Species-Specific Response to Climate Change: Evident through Retrospective Analysis Using Tree Ring Data
Abstract
1. Introduction
2. Materials and Methods
2.1. Selected Tree Species and Study Area
2.2. Sample Collection and Preparation
2.3. Tree Ring-Width Measurement and Analysis
2.4. Climate Data
2.5. Standardization and Chronology Building
2.6. Climate Growth Relationship
3. Results
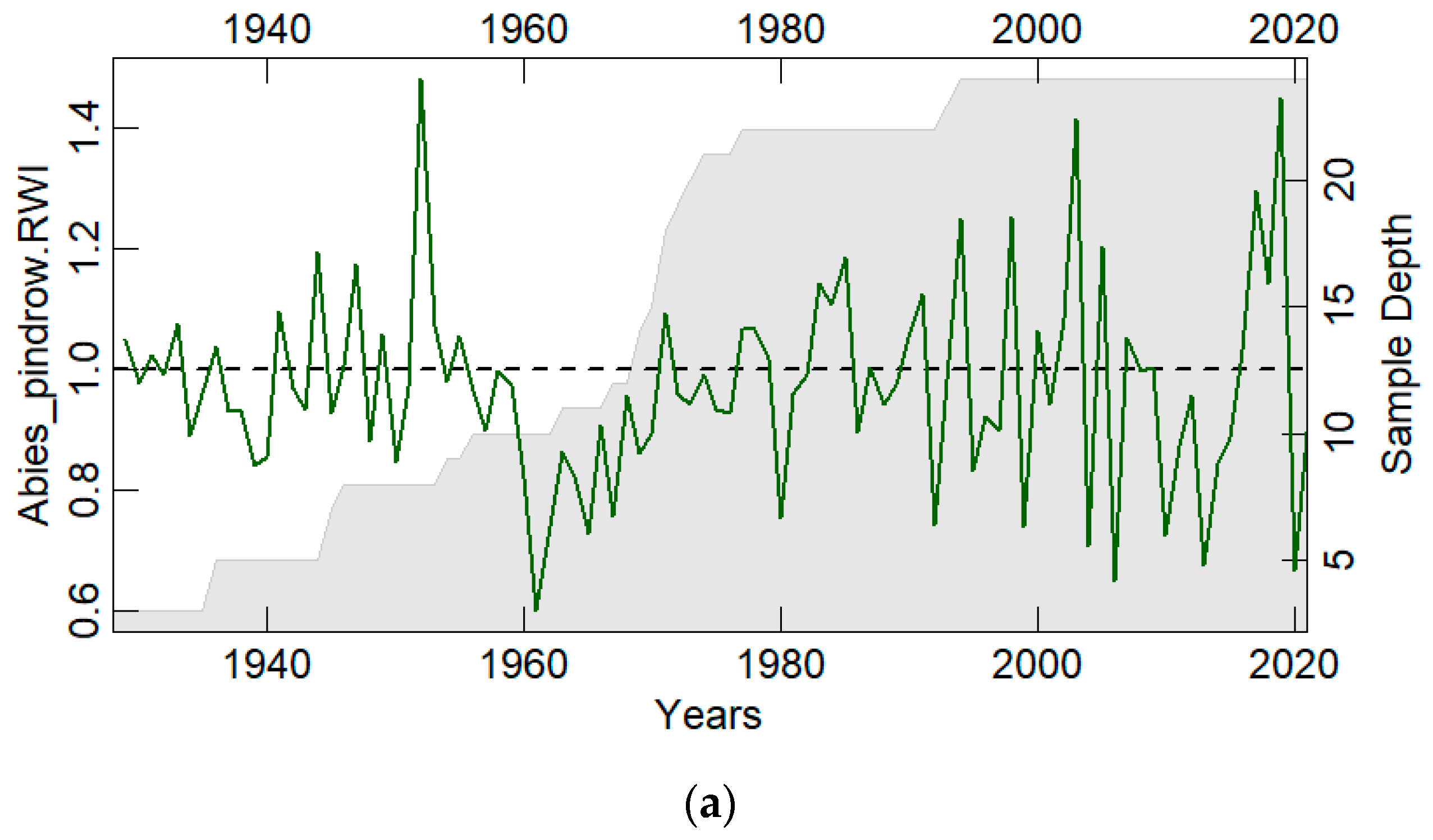
3.1. Chronology Characteristics
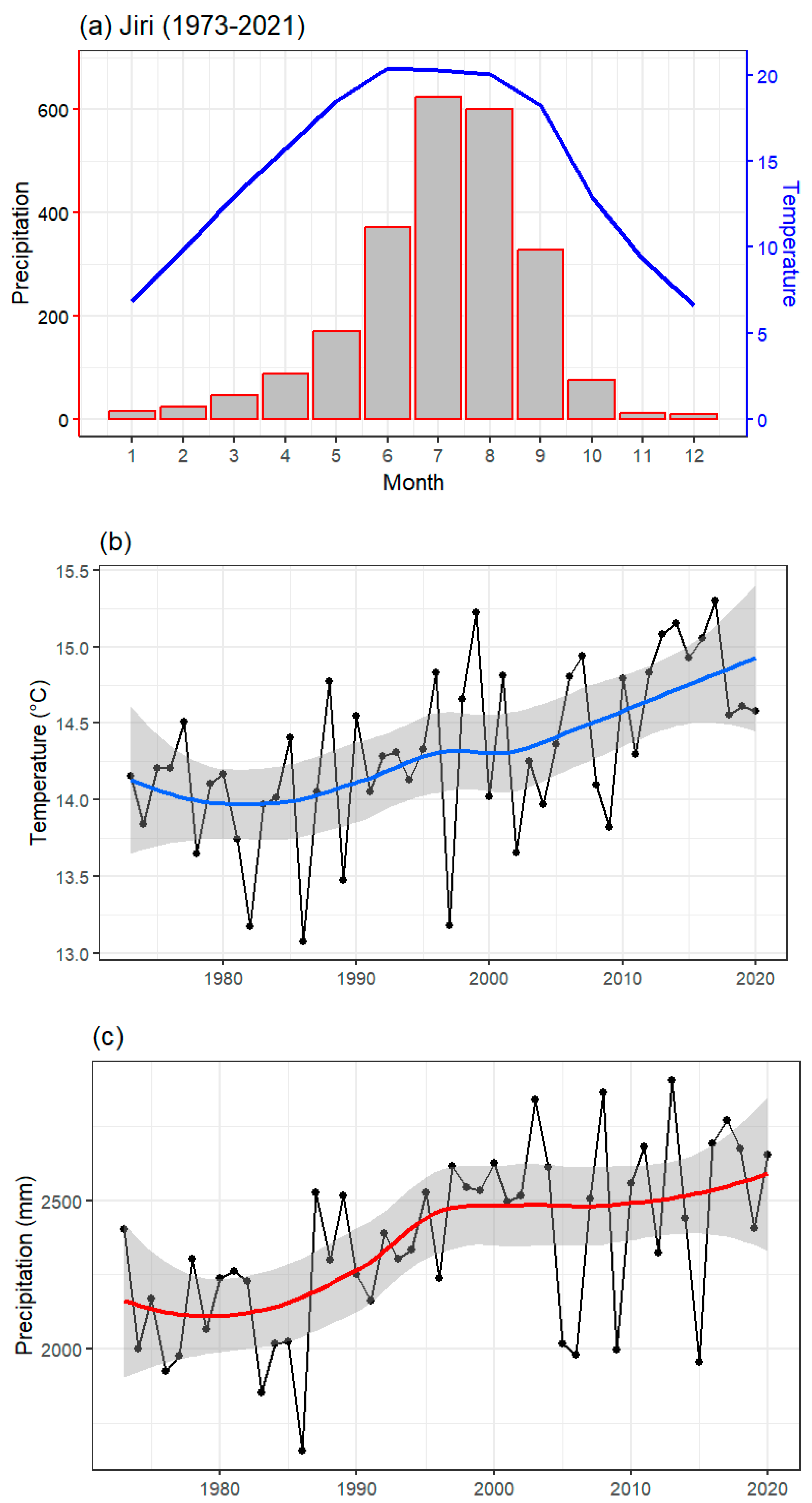
3.2. Local Climate
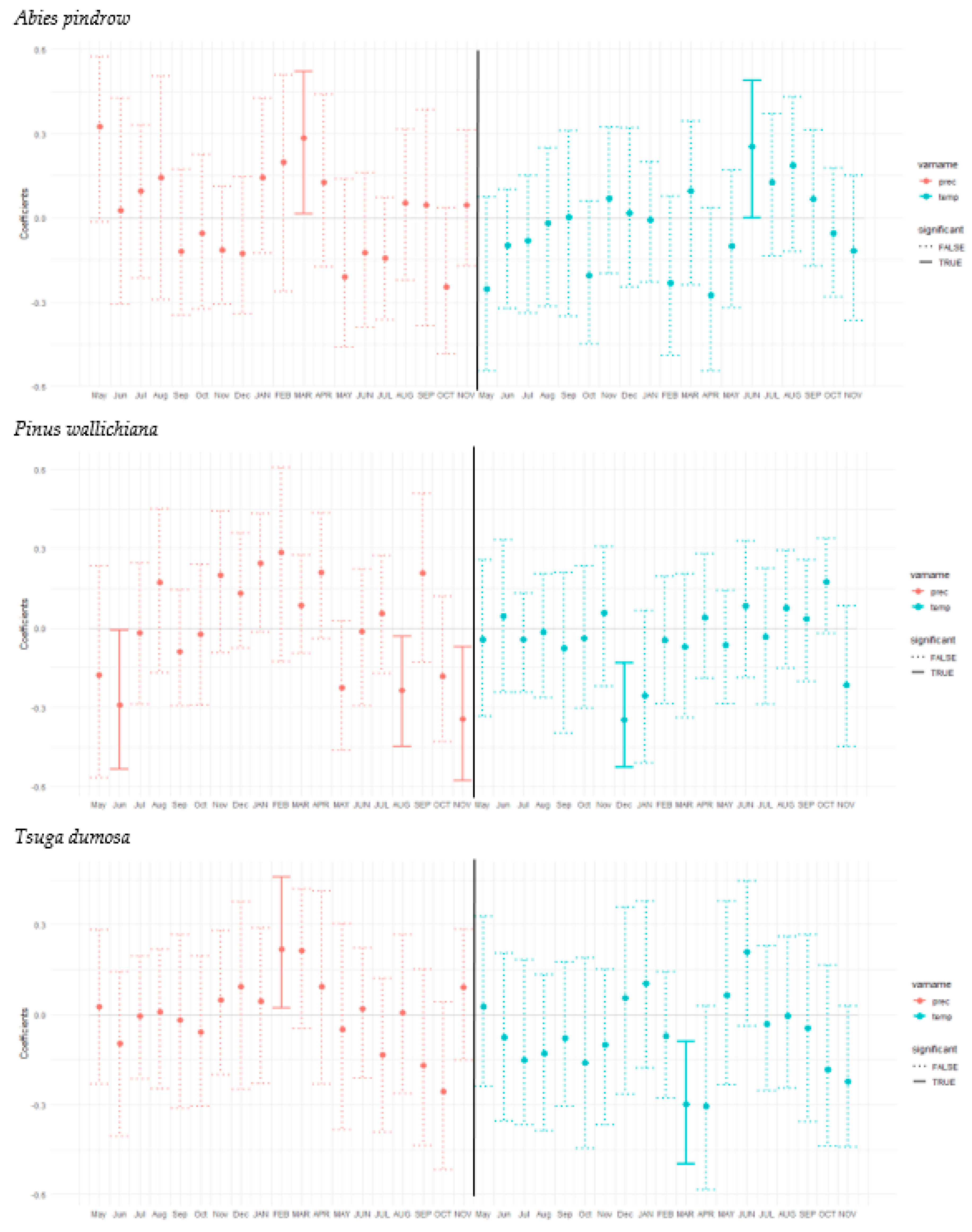
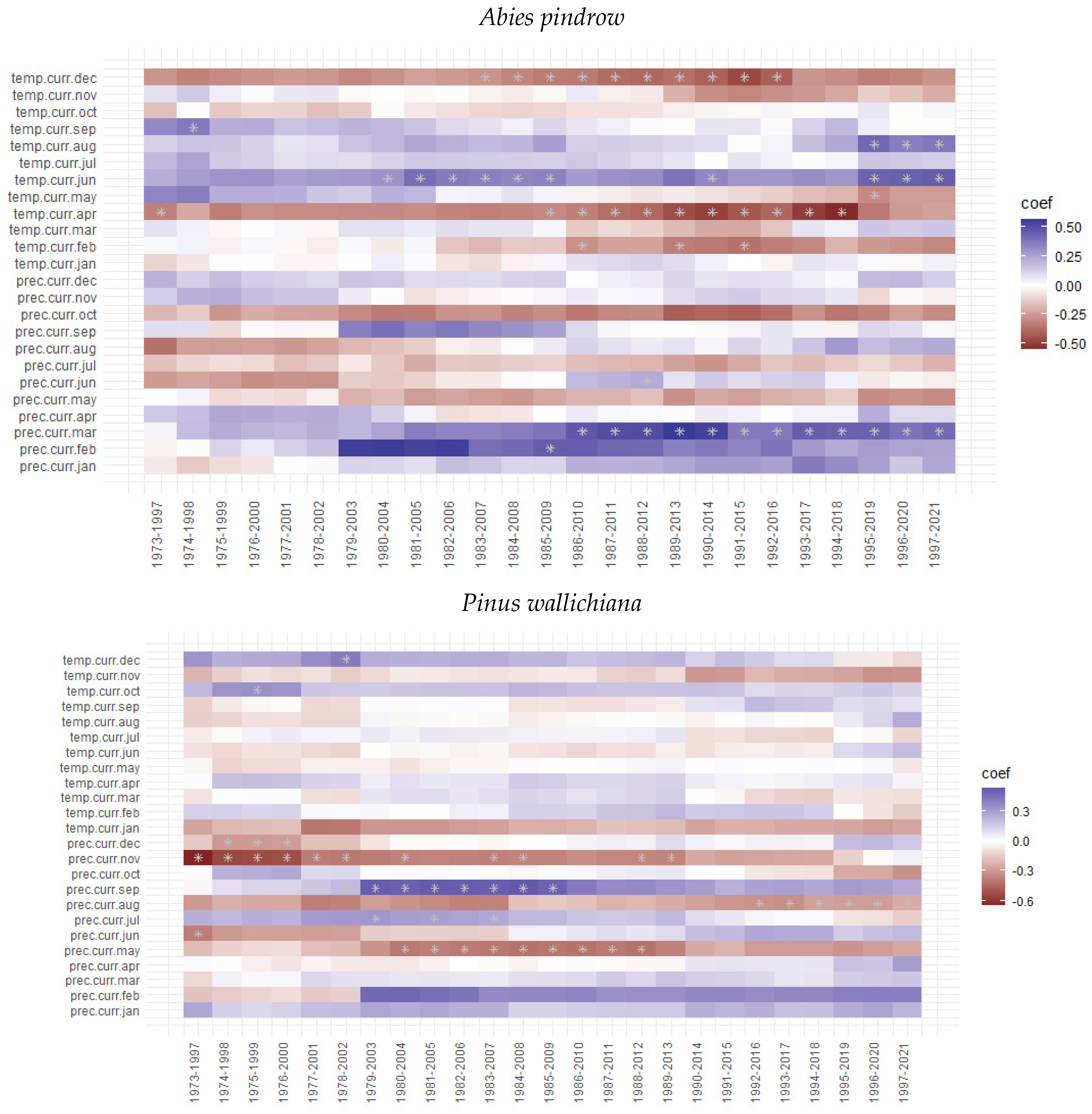
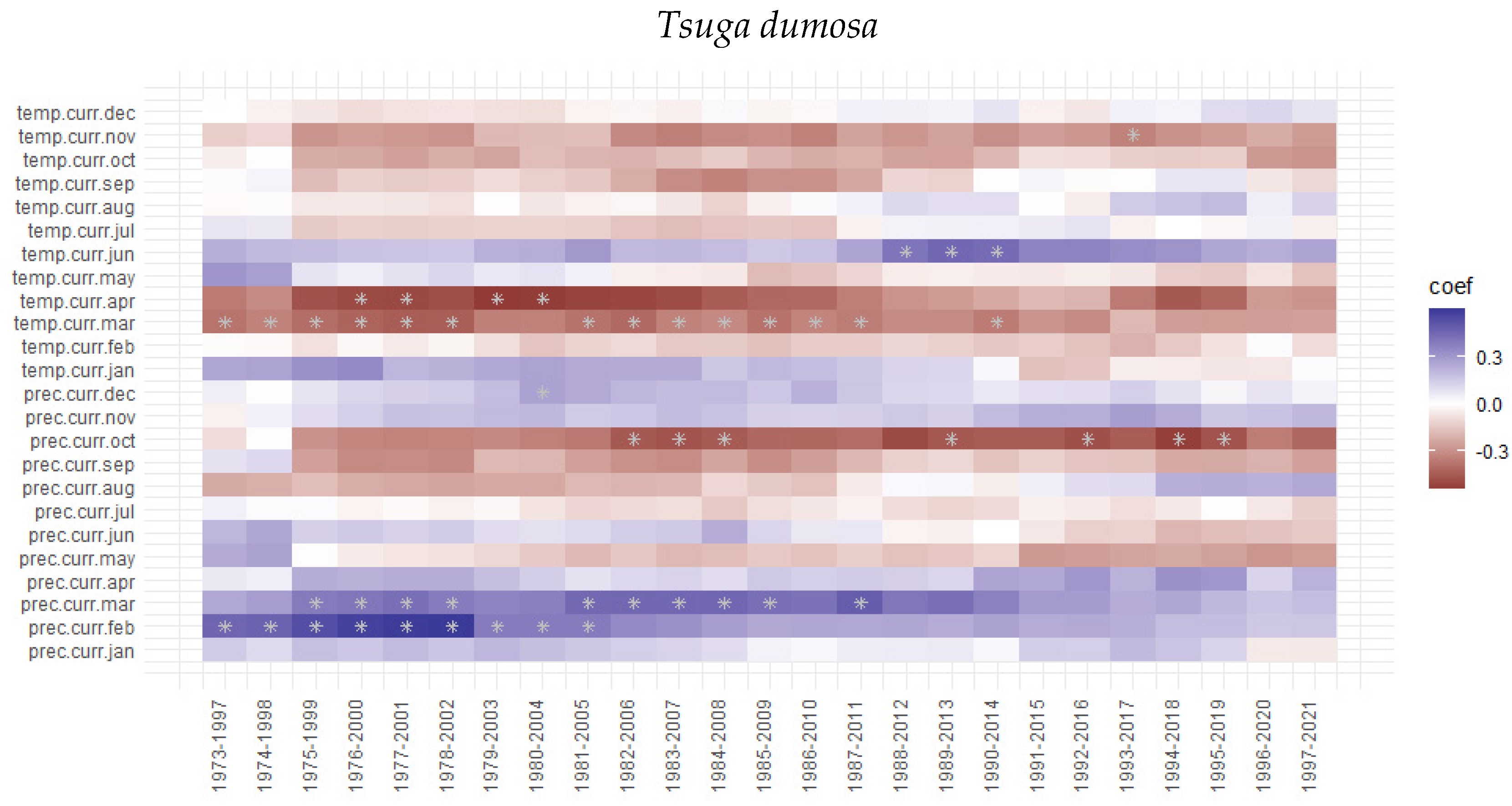
3.3. Tree Growth Response to Climatic Variable
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Summary for Policymakers. In Climate Change 2022: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Pörtner, H.-O., Roberts, D.C., Tignor, M., Poloczanska, E.S., Mintenbeck, K., Alegría, A., Craig, M., Langsdorf, S., Löschke, S., Möller, V., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022. [Google Scholar]
- IPCC. Summary for Policymakers. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; MassonDelmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021. [Google Scholar]
- Weiskopf, S.R.; Rubenstein, M.A.; Crozier, L.G.; Gaichas, S.; Griffis, R.; Halofsky, J.E.; Hyde, K.J.W.; Morelli, T.L.; Morisette, J.T.; Muñoz, R.C.; et al. Climate change effects on biodiversity, ecosystems, ecosystem services, and natural resource management in the United States. Sci. Total Environ. 2020, 733, 137782. [Google Scholar] [CrossRef] [PubMed]
- Román-Palacios, C.; Wiens, J.J. Recent responses to climate change reveal the drivers of species extinction and survival. Proc. Natl. Acad. Sci. USA 2020, 117, 4211–4217. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.B.; Brady, S.M. Plant developmental responses to climate change. Dev. Biol. 2016, 419, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Gauli, A.; Neupane, P.R.; Mundhenk, P.; Köhl, M. Effect of climate change on the growth of tree species: Dendroclimatological analysis. Forests 2022, 13, 496. [Google Scholar] [CrossRef]
