Abstract
To assess the impact of bamboo plantations on soil organic carbon (SOC) under prevailing climatic conditions, increase in temperature and soil amendments, the Roth C model was used. RothC is a promising model for the estimation of SOC changes in different land use systems. In the present study, the RothC model was used to predict the dynamics of SOC in the plantation of seven bamboo species under a usual scenario: increase temperature by 1 °C and 2 °C and farm yard manure (FYM) addition. The result revealed that RothC fairly predicts the SOC. The root mean square error (RMSE) value varied from 0.74 to 3.2 among seven bamboo species while comparing modeled and measured data. The increase in temperature resulted in a decrease in SOC. The decrease in SOC varied from 0.46 to 5.96 per cent as compared to the usual scenario, and the extent of the decrease varied from species to species. Among all species, the application of 9 t ha−1 FYM was found appropriate for maintaining the initial SOC level during the initial stage of bamboo growth.
1. Introduction
Soil organic carbon (SOC) has a major influence on soil structure, water holding capacity, CEC, and the soil’s ability to form complexes with metal ions and store nutrients and hence is vital for ecosystem functioning [1]. Variation in SOC content has a profound impact on soil functions. Soils are important carbon sinks to combat the challenges of climate and increasing atmospheric carbon dioxide concentration. The top 1 m soil profile has been estimated to store three times the amount stored in vegetation and double the amount in the atmosphere. However, this SOC is variable and is influenced by a number of factors. Land use systems, type of management practice, and age of the plantation affect SOC content in soil [2]. In the era of global warming and climate change, increasing concern about soil quality vis à vis soil organic carbon content and enhanced CO2 concentration is being felt [3,4,5]. Therefore, management practices which can add SOC and still provide economic benefits to farmers are of urgent need to offset carbon emissions and fulfill Kyoto commitments and more recent commitments, e.g., the United Nations Framework Convention on Climate Change (UNFCC) 27th Conference of the Parties in Egypt (2022).
Recently, there has been increased interest amongst researchers and policymakers in the role of bamboo in enhancing soil carbon and carbon sequestration. Bamboo is a fast growing-plant and has the potential to solve climate-related problems of resource-poor farmers. India is the second richest country in the world, after China, in terms of bamboo genetic resources [6]. The bamboo area of the country is estimated to be 15.69 million hectares, with a total standing stock of 189 million tons [7]. Bamboo plantations are considered cost-effective solutions for the mitigation of CO2 emissions and have the potential to modify climate change. Bamboo, due to fast growth, has a large capacity for biomass accumulation and a high potential for carbon storage from the atmosphere [8]. High litterfall and fine roots in bamboo also help in sequestering a large amount of carbon in soils [9]. Bamboo forest ecosystems store significant amounts of carbon ranging from 94 to 392 t C ha−1 [10]. Nathetal [9] and Yuen et al. [10] reported biomass and carbon sequestration rates as high as 13–24 Mg C ha−1 yr−1 for various types of bamboo worldwide. INBAR’s modeling study revealed that a managed moso bamboo forest could accumulate about 300 t of C ha−1 after 60 years. Another report [11] revealed that monopodial (P. pubescens) sequestered more carbon than the Chinese fir in the first 5 years due to rapid early growth in southeast China.
Bamboo plantations sequester carbon in the soil at rates which are comparable to tropical agroforestry systems 9. In northeast India, a sequestration rate of 0.59 Mg ha−1 yr−1 for bamboo-based agroforestry systems has been reported [12]. Yuen et al. [10] reported that soil organic carbon (SOC) and total ecosystem carbon (TEC) ranged from 70–200 Mg C ha−1 and 94–392 Mg C ha−1, respectively. Bamboo also produce phytolith occluded carbon, a stable form of carbon resulting from decomposing vegetation that remains in the soil for several thousand years. Parr et al. [13] estimated that the bio-sequestration of phytolith-occluded carbon by bamboo worldwide is equivalent to 11% of the current increase in atmospheric carbon dioxide. Huang et al. [14] reported that phytolith occluded carbon (PhytOC) produced by bamboo remains in the soil for thousands of years. The study of soil organic carbon dynamics in bamboo, therefore, is of immense importance.
Various soil models (Q, ROMUL, RothC, SoilCO2/RothC, and Yasso07) and plant–soil models (CENTURY, Coup-Model and Forest-DNDC) have been used for studying soil carbon stock dynamics. Management, fertilization, crop rotation [15,16], and climate change [17] have been incorporated into different simulation models for predicting long-term changes in SOC [18,19,20,21]. The availability of input data, their simplicity, and whether modelled and measured values are good or unsatisfactory dictates the use of these models [22].
RothC is a model for the turnover of organic carbon in non-waterlogged topsoil that allows for the effects of soil type, temperature, soil moisture, and plant cover on the turnover process. The RothC model has been demonstrated by many authors to have performed very well for SOC [21]. It has been used under different climates, including tropical and temperate (humid subtropical, Mediterranean, and maritime) in over 80 countries [15,18,23,24]. In India, the Roth C model was used by Panwar et al. [25] for SOC variation in Populus deltoides W.Bartram ex Marshall plantations and Pal et al. [26] to see the effect of tillage on SOC. Bhattacharyya et al. [27] used RothC for a long-term fertilizer experiment in different climatic conditions in India; Afzali et al. [28] used Roth C for rangelands of Iran and reported that it accurately simulated the changes in SOC. The present study was conducted using the RothC model with the objectives of ascertaining the SOC potential of bamboo plantations, how SOC varies under bamboo plantations under increased temperature regimes, and what quantity of farm yard manure (FYM) needs to be incorporated to maintain the SOC of soil in the initial stages of bamboo growth.
