Mechanism of Terrestrial Plant Community Assembly under Different Intensities of Anthropogenic Disturbance in Dianchi Lakeside
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling Sites
2.2. Soil Sampling and Measurement
2.3. Plant Trait Selection and Measurement
2.4. Phylogenetic Tree and Phylogenetic Signal Tests
2.5. Phylogenetic Community Structure Tests
2.6. Functional Community Structure Tests
2.7. Trade-Off Analysis of Leaf Economic Spectrum Traits
3. Results
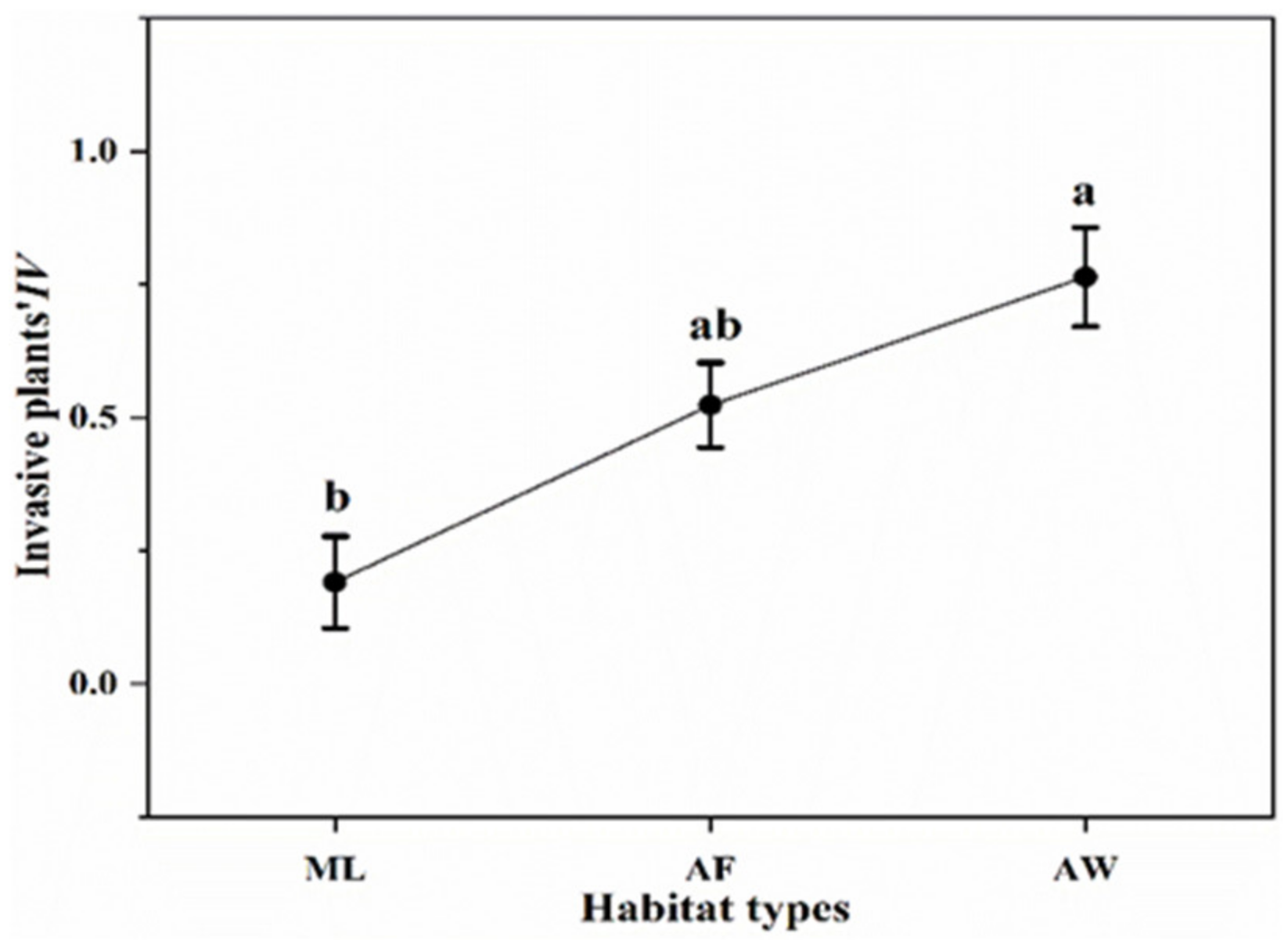
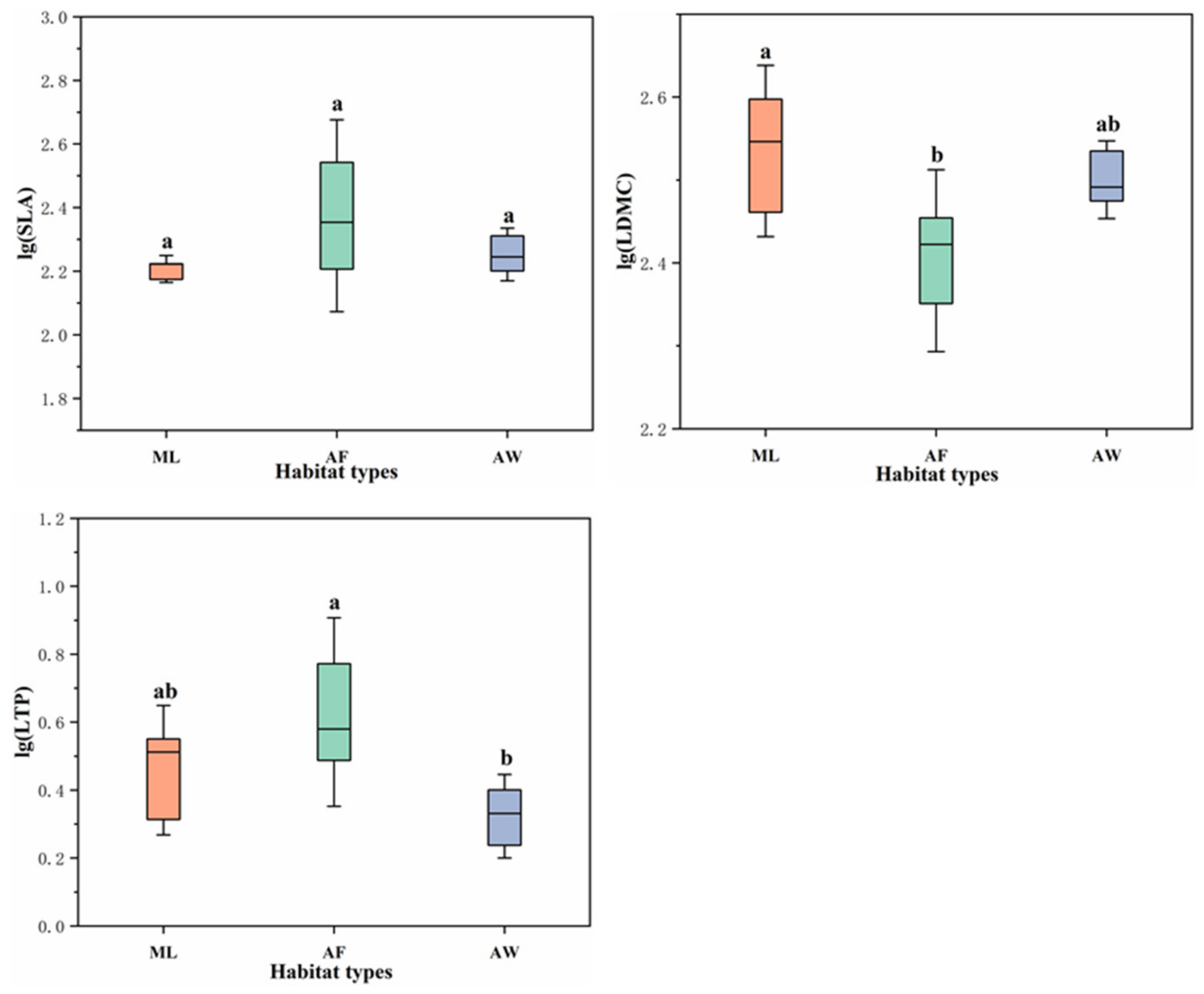
3.1. Community Characteristics in the Three Habitat Types
3.2. Phylogenetic Signal of Traits
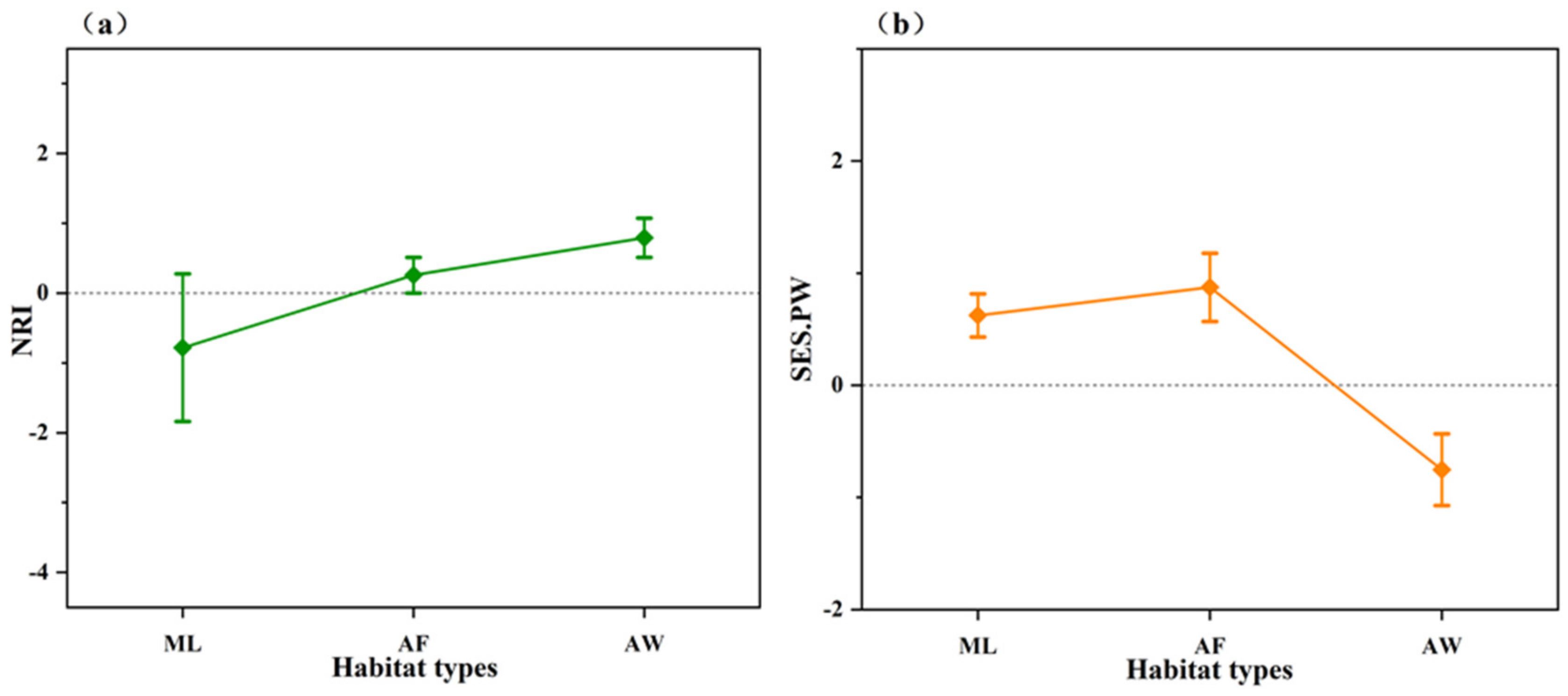
3.3. Community Phylogenetic Structure
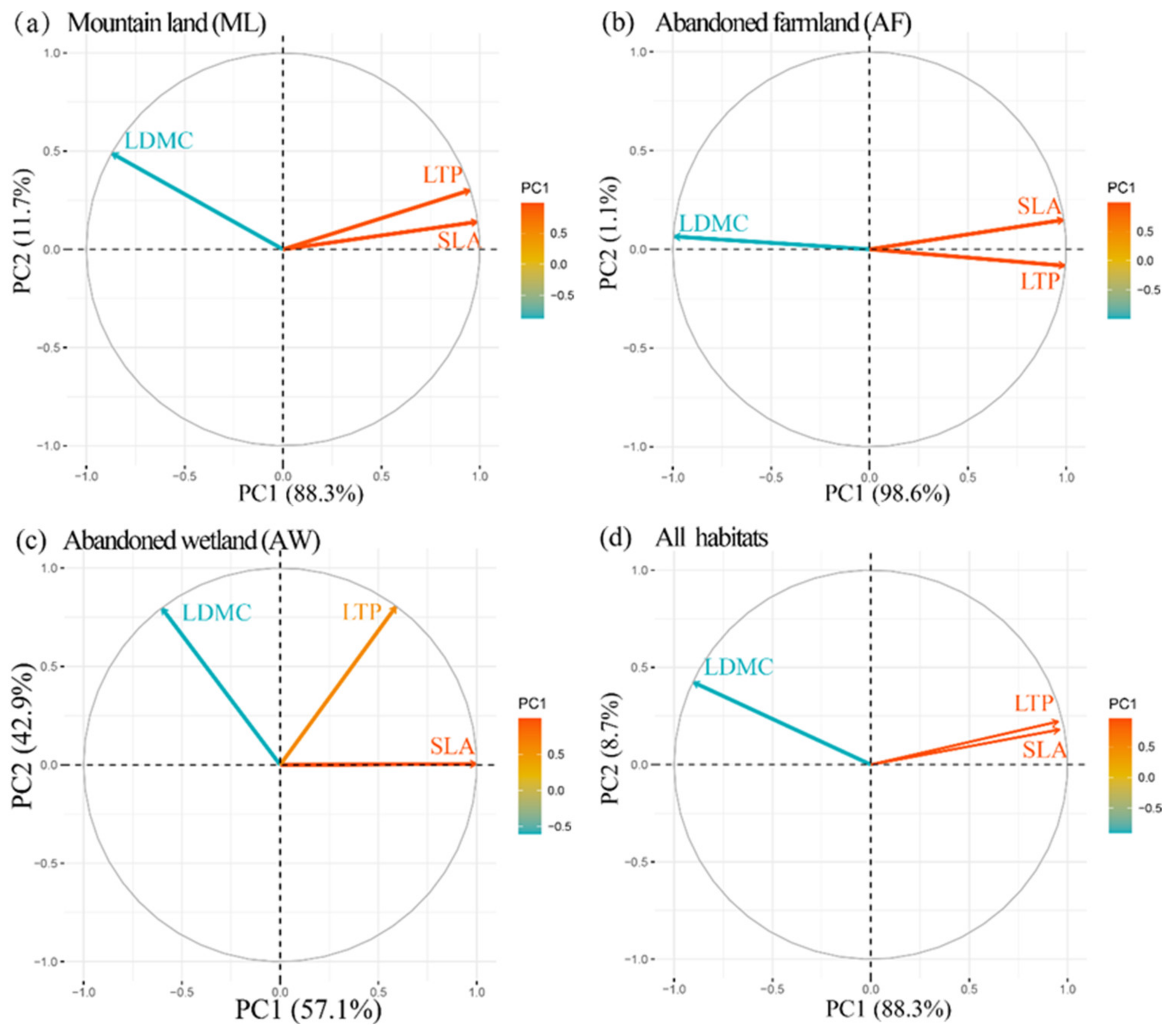
3.4. Community Functional Structure
3.5. Ecological Strategy of Plants in the Three Habitat Types
3.6. Relationship between Community Structure and Soil Factors
4. Discussion
4.1. Phylogenetic Signal and Trait Convergence in Different Habitat Types
4.2. Disturbance and Invasion Shaped the Phylogenetic Structure of a Community
4.3. Assembly Processes of Communities Reflected by Functional Traits
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Begon, M.C.R.; Townsend; Harper, J.L. Ecology: From Individuals to Ecosystems, 4th ed.; Blackwell Publishing: Oxford, UK, 2006. [Google Scholar]