- Margiorou, S.; Kastridis, A.; Sapountzis, M. Pre/post-fire soil erosion and evaluation of check-dams effectiveness in mediterranean suburban catchments based on field measurements and modeling. Land 2022, 11, 1705. [Google Scholar] [CrossRef]
- Deuffic, P.; Garms, M.; He, J.; Brahic, E.; Yang, H.; Mayer, M. Forest dieback, a tangible proof of climate change? A cross-comparison of forest stakeholders’ perceptions and strategies in the mountain forests of Europe and China. Environ. Manag. 2020, 66, 858–872. [Google Scholar] [CrossRef]
- Bugmann, H. Sensitivity of forests in the European Alps to future climatic change. Clim. Res. 1997, 8, 35–44. [Google Scholar] [CrossRef]
- Gonzalez, P.; Neilson, R.P.; Lenihan, J.M.; Drapek, R.J. Global patterns in the vulnerability of ecosystems to vegetation shifts due to climate change. Glob. Ecol. Biogeogr. 2010, 19, 755–768. [Google Scholar] [CrossRef]
- Paulsen, J.; Körner, C. A climate-based model to predict potential treeline position around the globe. Alp. Bot. 2014, 124, 1–12. [Google Scholar] [CrossRef]
- Telwala, Y.; Brook, B.W.; Manish, K.; Pandit, M.K. Climate-induced elevational range shifts and increase in plant species richness in a Himalayan biodiversity epicentre. PLoS ONE 2013, 8, e57103. [Google Scholar] [CrossRef]
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolström, M.; et al. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Speed, J.D.M.; Martinsen, V.; Hester, A.J.; Holand, Ø.; Mulder, J.; Mysterud, A.; Austrheim, G. Continuous and discontinuous variation in ecosystem carbon stocks with elevation across a treeline ecotone. Biogeosciences 2015, 12, 1615–1627. [Google Scholar] [CrossRef]
- Matskovsky, V.; Venegas-González, A.; Garreaud, R.; Roig, F.A.; Gutiérrez, A.G.; Muñoz, A.A.; Le Quesne, C.; Klock, K.; Canales, C. Tree growth decline as a response to projected climate change in the 21st century in Mediterranean mountain forests of Chile. Glob. Planet. Change 2021, 198, 103406. [Google Scholar] [CrossRef]
