Abstract
Fusarium circinatum causes pine pitch canker (PPC) disease and associated symptoms such as resinous lesions, mechanical weakness, and crown dieback that may lead to mortality in Pinus and Pseudotsuga spp. There are no ameliorative techniques available for the disease, and the genetic resistance among populations to support commercial plantation deployment has not been well characterized. In this study, we characterize the genetic control of PPC disease tolerance (and/or resistance) and predict the tolerance of families in existing breeding populations: open-pollinated (OP) half-sib and control-pollinated full-sib (FS) slash pine (Pinus elliottii var. elliottii Engelm.), OP loblolly pine (Pinus taeda L.), and advanced-generation OP hybrid slash × P. caribaea (Pinus elliottii var. elliottii Engelm. × Pinus caribaea var. bahamensis, caribaea, and hondurensis) using F. circinatum isolates obtained from three locations in Georgia and FL, USA. We describe a new experimental design that improves the accuracy of breeding value predictions, provides more precise genetic parameter estimates, and facilitates comparisons within and among taxa as well as comparisons among isolates. We found strong evidence for genetic control of the ratio of stem damage by F. circinatum, especially in slash pine and slash × P. caribaea hybrids. Loblolly and slash × P. caribaea hybrids exhibited less damage than slash pine. We observed a spectrum of virulence among F. circinatum isolate sources, which were not equally virulent in different pine taxa.
1. Introduction
Background and Review of Literature
Fusarium circinatum is a damaging necrotrophic fungal pathogen that causes pine pitch canker disease (PPC). The disease is characterized by heavy resinous lesions, crown dieback, mechanical weakness in stems, loss of vigor, and mortality [1]. Native to Central America, F. circinatum is naturalized to the Southeast region of the United States and many other regions of the world where North American Pinus species are commercially grown [2,3]. North American Pinus species are planted and managed for fast growth across their native ranges and are major species of the planted forest estates in South America, South Africa, Southeast Asia, Australia, and New Zealand. Pitch canker is especially devastating to the stands of P. radiata in Chile. Pine hybrids with resistance to PPC are now replacing the severely impacted P. patula forests of South Africa and elsewhere [2,4,5]. Although pitch canker is not presently found in New Zealand or Australia, its introduction is an issue of high concern based on its impacts in Europe, South America, and South Africa [2].
PPC infection is typically caused by the introduction of F. circinatum conidia into wounds and may be vectored by water, wind, or insects [6]. F. circinatum does not require an alternate host in its life cycle and can spread from tree to tree [3]. Symptoms of the disease are more frequently observed in trees with mechanical damage typically from high wind events and high nutrient availability from a silvicultural regime [7,8]. An overabundance of nitrogen is the most well-described factor that increases the risk of infection in production stands [9]. Forest landowners across the lower US Southeast and Gulf Coast regions have observed an increase in outbreak frequency and severity of PPC in commercial stands of loblolly pine (Pinus taeda L.) and slash pine (P. elliottii var. elliottii Engelm.). In nurseries, F. circinatum can severely damage a crop of seedlings without proper seed coat treatments, soil sanitation, and prophylactic fungicide treatments (e.g., Proline™) [10].
As there is no cost-effective chemical treatment for pitch canker remediation in commercial stands; genetic tolerance is the main approach to mitigate PPC damage. In the US Southeast, endemic fusiform rust (Cronartium quercuum f. sp. fusiforme) has traditionally been the most damaging pathogen in plantation forestry. Consequently, advances in genetic resistance captured in breeding programs have provided a robust response to rust infection risk, largely due to its prevalence and the proven utility of field-based phenotypes for resistance [11,12,13,14,15]. While screening for PPC tolerance has occurred in collaborative projects with academia, industry, and the United State Forest Service (USFS), there are no domestic loblolly or slash pine breeding programs producing seed from populations with verified pitch canker tolerance, principally because field data have not been useful in screening for pitch canker resistance.
Various studies have demonstrated differences in tolerance among Pinus species in the Australes, Oocarpa, and Patula sections as well as variation among species and families within species [16,17]. These and other inoculation studies show that varieties of P. caribaea are less susceptible to damage than P. elliottii var. elliottii or P. taeda. Among the species of the Patula and Oocarpa groups, there is a wide spectrum of tolerance. While inter-taxa variation in susceptibility to PPC response precipitating stem damage is reasonably well characterized, there is considerably less information concerning intra-species genetic variation, especially in Pinus spp. relevant to the US Southeast. Studies in the 1970s and 1980s reported significant genetic variation within slash pine populations, with heritability estimates for tolerance (variously defined as narrow-sense heritability and “repeatability”) near 25% [18]. Estimates in loblolly pine are limited to two studies by Quesada et al. and Kayihan et al. estimating clonal repeatability and narrow-sense heritability around 30%, respectively [12,19].
The UF|IFAS Cooperative Forest Genetics Research Program (CFGRP) has executed controlled screenings of slash pine with some success in identifying tolerant and susceptible families [18,20]. However, there are few descriptions of genetic control, including an affirmative delineation between resistance and tolerance as a response mechanism. These screenings, along with those from other Pinus spp. suggest that there is genetic variation in PPC tolerance and that some populations have a spectrum of inherent genetic tolerance [17]. The lack of information concerning genetic tolerance to F. circinatum limits our ability to guide deployment and improving tolerance has not been an explicit target for breeding populations of loblolly or slash pine. Although some groups have examined the merit of using genomic tools for characterizing pitch canker tolerance in loblolly pine, these analyses have not been utilized to improve tolerance in breeding programs [19,21].
In a preliminary study, we used the existing USFS Resistance Screening Center (RSC) methodology to assess the response to diseases affecting Pinus sp. The standard RSC design uses six experimental units per family or seed lot, each composed of twenty trees in a tray screened in two “runs” that represent independent screenings that are typically undertaken on sequential days. For this assay, the measured phenotype is the proportion of stems in the tray with 33% or more lesion coverage, which is used to infer the percent of “damaged” seedlings.