- Belyea, L.R.; Lancaster, J. Assembly Rules within a Contingent Ecology. Oikos 1999, 86, 402–416. [Google Scholar] [CrossRef]
- Yi, D.; Zang, R.; Letcher, S.G.; Liu, S.; He, F. Disturbance regime changes the trait distribution, phylogenetic structure and community assembly of tropical rain forests. Oikos 2012, 121, 1263–1270. [Google Scholar]
- Zhang, J.; Swenson, N.G.; Liu, J.; Liu, M.; Qiao, X.; Jiang, M. A phylogenetic and trait-based analysis of community assembly in a subtropical forest in central China. Ecol. Evol. 2020, 10, 8091–8104. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Cadotte, M.W.; Burgess, K.S.; Liu, J.; Gao, L. Forest community assembly is driven by different strata-dependent mechanisms along an elevational gradient. J. Biogeogr. 2019, 46, 2174–2187. [Google Scholar] [CrossRef]
- Diaz, S.; Cabido, M.; Casanoves, F. Plant functional traits and environmental filters at a regional scale. J. Veg. Sci. 1998, 9, 113–122. [Google Scholar] [CrossRef]
- Lavorel, S.; Garnier, E. Predicting changes in community composition and ecosystem functioning from plant traits: Revisiting the Holy Grail. Funct. Ecol. 2002, 16, 545–556. [Google Scholar] [CrossRef]