- Helama, S.; Sohar, K.; Läänelaid, A.; Mäkelä, H.M.; Raisio, J. Oak decline as illustrated through plant–climate interactions near the northern edge of species range. Bot. Rev. 2016, 82, 1–23. [Google Scholar] [CrossRef]
- FAO. The State of the World’s Forests 2022. Forest Pathways for Green Recovery and Building Inclusive, Resilient and Sustainable Economies; Food and Agriculture Organization of the United Nations: Rome, Italy, 2022. [Google Scholar]
- Neupane, P.R.; Gauli, A.; Maraseni, T.; Kübler, D.; Mundhenk, P.; Dang, M.V.; Köhl, M. A segregated assessment of total carbon stocks by the mode of origin and ecological functions of forests: Implication on restoration potential. Int. For. Rev. 2017, 19, 120–147. [Google Scholar]
- The World Bank Group. Climate Risk Country Profile: Nepal; The World Bank Group and the Asian Development Bank: Washington, DC, USA; Manila, Philippines, 2021; p. 28. [Google Scholar]
- Eckstein, D.; Künzel, V.; Schäfer, L. Global Climate Risk Index 2021; Germanwatch e. V.: Bonn, Germany, 2021; p. 50. [Google Scholar]
- Bohlinger, P.; Sorteberg, A. A comprehensive view on trends in extreme precipitation in Nepal and their spatial distribution. Int. J. Climatol. 2018, 38, 1833–1845. [Google Scholar] [CrossRef]
- Dahal, P.; Shrestha, N.S.; Shrestha, M.L.; Krakauer, N.Y.; Panthi, J.; Pradhanang, S.M.; Jha, A.; Lakhankar, T. Drought risk assessment in central Nepal: Temporal and spatial analysis. Nat. Hazards 2016, 80, 1913–1932. [Google Scholar] [CrossRef]
- Zhang, P.; Jeong, J.-H.; Yoon, J.-H.; Kim, H.; Wang, S.-Y.S.; Linderholm, H.W.; Fang, K.; Wu, X.; Chen, D. Abrupt shift to hotter and drier climate over inner East Asia beyond the tipping point. Science 2020, 370, 1095–1099. [Google Scholar] [CrossRef]
- Qi-Bin, Z.; Ouya, F. Tree rings circle an abrupt shift in climate. Science 2020, 370, 1037–1038. [Google Scholar] [CrossRef]
- GoN/MoFE. Nepal’sThird National Communication to the United Nations Framework Convention on Climate Change; Ministry of Forests and Environment: Kathmandu, Nepal, 2021; p. 262. [Google Scholar]
- Elsen, P.R.; Monahan, W.B.; Merenlender, A.M. Topography and human pressure in mountain ranges alter expected species responses to climate change. Nat. Commun. 2020, 11, 1974. [Google Scholar] [CrossRef]