2. Materials and Methods
2.1. Study Area and Experimental Details
The study was carried out at the Research farm of ICAR-Indian Institute of Soil and Water Conservation, Dehradun, India, located at 30°20′59″ N latitude and 77°53′05″ E longitude at 548 m above msl from 2012 to 2022 (Figure 1). The region has agroecological conditions typical of the Doon valley and lower Himalayan ranges. The soil contains 37% silt, 40% sand, and 23% clay and belongs to the silty clay loam type. The pH ranges within 5.4–5.6 with medium organic carbon (0.63%–0.73%), high bulk density (1.43–1.5 Mg m−3), and low hydraulic conductivity (0.9–1.8 cm h−1).
Figure 1.
Location of the study site.
Seven commercial bamboo species viz., Bambusa balcooa Roxb., Bambusa bambos (L.) Voss, Bambusa vulgaris Schrad ex Wendl., Bambusa nutans Wallich ex Munro, Dendrocalamus hamiltonii Nees & Arn. Ex Munro, Dendrocalamus stocksii (Munro.) Naithani and Dendrocalamus strictus (Roxb.) Neest. were planted at 5 m × 4 m in a randomized complete block design (RCBD) with three replications in the year 2012. In total, there were 21 plots of 180 m2, with 9 bamboo clumps in each block covering an area of 3780 m2. The experiment was conducted under rainfed conditions. The plantation raised for the experiment was cleaned regularly by adopting standard management practices recommended for bamboo, except for the use of manures and fertilizers. Soil samples were collected initially in each of the 21 plots from the 0–30 cm soil depth in the year 2012. Soil samples were again collected in the year 2018 (after 6 year of planting) and in the year 2022 (after 10 years of planting). All the collected samples were analyzed for soil organic carbon according to the method of Walkley and Black [29].
2.2. Description of the RothC Model
RothC-26.3 is a model for the turnover of organic carbon in non-waterlogged topsoils that allows for the effects of soil type, temperature, moisture content, and plant cover on the turnover process. It uses a monthly time step to calculate total organic carbon (t ha−1), microbial biomass carbon (t ha−1), and Δ14C (from which the equivalent radiocarbon age of the soil can be calculated) on a year to centuries timescale. The RothC model considers the effect of soil type, temperature, moisture content, and plant cover on the SOC conversion processes [30]. SOC stock comprises five compartments as a function of the speed of decomposition, four of these are considered active, (1) easily decomposed plant material (DPM, remains in the soil for 0.17 years), (2) microbial biomass (BIO, 1.69 years), (3) resistant plant material (RPM, 2.31 years), (4) humified organic matter (HUM, 49.5 years), and one passive, (5) inert organic matter (IOM), with a mean residence time of years. The DPM and RPM compartments decompose and become BIO and HUM and contribute CO2 to the atmosphere. The model includes a function that adjusts the proportions of BIO, HUM, and CO2 according to the soil clay content.
The input parameters for the model include mean temperature, monthly mean precipitation, mean monthly evaporation, clay content, soil depth, monthly C input from plant residues, organic fertilizers, and the number of months soil is covered. The details of the input parameters used in the present study are provided in Table 1. The model also requires plant residues that enter the soil at monthly intervals. The partition between the plant residues (PR) that enter the soil is given as a function of the default values originating from the DPM/RPM ratio. DPM/RPM is an estimate of the decomposability of the incoming plant material. Agriculture and cultivated grasses have a DPM/RPM ratio of 1.44; bushes and grasses, 0.67; tropical or deciduous forests, 0.25. The input of clay content in the model is used to calculate how much plant-available water the topsoil can hold; it also affects the way organic matter decomposes. It is necessary to indicate whether or not the soil is vegetated because decomposition has been found to be faster in fallow soil than in cropped soil, even when the cropped soil is not allowed to dry out. To access the impact of climate change on SOC, two increased temperature scenarios of 1 °C and 2 °C were created. The average monthly existing temperatures were arithmetically increased uniformly for all months in such a way that the average yearly temperature increased just by 1 °C and similarly for 2 °C. These two temperatures were taken as these are the likely increases in temperature up to 2050.

Table 1.
Data requirement for RothC.
2.3. RothC Model Simulations
RothC 26.3 model simulation was carried out in two stages: initialization and prediction. The initialization stage assumes that the SOC content measured in the systems achieves a condition of equilibrium [31,32]. RothC-26.3 is designed to run in two modes: ‘forward’, in which known inputs are used to calculate changes in soil organic matter, and ‘inverse’, when inputs are calculated from known changes in soil organic matter. In the present study, the RothC model was run in inverse mode to obtain the quantity of C that enters the soil yearly to maintain the specific initial SOC content (SOC) measured in the study. The equilibrium condition at the start of the experiment is achieved by running the RothC iteratively for 10,000 years. An equilibrium run requires an IOM value. This IOM value was calculated from Equation (1), provided by Falloon et al. [22].
where SOC = initial soil organic carbon (t ha−1).
IOM (t ha−1) = 0.049 × SOC 1.139,
The equilibrium run determines the initial distribution of C in four compartments viz. decomposable plant material (DPM), resistant plant material (RPM), microbial biomass carbon (BIO), and humified organic matter (HUM) are necessary for the model to function along with its radio carbon. The DPM/RPM ratio used was 0.67, as recommended by RothC (by default) for bushes and grasses. The inputs required for running equilibrium are provided in Table 1. The weather (monthly temperature, rainfall, and evaporation; clay % and soil depth) and land management (plant residue, FYM, and soil cover) DPM/RPM ratios were used in direct mode.
2.4. Evaluation of RothC Model Performance
The model’s performance was ascertained by comparing pairs of SOC data observed and predicted during the plantation years [21]. The statistics used were root mean square error (RMSE), Equation (2), and the efficiency of the model (EF), Equation (3). RMSE ranges from 0 to ∞ and EF from −∞ to 1.
where O = observed value, P = predicted value, and n = number.