- Gitay, W.H. Limitations to Species Coexistence: Evidence for competition from field observations, using a patch model. J. Veg. Sci. 1995, 6, 369–376. [Google Scholar]
- Webb, C.O.; Ackerly, D.D.; McPeek, M.A.; Donoghue, M.J. Phylogenies and community ecology. Annu. Rev. Ecol. Syst. 2002, 33, 475–505. [Google Scholar] [CrossRef]
- Chave, J. Neutral Theory and Community Ecology. Ecol. Lett. 2004, 7, 241–253. [Google Scholar] [CrossRef]
- Etienne, R.S.; Haegeman, B. The neutral theory of biodiversity with random fission speciation. Theor. Ecol. 2011, 4, 87–109. [Google Scholar] [CrossRef]
- Yi, D.; Zang, R.; Lu, X.H.; Huang, J.H. Functional features of tropical montane rain forests along a logging intensity gradient. Ecol. Indic. 2019, 97, 311–318. [Google Scholar]
- Feng, G.; Jens-Christian, S.; Mi, X.C.; Qi, J.; Mide, R.; Haibao, R.; Daniel, P.B.; Ma, K.P. Anthropogenic disturbance shapes phylogenetic and functional tree community structure in a subtropical forest. For. Ecol. Manag. 2014, 313, 188–198. [Google Scholar] [CrossRef]
- Díaz, S.; McIntyre, S.; Lavorel, S.; Pausas, J.G. Does Hairiness matter in harare? resolving controversy in global comparisons of plant trait responses to ecosystem disturbance. New Phytol. 2002, 154, 7–9. [Google Scholar] [CrossRef]