For taxa comparisons and genetic parameter estimation, the existing RSC method is inadequate. Inference on the experimental unit is information-poor: a count of trees meeting a threshold does not stratify relative stem damage (total damage and moderate damage count the same) and so does not account for intra-family variation. The traditional method also requires 120 seedlings per family and provides only 4 total degrees of freedom to estimate the significance of differences in stem damage (USFS RSC testing manual, unpublished). Internal studies found that the families with the highest and lowest damage scores, while demonstrably different on a visual basis, are not statistically different because of poor experiment power (Figure A1).
The screening we describe here was motivated not only by the need to construct more precise estimates of genetic merit in breeding populations but also in response to the increase in PPC outbreaks in the region and the recent discovery that the virulence of reference isolates used by the USFS RSC differ significantly and that some had become avirulent [22].
Consequently, we created and tested a new experiment design with greater power to differentiate among treatments and additional information to improve the accuracy of genetic parameter estimates. We screened slash pine families from the UF|IFAS CFGRP along with loblolly pine and slash × P. caribaea hybrids from industry breeding programs to provide comparisons among North American pine taxa. The goals of this screening include obtaining breeding value predictions to guide operational deployment and genetic parameter estimates to guide the development of pitch canker resistance. We used an experiment designed to improve the precision of breeding value estimates for pitch canker tolerance, measured as the percentage of seedling stems with lesions following inoculation. The design also provided genetic parameter estimates for pitch canker tolerance and for virulence comparisons among newly identified isolates [23]. In doing so, we provide a case study that provides supporting evidence for changes in the screening protocols currently used by the USFS RSC.
2. Materials and Methods
2.1. Genetic Resources
Families from slash pine (P. elliottii var. elliottii Engelm.), loblolly pine (P. taeda L.), and hybrid P. elliottii var. elliottii × P. caribaea (including bahamensis, caribaea, and hondurensis varieties) populations were screened for stem damage caused by F. circinatum.
Sixty slash pine families were tested, a sample of commercially relevant parents in orchards managed by members of the UF|IFAS CFGRP and the Texas A&M Western Gulf Cooperative Tree Improvement Programs (WGFTIP). There were fifty-three CFGRP and seven WGFTIP families. Of these, forty-seven were open-pollinated (OP), and all thirteen full sibs (FS) were CFGRP families. Six CFGRP OP families were collected from different orchards and one genetic entry represented an OP seed orchard mix. These genetic entries were used for internal gain predictions. While the FS and OP families from CFGRP populations are connected using a pedigree that tracks relatedness among seed lots, the OP WGFTIP families were treated as unrelated half-sib families.
The twelve loblolly pine families represent a small sample of commercially relevant OP material suitable for deployment in the US Southeast. Twenty hybrid slash × P. caribaea families represented OP seed lots collected from F2 selections that are commercially deployed in southeastern Queensland, Australia. This hybrid has also been established in South Africa, South Florida, and various tropical and subtropical regions worldwide. While the primary purpose of including slash × P. caribaea hybrid and loblolly families in the screen was to compare differences in symptom development among taxa, the use of pedigreed families also allowed for the comparison of genetic parameters from the different populations.
These isolates were collected in 2019 from Suwannee County, FL, Volusia County, FL, and Wilcox County, GA. The isolates were chosen because their pathogenicity varied on two susceptible slash pine families [22]. Further information concerning these isolates, including draft genomes, can be found in Fulton et al. [23]. The isolates have become standard for RSC pitch canker screening.
2.2. Experiment Design
A total of 103 seed lots were tested, along with known “tolerant” loblolly and “susceptible” slash pine families the USFS RSC uses as experiment controls. In all, 7200 trees were arranged into 1800 row plots of 4 seedlings each. These plots were randomized in an unbalanced nested incomplete block design. A total of 360 metal trays were used as incomplete blocks with trays accommodating 20 seedlings arranged as 5-row plots of 4 seedlings each. The dimensions of the screening trays were the determinate factor in the size of incomplete blocks. Twenty incomplete blocks were assigned to 1 of 18 replications and treatments were further nested within the 3-pitch canker isolates so that 6 replications of each pine treatment were inoculated with each of the 3-pitch canker isolates. The scheme for this design is found in Figure 1.
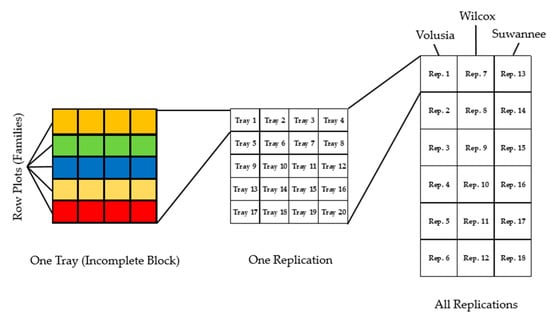
Figure 1.
A graphic representation of the experiment design. Each tray is designed as an incomplete block with five four-tree row plots. Each replication had 20 such trays (i.e., 20 incomplete blocks). Each of these replications was assigned to one of three isolate treatments.
Seeds from slash pine and slash × P. caribaea hybrid pine populations were directly planted with no prior stratification as recommended in the RSC protocol. Loblolly pine seed lots were stratified for 45 days prior to planting. Seedlings were grown in a 3:2:1 peat:vermiculite:perlite mixture and fertilized two times prior to inoculation (3 weeks and 5 weeks post-transplant) and then once (3 weeks) post-inoculation followed thereafter every 6 weeks. Fertilizer was a soluble 15-30-15 (N:P:K) dispensed at 1:100 using Dosatron injector into water stream. After 10 weeks of growth, a total of 7172 out of 7200 seedlings originally assigned in the experiment design were of sufficient size to be included in the screening. The remaining 28 cells (7-row plots) were filled with filler trees which were inoculated and then excluded from the analysis. Testing procedures followed the standard RSC protocol.
