exhibited a reactivation of cambial activity in December, one month after budding. This finding supported the idea that leaf emergence and young leaves are responsible for auxin supply and the stimulation of the cambial zone [65]. Bosio et al. (2016) investigated the periodicity and environmental drivers of apical and lateral growth in Kielmeyera grandiflora (Calophyllaceae), Cerrado areas, in Brazil [66]. They concluded that all of the phenological phases were modulated by the environmental pattern. Additionally, differentiation between the xylem and phloem was also seasonal, and these processes coincided with the beginning of cambium activity. The vascular cambium remained active for 7 months, and the differentiation of secondary xylem and phloem occurred for about 8–9 months, until May and June, about 1–2 months after the end of cambial activity. The dormancy of vascular cambium and the ending of xylem and phloem differentiation coincided with increasing senescent leaves and leaf shedding. Although the weather in the study area had a distribution of rain across two rainy seasons (the first rainy season occurred between October 2011 and June 2012 (1745 mm), and the second occurred between October and December 2012 (682.1 mm)) and two dry seasons (August–September 2011 (24.8 mm) and July–September 2012 (74.1 mm)), the onset of cambial activity occurred once. They confirmed that in K. grandiflora, a diffuse-porous deciduous species, the resumption of cambial activity and the formation of the first vessel element occurs after bud-opening. It is possible that the auxin produced in apical buds may be involved with the resumption of cambium activity [67].
Cardoso et al. (2012) investigated stem growth and phenology for two tropical trees (Citharexylum myrianthum (Verbenaceae) and Senna multijuga (Fabaceae)) in contrasting soil conditions. They found that some differed in the frequency, average timing, and intensity of their phenological phases. They proposed that, in addition to the climatic factors affecting phenology, soil characteristics also play an important role in determining phenological patterns and growth. Although some tropical trees have annual rings that can be determined through dendrochronology, the understanding of the development of individual cells from mitosis to lignification is still incomplete [67,68,69].
Shimamoto et al. (2015) examined 103 individuals of 10 species growing in the wet areas of the Atlantic Forest. They installed permanent dendrobands to assess monthly plant growth for 22 months [70]. In addition, the wood samples were observed at macroscopic and anatomic levels. Although all trees formed annual rings, those of trees from deciduous forests were more clearly visible than those of semi-deciduous and evergreen forests. Five out of 10 woody species showed increased growth in relation to temperature and day length. The researchers concluded that even in wet areas and less seasonal climates, warmer and longer days during the hot season resulted in increased cambium activity and stem expansion. Volland-Voigt et al. (2011) investigated the broadleaved deciduous tree species Tabebuia chrysantha (Bignoniaceae) growing in different environments, a tropical lower mountain forest and a dry forest in southern Ecuador. Stems of this species were measured using high-resolution dendrometers, and xylem features were analyzed by microscopic observations during a 3.5-year-long period from 2006 to 2009. T. chrysantha formed annual growth rings in both of the study areas. The length of the cambial active period varied from 3 to 7 months in the tropical lower montane forest and from 2 to 4 months in the dry forest. The annual growth boundary of T. chrysantha consists of a marginal parenchyma band. The results of this study were unable to account for the net growth rate; they provided only seasonal tree growth behavior in two different tropical areas. Both stem dehydration and rehydration in T. chrysantha indicated a low water storage capacity under dry conditions and a higher water storage capacity during humid periods [71].
The interesting study of Baker et al. (2017) introduced radiocarbon dating to examine Cedrela trees from four areas, Bolivia, Ecuador, Venezuela, and Suriname. They found that, in Suriname, Cedrela formed two rings each year, rainfall had a bimodal distribution, and it was much less pronounced than that at the other sites. In contrast, the other sites showed only wet and dry seasons per year [72].
In conclusion (Table 1), studying tree age, wood formation, and stem expansion in tropical trees in South America can involve the use of various methods and techniques:
- Dendrochronology: the study of tree rings. It can provide information on the age and growth of trees. In tropical regions, where growth rings may not be as distinct, researchers may use other tree ring parameters such as tree ring width, density, and isotopic composition to infer past climate conditions and tree growth [38,39,41,73].
- Radiocarbon dating can be used to estimate the age of trees by measuring the amount of radiocarbon in the wood [72].
- Wood anatomy: The microscopic examination of wood anatomy can provide information on the formation of growth rings, the distribution of different cell types, and the timing of wood formation [39,58].
- Remote sensing: Remote sensing techniques, such as light detection and ranging (LiDAR), can be used to measure the size and shape of trees and their growth patterns and structural characteristics [74].
- Field measurements: Researchers may take direct measurements of tree stem diameter, sap flow, and other physiological parameters to study stem expansion and wood formation [35,75,76].

Table 1.
Tree name/information, locations, methods, references in the studies in South America.
Table 1.
Tree name/information, locations, methods, references in the studies in South America.
| Tree-Name/Information | Family | Location/Climate | Methods | Cambial/Wood Formation | References |
|---|---|---|---|---|---|
| Well-drained site Brosimum lactescens Dipteryx panamensis Goethalsia meiantha Hampea appendiculata Hymenolobium mesoamericanum, Laetia procera Pentaclethra macroloba Virola sebifera Swamp site Carapa guianensis Hernandia didymantha Pentaclethra macroloba Virola koschnyi | Moraceae Fabaceae Malvaceae Malvaceae Fabaceae Salicaceae Fabaceae Myristicaceae Meliaceae Hernandiaceae Fabaceae Myristicaceae | La Selva, Atlantic lowlands of northeastern Costa Rica; wet and weakly seasonal | Radial dendrometer | Not directly studied. They considered cambial dormancy whenever no growth was registered for several days. | [35] |
| Bombacopsis quinate Cordia apurensis Terminalia guianensis Sapium styllare Cedrela odorata Swietenia macrophylla Pterocarpus vernalis Pinus caribaea Tectona grandis | Bombacaceae Boraginaceae Combretaceae Euphorbiaceae Meliaceae Meliaceae Papilionaceae Pinaceae Verbenaceae | Semi-deciduous forest of the Reserva forestall de Caparo, Venezuela; a dry season with mean monthly rainfall of <50 mm from January to March; the mean annual precipitation is 1700 mm per year | Tree-ring analysis, dendrometer, cambial marking | Distinctive growth zones appeared, except Cordia apurensis, annual growth period was related to rainfall pattern. | [38] |
| 139 species (see Table S1) | South America | Dendrochronology | [39] | ||
Cordia alliodora Luehea candida Randia sp. Tabebuia ochracea T. rosea
Astronium graveolens Hymenaea courbaril Licania arborea Samanea saman Sideroxylon tempisque Simarouba glauca Swietenia macrophylla Thouinidium pentandrum
Chlorophora tinctoria Dalbergia retusa Diospyros nicaraguensis Guazuma ulmifolia Lonchocarpus minimiflorum Myrospermum frutescens Piscidia carthaginensis
Bursera simatuba Cochlospermum vitifolium Plumeria rubra Spondias purpurea | Rubiaceae Boraginaceae Tiliaceae Rubiaceae Bignoniaceae Bignoniaceae Caesalpinaceae Anacardiaceae Caesalpinaceae Chrysobalanaceae Mimosaceae Sapotaceae Simaroubaceae Meliaceae Sapindaceae Caesalpinaceae Moraceae Fabaceae Ebenaceae Sterculiaceae Fabaceae Fabaceae Fabaceae Bombaceae Burseraceae Cochlospermaceae Apocynaceae Anacardiaceae | Hacienda La Pacifica, Cañas, Guanacaste, Costa Rica, dry forest area | Vegetative phenology | Not directly studied. | [53] |
| Cedrela fissilis | Meliaceae | São Paulo State, Brazil | Wood formation, phenology | The active period coincides with the wet season, growth rings are marked by parenchyma band, and the large early wood vessels of the growth rings are formed. | [58] |
| Aphananthe monoica Plearanthodendron lindenii Psychotria costivenia | Cannabaceae Salicaceae Rubiaceae | Sub-tropical rainforest located in central Veracruz, Mexico; total annual rainfall was 2217.2 mm, the rainy season lasts 4 months (June–September); there is a short dry season (March and April) | 1 ha plot, measured diameter increment. Generalized canonical correlation analysis (GCCA), three thermo-hygrometer data loggers | Not directly studied. Cambial activity periods were associated with maximum temperature and day length. | [75] |
| Schizolobium parahyba | Fabaceae | School of Agronomic Science, Sào Paulo; the mean annual rainfall for 2002–2003 was 1399 mm, the mean annual air temperature was 20.2 °C | Wood block, phenology | The reduction in cambial activity to a minimum correlates with the dry season and leaf fall. The higher cambial activity correlates to the wet season. | [77] |
| Astronium graveolens Aspidosperma polyneuron Tabebuia serratifolia Zeyheria tuberculate Savia dictyocarpa Ocotea porosa Cariniana estrellensis Cariniana legalis Caesalpinia ferrea Copaifera langsdorffii Hymenaea courbaril Peltophorum dubium Schizolobium parahyba Anadenanthera macrocarpa Piptadenia gonoacantha Centrolobium tomentosum Dipteryx alata Myroxylon balsamum Platycyamus regnellii Colubrina glandulosa Balfourodendron riedelianum Esenbeckia leiocarpa Guazuma ulmifolia Aegiphila sellowiana | Anacardiaceae Apocynaceae Bignoniaceae Bignoniaceae Euphorbiaceae Lauraceae Lecythidaceae Lecythidaceae Leguminosae Leguminosae Leguminosae Leguminosae Leguminosae Leguminosae Leguminosae Mimosaceae Mimosaceae Leguminosae. Leguminosae Rhamnaceae Rutaceae Rutaceae Sterculiaceae Verbenaceae | Southeast Brazil, semi-deciduous forest | Plant phenology observation, window method, and permanent dendrometer band | The semi-deciduous trees show a reduced rate of incremental growth during May and June (dry season) and faster rate during October and November (early rainy season). | [61] |