- Pan, X.; Chin, M.; Ichoku, C.M.; Field, R.D. Connecting Indonesian fires and drought with the type of El Niño and phase of the Indian Ocean dipole during 1979–2016. J. Geophys. Res. Atmos. 2018, 123, 7974–7988. [Google Scholar] [CrossRef]
- da Silva, S.S.; Fearnside, P.M.; de Alencastro Graça, P.M.L.; Brown, I.F.; Alencar, A.; de Melo, A.W.F. Dynamics of forest fires in the southwestern Amazon. For. Ecol. Manag. 2018, 424, 312–322. [Google Scholar] [CrossRef]
- Ometto, J.P.; Kalaba, K.; Anshari, G.Z.; Chacón, N.; Farrell, A.; Halim, S.A.; Neufeldt, H.; Sukumar, R. Cross-Chapter Paper 7: Tropical Forests. In Climate Change 2022: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Pörtner, H.-O., Roberts, D.C., Tignor, M., Poloczanska, E.S., Mintenbeck, K., Alegría, A., Craig, M., Langsdorf, S., Löschke, S., Möller, V., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022; pp. 2369–2410. [Google Scholar]
- Singh, S.P.; Singh, V.; Skutsch, M.M. Rapid warming in the Himalayas: Ecosystem responses and development options. Clim. Dev. 2010, 2, 221–232. [Google Scholar] [CrossRef]
- Cavaliere, C. The effects of climate change on medicinal and aromatic plants. Herbal. Gram. 2009, 81, 44–57. [Google Scholar]
- KC, A.; Ghimire, A. High-Altitude Plants in Era of Climate Change: A Case of Nepal Himalayas. In Climate Change Impacts on High-Altitude Ecosystems; Öztürk, M., Hakeem, K.R., Faridah-Hanum, I., Efe, R., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 177–187. [Google Scholar]
- Acharya, K.P. Post Fire Natural Regeneration of Dominant Tree Species in Pisang, Manang, Nepal; Tribhuvan University: Kathmandu, Nepal, 2004. [Google Scholar]
- Sousa-Silva, R. Adaptation to Climate Change: The Importance of Tree Diversity for the Resilience of Forest Ecosystems; Katholieke Universiteit Leuven: Leuven, Belgium, 2018. [Google Scholar]
- Speer, J.H. Fundamentals of Tree-Ring Research; The University of Arizona Press: Tucson, AZ, USA, 2012. [Google Scholar]