The fungal isolates were revived from the confetti pieces on PCNB agar. Spores produced from the confetti pieces were used to infect “storage” trees to rejuvenate the fungus using live trees before inoculating the test trees. The fungus was then cultured out of the infected storage trees with the use of several media including PCNB plates, APDA plates, Carnation agar plates, and water agar plates to produce spores for test inoculations. Spores were scraped from plates to produce independent aqueous spore suspensions for each isolate and were standardized to 100K spores/mL using a hemocytometer. Each seedling was cut and then hand sprayed once with a prescribed isolate from each of the 3 fusarium isolates. Seedlings were watered and monitored for 24 weeks. Total stem height and distance from the top of the seedling to the margin of the lesion were measured to estimate a damage ratio as the percentage of the stem that was damaged (ranging from 0 to 100%).
2.3. Linear Model and Variance Component Estimation
A linear mixed model was constructed to evaluate the screening data sets including and excluding seedlings with no lesion development that may have been escapes, i.e., where the inoculation process failed. Rather than a generalized linear mixed model assuming a binomial or truncated distribution, a more parsimonious and traditional linear mixed model was used [24]. The model was parameterized as shown in Equation (1):
where is the response or the proportion of total stem length that was damaged by PPC and defined as the ratio of the lesion length to the total height. is the mean, X is the design matrix for fixed effects with solutions in a vector denoted b, Z is the design matrix for random effects with solutions in a vector denoted u, and e is the error.
Fixed effects account for three taxa, three different F. circinatum isolates, the interaction of taxon and isolate, and a term for replication nested within the isolate. Random effects describe row plots nested within replicates and the double Kronecker product describes the implicit model including a pedigree-based numerator relationship matrix (A-matrix) nested within isolate that is disconnected along a block diagonal where block diagonal elements represent levels of pine taxa. This double-Kronecker product allows for taxon-specific variance component estimates with an internal covariance matrix that incorporates the A-matrix within each isolate. This model could account for numerous variance assumptions, including homogeneity or heterogeneity of variance component estimates for each pedigree within each of the isolates used for the screening.
Incomplete blocks were designed for the purpose of randomization and assignment of plots; however, they were excluded from the model because they are redundant and accounted for with the row plots. The incomplete block effect would be retained in the model if row plots were treated as experimental units instead of individual trees.
The model was assessed with multiple covariance parameterizations for the term describing the A matrix nested within F. circinatum isolate and convergence was assessed using loglikelihood solutions. A model with a common correlation and heterogenous variance estimates was found to minimize likelihood estimates. Diagonal elements of the covariance matrix (additive variance at each isolate) were assumed heterogeneous and off-diagonal elements were fixed as equal and heterogenous providing solutions for intra-isolate, inter-taxon pairwise, or group genetic correlations for predicted stem damage. The common correlation term was selected for parsimony based on relative log-likelihood. In all, this model requires estimates of nine additive variance components (one for each of three isolates in the three taxa) and three common correlations/covariances (one for each taxon). For a visual expression of the covariance model, see Figure 2.
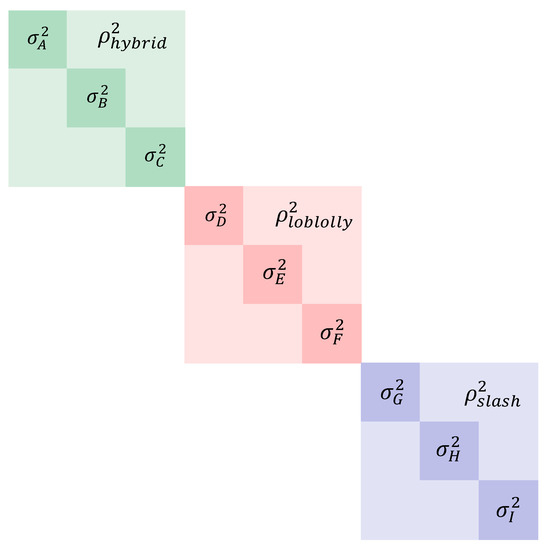
Figure 2.
A visualization of the covariance matrix specification to account for independent taxa, individual pedigree-based additive variances, and genetic correlations within taxa and among isolates. corresponds to the additive variance for slash × P. caribaea hybrids with the Suwanee isolate, corresponds to the additive variance for slash × P. caribaea hybrids with the Volusia isolate, and so on. Genetic correlations within a taxon are denoted and so on.
This model was evaluated with ASReml-R 4.1 [25]. Data arrangement, data summaries, and post-processing of variance components, statistics, and best linear unbiased predictions (BLUPs) were executed in R [26]. Breeding values of clones were calculated as BLUPs and extracted from respective model results.
Estimates of within-taxon narrow-sense heritability were calculated as shown in Equation (2):
where is the A-inverse numerated additive variance component (same for two other isolates), is the within-taxon group type-B genetic correlation, is the plot(block(rep)) variance component, and is the residual error. Estimating the heritability with the inclusion of the intra-isolate genetic correlation provides more conservative estimates.
3. Results
3.1. Summary Statistics
A sizeable proportion of seedlings (1182) had no visible damage and were either resistant seedlings or treatment escapes. A further 198 were excluded for mechanical damage or other reasons unrelated to screening: 7 slash × P. caribaea hybrid entries, 2 loblolly entries, and 189 slash pine entries. To evaluate the impact of escapes on genetic parameter estimates and breeding value predictions, data were analyzed including and excluding records with damage scores of 0%. For the reduced data set that excluded potential escapes, 511 out of 1874 slash × P. caribaea hybrid seedlings (27.3%, 20.4 seedlings/family), 222 out of 902 loblolly seedlings (24.6%, 17.3 seedlings/family), and 449 out of 4199 slash seedlings (10.7%, 6.7 seedlings/family) were removed.