- Dinnage, R. Disturbance Alters the Phylogenetic composition and structure of plant communities in an old field system. PLoS ONE 2009, 4, e7071. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Stephen, J.M.; He, F.L. Does disturbance regime change community assembly of angiosperm plant communities in the boreal forest? J. Plant Ecol. 2014, 7, 188–201. [Google Scholar] [CrossRef]
- Letcher, S.G.; Chazdon, R.L.; Andrade, A.C.S.; Bongers, F.; Breugel, M.V.; Finegan, B.; Laurance, S.G.; Mesquita, R.C.G.; Martínez-ramos, M.; Williamson, G.B. Phylogenetic community structure during succession: Evidence from three neotropical forest sites. J. PPEES Sources 2012, 14, 79–87. [Google Scholar] [CrossRef]
- Purschke, O.; Schmid, B.C.; Sykes, M.T.; Poschlod, P.; Winter, M.; Prentice, H.C.; Michalski, S.G.; Durka, W.; Ingolf, K. Contrasting changes in taxonomic, phylogenetic and functional diversity during a long-term succession: Insights into assembly processes. J. Ecol. 2013, 101, 857–866. [Google Scholar] [CrossRef]
- Bhaskar, R.; Dawson, T.E.; Balvanera, P. Community assembly and functional diversity along succession post-management. Funct. Ecol. 2014, 28, 1256–1265. [Google Scholar] [CrossRef]
- Lewis, R.J.; Marrs, R.H.; Pakeman, R.J. Inferring temporal shifts in landuse intensity from functional response traits and functional diversity patterns: A study of scotland’s machair grassland. Oikos 2014, 123, 334–344. [Google Scholar] [CrossRef]