- Forman, C.; Asher, C. Brave Green World: How Science Can Save Our Planet; The MIT Press: Cambridge, MA, USA, 2021. [Google Scholar]
- Biondi, F. Comparing tree-ring chronologies and repeated timber inventories as forest monitoring tools. Ecol. Appl. 1999, 9, 216–227. [Google Scholar] [CrossRef]
- Schweingruber, F.H. Tree Rings and Environment: Dendroecology; Swiss Federal Institute for Forest, Snow and Landscape Research: Birmensdorf, Switzerland, 1996; p. 609. [Google Scholar]
- Eckstein, D.; Krause, C. Dendroecologlcal studies on spruce trefs to monitor environmental changes around Hamburg. IAWA J. 1989, 10, 175–182. [Google Scholar] [CrossRef]
- Cavers, S.; Cottrell, J.E. The basis of resilience in forest tree species and its use in adaptive forest management in Britain. Forestry 2014, 88, 13–26. [Google Scholar] [CrossRef]
- Messier, C.; Bauhus, J.; Sousa-Silva, R.; Auge, H.; Baeten, L.; Barsoum, N.; Bruelheide, H.; Caldwell, B.; Cavender-Bares, J.; Dhiedt, E.; et al. For the sake of resilience and multifunctionality, let’s diversify planted forests! Conserv. Lett. 2022, 15, e12829. [Google Scholar] [CrossRef]
- Neupane, P.R. Viability Assessment of Jurisdictional Reduced Emissions from Deforestation and Forest Degradation (REDD+) Implementation in Vietnam; Universität Hamburg: Hamburg, Germany, 2015. [Google Scholar]
- Baral, S.; Gaire, N.; Aryal, S.; Pandey, M.; Rayamajhi, S.; Vacik, H. Growth ring measurements of Shorea robusta reveal responses to climatic variation. Forests 2019, 10, 466. [Google Scholar] [CrossRef]
- Baral, S.; Gaire, N.; Giri, A.; Maraseni, T.; Basnyat, B.; Paudel, A.; Kunwar, R.; Rayamajhi, S.; Basnet, S.; Sharma, S.; et al. Growth dynamics of Shorea robusta Gaertn in relation to climate change: A case study from tropical region of Nepal. Trees 2022, 36, 3. [Google Scholar] [CrossRef]
- Sigdel, S.R.; Dawadi, B.; Camarero, J.J.; Liang, E.; Leavitt, S.W. Moisture-limited tree growth for a subtropical Himalayan Conifer Forest in Western Nepal. Forests 2018, 9, 340. [Google Scholar] [CrossRef]
- Thapa, U.K.; Scott, S.G. Detecting the influence of climate and humans on pine forests across the dry valleys of eastern Nepal’s Koshi River basin. For. Ecol. Manag. 2019, 440, 12–22. [Google Scholar] [CrossRef]