Table 1 summarizes the average damage ratios stratified by taxon and inoculum. The data did not follow a normal distribution both because of the high number of end-range outcomes of 0% or 100% damage and because the response variable is bounded. Figure 3 and Figure 4 show the distribution of damage ratios stratified by taxon, family within taxon, and isolate.

Table 1.
Average damage ratios stratified by taxon and isolate for full and reduced data sets.
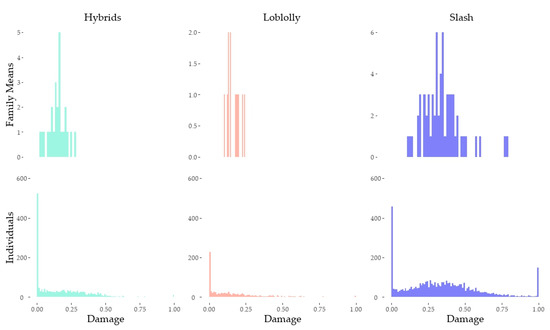
Figure 3.
Histograms of individual and raw family mean damage stratified by taxon. These his-to-grams include all data including records identified as potential escapes.
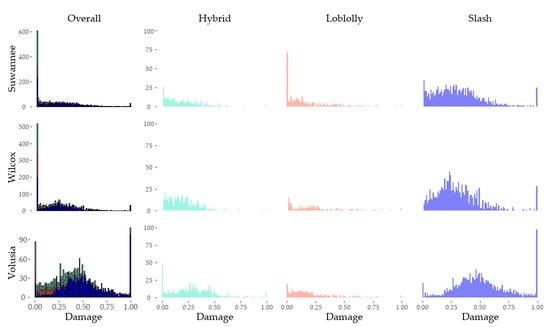
Figure 4.
Histograms of percent stem damage on individual trees stratified by isolate. The complete set of records (including all taxa) are seen in the first column. Records are stratified by taxa in the second (hybrid slash × P. caribaea), third (P. taeda), and fourth (P. elliottii var. elliottii) columns. These histograms include all data including records identified as potential escapes.
3.2. Model Effects and Genetic Parameters
3.2.1. Fixed Effects
Each fixed effect was significant when the models were fit with data that included and excluded potential escapes. From Figure 3 and Figure 4, it is evident that slash is the most susceptible pine species, and the Volusia isolate is the most virulent. The significance of the interaction between taxa and isolate seems to be largely attributable to changes in the loblolly linear predictions. Changes in the ranking of slash × P. caribaea hybrid families across taxa are negligible, and interactions of the slash families are small relative to loblolly. Although the Volusia isolate is clearly more virulent than the other two isolates, it is shown to be the least virulent isolate for a select set of loblolly families included in the screening. Solutions for the fixed effects of each model are found in Table 2:

Table 2.
Solutions for fixed effects from the model including all data and data with potential escapes excluded. Solutions indicate the predicted addition or subtraction of the predicted damage ratio. P-scores were functionally equivalent for fixed terms in each model, with every term including the replication–isolate nested interaction, a blocking effect, significant at p < 0.001.
3.2.2. Random Effects and Genetic Parameters
Random effects for respective models are listed in Table A1 with heritabilities and linear combinations of the random effects reported in Table 3. Heritability was calculated as defined in Equation (2). ASReml internally computes a common correlation as a linear combination of covariances and variances with the “corh” covariance specification option [25]. These internal estimates also include Taylor-series derived estimates of standard errors. Estimates are assumed homogeneous for each pairwise relationship of isolates within a taxon. These genetic correlation estimates correspond to both the tolerance among isolates for each taxon and a pseudo-type-B genetic correlation that describes a genotype-by-environment (GxE) interaction.

Table 3.
Estimates of narrow-sense individual tree heritability with standard errors and genetic correlations. Heritability was estimated following Equation (2). Genetic correlation is estimated directly in ASReml as a linear combination of the intra-taxon additive variances at each of the isolates and common intra-taxon covariance.
Narrow-sense heritabilities are strong for the slash × P. caribaea hybrid taxon and pure slash but low and statistically insignificant for loblolly. Each of these taxa represents samples from a deeply pedigreed population not selected for PPC tolerance, possibly contributing to narrow-sense heritability estimates higher than those typically observed in traits such as volume accumulation, stem straightness, or fusiform rust resistance.
As an additional verification, correlations among BLUP means specific to each isolate were calculated for each taxon considering the full and reduced data sets with isolates representing independent and different testing environments associated with PPC isolates Table 4. In the model where potential escapes were excluded, BLUPs were also estimated for individuals with no data to preserve the integrity of the model comparisons. These correlations were generally quite strong, indicating that information gleaned from predictions is not highly leveraged by the presence or absence of possible escapes in the experiment.

Table 4.
Correlations of BLUPs estimated for all selections in the pedigree and for parents (uniquely named clones in breeding programs or suitable for commercial deployment). Correlations are calculated for all records then stratified by taxon.
Figure 5 shows an interaction plots of the within-taxon genetic correlation among isolates. These predictions consider only the genetic effects estimated for a given parent tested with a different isolate, not the whole prediction including fixed effects for taxon, isolate, and the taxa-isolate interaction.
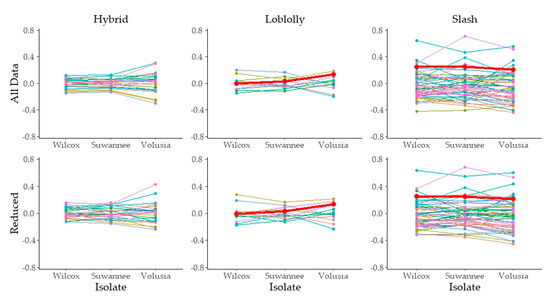
Figure 5.