| Aphananthe monoica Pleuranthodendron lindenii Psychotria costivenia | Cannabaceae Salicaceae Rubiaceae | 1 ha, a subtropical rainforest located in central Veracruz, Mexico; total annual rainfall was 2217.2 mm, the rainy season lasts 4 months (June–September), There is a short dry season (March and April) | Phenology, radial growth | Leaf initiation, flowering, and vascular cambium activity were the most closely related simultaneous events during the summer (April–August). | [75] |
| Citharexylum myrianthum Senna multijuga | Verbenaceae Fabaceae | Rio Cachoeira Reserve in Antonina, Parana State, southern coast of Brazil | Stem growth, phenology | Not directly studied. | [68] |
| Swietenia macrophylla Hymenaea courbaril Parkia pendula | Meliaceae Fabaceae Fabaceae | A seasonal dry tropical forest in southeast Parà, Brazil | Vernier dendrometer, phenology | Not directly studied. | [63] |
| Cedrela odorata Swietenia macrophylla Carapa guianensis | Meliaceae Meliaceae Meliaceae | Manaus-Amazȏnas, Aripuanã-Mato Grosso, Santarem-Paral | Stem discs, cambium samples | S. macrophylla had cambial dormancy from September to November and cambial reactivation in May; in C. odorata, cambium divided from January to April, and was dormant from September to November; in C. guianensis, cambium dormancy was not consistent. | [56] |
| Cedrela odorata Swietenia macrophylla | Meliaceae Meliaceae | Rio Branco, Brazil | Tree ring, discs | S. macrophylla, cambial activity occurred throughout almost the whole year. C. odorata, cambial activity occurred in the rainy season from September of the previous year to June of the current year. | [57] |
| Citharexylum myrianthum Schizolobium parahyba Senna multijuga Virola bicuhyba Handroanthus serratifolius Cabralea canjerana Cariniana estrellensis Inga edulis Inga marginata Myrsine coriacea | Verbenaceae Fabaceae Fabaceae Myristicaceae Bignoniaceae Meliaceae Lecythidaceae Fabaceae Fabaceae Primulaceae | The wet areas of the Atlantic Forest; an average rainfall of 1778 mm, no dry season and rare occurrence of frosts; the average temperature was 20.8 degrees Celsius | Permanent dendrobands | The girth increments of the 10 species were, in general, weakly or not related to rainfall, but strongly and positively related to temperature and day length. | [70] |
| Kielmeyera grandiflora | Calophyllaceae | In the Cerrado sensu stricto, Botucatu, Sào Paulo, Brazil | Bud and leaf phenology, wood block | The cambium was dormant in May, during the rainy season. Photoperiod and temperature may be important in controlling the growth of K. grandiflora. | [66] |
| Cordiera concolor | Rubiaceae | Botanical Garden of the Uni, Estadual Paulista (UNESP) | growth dynamics of an evergreen shrubby species | The cambium is dormant during the rainy season. Cambial activity was positively related to day length, and although it occurred in the rainy season. | [64] |
In conclusion, trees in tropical and subtropical South America have been extensively studied regarding the factors that regulate tree growth using a variety of methods. This paper reviewed studies on the factors contributing to tree growth in tropical and subtropical areas in South America, starting primarily from phenotypes, as there may be a link between phenotypes and environmental conditions, especially weather conditions. However, it was found that phenology studies alone may not be able to clearly demonstrate the tree growth process; therefore, the study of wood anatomy was also involved. Nonetheless, since all anatomical studies were concerned with broad-leaved trees, which have more complex cells than needle-leaved trees, only a few research articles that studied wood development have been found.
3. Tropical and Subtropical Africa
The cambium dynamics and xylogenesis of trees in Africa can be influenced by environmental factors (e.g., rainfall, temperature, and soil conditions) and management practices (e.g., selective logging and forest restoration). However, deforestation, climate change, and other pressures, which can affect tree growth and survival [78,79], also threaten many tropical tree species in Africa.
Despite these challenges, researchers have made progress in understanding the process of xylogenesis in some tropical tree species in Africa. For example, studies have shown that the weather in Munesa Forest in southeastern Ethiopia displays two rainy seasons. The first one covers from March to May, and the second from July to September. Krepkowski et al. (2010) investigated four tree species, the evergreen native conifer Podocarpus falcatus, the introduced evergreen conifer Pinus patula, the broadleaved evergreen tree Prunus africana, and the deciduous broadleaved tree Celtis africana [80]. They used a combination of high-resolution electronic dendrometers and wood samples. They concluded that evergreen conifer tree species could have an initial wood formation during the short rainy season, while the broadleaf tree cambium begins to divide during the more extended rainy season. The two study techniques yielded different results because stem expansion is due to water uptake, which may not be the result of wood formation. The same researchers (Krepkowski et al., 2012) have studied Podocarpus falcatus further by using wood anatomy and carbon-14. They still could not determine the tree’s exact age, but it was estimated to be about 500 years old [81]. It is possible that each lobe of meristematic tissue in the non-round lobe trunk of P. falcatus does not divide simultaneously, as suggested by other studies on this species. The cambium tissue in each lobe of the trunk may be influenced by different environmental or physiological factors, leading to variations in the rate of cell division and the timing of wood formation. This could result in the formation of distinct growth rings in each lobe of the trunk, with variations in ring width or clarity. Based on this observation, the researchers recommend future studies of P. falcatus in areas with strong seasonality and in conjunction with carbon-14 dating to determine the age and/or stable isotope measurements to better understand how this species grows and if there is potential for the study of annual rings.
Forest ecosystems are complex and multifaceted, and a range of environmental and biological factors can affect the growth and survival of tree species. Stahle et al. (1999) used woody disk samples of Pterocarpus angolensis to investigate the annual growth rings of this tree species in Zimbabwe [8]. Their goal was to determine whether P. angolensis had distinct annual growth rings that could be used to track the growth and age of individual trees and whether the formation of these rings was consistent with the seasonal rainfall patterns in the region. Based on their analysis, they found that P. angolensis had distinct annual growth rings visible in the tree’s wood [8]. These rings were formed by the activity of the vascular cambium, which produces new xylem cells each year. The researchers also found that the formation of these rings was closely tied to the seasonal rainfall patterns in western Zimbabwe, with ring width varying depending on the amount and timing of rainfall. The results of this study have important implications for forest management in the region, as they suggest that the growth and productivity of P. angolensis may be influenced by variations in rainfall patterns. By tracking the growth rings of individual trees, forest managers may better understand the long-term growth and productivity of P. angolensis and make more informed decisions about forest management and conservation strategies.
Couralet et al. (2010) conducted research on three common, evergreen understory tree species, Aidia ochroleuca, Corynanthe paniculata (both Rubiaceae), and Xylopia wilwerthii (Annonaceae) from the Mayombe forest, west of the Democratic Republic of Congo. The characteristics of the wood are as follows: A. ochroleuca shows a frequency of vessel decreases to the boundary of the annual rings; C. paniculata has a ring boundary marked by one or two lines of radially flattened fibers; X. wilwerthii has a ring boundary marked by two to three lines of very-thick-walled latewood fibers. Overall, trees of the same species synchronized their radial stems [82]. The results showed that the three species exhibited distinct growth rings that were related to the rainy season in different months. The cambial activity of A. ochroleuca appeared at the beginning of the rainy season, while that of C. paniculata and X. wilwerthii resumed only later in the wet season. The researchers suggested that for A. ochroleuca, which has a shallow root system, the moisture from the first rain encourages cambium to divide and stem to expand, in contrast to the other two species, which have deeper root systems. Therefore, stem growth of A. ochroleuca would mostly occur at the beginning of the wet season, whereas the stem growth of the other two species occurred in response to rainfall at the end of the rainy season. Trouet et al. (2012) investigated six Brachystegia spiciformis trees, a dominant species of the Miombo woodland in western Zambia [83]. The results showed that the beginning and end of cambial division for all six trees occurred during the same period but not following the rainy season; the onset of cambial reactivation coincided mainly with the rainy season. All six trees had three to four months of cambium growth.