3. Results
3.1. Measured and Modeled SOC under Prevailing Climatic Conditions
Modeled SOC in all the bamboo species decreased in the initial four years, and after the fifth year onwards, the SOC started increasing (Figure 2). The observed SOC values also reflected the same trend. The SOC under B. nutans was initially 17.80 Mg ha−1, which reduced to 15.56 Mg ha−1 after 5 years of plantation and then increased to the initial level of 17.76 Mg ha−1 after 10 years of plantation (Figure 2). In a similar way, other species also had similar trends for observed SOC values. The CO2 evolution from the bamboo plantation during the simulated period followed the trend of the SOC variation (Figure 3). Statistics were employed to describe the relationship between measured and modeled values (Table 2). The agreement between the modeled and measured data is satisfactory, as the RMSE value for bamboo species varied from 0.74 to 3.2. This signifies that modeled SOC data was much closer to measured data. Model efficiency (EF) varied from −20.32 to 0.94. The EF for B. vulgaris was much less, indicating RothC is not suitable for the species. Hence, for further scenarios, the species was not taken. The data for other species signify near prediction of SOC using RothC (Table 2).
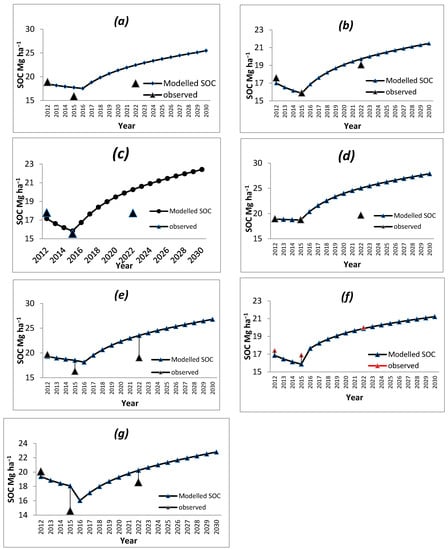
Figure 2.
Observed and modeled SOC under a usual scenario in (a) B. balcooa, (b) B. bambos, (c) B. nutans, (d) B. vulgaris, (e) D. hamiltonii, (f) D. stocksi and (g) D. strictus.
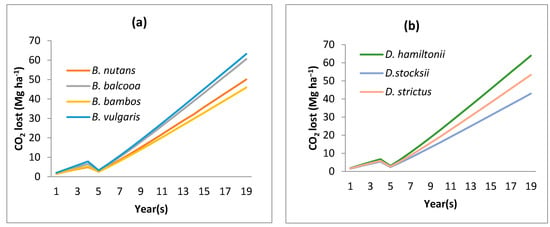
Figure 3.
CO2 evolution under a usual scenario in (a) Bambusa species and (b) Dendrocalamus species.

Table 2.
Statistics describing performance of model for each treatment.
3.2. Effect of Climate Change on SOC
The impact on SOC level under bamboo species with a likely climate change of 1 °C and 2 °C increase in temperature was modeled considering that RothC model fairly predicted the results under prevailing climatic conditions. It was observed that the SOC decreased with an increase in temperature (Figure 4). The range of decrease in SOC varied from 0.46 (D. hamiltonii) in the first year to 3.04 per cent (B. balcooa) after 15 years of simulation, when 1 °C increase in temperature was modeled. With the increase in temperature to 2 °C, the range of SOC decrease was 0.95 (B. bambos and D. stocksii) in the first year to 5.97 per cent (B. nutans) after 15 years of simulation.
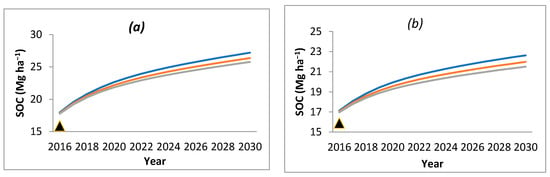
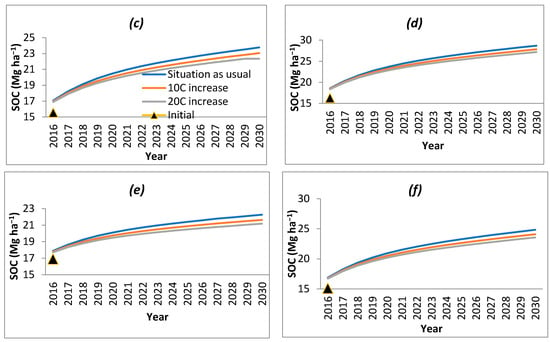
Figure 4.
SOC variation as a result of increase in temperature in (a) B. balcooa, (b) B. bambos, (c) B. nutans, (d) D. hamiltonii, (e) D. stocksii and (f) D. strictus.
3.3. Maintaining SOC: Addition of Organic Inputs
The observed data for all the bamboo species and consequently modeled data for the corresponding period revealed that in the initial stages of bamboo plantation, SOC reduces. Through the RothC model, it was ascertained at what rate organic manures (farm yard manures; FYM) should be applied to the bamboo so that the SOC levels do not reduce and are retained to the near-original SOC values. Different rates of FYM application (@ 5 t ha−1, 7 t ha−1, 8 t ha−1, and 9 t ha−1) were modeled, and it was observed that FYM applied @ 9 t ha−1 helps in maintaining the initial SOC level (Figure 5). However, this will also result in an increase in CO2 (Figure 6).
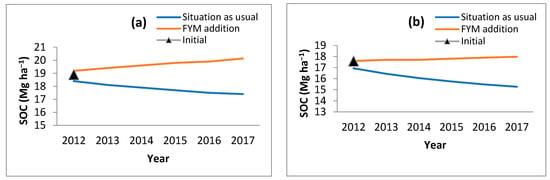
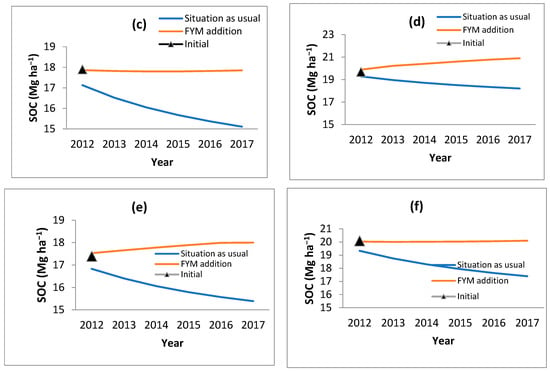
Figure 5.