Interaction plot of BLUPs for parent and ancestors (i.e., excluding progenies) stratified by taxon for both the full and reduced (i.e., potential escapes excluded) data sets. Thick red bars indicate the internal RSC check lots: MVLB for loblolly and U17 for slash.
4. Discussion
4.1. PPC Tolerance across Taxa and Isolates
We found that slash pine is significantly less tolerant than loblolly or the slash × P. caribaea hybrids to the three F. circinatum isolates used for this screening. The mean damage ratio in slash pine seedlings was significantly greater, approximately 2-fold in data sets including and excluding potential escapes (Table 1 and Table 2). The significant fixed effects for taxa from each model support Hodge and Dvorak’s findings that pure P. elliottii var. elliottii is more susceptible to PPC than P. caribaea varieties, as the hybrid of the two was significantly more tolerant [16,17]. The P. taeda families were more tolerant than both slash × P. caribaea hybrids and pure slash pine. Although these differences are statistically significant, the relatively small number of loblolly and slash × P. caribaea hybrid pine families preclude broad inter-taxa conclusions until more families are screened.
Seedlings from all three taxa were assigned randomly to trays, and there was no prescribed pattern of row plots of one taxon being near or far from another. The analysis to affirm data integrity revealed no differences among locations in trays; escape rates were functionally uniform among each of the rows (plots) and columns (seedling position in row plot). While we cannot rule out that some unknown factor in execution lead to this difference in potential escapes between the taxa, it is also possible that the data support the hypothesis that loblolly and slash × P. caribaea hybrids are not only more tolerant when infected but also more resistant to infection by F. circinatum than slash pine. Future experiments are required to test this hypothesis.
The significance of the taxa–isolate interaction limits inferences of main effects for taxa and isolate. There is some level of theoretical confounding between the fixed-effect interaction and the random effect corresponding to genetic correlation within a taxon among isolates. However, these terms are distinct in an important way: the fixed effect accounts for broad species/taxon level interactions, and the random term accounts for differences in how individual families might perform. In future analyses, it might be more appropriate to screen more families in a single taxon and to define a heterogeneous covariance structure (or a factor-analytic approximation) to account for differences between isolates.
The largest differences in taxa performance when different isolates were used are associated with the performances of loblolly pine families, where there was relatively little PPC damage and there were few families tested. Results indicate that including loblolly in the analysis to account for all records and analysis excluding escapes provides estimates for taxa and isolates that have useful interpretations. The interaction of loblolly with isolate warrants further investigation as the two isolates sourced from the extreme end range of natural loblolly distribution were demonstrably less pathogenic (Table 2, Figure 6).
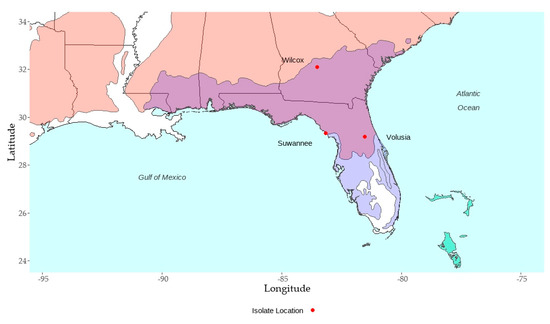
Figure 6.
Map of isolate collection locations overlaid on a map of the natural range of P. caribaea (light green, P. taeda (salmon, light purple zone overlapping with slash pine), and P. elliottii var. elliottii (overlapping light purple zone and lilac). Note that Wilcox, the northern-most isolate was demonstrated to be the most pathogenic for loblolly pine.
The Volusia isolate was most virulent for slash, least virulent for loblolly, and had the most heterogeneous response for slash × P. caribaea hybrids (Table 2, Figure 4). The Volusia isolate was the most southerly and farthest along the proposed path of Fusarium circinatum invasion from Central America [2,3,27]. The significant taxa–isolate interaction, along with observed variation in isolate performance and the stem damage ratio, motivates further study of the genetic architecture of response and virulence, including a study of longitudinal and latitudinal gradations in tolerance and virulence for tree hosts and F. circinatum isolates, respectively. This suggests that a gradation in both fungal pathogenicity and genetic tolerance (or resistance) may exist and should be explored with additional screening of both slash and loblolly families. Pathogenicity would be a more supported hypothesis given the very recent introduction of F. circinatum to the eastern end of the slash and loblolly pine ranges, following Gordon’s assertion about F. circinatum originating principally from Mexico [27]. Given that slash × P. caribaea hybrids exhibited the most heterogeneous response to the Volusia isolate, it could mean that this isolate had evolved to recognize P. caribaea less effectively than isolates taken from sources closer to its proposed Central American origin. These hypotheses support conclusions drawn by Schmidtling et al. concerning refugia of P. taeda and would further explain the putative co-evolution of P. taeda and P. caribaea varieties and pitch canker [28].
4.2. Genetic Parameters
Genetic correlations among isolates within taxa may be interpreted as a measure of the genotype-by-environment (GxE) interaction. In tree breeding, developing distinct populations for different environments is suggested if genetic correlation estimates are less than 0.66 [29]. In this study, for both data sets and among all three taxa, estimates are relatively modest (0.51–0.78) (Table 3). The strength of correlations varied among taxa and data sets, indicating that, like the case of the fixed taxa by isolate interaction term, pedigrees in different taxa relate in different ways to the virulence of a given isolate.
Estimates of narrow-sense heritability of PPC tolerance in slash pine have not been reported frequently or recently. Here, we show that heritability is strong in slash pine and hybrid P. caribaea × slash pine (Table 3). Genetic parameter estimates differ little in the analysis using both the full data set and the data set with potential escapes excluded. Heritabilities were shown to be marginally higher for the data set including potential escapes. This could be simply associated with a better normality approximation of the data or could be associated with true escapes being excluded.