Martin and Moss (1997) studied the age of Acacia tortilis from northern Kenya. They divided trees by height into three groups and cut the wood into discs to count the rings [84]. Ring counting was unsuccessful due to unclear ring boundaries. These researchers recommended the use of a tree of known age in conjunction with pinning techniques. However, Prins and van der Jeugd (1993) successfully counted the rings of Acacia tortilis [85]. Anderson and Krzywinski (2007) examined the age of Acacia tortilis in the hyper-arid eastern desert of Egypt [86]. They realized that A. tortilis’s life history, longevity, and growth are poorly known; therefore, dendrochronological studies based on this species from the eastern desert are scarce.
In contrast, Gebrekirstos et al. (2008) examined Acacia senegal, Acacia seyal, Acacia tortilis, and Balanites aegyptiaca from semi-arid savanna woodlands in Ethiopia [87]. They found that all four tree species formed distinct growth boundaries. They all displayed a highly positive correlation with precipitation, and their annual rings exhibited a substantial decline correlated with El Niño–Southern Oscillation events. Van Camp et al. (2018) introduced a combination of pinning techniques (cambial wound) with continuous measurement of sap flow and stem diameter variation to investigate the seeding of the young African tropical tree Maesopsis eminii [88]. They found that wood formation completely stopped during drought and was associated with stem shrinkage. An unexpected increase in stem diameter was observed later, but it was not accompanied by wood formation. The authors explained those changes by alluding to the stress caused by consecutive droughts; a first drought episode does not considerably affect a tree’s growth, because internal water reserves are still adequate, but a second drought episode is harsher because internal water or carbon reserves are not replenished [89,90].
Battipaglia et al. (2015) developed three tree ring indexes for Entandrophragma cylindricum (Meliaceae), Triplochiton scleroxylon (Sterculiaceae), and Erythrophloeum ivorense (Caesalpiniaceae) growing in southeastern Cameroon. They found that increased CO2 had not stimulated tree growth in the studied area. However, the tree ring widths correlated to the increase in local temperature [91].
Dié et al. (2012) analyzed cambial activity in relation to rainfall regarding the annual rings and intra-annual growth zones of xylem and phloem in teak (Tectona grandis), which is not a native species in Africa, in a plantation on the Ivory Coast [92]. They found that a 3-month dry season resulted in cambial dormancy, leading to the formation of annual growth rings. Teak sheds leaves during the dry period, but trees do not stand completely leafless; sprouting occurs with the start of the major wet season and dormancy in November. Resumption of cambium activity occurs in February and peaks in May. The formation of phloem in teak takes place simultaneously with the formation of xylem. The authors suggested that the xylem growth zone function for water transport must be developed early in the growing season in order to supply sufficient water for increased transpiration, photosynthesis, and radial growth. The authors proposed that teak trees on Ivory Coast begin the growth of annual rings in physical response to new leaves, similarly to temperate ring porous trees; the early development of new functional xylem takes place before new leaves begin growing.
For wood in tropical and subtropical Africa (Table 2), the study method most likely to be successful is to start with trees of known age and look at annual ring patterns and then study larger or presumably older trees.

Table 2.
Tree name/information, locations, methods, references in the studies in Subtropical Africa.
4. Southwest Asia
The study of age, cambial dynamics, and wood formation of tropical trees in India has been conducted using various methods. Venugopal and Krishmanurthy (1994) studied cell division in the vascular cambium of Albizzia lebbeck, Dalbergia sissoo, Tectona grandis, Terminalia crenulata, Calophyllum inophyllum, Mangifera indica, and Morinda tinctoria using 0.5 cm stem twigs of 3–5-year-old trees [93]. For A. lebbeck, D. sissoo, and T. crenulata, they registered two annual peaks of cambial activity that alternated with two dormancy periods, whereas one annual peak of cambial activity was found for the other species. Rao and Rajput (1999) investigated vascular cambium during the growth of T. grandis in a moist deciduous forest (MDF) and a dry deciduous forest (DDF) in western India [94]. In both areas, cambial activity began simultaneously with xylem and phloem differentiation in June at a maximum temperature of 35 °C. New leaf sprouting occurred before cambial activation in June in the MDF and one month later in the DDF. Cambial cells divided, and their differentiation peaked when the rain was heavy. The differentiation of Thai teak begins during the transition from summer to the rainy season, and we suspect that high temperatures in the summer and the first moisture at the beginning of the rainy season trigger cambium reactivation [95,96].
Nanda et al. (2014) observed a dry deciduous forest’s phenology (leafing, flowering, and fruiting) in central Western Ghats, India [97]. They concluded that leafing and flowering occur in the summer or pre-monsoon, and fruiting occurs from monsoon to post-monsoon. These phenological activities may be associated with the alteration in rainfall, temperature, relative humidity, and hours of sunshine. Singh and Venugopal (2011) investigated the cambial activity and the annual rhythm of xylem production of Pinus kesiya, growing in a sub-tropical wet forest of northeast India [98]. They found that cambium reactivation and dormancy occur after the sprouting of new leaves in mid-February, simultaneously with cone formation. They confirmed that cone formation could enhance cambial activity and that the low temperatures and precipitation induce dormancy. These factors were similar to those involved in the inactivation of the meristem activity of Pinus kesiya in Thailand [32].
In conifers, one trigger of cambial reactivation is the production of cones. This is because the production of cones requires a significant amount of energy and resources, which must be obtained from the tree’s vascular system. As a result, the production of cones can stimulate the cambium to become active and start producing new growth. On the other hand, cool temperatures and low precipitation can cause the cambium to become dormant. The cambium requires warmth and moisture to remain active and produce new growth. Without these conditions, the cambium can become dormant and cease growth until conditions become favorable.
Swaminathan and Anbarasu (2022) caused injuries on 11 tree species: Albizzia lebbek, Azadirachta indica, Delonix regia, Lannea coromandelica, Madhuca longifolia, Pongamia glabra, Peltophorum pterocarpum, Terminalia catappa, Senna siamea, Syzygium cumini, and Terminalia arjuna, in India [99]. They wanted to observe tissue recovery one year after the injury. They reported that only the fast-growing L. Coromandelica, a deciduous hardwood, exhibited cambial growth—or callus formation. In India, branches of L. Coromandelica were heated to test whether the cambium would divide as it did in temperate plants [100]. In the heated branches, reactive and dormant cambium were found. The branches exposed to heating exhibited a wide cambial zone with fewer differentiated xylem elements than the control branches, a disappearance of storage starch in xylem elements, and the removal of dormancy calluses from the sieve element. The results of this study are consistent with those of studies on another deciduous hardwood [101]. Table 3 lists tree species, locations, and the methods of study in Southwest Asia.

Table 3.
Tree name/information, locations, methods, references in studies in Southwest Asia.
5. Southeast Asia
The Dutch meteorologist C.A.W. Coster [102,103] came to Indonesia interested in studying Java trees that may have clear annual rings that could be used to construct a long-term climate record [103]. He was influenced by the work of the German climatologist W. Köppen, who suggested that the growth rings of tropical trees could be used to study the region’s climate. Coster (1927, 1928) showed a clear relationship between teak tree ring width, seasonal precipitation, and phenology [103]. This is because Java has two different climatic conditions: the east is influenced by seasonal monsoons, and the west is an ever-wet zone. In addition, due to these different climates, the trees growing in the east have distinct rings, while those in the west have indistinct rings. In addition, Coster (1927, 1928) demonstrated the synchronization of phenology and wood anatomy [103].
In addition, Rahman et al. (2019) investigated cambial activities in four tropical hardwoods grown in Indonesia: Acacia mangium, Eucalyptus urophylla, Neolamarkia cadamba, and Tectona grandis [104]. They concluded that absent or low precipitation for 3–4 months induces a pause in cambial activities. On the contrary, cambial reactivation may continue throughout the year in the four tree species if precipitation is continuous.
Cambium activity and xylem formation in tropical trees in Malaysia can vary depending on the species, environmental conditions, and other factors [105].