- Liu, X.; Wang, H. Dianchi lake, China: Geological formation, causes of eutrophication and recent restoration efforts. Aquat. Ecosyst. Health Manag. 2016, 19, 40–48. [Google Scholar] [CrossRef]
- Du, L.N.; Li, Y.; Chen, X.Y.; Yang, J.X. Effect of eutrophication on molluscan community composition in the lake Dianchi (China, Yunnan). Limnologica 2011, 41, 213–219. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Z.; Zhang, J.Q.; Zhang, Y.Y.; Liu, H.Q.; Yan, S.H. Large-scale utilization of water hyacinth for nutrient removal in Lake Dianchi in China: The effects on the water quality, macrozoobenthos and zooplankton. Chemosphere 2012, 89, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.B.; Wang, S.R.; Zhang, L.; Ni, Z.K. Water pollution characteristics of Dianchi Lake and the course of protection and pollution management. Environ. Earth Sci. 2015, 74, 3767–3780. [Google Scholar] [CrossRef]
- Li, T.; Gao, X. Ecosystem services valuation of lakeside wetland park beside Chaohu Lake in China. Water 2016, 8, 301. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, S.K.; Wu, F.Q.; Yang, G.S.; Dong, W.J.; Liu, F.L.; He, S.Z.; Wang, Y.H. Species compositions and flora analysis of spermatophyta in Dianchi Lakeside. Chin. J. Ecol. 2017, 36, 359–366. (In Chinese) [Google Scholar]