In loblolly pine, estimates for narrow-sense heritability are moderate to low and are within the expected ranges for other traits (~0.15). However, the small number of tested families, generally high PPC tolerance, high standard errors, and observed interaction with isolates make population inferences inappropriate for the loblolly pine population. This experiment demonstrates considerably lower estimates than those reported by Kayihan et al. and Quesada et al. [12,19]. Assuming that there is no factor uniquely affecting experimental integrity for experimental units in loblolly, it seems that the relatively low spectrum of susceptibility limits inference on genetic differences in this study. There is evidence for a spectrum of tolerance among loblolly families, but to obtain estimates appropriate for inference, many more families would need to be screened as in Kayihan et. al. and Quesada et al. While these studies demonstrate that loblolly has a spectrum of genetic tolerance, the families screened here were so tolerant that there is little evidence that the differences are meaningful or practical to describe. The principal inference from these data concerning loblolly pine is that it is highly tolerant to PPC [16,17]. Further inferences concerning genetic control and GxE would require further specific examination.
4.3. Informing Deployment Decisions in Slash Pine
The PPC response is nearly impossible to test using traditional multi-year progeny tests for many reasons. Conditions leading to PPC infection in progeny trials offset the assessment of other objectives including stem health, environmental homogeneity, and expression of other traits under genetic control such as stem form and growth. The environmental density of spore loads of F. circinatum is functionally unpredictable with currently available tools and would not reach each tree in a test uniformly. Perhaps most importantly, PPC is often observed in stands 15 years and older, by which time the integrity of progeny trial design is compromised. The correlation between results from controlled inoculations of seedlings in the greenhouse with field resistance/tolerance is unknown for PPC disease. Significant interactions between host and isolate add complexity because it is impractical to produce estimates for all possible F. circinatum isolates. However, this limited study suggests that rank changes in the performance of families may not be practically meaningful with different isolates (Figure 5).
We report the only published effort in which rigorous screening techniques have been used to guide deployment decisions with respect to PPC in the US Southeast. For commercial deployment in the US Southeast, GxE has largely been ignored for most traits beyond broad adaptation for winter minimum temperatures [30]. In deployment, the typical commercial solution for GxE is to use the mean predicted response across the environment multiplied by intra-environment genetic correlations centered on the predicted mean for breeding value estimates, assuming a single meta-environment with isolates of similar virulence. Interactions do impact predictions; however, BLUP regression across all environments prevents families with a heterogeneous predicted performance from being deployed in risky areas and favors families with homogeneously favorable predicted performance. For commercial use in slash pine, breeding values for families that are derived from the full data set and commensurate gain calculations will be used to match tested families to sites based on historical and predicted risk for PPC infection. In slash pine, this information was integrated into the CFGRP GRADE™ tool. BLUP estimates and corresponding genetic gains cannot be described as direct predictions but are instead measures of relative risk. Gain estimates should be used to characterize and identify the most appropriate families for deployment based on perceived acceptable risk thresholds for PPC, as would be the case for other disease traits.
As in the case of other commercially relevant traits, there are many difficulties in interpreting the importance and magnitude of interactions between genotypes and environments. On one side, rigorous statistical analysis demonstrates that there are meaningful differences between predicted performances when the environment is changed by exposing a different member of a pedigree to different environments. On the other, these different predictions cannot account for how environments vary over the life of a test and indeed a commercial stand.
5. Conclusions
These data demonstrate strong evidence for genetic variation and potential for capture of genetic gain in pitch canker tolerance that may be deployed from commercially relevant southern yellow pine populations. They also confirm that meaningful differences exist in the virulence of isolates and agree with previous studies indicating tolerance to PPC varies among and within Pinus taxa. We further find that the new screening procedure offers a much more powerful design to discriminate amongst pine families for PPC tolerance and to also quantify the virulence of PPC isolates.
Author Contributions
The authors contributed to the following associated categories: Conceptualization, A.D.S., J.T.B., and G.F.P.; methodology, A.D.S. and J.T.B.; analysis, A.D.S. and J.T.B.; resources, W.P.C., D.K., and W.P.C.; data collection and curation, A.D.S., J.T.B., and K.S.; writing—original draft preparation, A.D.S.; writing—review and editing, G.F.P. and J.T.B.; visualization, A.D.S.; supervision, G.F.P.; project administration, J.T.B.; funding acquisition, J.T.B. and G.F.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by members of the UF|IFAS Cooperative Forest Genetics Research Program: ArborGen, Florida Forest Service, Four Rivers Land and Timber, the Georgia Forestry Commission, IFCO Seedlings, Rayonier, and Weyerhaeuser, and the University of Florida; the United States Department of Agriculture–National Institute of Food and Agriculture Tactical Sciences for Agricultural Biosecurity Institute of Food and Agricultural Sciences project 13117320; and McIntire-Stennis grant number FLA-PLP-005931, “Developing and scaling up the next generation of healthy forests.”
Data Availability Statement
Data will be made available by request.
Acknowledgments
We would like to thank Katie McKeever with the USDA Forest Service for assistance in implementation of this experiment at the USFS RSC and for willingness to allow implementation and publication of new screening designs.
Conflicts of Interest
The funders had no role in the design of the study or in the collection, analyses, interpretation of data and writing of the manuscript. Commercially relevant results from the loblolly pine population are limited to use by ArborGen; results for hybrid slash × P. caribaea are limited to use by HQPlantations; results from this analysis relevant to slash pine are open for equal use by all CFGRP members.
Appendix A

Table A1.
Variance components for random effects of respective models, along with standard errors (italicized), genetic correlations in bold, and their Z ratios. Each effect is statistically significant.
Table A1.