Fujii et al. (1999) investigated xylem development using cambial blocks in three species growing in Sarawak: Shorea patoiensis, Shorea pinanga, and Shore dasyphylla. A year-and-a-half-long study spanning two rainy seasons and one dry season showed that the studied cambium was dormant [106]. They concluded that the xylem development of these three species was not simply related to the alternation between rainy and indistinct dry seasons. The cambial activity of these sample trees is not related to climatic conditions.
Furthermore, Wang and Hamzah (2018) explored the cambium activities of Shorea leoprosula and Shorea acuminata trees, with different stem diameters growing in natural lowland dipterocarp forests in Malaysia [107]. The cambium of the two Shorea species exhibited periods of active and less active growth. The monthly mean relative humidity and monthly mean vapor pressure deficit were crucial factors affecting the number of cambial cells of S. acuminata with larger stem diameters and S. parvifolia with different stem diameters. The total monthly precipitation had a significant positive correlation with the number of cambial cells in S. parvifolia with different stem diameters. It is generally thought that larger trees tend to have more cambial cell layers than smaller trees, since the cambium is responsible for producing new layers of xylem and phloem tissues that contribute to the growth in stem diameter [108]. The two Shorea species respond in agreement with this generalization. However, it is important to note that the relationship between stem diameter and the number of cambial cell layers is not always straightforward. The number of cambial cells can also be influenced by other factors, such as environmental conditions, genetic factors, and growth rates [109,110].
Wang and Hamzah (2019) monitored the cambial activity of Macaranga gigantea and Endospermum diadenum growing in tropical forests [111]. The rhythm of cambial activities was found to be different between the two species, even if stem diameter was similar. M. gigantea examples with smaller stem diameters exhibited higher annual diameter increments, had longer active cambial growth periods, and produced a higher number of cell layers than those with larger stem diameters. In contrast, E. diadenum examples with larger stem diameters showed higher diameter growth increments, longer active growth periods, and a higher number of cell layers than trees with smaller stem diameters. This study concluded that there was no correlation between cambial activities and the number of cambial cells with climatic data. Based on the information provided, the study on the two pioneer species M. gigantean and E. diadenum growing in tropical forests in Malaysia indicated that the pattern of wood formation and tree size within a single year (intra-annual) varied depending on the species and the size of the tree. It is important to note that it is difficult to provide a more comprehensive understanding of the study or its implications without further context or details. However, the conclusion suggests that there may be important species-specific and size-specific factors to consider when studying or managing tropical forests, particularly concerning tree growth and productivity.
Nobuchi et al. (1995) applied the pinning method by using a nail on Hopea odorata (Dipterocarpaceae) growing in subtropical forests [100], but the amount of radial growth was not correlated to rainfall. Palakit et al. (2016) investigated the leaf phenology and wood increment of Melia azedarach (Meliaceae). M azedarach formed annual rings with wood increments from February to November, corresponding to the emergence of new leaves, and remained dormant from December to January, corresponding to older and later fallen leaves [112]. The researchers reported that the woody cambium begins to divide in February under the marginal parenchyma layer and the larger vessel trail and ends in August. Soil moisture and abundances of mature dark leaves were related to leaf phenology and stem increment, while temperature and rainfall were not shown to be significant concerning growth increments. Furthermore, Palakit et al. (2021) studied the wood increment and leaf phenology of Afzelia xylocarpa (Fabaceae) and Lagerstroemia duperrana (Lythraceae) growing naturally in central Thailand [113]. Both tree species formed annual rings. The appearance of axial parenchyma was indicated by the marked annual ring boundary of A. xylocarpa, while the variation in vessel size and fiber cell wall thickness was indicated by the annual ring boundary in L. duperrana. The monthly wood increment and leaf flush in these tree species occurred at the onset of the rainy season or one month later. Vlam et al. (2013) studied the tree ring width of five deciduous tree species in western Thailand: Afzelia xylocarpa (Fabaceae), Chukrasia tabularis (Meliaceae), Melia azedarach (Meliaceae), Neolitsea obtusifolia (Lauraceae), and Toona ciliata (Meliaceae). They found that growth ring width was significantly negatively correlated with current-year maximum and minimum temperatures and positively correlated with dry-season rainfall. The authors concluded that the negative correlation between growth ring width and temperature might be attributed to a positive relationship between temperature and autotrophic respiration rate [114]. The positive relationship between growth ring width and dry-season rainfall may be reflected in the strong water demand during leaf flush.
A combination of studies of wood anatomy and stable carbon isotopes (δ13C) has been applied to Dipterocapace (three Shorea, two Dipterocarpus, and Hopea) in Thailand. The δ13C value variation in all species appeared to be annually cyclic. Dipterocarpaceae species exhibited a significant correlation between δ13C value and vessel morphology. This correlation implied that the changes in vessel traits were caused by the seasonal variation in the moisture available to the trees [42].
Pumijumnong et al. (2019) studied cambial activities and radial growth dynamics of three tropical trees, Hopea pierrei (Dipterocarpaceae), Cleidion spiciflorum (Euphorbiaceae), and Tetrameles nudiflora (Tetramelaceae), on Chang Island, Thailand [115]. The study area is an island with a relatively high average annual rainfall compared to the average inland rainfall. Cambial activity was determined by counting the number of undifferentiated cell layers between the mature xylem and phloem. They found that H. pierrei displayed the highest cambial activities through the wet and dry seasons, peaking in July. The cambium of T. mudiflora began to divide in May, the start of the rainy season, reached its peak in July, and gradually decreased and remained relatively stable from November to April (dry season). C. spiciflorum displayed a relatively uniform number of cambium layers throughout the dry season, from November to April. The number of cambium layers peaked in June and decreased until November. However, the cambium cell numbers of the three species were correlated with air relative humidity and soil moisture. Only the cambium cell number of T. nudiflora was significantly positively correlated with the relative air humidity. In addition, this study found no correspondence between radial expansion and cambium cell division. Since the studied area is notably humid, it is likely that the expansion and contraction of the stem is the result of moisture accumulation in the stem rather than of actual growth.
Another example of a subtropical tree commonly found in Thailand is the teak tree (Tectona grandis; Lamiaceae). Teak trees are known for their high-quality wood, used for furniture and other decorative purposes. Teak trees typically produce xylem with large vessels, which helps them transport water efficiently and support their tall stature. Buajan et al. (2023) investigated the cambium cell layers, cambium width, and xylem differentiation of a teak tree for 2 years [96]. They found that the activation of cambium cells started in May during the rainy season and ended in October (late rainy season), peaking in July and August (mid-rainy season). The development of xylem cells takes about 6 months, beginning in May and peaking in June, the vessels being the first formed. Vessels in the early wood are larger than in the latewood. In this study, the number of cambium cells, cambium zone width, and xylem differentiation zone width significantly correlated with all climatic variables except the mean temperature. The previous year’s mean temperature was significantly and positively correlated with each of the dependent variables. The division of cambium prior to leaf budding, as studied by Buajan et al., was consistent with that of teak from the Ivory Coast but differed from Indian teak, in which the buds emerge first and then stimulate cambium division. Tanaka et al. (2011) studied the teak leaf expansion and outbreaks of a teak defoliator during 2001–2008 [116]. They observed that the teak leaves began to expand from late March to May and that soil moisture at 1–40 cm depth stimulated the teak leaf expansion but did not exceed 60 cm depth. The moisture in the soil at 1–20 cm depth was not sufficient to stimulate teak leaf growth. Tanaka’s research used the leaf area index as a parameter but yielded results inconsistent with the study by Buajan et al. (2023), wherein teak leaves began expansion in the late dry season (March) to the rainy season (May), and it explained that the teak leaves appear first, followed by reactive cambium cells, and soil moisture content is very important for new leaf formation. Pumijumnong and Buajan (2013) studied the cambium activity of five evergreen species, Tetrameles nudiflora (Tetramelaceae), Magnolia baillonii (Magnoliaceae), Canarium euphyllum (Burseraceae), Toona ciliata (Meliaceae), and Spondias axillaris (Anacardiaceae) growing in the Khao Yai National Park [117]. They found that the number of cambium layers of all five species was significantly correlated with the monthly rainfall during both the rainy (May to October) and dry (November to April) seasons, except for T. nudiflora, for which the monthly rainfall was not significantly related to the number of layers of cambium during the dry season.