Change in SOC with addition of FYM @ 9 t ha−1 in (a) B. balcooa, (b) B. bambos, (c) B. nutans, (d) D. hamiltonii, (e) D. stocksii and (f) D. strictus.
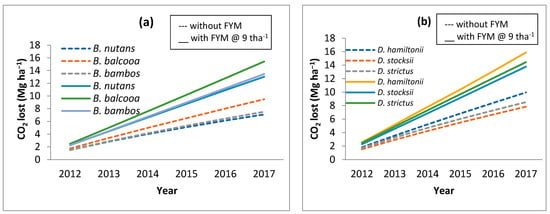
Figure 6.
CO2 evolved as a result of addition of FYM @ 9 t ha−1 in (a) Bambusa species and (b) Dendrocalamus species.
3.4. Effect of Increase in Temperature and FYM Addition on SOC
Keeping 9 t ha−1 FYM additions to maintain SOC level in the initial stage of bamboo growth (as modeled above) and increase in temperature by 1 °C and 2 °C (the likely climate scenario), the SOC was modeled through RothC (Figure 7). It was observed that with an increase in temperature, the SOC decreased. The decrease in SOC with 1 °C increase in temperature varied from 1.17 in D. stocksii to 1.79 per cent in B. nutans after the 6th year of plantation. When 2 °C simulated an increase in temperature, the decrease in SOC varied from 2.44 in D. stocksii to 3.03 per cent in B. balcooa after the 6th year of plantation. The CO2 also increased correspondingly with an increase in temperature.
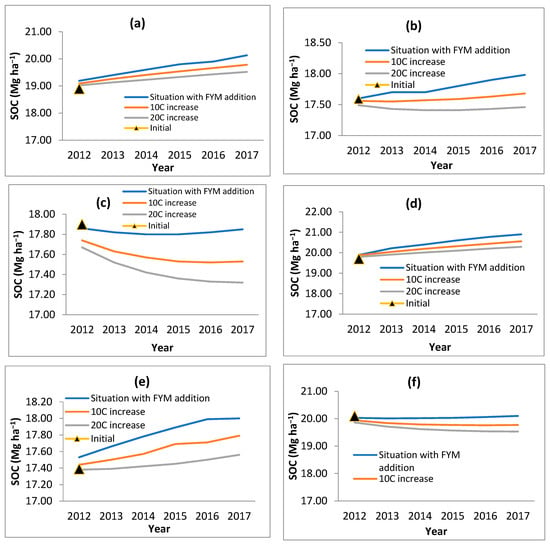
Figure 7.
Effect of temperature increase on SOC with FYM addition @ 9 t ha−1 (a) B. balcooa, (b) B. bambos, (c) B. nutans, (d) D. hamiltonii, (e) D. stocksii and (f) D. strictus.
4. Discussion
4.1. Measured and Modeled SOC under Prevailing Climatic Conditions
The dynamics of soil carbon are critical and contribute significantly to carbon cycling and the sustainability of terrestrial ecosystems [33]. Though the C sequestration potential of bamboo is well established [34,35], there are few studies that reflect the changes in the SOC pools under bamboo plantations, which are significant from a C storage point of view since a minute change in the SOC pools could change the atmospheric CO2 concentration [36].
It was observed that the SOC initially decreased and then increased after the 5th year of plantation (Figure 2). The buildup of soil organic matter and nutrient turnover is affected by the input of nutrients through the leaf, stem, branch, and root [37]. The initial dip in SOC under bamboo may be ascribed to less amount of leaf litter addition during the establishment phase (1–4th year), due to which they could not exert a remarkable effect on SOC [38]. Additionally, during the establishment phase, the interspaces were ploughed mechanically to check the weed growth, which might have resulted in the loss of accumulated carbon to the atmosphere. Sainju et al. [39] reported that deep tillage applied in the intensive management regime would break up the soil profile and improve the aeration condition, and subsequently increase the exposure chance of SOC to decomposers. Wang et al. [40] also reported that the removal of forest understory vegetation decreased the C input into the soil, which may have contributed to the decrease in SOC storage. Removal of weeds and understory growth also may have resulted in an increase in soil temperature, thereby enhancing mineralization. Additionally, the fast growth rate of bamboo (the ability to complete the growth cycle within a short temporal scale of 120 to 150 days) makes it a highly potent species for SOC and nutrient exploitation initially [41].
However, overlooking initial disturbance, bamboo enhanced the active pool of SOC, and land use changes induced C losses and thus could easily restore the degraded lands under bamboo cultivation [42]. The projected increase in soil organic carbon content under various bamboo species may be attributed to the addition of varying amounts of leaf litter (Figure 2) and different decomposition rates. In general, perennials have the ability to maintain soil organic matter through the supply of litter and root residues. Fine root turnover is another important flux of soil organic C with an amount of C equal to leaf litter [43]. Bamboo species, due to fast growth, may allocate more biomass to their roots, which can transfer more root detritus to the soil and hence higher SOC accumulation in the long term. The fine roots and root hairs of the bamboo root system play a significant role in supporting high productivity and buildup of soil organic carbon [44]. Singh and Singh [45] reported that the introduction of bamboo resulted in a progressive buildup of soil organic carbon and increasing nutrients being immobilized in microbial biomass with increasing age of the plantation. Similar buildup in soil OC was also reported for Bambusa pallida [46], Gigantochloa spp., Bambusa vulgaris, and seven 12-year-old commercial bamboo species [38]. Christanty et al. [47] also reported that bamboo resulted in an increase in soil organic matter of approximately 7 tons ha−1 to 25 cm depth during the 4-year fallow period in the rotation cycle of shifting cultivation.