Variance components for random effects of respective models, along with standard errors (italicized), genetic correlations in bold, and their Z ratios. Each effect is statistically significant.
| Full Data Set | Reduced Data Set | |||||
|---|---|---|---|---|---|---|
| Effect | Component | s.e. | Z Ratio | Component | s.e. | Z Ratio |
| Rep*Block*Plot | 0.003 | 0.00 | 6.76 | 0.004 | 0.00 | 8.02 |
| Hybrid Correlation | 0.784 | 0.15 | 5.35 | 0.581 | 0.18 | 3.21 |
| Hybrid(Suwanee) | 0.006 | 0.00 | 2.86 | 0.015 | 0.00 | 4.49 |
| Hybrid(Volusia) | 0.028 | 0.00 | 7.85 | 0.031 | 0.00 | 8.29 |
| Hybrid(Wilcox) | 0.007 | 0.00 | 3.07 | 0.010 | 0.00 | 3.49 |
| Loblolly Correlation | 0.517 | 0.25 | 2.04 | 0.555 | 0.24 | 2.30 |
| Loblolly(Suwanee) | 0.024 | 0.00 | 5.72 | 0.031 | 0.00 | 6.28 |
| Loblolly(Volusia | 0.026 | 0.00 | 5.97 | 0.032 | 0.00 | 6.91 |
| Loblolly(Wilcox) | 0.027 | 0.00 | 6.03 | 0.043 | 0.01 | 6.29 |
| Slash Correlation | 0.623 | 0.08 | 7.95 | 0.707 | 0.07 | 9.83 |
| Slash(Suwanee) | 0.034 | 0.00 | 10.95 | 0.032 | 0.00 | 9.45 |
| Slash(Volusia) | 0.033 | 0.00 | 10.96 | 0.035 | 0.00 | 10.40 |
| Slash(Wilcox) | 0.027 | 0.00 | 9.61 | 0.026 | 0.00 | 8.29 |
| Error | 0.014 | 0.00 | 8.30 | 0.007 | 0.00 | 3.54 |
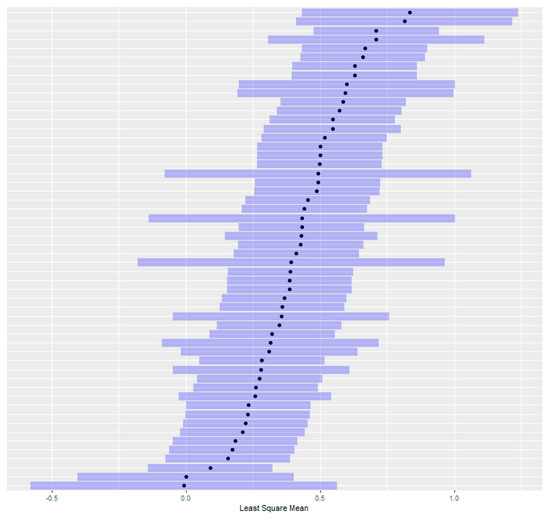
Figure A1.
Least square means of full-sib CFGRP slash pine families screened using traditional USFS RSC protocols with confidence intervals. Note that the most tolerant (lower least square mean) families have overlapping confidence intervals with the least tolerant, indicating a lack of statistical differences.
References
- Zamora-Ballesteros, C.; Diez, J.J.; Martín-García, J.; Witzell, J.; Solla, A.; Ahumada, R.; Capretti, P.; Cleary, M.; Drenkhan, R.; Dvořák, M.; et al. Pine Pitch Canker (PPC): Pathways of Pathogen Spread and Preventive Measures. Forests 2019, 10, 1158. [Google Scholar] [CrossRef]
- Wingfield, M.J.; Hammerbacher, A.; Ganley, R.J.; Steenkamp, E.T.; Gordon, T.R.; Wingfield, B.D.; Coutinho, T.A. Pitch Canker Caused by Fusarium Circinatum—A Growing Threat to Pine Plantations and Forests Worldwide. Australas. Plant Pathol. 2008, 37, 319. [Google Scholar] [CrossRef]
- Gordon, T.R. Pitch Canker Disease of Pines. Phytopathology 2006, 96, 657–659. [Google Scholar] [CrossRef] [PubMed]
- Starkey, D.; Meeker, J.; Mangini, A. Pitch Canker of Southern Pines and Recent Cases in Florida, Louisiana, Mississippi, and Texas. In National Proceedings: Forest and Conservation Nursery Associations—2006. Proc. RMRS-P-50; Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2007; pp. 97–103. [Google Scholar]
- Coutinho, T.A.; Steenkamp, E.T.; Mongwaketsi, K.; Wilmot, M.; Wingfield, M.J. First Outbreak of Pitch Canker in a South African Pine Plantation. Australas. Plant Pathol. 2007, 36, 256–261. [Google Scholar] [CrossRef]
- Hoover, K.; Wood, D.L.; Storer, A.J.; Fox, J.W.; Bros, W.E. Transmission of the Pitch Canker Fungus, Fusarium Subglutians F. Sp. Pini, to Monterey Pine, Pinus Radiata, by Cone- and Twig-Infesting Beetles. Can. Entomol. 1996, 128, 981–994. [Google Scholar] [CrossRef]
- Fisher, R.F.; Garbett, W.S.; Underhill, E.M. Effects of Fertilization on Healthy and Pitch Canker-Infected Pines. South. J. Appl. For. 1981, 5, 77–79. [Google Scholar] [CrossRef]
- Solel, Z.; Bruck, R.I. Effect of Nitrogen Fertilization and Growth Suppression on Pitch Canker Development on Loblolly Pine Seedlings. J. Phytopathol. 1989, 125, 327–335. [Google Scholar] [CrossRef]
- Lopez-Zamora, I.; Bliss, C.; Jokela, E.J.; Comerford, N.B.; Grunwald, S.; Barnard, E.; Vasquez, G.M. Spatial Relationships between Nitrogen Status and Pitch Canker Disease in Slash Pine Planted Adjacent to a Poultry Operation. Environ. Pollut. 2007, 147, 101–111. [Google Scholar] [CrossRef]
- Barnard, E.L.; Blakeslee, G.M. Pitch Canker of Slash Pine Seedlings: A New Disease in Forest Tree Nurseries. Plant Dis. 1980, 64, 695–696. [Google Scholar] [CrossRef]
- McKeand, S.; Li, B.; Amerson, H.V. Genetic Variation in Fusiform Rust Resistance in Loblolly Pine Across a Wide Geographic Range. Silvae Genet. 1999, 48, 255–260. [Google Scholar]