- Chen, Q.Y.; Ni, Z.K.; Wang, S.R.; Guo, Y.; Liu, S.R. Climate Change and Human Activities Reduced the Burial Efficiency of Nitrogen and Phosphorus in Sediment from Dianchi Lake, China. J. Clean. Prod. 2020, 274, 122839. [Google Scholar] [CrossRef]
- Hou, Y.; Li, B.; Müller, F.; Chen, W.P. Ecosystem services of human-dominated watersheds and land use influences: A case study from the Dianchi Lake watershed in China. Environ. Monit. Assess. 2016, 188, 652. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Liang, R.F.; Zhao, P.X.; Liu, Q.Y.; Li, Y.; Wang, K.L.; Li, K.F.; Liu, Y.; Wang, P. The hydraulic driving mechanisms of cyanobacteria accumulation and the effects of flow pattern on ecological restoration in Lake Dianchi Caohai. Int. J. Environ. Res. Public Health 2019, 16, 361. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Wu, Z.L.; Wu, L.Z.; Shi, H.L.; Bai, H.T.; Fu, W.; Ye, Y. Interspecific correlation between exotic and native plants under artificial wetland forests on the Dianchi Lakeside, South-West China. Mar. Freshw. Res. 2018, 69, 669–676. [Google Scholar] [CrossRef]
- Ministry of Ecology and Environment of the People’s Republic of China. 2016. Available online: https://www.mee.gov.cn/ (accessed on 24 September 2020).