Pumijumnong and Wanyaphet (2006) investigated the cambium of two native pine species, Pinus merkusii and Pinus kesiya. Both species were divided into three groups: 30–60 (young), 60–90 (middle age), and >90 (old) years old [118]. They found that monthly precipitation and average temperature had no significant effect on the cambium activity of the two species. Only soil moisture content had a significant positive correlation with the number of cambium layers for both pine species and for all ages. The researchers explained that although soil moisture was correlated with rainfall, in the study area, the wilting point even in the absence of rain meant that moisture could still accumulate in the soil. It is an essential factor in stimulating the division of cambium. Later, Pumijumnong et al. (2021) studied the climate control of cambial dynamics of the two pine species in Thailand. P. latteri and P. kesiya grew in the same area, so differences in physical parameters were irrelevant [32]. The researchers found that total monthly rainfall and relative humidity were the main factors stimulating cambial activity. In addition, they found that P. latteri might have a half a month longer growth period than P. kesiya.
Table 4 indicates trees names, location, and methods of study in Southeast Asia, and Figure 2 shows the study of timber extraction and cambium activity in some Thai timber species and Walter and Lieth climate diagram [11].

Table 4.
Tree names, locations, methods, and references in the studies in Southeast Asia.
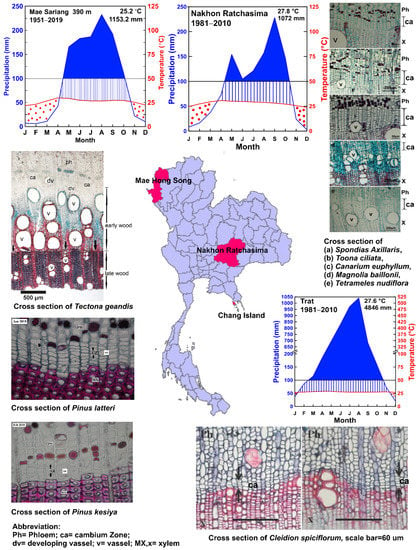
Figure 2.
Study of timber extraction and cambium activity in some Thai timber species. Ph = phloem; Ca = cambium zone; dv = developing vessel; V= vessel; X= xylem. Walter and Lieth climate diagram [11]. The blue line indicates the precipitation curve, the red line indicates the temperature, the red dots indicate the dry period, blue stripes indicate the humid period, and the blue area shows the super humid period. The average maximum temperature of the warmest month and the average minimum temperature of the coldest month are indicated on the right axis. The annual average temperature and annual precipitation are shown in the upper right corner of the diagram.
6. Future Research
Xylogenetic studies have largely focused on conifers, with their relatively simple wood structure; therefore, an important objective will be to extend this balanced source sink to other species in both warm and cold regions. Future studies need to study across a range of tree types to cover more areas and various environmental conditions, with emphasis on hydraulic properties. There is still much to be learned about the complex interactions between climate, soil, and other environmental factors that influence tree growth and wood formation in tropical and subtropical regions. Future research should focus on developing more precise and accurate methods for measuring cambial dynamics and wood formation and reaching a better understanding of the role of soil and nutrient availability on tree growth. Long-term monitoring efforts are also essential for developing a more comprehensive understanding of how these important ecosystems change over time.
In conclusion, understanding the dynamics of cambial activity and wood formation in tropical and subtropical trees is crucial for developing effective strategies for conserving and managing these ecosystems. Continued research efforts are essential for improving our comprehension of the complex factors that influence tree growth and wood formation in these regions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f14051025/s1, Table S1: Tree name/information, Location, Methods, references in the study in South America.
Author Contributions
Conceptualization, N.P. and S.B.; methodology, P.S.; software, C.M.; validation, S.B. and N.P.; formal analysis, P.S.; investigation, U.C.; resources, N.P.; data curation, N.P.; writing—original draft preparation, C.M.; writing—review and editing, S.B. and R.C.; visualization, U.C.; supervision, N.P.; project administration, N.P.; funding acquisition, N.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research is support by Mahidol University (Basic Research Fund: fiscal year 2022), grant number BRF1-070/2565 to Pumijumnong, N., Mahidol University (Basic Research Fund: fiscal year 2023), grant number FF-121/2566 to Muangsong, C., and Mahidol University (Basic Research Fund: fiscal year 2023), grant number FRB660042/0185 to Pumijumnong, N.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- D’Alessandro, S. Non-linear dynamics of population and natural resources: The emergence of different patterns of development. Ecol. Econ. 2007, 62, 473–481. [Google Scholar] [CrossRef]
- Wagner, A.O.; Lackner, N.; Mutschlechner, M.; Prem, E.M.; Markt, R.; Illmer, P. Biological pretreatment strategies for second-generation lignocellulosic resources to enhance biogas production. Energies 2018, 11, 1797. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef]
- Brooks, T.M.; Mittermeier, R.A.; Da Fonseca, G.A.; Gerlach, J.; Hoffmann, M.; Lamoreux, J.F.; Mittermeier, C.G.; Pilgrim, J.D.; Rodrigues, A.S. Global biodiversity conservation priorities. Science 2006, 313, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Fu, Y.; Zhou, L.; Li, B.; Luo, Y. An imperative need for global change research in tropical forests. Tree Physiol. 2013, 33, 903–912. [Google Scholar] [CrossRef]
- Artaxo, P.; Hansson, H.C.; Machado, L.A.T.; Rizzo, L.V. Tropical forests are crucial in regulating the climate on Earth. PLoS Clim. 2022, 1, e0000054. [Google Scholar] [CrossRef]
- Crowther, T.W.; Glick, H.B.; Covey, K.R.; Bettigole, C.; Maynard, D.S.; Thomas, S.M.; Smith, J.R.; Hintler, G.; Duguid, M.C.; Amatulli, G. Mapping tree density at a global scale. Nature 2015, 525, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Stahle, D.; Mushove, P.; Cleaveland, M.; Roig, F.; Haynes, G. Management implications of annual growth rings in Pterocarpus angolensis from Zimbabwe. For. Ecol. Manag. 1999, 124, 217–229. [Google Scholar] [CrossRef]
- Plomion, C.; Leprovost, G.; Stokes, A. Wood formation in trees. Plant Physiol. 2001, 127, 1513–1523. [Google Scholar] [CrossRef]
- Smith, C.R.; De Leo, F.C.; Bernardino, A.F.; Sweetman, A.K.; Arbizu, P.M. Abyssal food limitation, ecosystem structure and climate change. Trends Ecol. Evol. 2008, 23, 518–528. [Google Scholar] [CrossRef]
- Walter, H.; Lieth, H. Klimadiagramm-Weltatlas; VEB Gustav Fischer Verlag: Jena, Germany, 1967. [Google Scholar]
- Wright, S.J. Tropical forests in a changing environment. Trends Ecol. Evol. 2005, 20, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Deb, J.; Phinn, S.; Butt, N.; McAlpine, C. Climate change impacts on tropical forests: Identifying risks for tropical Asia. J. Trop. For. Sci. 2018, 30, 182–194. [Google Scholar]
- Wang, G.; Mang, S.L.; Riehl, B.; Huang, J.; Wang, G.; Xu, L.; Huang, K.; Innes, J. Climate change impacts and forest adaptation in the Asia–Pacific region: From regional experts’ perspectives. J. For. Res. 2019, 30, 277–293. [Google Scholar] [CrossRef]
- Zimmerman, B.L.; Kormos, C.F. Prospects for sustainable logging in tropical forests. Bioscience 2012, 62, 479–487. [Google Scholar]
- Carvalho, E.A., Jr.; Mendonça, E.N.; Martins, A.; Haugaasen, T. Effects of illegal logging on Amazonian medium and large-sized terrestrial vertebrates. For. Ecol. Manag. 2020, 466, 118105. [Google Scholar] [CrossRef]