On the contrary, Friggens et al. [48] did not record any increase in net ecosystem C stock 12 or 39 years after planting in two native species (Betula pubescens and Pinus sylvestris). Contributing factors for SOC increase are temperature, soil condition, clay fraction, management practices, and the species itself, either alone or in combination with all these factors. Dwivedi et al. [49] reported that the stable fraction of C is strongly bound with the soil mineral matrix to form mineral–humus complexes and thus are shielded from microbial action and least decomposed. Bamboos are known to produce phytolith occluded carbon (PhytOC) from decomposing vegetation which is highly stable and remains in the soil for several thousand years [13,14]; hence, long-term bamboo plantation can add to carbon sequestration. If current trends continue, bamboo plantations may show significantly higher soil C storage.
4.2. Scenario for Climate Change and FYM Addition
By modeling the scenario of the impact of a likely increase in temperature, it was found that SOC decreases with an increase in temperature (Figure 4). Climate factors, such as precipitation and temperature, significantly affect SOC content which is the basic component of the global carbon cycle, particularly in the context of climate change [50,51,52,53]. Under climate change scenarios, most studies [54,55] have reported losses from the soil organic carbon. Increased temperatures might enhance the release of CO2 to the atmosphere from SOC. Temperature increases will accelerate decomposition and, consequently, increase the loss of SOC stock in the upper layer of the soil [56]. As reported by Xu et al. [57] using the RothC model, results indicated that SOC stock would decrease by 2%–6% over 40 years under the climate change scenarios in the rangelands of southern Ireland. Using the CarboSOIL model, Muñoz Rojas et al. [55] also showed that climate change had a negative impact on SOC contents in the upper layers of the soil section in Andalusia. Jebari et al. [51], also using the RothC model, predicted that SOC content will decrease in the different climate change scenarios in comparison with no climate change scenario for future decades overall in Spain.
A series of studies have shown that the environmental factors controlling soil organic carbon differ depending on the scale [58,59]. With an increase in temperature, soil microbial decomposition activity is enhanced, which leads to a higher soil organic carbon output. The soil organic carbon content depends on the balance between the carbon input and output, and environmental factors affect soil organic carbon by impacting the input and/or output of carbon [60,61]. In terrestrial ecosystems, the carbon input is related to plant productivity, whereas the carbon output mainly depends on microbial organic matter decomposition [58,62].
RMSE values with the RothC model are reported in the range of 2%–30% [21,23,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62]. EF was −20.32 to 0.94. González-Molina et al. [63] also reported an EF range from 0.32 (grasslands) to 0.90 (forests) and concluded that RothC performs better in agriculture and forest land compared to grasslands and rangelands. Barančíková et al. [64] reported that the RothC modeled and observed values are fairly close to each other, and RothC is a good model for the prediction of SOC. The model simulated an increase in CO2 with an increase in temperature and application of FYM (Figure 6). The increasing temperature will expedite the decomposition of soil organic matter and tend to increase SOC loss in the future.
5. Conclusions
The results obtained conclude that the RothC 26.3 model is suitable for the estimation of SOC stock changes under different bamboo species. The RMSE value range (0.74 to 3.2), and the EF value range (−20.32 to 0.94), suggest that RothC modeled values are near to the observed values of SOC for the bamboo plantations. In India and other developing countries, bamboo are seldom raised in fertile soil, and degraded soils are allotted for bamboo plantations. The observed Soc values suggest a decrease in SOC; such trends warrant external inputs to maintain soil fertility. Simulation with FYM addition suggests that 9 t ha−1 FYM should be added at the time of plantation or within three years of plantation to avoid a decrease in initial SOC content due to vigorous bamboo growth. An increase in temperature (simulated at 1 °C and 2 °C) in the event of climate change would reduce SOC content ranging from 0.46 per cent to 5.97 per cent over 15 years of simulation (up to the year 2030) compared to initial SOC status. This suggests taking appropriate soil management measures to maintain SOC under changing climate scenarios.
Author Contributions
Conceptualization, P.P., R.K. and J.D.; methodology, R.K., J.M.S.T. and P.P.; software, P.P.; validation, R.K., P.P.; formal analysis, P.P.; investigation, R.K.; resources, J.D. and S.R.; data curation, P.D., D.M. and A.G.; writing—original draft preparation, P.P.; writing—review and editing, P.P., J.D., S.R. and R.K.; visualization, C.S.; supervision, M.M.; project administration, R.K.; funding acquisition, R.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the State Forest Department, Uttarakhand, India, and the International Bamboo and Rattan Organization (INBAR), with its project funded by the International Fund for Agriculture Development (IFAD) and European Union (EU).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The financial help received is duly acknowledged. The authors are thankful to the ICAR and the Director of the Institute for providing the necessary facilities to conduct the work. The corresponding author is highly grateful to Kevin Coleman (UK), who provided the RothC Model software.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Van Keulen, H. Tropical soil organic matter modelling: Problems and prospects. Nutr. Cycl. Agroecosyst. 2001, 61, 33–39. [Google Scholar] [CrossRef]
- Rani, S.; Benbi, D.K.; Rajasekaran, A.; Chauhan, S.K. Litterfall, decomposition and nutrient release patterns of different tree species in TaranTaran district of Punjab, India. J. Appl. Nat. Sci. 2016, 8, 1260–1266. [Google Scholar] [CrossRef]
- Farage, P.K.; Ardö, J.; Olsson, L.; Rienzi, E.A.; Ball, A.S.; Pretty, J.N. The potential for soil carbon sequestration in the tropic dryland farming systems of Africa and Latin America: A modelling approach. Soil Tillage Res. 2007, 94, 457–472. [Google Scholar] [CrossRef]
- Jones, C.; McConnell, C.; Coleman, K.; Cox, P.; Falloon, P.; Jenkinson, D.; Powlson, D. Global climate change and soil carbon stock; predictions from two contrasting models for turnover of organic carbon in soil. Glob. Change Biol. 2005, 11, 154–166. [Google Scholar] [CrossRef]
- Paustian, K.; Andren, O.; Janzen, H.; Lal, R.; Tian, G.; Tiessen, H.; van Noordwijk, M.; Woomer, P. Agricultural soil as a C sink to offset CO2 emission. Soil Use Manag. 1997, 13, 230–244. [Google Scholar] [CrossRef]
- ISFR. Indian State of Forest Report; Forest Survey of India, Ministry of Environment & Forests, Government of India: Dehradun, India, 2017.