- Aryal, S.; Gaire, N.P.; Pokhrel, N.R.; Rana, P.; Sharma, B.; Kharal, D.K.; Poudel, B.S.; Dyola, N.; Fan, Z.-X.; Grießinger, J.; et al. Spring Season in Western Nepal Himalaya is not yet Warming: A 400-Year Temperature Reconstruction Based on Tree-Ring Widths of Himalayan Hemlock (Tsuga dumosa). Atmosphere 2020, 11, 132. [Google Scholar] [CrossRef]
- Thapa, U.K.; Shah, S.K.; Gaire, N.; Bhuju, D.; Bhattacharyya, A.; Thaguna, G.S. Influence of climate on radial growth of Abies pindrow in Western Nepal Himalaya. Banko Janakari 2013, 23, 14–19. [Google Scholar] [CrossRef]
- Jackson, J.K. Manual of Afforestation in Nepal; Forest Research and Survey Centre, Ministry of Forests and Soil Conservation: Kathmandu, Nepal, 1994; Volume 2. [Google Scholar]
- Ministry of Environment. Climate Change Vulnerability Mapping for Nepal; Ministry of Environment: Kathmandu, Nepal, 2010. [Google Scholar]
- Khezri, A.; Bennett, R.; Zevenbergen, J. Defining the requirements of an information system for climate change adaptation in the mountain communities of Dolakha, Nepal. Climate 2018, 6, 47. [Google Scholar] [CrossRef]
- World Weather Online. Dolakha Annual Weather Averages. 2022. Available online: https://www.worldweatheronline.com/dolakha-weather-averages/np.aspx (accessed on 15 March 2023).
- Pokharel, R.K.; Neupane, P.R.; Tiwari, K.R.; Köhl, M. Assessing the sustainability in community based forestry: A case from Nepal. For. Policy Econ. 2015, 58, 75–84. [Google Scholar] [CrossRef]
- Rinn, F. Products and Services for Tree and Wood Analysis. 2003. Available online: http://www.rinntech.com/ (accessed on 14 September 2020).
- Grissino-mayer, H. Evaluating crossdating accuracy: A manual and tutorial for the computer program COFECHA. Tree-Ring Res. 2001, 57, 205–221. [Google Scholar]
- Bunn, A.G. A dendrochronology program library in R (dplR). Dendrochronologia 2008, 26, 115–124. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. URL. 2016. Available online: https://www.R-project.org/ (accessed on 1 February 2022).