- Murphy, B.P.; Bowman, D.M. What controls the distribution of tropical forest and savanna? Ecol. Lett. 2012, 15, 748–758. [Google Scholar] [CrossRef]
- Feeley, K.J.; Joseph Wright, S.; Nur Supardi, M.; Kassim, A.R.; Davies, S.J. Decelerating growth in tropical forest trees. Ecol. Lett. 2007, 10, 461–469. [Google Scholar] [CrossRef]
- Chambers, J.Q.; Asner, G.P.; Morton, D.C.; Anderson, L.O.; Saatchi, S.S.; Espírito-Santo, F.D.; Palace, M.; Souza, C. Regional ecosystem structure and function: Ecological insights from remote sensing of tropical forests. Trends Ecol. Evol. 2007, 22, 414–423. [Google Scholar] [CrossRef]
- Hättenschwiler, S.; Jørgensen, H.B. Carbon quality rather than stoichiometry controls litter decomposition in a tropical rain forest. J. Ecol. 2010, 98, 754–763. [Google Scholar] [CrossRef]
- Putz, F.E.; Blate, G.M.; Redford, K.H.; Fimbel, R.; Robinson, J. Tropical forest management and conservation of biodiversity: An overview. Conserv. Biol. 2001, 15, 7–20. [Google Scholar] [CrossRef]
- Worbes, M.; Herawati, H.; Martius, C. Tree growth rings in tropical peat swamp forests of Kalimantan, Indonesia. Forests 2017, 8, 336. [Google Scholar] [CrossRef]
- Wright, J.S. Plant diversity in tropical forests: A review of mechanisms of species coexistence. Oecologia 2002, 130, 1–14. [Google Scholar] [CrossRef]
- Zuidema, P.A.; Baker, P.J.; Groenendijk, P.; Schippers, P.; van der Sleen, P.; Vlam, M.; Sterck, F. Tropical forests and global change: Filling knowledge gaps. Trends Plant Sci. 2013, 18, 413–419. [Google Scholar] [CrossRef]
- Waisel, Y.; Fahn, A. The Effects of Environment on Wood Formation and Cambial Activity in Robina pseudacacia L. New Phytol. 1965, 64, 436–442. [Google Scholar] [CrossRef]
- Baas, P.; Vetter, R.E. Growth Rings in Tropical Trees; Rijksherbarium: Leiden, The Netherlands, 1989. [Google Scholar]
- Sheppard, P.R. Dendroclimatology: Extracting climate from trees. Wiley Interdiscip. Rev. Clim. Chang. 2010, 1, 343–352. [Google Scholar] [CrossRef]
- Kirdyanov, A.V.; Saurer, M.; Siegwolf, R.; Knorre, A.A.; Prokushkin, A.S.; Churakova, O.V.; Fonti, M.V.; Büntgen, U. Long-term ecological consequences of forest fires in the continuous permafrost zone of Siberia. Environ. Res. Lett. 2020, 15, 034061. [Google Scholar] [CrossRef]
- Boulanger, Y.; Arseneault, D.; Morin, H.; Jardon, Y.; Bertrand, P.; Dagneau, C. Dendrochronological reconstruction of spruce budworm (Choristoneura fumiferana) outbreaks in southern Quebec for the last 400 years. Can. J. For. Res. 2012, 42, 1264–1276. [Google Scholar] [CrossRef]
- Preechamart, S.; Pumijumnong, N.; Bräuning, A.; Muangsong, C.; Cai, B.; Payomrat, P.; Buajan, S.; Wang, F.; Li, M. Tree-ring oxygen isotope chronology of teak log coffins in northwestern Thailand and its relationship with Pacific Decadal Oscillation and El Niño-Southern Oscillation. Quat. Int. 2022, 629, 81–92. [Google Scholar] [CrossRef]
- Root, T.L.; Price, J.T.; Hall, K.R.; Schneider, S.H.; Rosenzweig, C.; Pounds, J.A. Fingerprints of global warming on wild animals and plants. Nature 2003, 421, 57–60. [Google Scholar] [CrossRef]
- Pumijumnong, N.; Songtrirat, P.; Buajan, S.; Preechamart, S.; Chareonwong, U.; Muangsong, C. Climate control of cambial dynamics and tree-ring width in two tropical pines in Thailand. Agric. For. Meteorol. 2021, 303, 108394. [Google Scholar] [CrossRef]
- Uggla, C.; Magel, E.; Moritz, T.; Sundberg, B. Function and dynamics of auxin and carbohydrates during earlywood/latewood transition in Scots pine. Plant Physiol. 2001, 125, 2029–2039. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.-W.; Eckstein, D.; Jalkanen, R.; Rickebusch, S.; Schmitt, U. Estimating the onset of cambial activity in Scots pine in northern Finland by means of the heat-sum approach. Tree Physiol. 2008, 28, 105–112. [Google Scholar] [CrossRef]
- Breitsprecher, A.; Bethel, J. Stem-growth periodicity of trees in a tropical wet forest of Costa Rica. Ecology 1990, 71, 1156–1164. [Google Scholar] [CrossRef]
- Rossi, S.; Morin, H.; Deslauriers, A. Causes and correlations in cambium phenology: Towards an integrated framework of xylogenesis. J. Exp. Bot. 2012, 63, 2117–2126. [Google Scholar] [CrossRef] [PubMed]
- Worbes, M. Growth rings, increment and age of trees in inundation forests, savannas and a mountain forest in the Neotropics. IAWA J. 1989, 10, 109–122. [Google Scholar] [CrossRef]
- Worbes, M. Annual growth rings, rainfall-dependent growth and long-term growth patterns of tropical trees from the Caparo Forest Reserve in Venezuela. J. Ecol. 1999, 87, 391–403. [Google Scholar] [CrossRef]
- Worbes, M. One hundred years of tree-ring research in the tropics–A brief history and an outlook to future challenges. Dendrochronologia 2002, 20, 217–231. [Google Scholar] [CrossRef]
- Suzuki, E. Diversity in specific gravity and water content of wood among Bornean tropical rainforest trees. Ecol. Res. 1999, 14, 211–224. [Google Scholar] [CrossRef]
- Worbes, M. How to measure growth dynamics in tropical trees a review. IAWA J. 1995, 16, 337–351. [Google Scholar] [CrossRef]
- Ohashi, S.; Okada, N.; Nobuchi, T.; Siripatanadilok, S.; Veenin, T. Detecting invisible growth rings of trees in seasonally dry forests in Thailand: Isotopic and wood anatomical approaches. Trees 2009, 23, 813–822. [Google Scholar] [CrossRef]
- Norstrom, E.; Holmgren, K.; Morth, C.-M. Rainfall-driven variations in δ13C composition and wood anatomy of Breonadia salicina trees from South Africa between AD 1375 and 1995. S. Afr. J. Sci. 2005, 101, 162–168. [Google Scholar]
- Fichtler, E.; Helle, G.; Worbes, M. Stable-carbon isotope time series from tropical tree rings indicate a precipitation signal. Tree-Ring Res. 2010, 66, 35–49. [Google Scholar] [CrossRef]
- Van Der Sleen, P.; Groenendijk, P.; Vlam, M.; Anten, N.P.; Boom, A.; Bongers, F.; Pons, T.L.; Terburg, G.; Zuidema, P.A. No growth stimulation of tropical trees by 150 years of CO2 fertilization but water-use efficiency increased. Nat. Geosci. 2015, 8, 24–28. [Google Scholar] [CrossRef]
- Nock, C.A.; Baker, P.J.; Wanek, W.; Leis, A.; Grabner, M.; Bunyavejchewin, S.; Hietz, P. Long-term increases in intrinsic water-use efficiency do not lead to increased stem growth in a tropical monsoon forest in western Thailand. Glob. Chang. Biol. 2011, 17, 1049–1063. [Google Scholar] [CrossRef]
- Dansgaard, W. Stable isotopes in precipitation. Tellus 1964, 16, 436–468. [Google Scholar] [CrossRef]
- Tharammal, T.; Bala, G.; Noone, D. Impact of deep convection on the isotopic amount effect in tropical precipitation. J. Geophys. Res. Atmos. 2017, 122, 1505–1523. [Google Scholar] [CrossRef]
- Villegas, P.; Paredes, V.; Betancur, T.; Taupin, J.D.; Toro, L.E. Groundwater evolution and mean water age inferred from hydrochemical and isotopic tracers in a tropical confined aquifer. Hydrol. Process. 2018, 32, 2158–2175. [Google Scholar] [CrossRef]
- Slik, J.F.; Arroyo-Rodríguez, V.; Aiba, S.-I.; Alvarez-Loayza, P.; Alves, L.F.; Ashton, P.; Balvanera, P.; Bastian, M.L.; Bellingham, P.J.; Van Den Berg, E. An estimate of the number of tropical tree species. Proc. Natl. Acad. Sci. USA 2015, 112, 7472–7477. [Google Scholar] [CrossRef]
- Hubbell, S.P. Estimating the global number of tropical tree species, and Fisher’s paradox. Proc. Natl. Acad. Sci. USA 2015, 112, 7343–7344. [Google Scholar] [CrossRef]