- ISFR. Chapter 8 Bamboo resource of country. In Indian State of Forest Report; Forest Survey of India, Ministry of Environment & Forests, Government of India: Dehradun, India, 2019. [Google Scholar]
- Kleinhenz, V.; Midmore, D. Aspects of Bamboo Agronomy. Adv. Agron. 2001, 94, 99–144. [Google Scholar]
- Nath, A.J.; Lal, R.; Das, A.K. Managing woody bamboos for carbon farming and carbon trading. Glob. Ecol. Conserv. 2015, 3, 654–663. [Google Scholar] [CrossRef]
- Yuen, J.Q.; Fung, T.; Ziegler, A.D. Carbon stocks in bamboo ecosystems worldwide: Estimates and uncertainties. For. Ecol. Manag. 2017, 393, 113–138. [Google Scholar] [CrossRef]
- Lou, Y.; Li, Y.; Kathler, B.; Giles, H.; Zhou, G. Bamboo and Climate Change Mitigation: Comparative Analysis of Carbon Sequestration; Technical Report No. 32; INBAR: Beijing, China, 2010; pp. 1–20. [Google Scholar]
- Nath, A.J.; Lal, R.; Das, A.K. Ethnopedology and soil properties in bamboo (Bambusa sp.) based agroforestry system in North East India. Catena 2015, 135, 92–99. [Google Scholar] [CrossRef]
- Parr, J.; Sullivan, L.; Chen, B.; Ye, G.; Zheng, W. Carbon bio-sequestration within the phytoliths of economic bamboo species. Glob. Chang. Biol. 2010, 16, 2661–2667. [Google Scholar] [CrossRef]
- Huang, Z.-T.; Li, Y.-F.; Jiang, P.-K.; Chang, S.X.; Song, Z.-L.; Liu, J.; Zhou, G.-M. Long-term intensive management increased carbon occluded in phytolith (PhytOC) in bamboo forest soils. Sci. Rep. 2014, 4, 3602. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, B.; Schulz, E.; Rethemeyer, J.; Merbach, I.; Flessa, H. Predictive modelling of C dynamics in the long-term fertilization experiment at Bad Lauchstadt with the Rothamsted Carbon Model. Eur. J. Soil Sci. 2007, 58, 1155–1163. [Google Scholar] [CrossRef]
- Dendoncker, N.; Van Wesemael, B.; Smith, P.; Lettens, S.; Roelandt, C.; Rounsevell, M. Assessing scale effects on modelled soil organic carbon contents as a result of land use change in Belgium. Soil Use Manag. 2008, 24, 8–18. [Google Scholar] [CrossRef]
- Falloon, P.; Jones, C.D.; Cerri, C.E.; Al-Adamat, R.; Kamoni, P.; Bhattacharyya, T.; Easter, M.; Paustian, K.; Killian, K.; Coleman, K.; et al. Climate change and its impact on soil and vegetation carbon storage in Kenya, Jordan, India and Brazil. Agric. Ecosyst. Environ. 2007, 122, 114–124. [Google Scholar] [CrossRef]
- Coleman, K.; Jenkinson, D.S.; Crocker, G.J.; Grace, P.R.; Klir, J.; Körschens, M.; Poulton, P.R.; Richter, D.D. Simulating trends in soil organic carbon in long-term experiments using RothC-26.3. Geoderma 1997, 81, 29–44. [Google Scholar] [CrossRef]
- Jenkinson, D.S.; Meredith, J.; Kinyamario, J.I.; Warren, G.P.; Wong, M.T.; Harkness, D.D.; Bol, R.; Coleman, K. Estimating net primary production from measurements made on soil organic matter. Ecology 1999, 80, 2762–2773. [Google Scholar] [CrossRef]
- Kelly, R.H.; Parton, W.J.; Crocker, G.J.; Graced, P.R.; Klir, J.; Körschens, M.; Poulton, P.R.; Richter, D.D. Simulating trends in soil organic carbon in long-term experiments using the century model. Geoderma 1997, 81, 75–90. [Google Scholar] [CrossRef]
- Smith, P.; Smith, J.U.; Powlson, D.S.; McGill, W.B.; Arah, J.R.; Chertov, O.G.; Coleman, K.; Franko, U.; Frolking, S.; Jenkinson, D.S.; et al. A comparison of the performance of nine soil organic matter models using datasets from seven long-term experiments. Geoderma 1997, 81, 153–225. [Google Scholar] [CrossRef]
- Falloon, P.; Smith, P.; Coleman, K.; Marshall, S. Estimating the size of the inert organic matter pool from total soil organic carbon content for use in the Rothamsted carbon model. Soil Biol. Biochem. 1998, 30, 1207–1211. [Google Scholar] [CrossRef]
- Falloon, P.; Smith, P. Simulating SOC changes in long-term experiments with RothC and CENTURY: Model evaluation for a regional scale application. Soil Use Manag. 2002, 18, 101–111. [Google Scholar] [CrossRef]
- Zimmermann, M.; Leifeld, J.; Schmidt, M.W.; Smith, P.; Fuhrer, J. Measured soil organic matter fractions can be related to pools in the RothC model. Eur. J. Soil Sci. 2006, 58, 658–667. [Google Scholar] [CrossRef]
- Panwar, P.; Chauhan, S.; Das, D.K.; Kaushal, R.; Arora, G.; Chaturvedi, S. Soil organic carbon dynamics in Populus deltoids plantations using RothC-model in the Indo-Gangetic region of India. Curr. Sci. 2021, 121, 1623–1627. [Google Scholar] [CrossRef]
- Pal, S.; Panwar, P.; Yadav, R.P. Modelling dynamics of soil organic carbon under different tillage systems. Agrochimica 2022, 66, 3–14. [Google Scholar] [CrossRef]