- Cook, E.R.; Peters, K. The Smoothing Spline: A New Approach to Standardizing Forest Interior Tree-Ring Width Series for Dendroclimatic Studies. Tree-Ring Bull. 1981, 41, 45–53. [Google Scholar]
- Torrence, C.; Compo, G.P. A Practical Guide to Wavelet Analysis. Bull. Am. Meteorol. Soc. 1998, 79, 61–78. [Google Scholar] [CrossRef]
- Cook, E.R.; Buckley, B.M.; D’Arrigo, R.D.; Peterson, M.J. Warm-season temperatures since 1600 BC reconstructed from Tasmanian tree rings and their relationship to large-scale sea surface temperature anomalies. Clim. Dyn. 2000, 16, 79–91. [Google Scholar] [CrossRef]
- Mann, H.B. Nonparametric tests against trend. Econometrica 1945, 13, 245–259. [Google Scholar] [CrossRef]
- Kendall, M.G. Rank Correlation Methods; Oxford University Press: New York, NY, USA, 1975. [Google Scholar]
- Sen, P.K. Estimates of the regression coefficient based on Kendall’s tau. J. Am. Stat. Assoc. 1968, 63, 1379–1389. [Google Scholar] [CrossRef]
- Rahman, M.; Islam, M.; Wernicke, J.; Bräuning, A. Changes in Sensitivity of Tree-Ring Widths to Climate in a Tropical Moist Forest Tree in Bangladesh. Forests 2018, 9, 761. [Google Scholar] [CrossRef]
- Fritts, H.C. Tree Rings and Climate; Academic Press: London, UK, 1976; pp. 434–505. [Google Scholar]
- Zang, C.; Biondi, F. treeclim: An R package for the numerical calibration of proxy-climate relationships. Ecography 2015, 38, 431–436. [Google Scholar] [CrossRef]
- Malik, R.; Sukumar, R. June–July Temperature Reconstruction of Kashmir Valley from Tree Rings of Himalayan Pindrow Fir. Atmosphere 2021, 12, 410. [Google Scholar] [CrossRef]
- Bukhari, S.; Bajwa, G.; Rehman, S. Climate-Growth Response Function of the Blue Pine (Pinus wallichiana) in Galies Forest Division-Abbottabad, KP, Pakistan. Sarhad J. Agric. 2019, 35, 116–125. [Google Scholar] [CrossRef]
- Chhetri, P.K.; Cairns, D.M. Dendroclimatic response of Abies spectabilis at treeline ecotone of Barun Valley, eastern Nepal Himalaya. J. For. Res. 2016, 27, 1163–1170. [Google Scholar] [CrossRef]
- Fan, Z.-X.; Bräuning, A.; Cao, K.-F.; Zhu, S.-D. Growth–climate responses of high-elevation conifers in the central Hengduan Mountains, southwestern China. For. Ecol. Manag. 2009, 258, 306–313. [Google Scholar] [CrossRef]
- Way, D.A.; Oren, R. Differential responses to changes in growth temperature between trees from different functional groups and biomes: A review and synthesis of data. Tree Physiol. 2010, 30, 669–688. [Google Scholar] [CrossRef]
- Malik, R.; Rossi, S.; Sukumar, R. Variations in the timing of different phenological stages of cambial activity in Abies pindrow (Royle) along an elevation gradient in the north-western Himalaya. Dendrochronologia 2020, 59, 125660. [Google Scholar] [CrossRef]
- Kastridis, A.; Kamperidou, V.; Stathis, D. Dendroclimatological analysis of Fir (A. borisii-regis) in Greece in the frame of climate change investigation. Forests 2022, 13, 879. [Google Scholar] [CrossRef]
- Pasho, E.; Toromani, E.; Alla, A.Q. Climatic impact on tree-ring widths in Abies Borisii-regis forests from South-East Albania. Dendrochronologia 2014, 32, 237–244. [Google Scholar] [CrossRef]
- Yadav, R.; Bhattacharyya, A. Climate and Growth Relationship in Blue Pine (Pinus wallichiana) from the Western Himalaya, India. Korean J. Ecology 1997, 20, 95–102. [Google Scholar]
- Guo, G.; Li, Z.; Zhang, Q.-B.; Ma, K.; Mu, C. Dendroclimatological studies of Picea likiangensis and Tsuga dumosa in Lijiang, China. IAWA J. 2009, 30, 435–441. [Google Scholar] [CrossRef]