- Borchert, R. Climatic periodicity, phenology, and cambium activity in tropical dry forest trees. IAWA J. 1999, 20, 239–247. [Google Scholar] [CrossRef]
- Borchert, R.; Robertson, K.; Schwartz, M.D.; Williams-Linera, G. Phenology of temperate trees in tropical climates. Int. J. Biometeorol. 2005, 50, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Nepstad, D.C.; de Carvalho, C.R.; Davidson, E.A.; Jipp, P.H.; Lefebvre, P.A.; Negreiros, G.H.; da Silva, E.D.; Stone, T.A.; Trumbore, S.E.; Vieira, S. The role of deep roots in the hydrological and carbon cycles of Amazonian forests and pastures. Nature 1994, 372, 666–669. [Google Scholar] [CrossRef]
- Schöngart, J.; Piedade, M.T.F.; Ludwigshausen, S.; Horna, V.; Worbes, M. Phenology and stem-growth periodicity of tree species in Amazonian floodplain forests. J. Trop. Ecol. 2002, 18, 581–597. [Google Scholar] [CrossRef]
- Dünisch, O.; Bauch, J.; Gasparotto, L. Formation of increment zones and intraannual growth dynamics in the xylem of Swietenia macrophylla, Carapa guianensis, and Cedrela odorata (Meliaceae). IAWA J. 2002, 23, 101–119. [Google Scholar] [CrossRef]
- Dünisch, O.; Montóia, V.R.; Bauch, J. Dendroecological investigations on Swietenia macrophylla King and Cedrela odorata L. (Meliaceae) in the central Amazon. Trees 2003, 17, 244–250. [Google Scholar] [CrossRef]
- Marcati, C.R.; Angyalossy, V.; Evert, R.F. Seasonal variation in wood formation of Cedrela fissilis (Meliaceae). IAWA J. 2006, 27, 199–211. [Google Scholar] [CrossRef]
- Bräuning, A.; Volland-Voigt, F.; Burchardt, I.; Ganzhi, O.; Nauss, T.; Peters, T. Climatic control of radial growth of Cedrela montana in a humid mountain rainforest in southern Ecuador. Erdkunde 2009, 64, 337–345. [Google Scholar] [CrossRef]
- Rodríguez-Ramírez, E.C.; Ferrero, M.E.; Acevedo-Vega, I.; Crispin-DelaCruz, D.B.; Ticse-Otarola, G.; Requena-Rojas, E.J. Plastic adjustments in xylem vessel traits to drought events in three Cedrela species from Peruvian Tropical Andean forests. Sci. Rep. 2022, 12, 21112. [Google Scholar] [CrossRef]
- Lisi, C.S.; Fo, M.T.; Botosso, P.C.; Roig, F.A.; Maria, V.R.; Ferreira-Fedele, L.; Voigt, A.R. Tree-ring formation, radial increment periodicity, and phenology of tree species from a seasonal semi-deciduous forest in southeast Brazil. IAWA J. 2008, 29, 189–207. [Google Scholar] [CrossRef]
- Ferrero, M.E.; Villalba, R.; Rivera, S.M. An assessment of growth ring identification in subtropical forests from northwestern Argentina. Dendrochronologia 2014, 32, 113–119. [Google Scholar] [CrossRef]
- Grogan, J.; Schulze, M. The impact of annual and seasonal rainfall patterns on growth and phenology of emergent tree species in Southeastern Amazonia, Brazil. Biotropica 2012, 44, 331–340. [Google Scholar] [CrossRef]
- de Lara, N.O.T.; Marcati, C.R. Cambial dormancy lasts 9 months in a tropical evergreen species. Trees 2016, 30, 1331–1339. [Google Scholar] [CrossRef]
- Bhalerao, R.P.; Fischer, U. Environmental and hormonal control of cambial stem cell dynamics. J. Exp. Bot. 2017, 68, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Bosio, F.; Rossi, S.; Marcati, C.R. Periodicity and environmental drivers of apical and lateral growth in a Cerrado woody species. Trees 2016, 30, 1495–1505. [Google Scholar] [CrossRef]
- Aloni, R.; Schwalm, K.; Langhans, M.; Ullrich, C.I. Gradual shifts in sites of free-auxin production during leaf-primordium development and their role in vascular differentiation and leaf morphogenesis in Arabidopsis. Planta 2003, 216, 841–853. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; Marques, R.; Botosso, P.; Marques, M. Stem growth and phenology of two tropical trees in contrasting soil conditions. Plant Soil 2012, 354, 269–281. [Google Scholar] [CrossRef]
- Valdez-Hernández, M.; Andrade, J.L.; Jackson, P.C.; Rebolledo-Vieyra, M. Phenology of five tree species of a tropical dry forest in Yucatan, Mexico: Effects of environmental and physiological factors. Plant Soil 2010, 329, 155–171. [Google Scholar] [CrossRef]
- Shimamoto, C.Y.; Botosso, P.C.; Amano, E.; Marques, M.C. Stem growth rhythms in trees of a tropical rainforest in Southern Brazil. Trees 2016, 30, 99–111. [Google Scholar] [CrossRef]
- Volland-Voigt, F.; Bräuning, A.; Ganzhi, O.; Peters, T.; Maza, H. Radial stem variations of Tabebuia chrysantha (Bignoniaceae) in different tropical forest ecosystems of southern Ecuador. Trees 2011, 25, 39–48. [Google Scholar] [CrossRef]
- Baker, J.C.; Santos, G.M.; Gloor, M.; Brienen, R.J. Does Cedrela always form annual rings? Testing ring periodicity across South America using radiocarbon dating. Trees 2017, 31, 1999–2009. [Google Scholar] [CrossRef]
- Roel-Valdes, J.; Arizo-Luque, V.; Ronda-Perez, E. Epidemiology of occupationally-caused carpal tunnel syndrome in the province of Alicante, Spain 1996–2004. Rev. Esp. Salud Publica 2006, 80, 395–409. [Google Scholar]
- Xiao, X.; Hagen, S.; Zhang, Q.; Keller, M.; Moore, B., III. Detecting leaf phenology of seasonally moist tropical forests in South America with multi-temporal MODIS images. Remote Sens. Environ. 2006, 103, 465–473. [Google Scholar] [CrossRef]
- Yáñez-Espinosa, L.; Terrazas, T.; López-Mata, L. Phenology and radial stem growth periodicity in evergreen subtropical rainforest trees. IAWA J. 2010, 31, 293–307. [Google Scholar] [CrossRef]
- Lavrič, M.; Eler, K.; Ferlan, M.; Vodnik, D.; Gričar, J. Chronological sequence of leaf phenology, xylem and phloem formation and sap flow of Quercus pubescens from abandoned karst grasslands. Front. Plant Sci. 2017, 8, 314. [Google Scholar] [CrossRef] [PubMed]
- Marcati, C.R.; Milanez, C.R.D.; Machado, S.R. Seasonal development of secondary xylem and phloem in Schizolobium parahyba (Vell.) Blake (Leguminosae: Caesalpinioideae). Tree 2008, 22, 3–12. [Google Scholar] [CrossRef]
- Sintayehu, D.W. Impact of climate change on biodiversity and associated key ecosystem services in Africa: A systematic review. Ecosyst. Health Sustain. 2018, 4, 225–239. [Google Scholar] [CrossRef]
- Leal Filho, W.; Azeiteiro, U.M.; Balogun, A.-L.; Setti, A.F.F.; Mucova, S.A.; Ayal, D.; Totin, E.; Lydia, A.M.; Kalaba, F.K.; Oguge, N.O. The influence of ecosystems services depletion to climate change adaptation efforts in Africa. Sci. Total Environ. 2021, 779, 146414. [Google Scholar] [CrossRef] [PubMed]
- Krepkowski, J.; Bräuning, A.; Gebrekirstos, A.; Strobl, S. Cambial growth dynamics and climatic control of different tree life forms in tropical mountain forest in Ethiopia. Trees 2011, 25, 59–70. [Google Scholar] [CrossRef]
- Krepkowski, J.; Bräuning, A.; Gebrekirstos, A. Growth dynamics and potential for cross-dating and multi-century climate reconstruction of Podocarpus falcatus in Ethiopia. Dendrochronologia 2012, 30, 257–265. [Google Scholar] [CrossRef]
- Couralet, C.; Sterck, F.J.; Sass-Klaassen, U.; Van Acker, J.; Beeckman, H. Species-specific growth responses to climate variations in understory trees of a Central African rain forest. Biotropica 2010, 42, 503–511. [Google Scholar] [CrossRef]
- Trouet, V.; Mukelabai, M.; Verheyden, A.; Beeckman, H. Cambial growth season of brevi-deciduous Brachystegia spiciformis trees from south central Africa restricted to less than four months. PLoS ONE 2012, 7, e47364. [Google Scholar] [CrossRef] [PubMed]
- MARTIN, D.; MOSS, J. Age determination of Acacia tortilis (Forsk.) Hayne from northern Kenya. Afr. J. Ecol. 1997, 35, 266–277. [Google Scholar] [CrossRef]