- Dewi, C.S.U.; Sukandar. Important value index and biomass (estimation) of seagrass on Talango Island, Sumenep, Madura. AIP Conf. Proc. 2017, 1908, 030005. [Google Scholar]
- Sherrod, L.A.; Dunn, G.; Peterson, G.A.; Kolberg, R.L. Inorganic carbon analysis by modified pressure-calcimeter method. Soil Sci. Soc. Am. J. 2002, 66, 299–305. [Google Scholar] [CrossRef]
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E. New handbook for standardised measurement of plant functional traits worldwide corrigendum. Aust. J. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- Webb, C.O. Exploring the phylogenetic structure of ecological communities: An example for rain forest trees. Am. Nat. 2000, 156, 145–155. [Google Scholar] [CrossRef]
- Zanne, A.E.; David, C.T.; William, K.C.; Jonathan, M.E.; Stephen, A.S.; Richard, G.F.; Daniel, J.M.; O’Meara, B.C.; Moles, A.T.; Reich, P.B.; et al. Three keys to the radiation of angiosperms into freezing environments. Nature 2014, 506, 89–92. [Google Scholar] [CrossRef]
- Blomberg, S.P.; Garland, T.; Anthony, R.I. Testing for phylogenetic signal in comparative data: Behavioral traits are more labile. Evolution 2003, 57, 717–745. [Google Scholar] [PubMed]
- Duarte, L.D.S.; Debastiani, V.J.; Carlucci, M.B.; Diniz-Filho, J.A.F. Analyzing community-weighted trait means across environmental gradients: Should phylogeny stay or should it go? Ecology 2018, 99, 385–398. [Google Scholar] [CrossRef]
- Ackerly, D.D.; Cornwell, W.K. A trait-based approach to community assembly: Partitioning of species trait values into within- and among-community components. Ecol. Lett. 2007, 10, 135–145. [Google Scholar] [CrossRef]
- Kembel, S.W.; Cowan, P.D.; Helmus, M.R.; Cornwell, W.K.; Morlon, H.; Ackerly, D.D.; Blomberg, S.P.; Webb, C.O. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 2010, 26, 1463–1464. [Google Scholar] [CrossRef] [PubMed]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The Worldwide Leaf Economics Spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Díaz, S.; Kattge, J.; Cornelissen, J.H.C.; Wright, I.J.; Lavorel, S.; Dray, S.; Reu, B.; Kleyer, M.; Wirth, C.; Prentice, I.C.; et al. The global spectrum of plant form and function. Nature 2016, 529, 167–171. [Google Scholar] [CrossRef]
- Riva, E.G.D.; Tosto, A.; Perez-Ramos, C.M.; Navarro-Fernandez, I.M.; Olmo, M.; Anten, N.P.R.; Maranon, T.; Villar, R. A plant economics spectrum in Mediterranean Forests along environmental gradients: Is there coordination among leaf, stem and root traits? J. Veg. Sci. 2016, 27, 187–199. [Google Scholar] [CrossRef]
- Josse, L.S.J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar]
- Knapp, S.; Kühn, I.; Schweiger, O.; Klotz, S. Challenging urban species diversity: Contrasting phylogenetic patterns across plant functional groups in Germany. Ecol. Lett. 2008, 11, 1054–1064. [Google Scholar] [CrossRef] [PubMed]
- Pipenbaher, N.; Skornik, S.; Carvalho, G.H.D.; Batalha, M.A. Phylogenetic and functional relationships in pastures and meadows from the North Adriatic Karst. Plant Ecol. 2013, 214, 501–519. [Google Scholar] [CrossRef]
- DeFerrari, C.M.; Naiman, R.J. A Multi-Scale Assessment of the occurrence of exotic plants on the Olympic Peninsula, Washington. J. Veg. Sci. 1994, 5, 247–258. [Google Scholar] [CrossRef]
- Daleo, P.; Alberti, J.; Iribarne, O. Biological invasions and the neutral theory. Divers. Distrib. 2009, 15, 547–553. [Google Scholar] [CrossRef]