- Dawadi, B.; Liang, E.; Tian, L.; Devkota, L.P.; Yao, T. Pre-monsoon precipitation signal in tree rings of timberline Betula utilis in the central Himalayas. Quat. Int. 2013, 283, 72–77. [Google Scholar] [CrossRef]
- Yadav, R.R.; Park, W.-K.; Singh, J.; Dubey, B. Do the western Himalayas defy global warming? Geophys. Res. Lett. 2004, 31, L17201. [Google Scholar] [CrossRef]
- Borgaonkar, H.P.; Sikder, A.B.; Ram, S. High altitude forest sensitivity to the recent warming: A tree-ring analysis of conifers from Western Himalaya, India. Quat. Int. 2011, 236, 158–166. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Z.-S.; Fan, Z.-X. Tree-ring based February–April relative humidity reconstruction since A.D. 1695 in the Gaoligong Mountains, southeastern Tibetan Plateau. Asian Geogr. 2017, 34, 59–70. [Google Scholar] [CrossRef]
- Ram, S.; Borgaonkar, H.P. Climatic response of various tree ring parameters of fir (Abies pindrow) from Chandanwadi in Jammu and Kashmir, western Himalaya, India. Curr. Sci. 2014, 106, 1568–1576. [Google Scholar]
- Malla, R.; Neupane, P.R.; Köhl, M. Modelling soil organic carbon as a function of topography and stand variables. Forests 2022, 13, 1391. [Google Scholar] [CrossRef]
- Sankey, T.; Tatum, J. Thinning increases forest resiliency during unprecedented drought. Sci. Rep. 2022, 12, 9041. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, I.; Acharya, K.; Juno, E.; Karounos, C.; Lee, B.R.; McCollum, C.; Schaffer-Morrison, S.; Tourville, J. Forest resilience under global environmental change: Do we have the information we need? A systematic review. PLoS ONE 2019, 14, e0222207. [Google Scholar] [CrossRef] [PubMed]
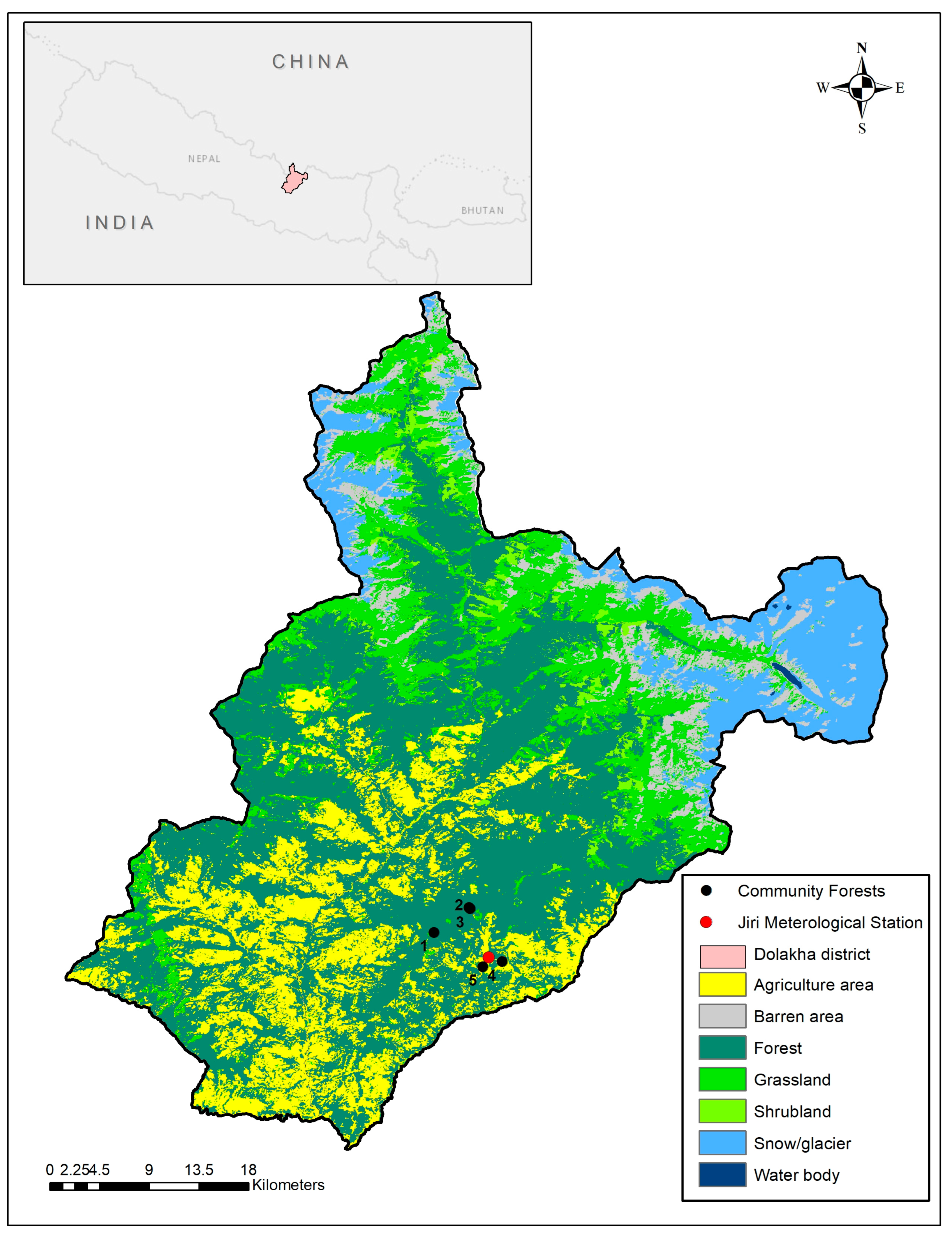

| Forest Name | Elevation (m) | Latitude (N) | Longitude (E) |
|---|---|---|---|
| Hanumante Community Forest | 2833 | 27.6508 | 86.1873 |
| Bhatekhola Community Forest | 2742 | 27.6712 | 86.2160 |
| Kalobhir Community Forest | 2769 | 27.6703 | 86.2169 |
| Jireshwori Community Forest | 1953 | 27.6269 | 86.2429 |
| Debithan Kimane Community Forest | 1903 | 27.6227 | 86.2271 |
| Parameters | Abies pindrow | Pinus wallichiana | Tsuga dumosa |
|---|---|---|---|
| No. of trees/cores | 24/45 | 23/41 | 24/44 |
| Overall period | 1928–2021 | 1973–2021 | 1961–2021 |
| Age range (years) | 94 | 49 | 61 |
| Mean tree ring width (mm) | 3.50 | 4.578 | 5.31 |
| Standard deviation (mm) | 1.80 | 2.96 | 2.10 |
| Mean sensitivity | 0.30 | 0.34 | 0.24 |
| Series intercorrelation | 0.472 | 0.517 | 0.586 |
| Autocorrelation | 0.24 | 0.175 | 0.247 |
| Expressed Population Signal (EPS) | 0.853 | 0.888 | 0.868 |
| Rbar | 0.268 | 0.284 | 0.304 |
| Mean GLK * | 0.624 | 0.98 | 0.928 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neupane, P.R.; Gauli, A.; KC, R.; Poudel, B.S.; Köhl, M. Species-Specific Response to Climate Change: Evident through Retrospective Analysis Using Tree Ring Data. Forests 2023, 14, 737. https://doi.org/10.3390/f14040737
Neupane PR, Gauli A, KC R, Poudel BS, Köhl M. Species-Specific Response to Climate Change: Evident through Retrospective Analysis Using Tree Ring Data. Forests. 2023; 14(4):737. https://doi.org/10.3390/f14040737
Chicago/Turabian StyleNeupane, Prem Raj, Archana Gauli, Rajendra KC, Buddi Sagar Poudel, and Michael Köhl. 2023. "Species-Specific Response to Climate Change: Evident through Retrospective Analysis Using Tree Ring Data" Forests 14, no. 4: 737. https://doi.org/10.3390/f14040737
APA StyleNeupane, P. R., Gauli, A., KC, R., Poudel, B. S., & Köhl, M. (2023). Species-Specific Response to Climate Change: Evident through Retrospective Analysis Using Tree Ring Data. Forests, 14(4), 737. https://doi.org/10.3390/f14040737