- Kayihan, G.C.; Huber, D.A.; Morse, A.M.; White, T.L.; Davis, J.M. Genetic Dissection of Fusiform Rust and Pitch Canker Disease Traits in Loblolly Pine. Theor. Appl. Genet. 2005, 110, 948–958. [Google Scholar] [CrossRef]
- Isik, F.; Amerson, H.V.; Whetten, R.W.; Garcia, S.A.; Li, B.; McKeand, S.E. Resistance of Pinus taeda Families under Artificial Inoculations with Diverse Fusiform Rust Pathogen Populations and Comparison with Field Trials. Can. J. For. Res. 2008, 38, 2687–2696. [Google Scholar] [CrossRef]
- Randolph, K.C.; Cowling, E.B.; Starkey, D.A. Long-Term Changes in Fusiform Rust Incidence in the Southeastern United States. J. For. 2015, 113, 381–392. [Google Scholar] [CrossRef]
- Walker, T.D.; McKeand, S.E. Fusiform Rust Hazard Mapping for Loblolly Pine in the Southeastern United States Using Progeny Test Data. J. For. 2018, 116, 117–122. [Google Scholar] [CrossRef]
- Hodge, G.R.; Dvorak, W.S. Differential Responses of Central American and Mexican Pine Species and Pinus Radiata to Infection by the Pitch Canker Fungus. New For. 2000, 19, 241–258. [Google Scholar] [CrossRef]
- Hodge, G.R.; Dvorak, W.S. Variation in Pitch Canker Resistance among Provenances of Pinus Patula and Pinus Tecunumanii from Mexico and Central America. New For. 2007, 33, 193–206. [Google Scholar] [CrossRef]
- McRae, C.H.; Rockwood, D.L.; Blakeslee, G.M. Evaluation of Slash Pine For Resistance To Pitch Canker. In Proceedings of the 18th Southern Forest Tree Improvement Conference; Forgotten Books: London, UK, 1985. [Google Scholar]
- Quesada, T.; Gopal, V.; Cumbie, W.P.; Eckert, A.J.; Wegrzyn, J.L.; Neale, D.B.; Goldfarb, B.; Huber, D.A.; Casella, G.; Davis, J.M. Association Mapping of Quantitative Disease Resistance in a Natural Population of Loblolly Pine (Pinus taeda L.). Genetics 2010, 186, 677–686. [Google Scholar] [CrossRef]
- Rockwood, D.L.; Blakeslee, G.M.; Lowerts, G.A.; Underhill, E.M.; Oak, S.W. Genetic Strategies for Reducing Pitch Canker Incidence in Slash Pine. South. J. Appl. For. 1988, 12, 28–32. [Google Scholar] [CrossRef]
- Torre, A.R.D.L.; Puiu, D.; Crepeau, M.W.; Stevens, K.; Salzberg, S.L.; Langley, C.H.; Neale, D.B. Genomic Architecture of Complex Traits in Loblolly Pine. New Phytol. 2019, 221, 1789–1801. [Google Scholar] [CrossRef]
- Quesada, T.; Lucas, S.; Smith, K.; Smith, J. Response to Temperature and Virulence Assessment of Fusarium circinatum Isolates in the Context of Climate Change. Forests 2019, 10, 40. [Google Scholar] [CrossRef]
- Fulton, J.C.; Huguet-Tapia, J.C.; Adams, S.M.; Dufault, N.S.; Quesada, T.; Brawner, J.T. Draft Genome Sequences of Three Fusarium circinatum Isolates Used to Inoculate a Pedigreed Population of Pinus elliottii Seedlings. Microbiol. Resour. Announc. 2020, 9, e00631-20. [Google Scholar] [CrossRef] [PubMed]
- Brawner, J.T.; Lee, D.J.; Hardner, C.M.; Dieters, M.J. Relationships between Early Growth and Quambalaria Shoot Blight Tolerance in Corymbia Citriodora Progeny Trials Established in Queensland, Australia. Tree Genet. Genomes 2011, 7, 759–772. [Google Scholar] [CrossRef]
- Gilmour, A.R.; Gogel, B.J.; Cullis, B.R.; Welham, S.J.; Thompson, R.; Butler, D.; Cherry, M.; Collins, D.; Dutkowski, G.; Harding, S.A.; et al. ASReml User Guide. Release 4.1 Structural Specification; VSN International Ltd.: Hemel Hempstead, UK, 2014. [Google Scholar]
- Venables, W.N.; Smith, D.M.; The R Core Team. An Introduction to R. 2014. Available online: https://cran.r-project.org/doc/manuals/r-release/R-intro.pdf (accessed on 2 January 2015).
- Gordon, T.R.; Storer, A.J.; Wood, D.L. The Pitch Canker Epidemic in California. Plant Dis. 2001, 85, 1128–1139. [Google Scholar] [CrossRef] [PubMed]
- Schmidtling, R.C.; Hipkins, V.; Carroll, E. Pleistocene Refugia for Longleaf and Loblolly Pines. In Frontiers of Forest Biology; CRC Press: Boca Raton, FL, USA, 2021; pp. 349–354. ISBN 978-1-00-321064-1. [Google Scholar]
- Burdon, R.D. Genetic Correlation as a Concept for Studying Genotype-Environment Interaction in Forest Tree Breeding. Silvae Genet. 1977, 26, 168–175. [Google Scholar]
- Lambeth, C.; McKeand, S.; Rousseau, R.; Schmidtling, R. Planting Nonlocal Seed Sources of Loblolly Pine—Managing Benefits and Risks. South. J. Appl. For. 2005, 29, 96–104. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).