- Prins, H.H.; van der Jeugd, H.P. Herbivore population crashes and woodland structure in East Africa. J. Ecol. 1993, 81, 305–314. [Google Scholar] [CrossRef]
- Andersen, G.L.; Krzywinski, K. Longevity and growth of Acacia tortilis; insights from 14 C content and anatomy of wood. BMC Ecol. 2007, 7, 1–14. [Google Scholar] [CrossRef]
- Gebrekirstos, A.; Mitlöhner, R.; Teketay, D.; Worbes, M. Climate–growth relationships of the dominant tree species from semi-arid savanna woodland in Ethiopia. Trees 2008, 22, 631–641. [Google Scholar] [CrossRef]
- Van Camp, J.; Hubeau, M.; Van den Bulcke, J.; Van Acker, J.; Steppe, K. Cambial pinning relates wood anatomy to ecophysiology in the African tropical tree Maesopsis eminii. Tree Physiol. 2018, 38, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Steppe, K.; Sterck, F.; Deslauriers, A. Diel growth dynamics in tree stems: Linking anatomy and ecophysiology. Trends Plant Sci. 2015, 20, 335–343. [Google Scholar] [CrossRef]
- O’Brien, M.J.; Leuzinger, S.; Philipson, C.D.; Tay, J.; Hector, A. Drought survival of tropical tree seedlings enhanced by non-structural carbohydrate levels. Nat. Clim. Chang. 2014, 4, 710–714. [Google Scholar] [CrossRef]
- Battipaglia, G.; Zalloni, E.; Castaldi, S.; Marzaioli, F.; Cazzolla-Gatti, R.; Lasserre, B.; Tognetti, R.; Marchetti, M.; Valentini, R. Long tree-ring chronologies provide evidence of recent tree growth decrease in a central African tropical forest. PLoS ONE 2015, 10, e0120962. [Google Scholar] [CrossRef]
- Dié, A.; Kitin, P.; Kouamé, F.N.G.; Van den Bulcke, J.; Van Acker, J.; Beeckman, H. Fluctuations of cambial activity in relation to precipitation result in annual rings and intra-annual growth zones of xylem and phloem in teak (Tectona grandis) in Ivory Coast. Ann. Bot. 2012, 110, 861–873. [Google Scholar] [CrossRef]
- Venugopal, N.; KV, K. Seasonal pattern of cell division in the vascular cambium of some tropical timber trees. Cytologia 1994, 59, 323–332. [Google Scholar] [CrossRef]
- Rao, K.; Rajput, K.S. Seasonal behaviour of vascular cambium in teak (Tectona grandis) growing in moist deciduous and dry deciduous forests. IAWA J. 1999, 20, 85–93. [Google Scholar] [CrossRef]
- Pumijumnong, N. Dendrochrnologie mit Teak (Tectona grandis L.) in Nord-Thailand. Jahrringbildung—Chronol.—Klimasignal. Diss. Univ. Hambg. 1995, 104. [Google Scholar]
- Buajan, S.; Pumijumnong, N.; Songtrirat, P.; Muangsong, C. Relationship of cambial activity and xylem production in Teak (Tectona geandis) to phenology and climate variable in North western Thailand. J. Trop. For. Sci. 2023, 35, 141–156. [Google Scholar]
- Nanda, A.; Suresh, H.S.; Krishnamurthy, Y.L. Phenology of a tropical dry deciduous forest of Bhadra wildlife sanctuary, southern India. Ecol. Process. 2014, 3, 1–12. [Google Scholar] [CrossRef]
- Singh, N.D.; Venugopal, N. Cambial activity and annual rhythm of xylem production of Pinus kesiya Royle ex. Gordon (Pinaceae) in relation to phenology and climatic factors growing in sub-tropical wet forest of North East India. Flora-Morphol. Distrib. Funct. Ecol. Plants 2011, 206, 198–204. [Google Scholar] [CrossRef]
- Swaminathan, C.; Anbarasu, M. Response of cambium growth to mechanical injuries in tree species. J. Trop. For. Sci. 2022, 34, 170–175. [Google Scholar] [CrossRef]
- Nobuchi, T.; Ogata, Y.; Siripatanadilok, S. Seasonal characteristics of wood formation in Hopea odorata and Shorea henryana. IAWA J. 1995, 16, 361–369. [Google Scholar] [CrossRef]
- Begum, S.; Nakaba, S.; Oribe, Y.; Kubo, T.; Funada, R. Induction of cambial reactivation by localized heating in a deciduous hardwood hybrid poplar (Populus sieboldii × P. grandidentata). Ann. Bot. 2007, 100, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Geiger, F. Anatomische Untersuchungen über Die Jahresringbildung von Tectona Grandis; Borntraeger: Beeville, TX, USA, 1915. [Google Scholar]
- Coster, C. Zur Anatomie und Physiologie der Zuwachszonen-und Jahresringbildung in den Tropen. Ph.D Thesis, Wageningen University, Wageningen, The Netherlands, Brill, Leiden, The Netherlands, 1927. [Google Scholar]
- Rahman, M.H.; Nugroho, W.D.; Nakaba, S.; Kitin, P.; Kudo, K.; Yamagishi, Y.; Begum, S.; Marsoem, S.N.; Funada, R. Changes in cambial activity are related to precipitation patterns in four tropical hardwood species grown in Indonesia. Am. J. Bot. 2019, 106, 760–771. [Google Scholar] [CrossRef]
- Sass, U.; Killmann, W.; Eckstein, D. Wood formation in two species of Dipterocarpaceae in peninsular Malaysia. IAWA J. 1995, 16, 371–384. [Google Scholar] [CrossRef]
- Fujii, T. Growth periodicity in relation to the xylem development in three Shorea spp.(Dipterocarpaceae) growing in Sarawak. In Tree-Ring Anal.; Wimmer, R., Vetter, R.E., Eds.; CABI Publishing: Oxon, UK, 1999; pp. 169–183. [Google Scholar]
- Wang, K.H.; Hamzah, M.Z. Different cambial activities in response to climatic factors of three Malaysian rainforest Shorea species with different stem diameters. Trees 2018, 32, 1519–1530. [Google Scholar] [CrossRef]
- Nabeshima, E.; Kubo, T.; Hiura, T. Variation in tree diameter growth in response to the weather conditions and tree size in deciduous broad-leaved trees. For. Ecol. Manag. 2010, 259, 1055–1066. [Google Scholar] [CrossRef]
- Qaderi, M.M.; Martel, A.B.; Dixon, S.L. Environmental factors influence plant vascular system and water regulation. Plants 2019, 8, 65. [Google Scholar] [CrossRef]
- Begum, S.; Kudo, K.; Rahman, M.H.; Nakaba, S.; Yamagishi, Y.; Nabeshima, E.; Nugroho, W.D.; Oribe, Y.; Kitin, P.; Jin, H.-O. Climate change and the regulation of wood formation in trees by temperature. Trees 2018, 32, 3–15. [Google Scholar] [CrossRef]
- Wang, K.H.; Hamzah, M.Z. Annual wood formation of tropical pioneer species related to stem diameters. J. Wood Sci. 2019, 65, 1–12. [Google Scholar] [CrossRef]
- Palakit, K.; Siripatanadilok, S.; Lumyai, P.; Duangsathaporn, K. Leaf phenology and wood formation of white cedar trees Melia azedarach L. and their responses to climate variability. Songklanakarin J. Sci. Technol. 2018, 40, 61–68. [Google Scholar]
- Palakit, K.; Lumyai, P.; Duangsathaporn, K. Periodic growth of Afzelia xylocarpa (Kurz) Craib and Lagerstroemia duperreana Pierre ex Gagnep. and their responses to climatic variability. Agric. Nat. Resour. 2021, 55, 282–291. [Google Scholar]
- Vlam, M.; Baker, P.J.; Bunyavejchewin, S.; Zuidema, P.A. Temperature and rainfall strongly drive temporal growth variation in Asian tropical forest trees. Oecologia 2014, 174, 1449–1461. [Google Scholar] [CrossRef]
- Pumijumnong, N.; Danpradit, S.; Tadang, N.; Buajan, S.; Muangsong, C. Cambial activity and radial growth dynamics of three tropical tree species at Chang Island, Thailand. J. Trop. For. Sci. 2019, 31, 404–414. [Google Scholar] [CrossRef]
- Tanaka, K.; Tantasirin, C.; Suzuki, M. Interannual variation in leaf expansion and outbreak of a teak defoliator at a teak stand in northern Thailand. Ecol. Appl. 2011, 21, 1792–1801. [Google Scholar] [CrossRef] [PubMed]
- Pumijumnong, N.; Buajan, S. Seasonal cambial activity of five tropical tree species in central Thailand. Trees 2013, 27, 409–417. [Google Scholar] [CrossRef]
- Pumijumnong, N.; Wanyaphet, T. Seasonal cambial activity and tree-ring formation of Pinus merkusii and Pinus kesiya in Northern Thailand in dependence on climate. For. Ecol. Manag. 2006, 226, 279–289. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).