- Čeplová, N.; Lososová, Z.; Zelený, D.; Chytrý, M.; Danihelka, J.; Fajmon, K.; Láníková, D.; Preislerová, Z.; Řehořek, V.; Tichý, L. Phylogenetic diversity of central-European urban plant communities: Effects of alien species and habitat types. Preslia 2015, 87, 1–16. [Google Scholar]
- Winter, M.; Schweiger, O.; Klotz, S.; Nentwig, W.; Andriopoulos, P.; Arianoutsou, M.; Basnou, C.; Delipetrou, P.; Didžiulis, V.; Hejda, M.; et al. Plant extinctions and introductions lead to phylogenetic and taxonomic homogenization of the European Flora. Proc. Natl. Acad. Sci. USA 2009, 106, 21721–21725. [Google Scholar] [CrossRef] [PubMed]
- Mouillot, D.; Graham, N.A.J.; Villéger, S.; Mason, N.W.H.; Bellwood, D.R. A functional approach reveals community responses to disturbances. Trends Ecol. Evol. 2013, 28, 167–177. [Google Scholar] [CrossRef]
- Weiher, E.; Clarke, G.D.P.; Keddy, P.A.; Weiher, E.; Clarke, G.D.P.; Keddy, P.A. Community assembly rules, morphological dispersion, and the coexistence of plant species. Oikos 1998, 81, 309–322. [Google Scholar] [CrossRef]
- Prommer, J.; Walker, T.W.N.; Wanek, W.; Braun, J.; Zezula, D.; Hu, Y.; Hofhansl, F.; Richter, A. Long-term functional structure and functional diversity changes in scottish grasslands. Agric. Environ. 2017, 247, 352–362. [Google Scholar]

| Plots | Habitat Types | Human Disturbance | Duration of Ecological Restoration |
|---|---|---|---|
| DW | Mountain land | Weak | ≥10 a |
| HYC | Mountain land | Weak | 5~10 a |
| LNW | Mountain land | Weak | ≥10 a |
| WLC | Abandoned farmland | Medium | 5~10 a |
| XHG | Abandoned farmland | Medium | 5~10 a |
| SP | Abandoned farmland | Medium | 5~10 a |
| FB | Abandoned farmland | Medium | 5~10 a |
| HF | Abandoned wetland | Strong | 5~10 a |
| XH | Abandoned wetland | Strong | 5~10 a |
| PLJ | Abandoned wetland | Strong | 5~10 a |
| Functional Traits | K | P |
|---|---|---|
| SLA | 0.073 | 0.371 |
| LDMC | 0.209 | 0.090 |
| LA | 0.111 | 0.501 |
| LT | 0.509 * | 0.017 |
| LTN | 0.109 | 0.164 |
| LTP | 0.130 | 0.095 |
| Chl | 0.062 | 0.401 |
| LOC | 0.408 * | 0.009 |
| NRI | S.E.S PW | |||||
|---|---|---|---|---|---|---|
| Habitat Types | Mean | SE | p | Mean | SE | p |
| ML | −0.78 | 1.057 | 0.620 | 0.62 | 0.193 | 0.370 |
| AF | 0.26 | 0.255 | 0.671 | 0.87 | 0.304 | 0.151 |
| AW | 0.79 | 0.280 | 0.194 | −0.75 | 0.320 | 0.154 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.-D.; Zhou, X.-L.; Tian, J.-J.; Yang, L.; Wang, Y.-H.; Shen, S.-K. Mechanism of Terrestrial Plant Community Assembly under Different Intensities of Anthropogenic Disturbance in Dianchi Lakeside. Forests 2023, 14, 670. https://doi.org/10.3390/f14040670
Liu Z-D, Zhou X-L, Tian J-J, Yang L, Wang Y-H, Shen S-K. Mechanism of Terrestrial Plant Community Assembly under Different Intensities of Anthropogenic Disturbance in Dianchi Lakeside. Forests. 2023; 14(4):670. https://doi.org/10.3390/f14040670
Chicago/Turabian StyleLiu, Zhen-Dian, Xiong-Li Zhou, Jian-Juan Tian, Liu Yang, Yue-Hua Wang, and Shi-Kang Shen. 2023. "Mechanism of Terrestrial Plant Community Assembly under Different Intensities of Anthropogenic Disturbance in Dianchi Lakeside" Forests 14, no. 4: 670. https://doi.org/10.3390/f14040670
APA StyleLiu, Z.-D., Zhou, X.-L., Tian, J.-J., Yang, L., Wang, Y.-H., & Shen, S.-K. (2023). Mechanism of Terrestrial Plant Community Assembly under Different Intensities of Anthropogenic Disturbance in Dianchi Lakeside. Forests, 14(4), 670. https://doi.org/10.3390/f14040670



_Sun.png)