- Bhattacharyya, T.; Pal, D.K.; Deshmukh, A.S.; Deshmukh, R.R.; Ray, S.K.; Chandran, P.; Mandal, C.; Telpande, B.; Nimje, A.M.; Tiwary, P. Evaluation of RothC model using four Long Term Fertilizer Experiments in blacksoils, India. Agric. Ecosyst. Environ. 2011, 144, 222–234. [Google Scholar] [CrossRef]
- Afzali, S.F.; Azad, B.; Golabi, M.H.; Francaviglia, R. Using RothC Model to Simulate Soil Organic Carbon Stocks under Different Climate Change Scenarios for the Rangelands of the Arid Regions of Southern Iran. Water 2019, 11, 2107. [Google Scholar] [CrossRef]
- Walkley, A.J.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Coleman, K.; Jenkinson, D.S. Rothc-26.3: A Model for the Turnover of Carbon in Soil. Model Description and Windows Users’ Guide; Institute of Arable Crops Research: Rothamsted, UK, 2005. [Google Scholar]
- Jenkinson, D.S.; Harris, H.C.; Ryan, J. Organic matter turnoverin a calcareous clay soil from Syria under a two-course cerealrotation. Soil Biol. Biochem. 1999, 31, 687–693. [Google Scholar] [CrossRef]
- Roberta, F.; Coleman, K.; Whitmore, A.P. Modification of the RothC model for simulations of soil organic C dynamics in dryland regions. Geoderma 2013, 200–201, 18–30. [Google Scholar]
- Chen, C.R.; Xu, Z.H.; Mathers, N.J. Soil carbon pools in adjacent natural and plantation forests of subtropical Australia. Soil Sci. Soc. Am. J. 2004, 68, 282–291. [Google Scholar] [CrossRef]
- Nath, A.; Das, A. Carbon storage and sequestration in bamboo-based smallholder homegardens of Barak Valley, Assam. Curr. Sci. 2011, 100, 229–233. [Google Scholar]
- Kaushal, R.; Subbulaksmi, V.; Tomar, J.M.S.; Alam, N.M.; Jayaprakash, J.; Mehta, H.; Chaturvedi, O.P. Predictive models for biomass and carbon stock estimation in Male bamboo (Dendrocalamus strictus L.) in Doon valley, India. Acta Ecol. Sin. 2016, 36, 469–476. [Google Scholar] [CrossRef]
- Guo, L.B.; Gifford, R.M. Soil carbon stocks and land use change. Glob. Chang. Biol. 2002, 8, 345–360. [Google Scholar] [CrossRef]
- Koul, D.N.; Panwar, P. Opting different land use for carbon buildup in soils and their bioeconomics in humid subtropics of West Bengal, India. Ann. For. Res. 2012, 55, 253–264. [Google Scholar]
- Kaushal, R.; Singh, I.; Thapliyal, S.D.; Gupta, A.K.; Mandal, D.; Tomar, J.M.S.; Kumar, A.; Alam, N.M.; Kadam, D.; Singh, D.V.; et al. Rooting behavior and soil properties in different bamboo species of Western Himalayan Foothils, India. Sci. Rep. 2020, 10, 4966. [Google Scholar] [CrossRef]
- Sainju, U.M.; Jabro, J.D.; Stevens, W.B. Soil carbon dioxide emission and carbon content as affected by irrigation, tillage, cropping system, and nitrogen fertilization. J. Environ. Qual. 2008, 37, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Zhao, J.; Wu, J.P.; Chen, H.; Lin, Y.B.; Zhou, L.X.; Fu, S.L. Impacts of understory species removal and/or addition on soil respiration in a mixed forest plantation with native species in southern China. For. Ecol. Manag. 2011, 261, 1053–1060. [Google Scholar] [CrossRef]
- Zhou, B.-Z.; Fu, M.-Y.; Xie, J.-Z.; Yang, X.-S.; Li, Z.-C. Ecological functions of bamboo forest: Research and application. J. For. Res. 2005, 16, 143–147. [Google Scholar]
- Sahoo, U.K.; Singh, S.L.; Gogoi, A.; Kenye, A.; Sahoo, S.S. Active and passive soil organic carbon pools as affected by different land use types in Mizoram, Northeast India. PLoS ONE 2019, 14, e0219969. [Google Scholar] [CrossRef]
- Rasse, D.P.; Rumpel, C.; Dignac, M.F. Is soil carbon mostly root carbon? Mechanisms for a specific stabilisation. Plant Soil 2005, 269, 341–356. [Google Scholar] [CrossRef]
- Tripathi, S.K.; Singh, K.P. Productivity and nutrient cycling in recently harvested and mature bamboo savannas in the dry tropics. J. Appl. Ecol. 1994, 31, 109–124. [Google Scholar] [CrossRef]
- Singh, A.N.; Singh, J.S. Biomass net primary production and impact of bamboo plantation on soil redevelopment in a dry tropicalregion. For. Ecol. Manag. 1999, 119, 195–207. [Google Scholar] [CrossRef]
- Upadhyaya, K.; Arunachalam, A.; Arunachalam, K. Microbial biomass and physico-chemical properties of soil under the canopy of Bambusa balcooa Roxb. and Bambusa pallida Munro. Indian J. Soil Conserv. 2003, 31, 152–156. [Google Scholar]
- Christanty, L.; Mailly, D.; Kimmins, J.P. ‘Without bamboo, the land dies’: Biomass, litterfall, and soil organic matter dynamics of a Javanese bamboo talun-kebunsystem. For. Ecol. Manag. 1996, 87, 75–88. [Google Scholar] [CrossRef]
- Friggens, N.L.; Hester, A.J.; Mitchell, R.J.; Parker, T.C.; Subke, J.A.; Wookey, P.A. Tree planting in organic soils does not result in net carbon sequestration on decadal timescales. Glob. Change Biol. 2020, 26, 5178–5188. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, D.; Tang, J.; Bouskill, N.; Georgiou, K.; Chacon, S.S.; Riley, W.J. Abiotic and biotic controls on soil organo–mineral interactions: Developing model structures to analyze why soil organic matter persists. Rev. Mineral. Geochem. 2019, 85, 329–348. [Google Scholar] [CrossRef]
- Azad, B.; Afzali, S.F.; Ghanbarian, G.A. Modeling the effect of vegetation conversion and climate change on soil organic carbon stock dynamics in a complex ecosystem. J. Soil Manag. Sustain. 2019, 9, 83–99. [Google Scholar]
- Jebari, A.; Del Prado, A.; Pardo, G.; Martín, J.A.R.; Álvaro-Fuentes, J. Modeling Regional Effects of Climate Change on Soil Organic Carbon in Spain. J. Environ. Qual. 2018, 47, 644–653. [Google Scholar] [CrossRef]
- Mondini, C.; Coleman, K.; Whitmore, A.; Whitmore, A. Spatially explicit modelling of changes in soil organic C in agricultural soils in Italy, 2001–2100: Potential for compost amendment. Agric. Ecosyst. Environ. 2012, 153, 24–32. [Google Scholar] [CrossRef]
- Soleimani, A.; Hosseini, S.M.; Bavani, A.R.M.; Jafari, M.; Francaviglia, R. Simulating soil organic carbon stock as affected by land cover change and climate change, Hyrcanian forests (northern Iran). Sci. Total Environ. 2017, 599, 1646–1657. [Google Scholar] [CrossRef]
- Muñoz-Rojas, M.; Jordan, A.; Zavala, L.M.; González-Peñaloza, F.A.; De La Rosa, D.; Anaya-Romero, M. Modelling soil organic carbon stocks in global change scenarios: A CarboSOIL application. Biogeosci. Discuss. 2013, 10, 10997–11035. [Google Scholar] [CrossRef]
- Muñoz-Rojas, M.; Doro, L.; Ledda, L.; Francaviglia, R. Application of CarboSOIL model to predict the effects of climate change on soil organic carbon stocks in agro-silvo-pastoral Mediterranean management systems. Agric. Ecosyst. Environ. 2015, 202, 8–16. [Google Scholar] [CrossRef]
- Wan, Y.; Lin, E.; Xiong, W.; Li, Y.; Guo, L. Modeling the impact of climate change on soil organic carbon stock in upland soils in the 21st century in China. Agric. Ecosyst. Environ. 2011, 141, 23–31. [Google Scholar] [CrossRef]
- Xu, X.; Liu, W.; Kiely, G. Modeling the change in soil organic carbon of grassland in response to climate change: Effects of measured versus modelled carbon pools for initializing the Rothamsted Carbon model. Agric. Ecosyst. Environ. 2011, 140, 372–381. [Google Scholar] [CrossRef]
- Hobley, E.; Wilson, B.; Wilkie, A.; Gray, J.; Koen, T. Drivers of soil organic carbon storage and vertical distribution in Eastern Australia. Plant Soil 2015, 390, 111–127. [Google Scholar] [CrossRef]
- Longbottom, T.L.; Townsend-Small, A.; Owen, L.A.; Murari, M.K. Climatic and topographic controls on soil organic matter storage and dynamics in the Indian Himalaya: Potential carbon cycle-climate change feedbacks. Catena 2014, 119, 125–135. [Google Scholar] [CrossRef]
- Rabbi, S.M.; Tighe, M.; Delgado-Baquerizo, M.; Cowie, A.; Robertson, F.; Dalal, R.; Page, K.; Crawford, D.; Wilson, B.R.; Schwenke, G.; et al. Climate and soil properties limit the positive effects of land use reversion on carbon storage in Eastern Australia. Sci. Rep. 2015, 5, 17866. [Google Scholar] [CrossRef]
- Silva Araujo, J.K.; de Souza Junior, V.S.; Marques, F.A.; Voroney, P.; da Silva Souza, R.A. Assessment of carbon storage under rainforests in Humic Hapludox along a climosequence extending from the Atlantic coast to the highlands of northeastern Brazil. Sci. Total Environ. 2016, 568, 339–349. [Google Scholar] [CrossRef]
- McSherry, M.E.; Ritchie, M.E. Effects of grazing on grassland soil carbon: A global review. Glob. Change Biol. 2013, 19, 1347–1357. [Google Scholar] [CrossRef]
- González-Molina, L.; Etchevers-Barra, J.D.; Paz-Pellat, F. Performance of the rothc-26.3 model in short-term experiments in Mexican sites and systems. J. Agric. Sci. 2011, 149, 415–425. [Google Scholar] [CrossRef]
- Barančíková, G.; Halas, J.; Guttekova, M.; Makovnikova, J.; Novakova, M.; Skalský, R.; Tarasovičová, Z. Application of RothC model to predict soil organic carbon stock on agricultural soils of Slovakia. Soil Water Res. 2010, 5, 1–9. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).