Tissue Metabolic Responses to Artificial Bending and Gravitation Stimuli in Betula platyphylla
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. Anatomical Analysis
2.3. Extraction of Metabolites
2.4. Detection of Metabolites
2.5. Analytical Methods
3. Results
3.1. Growth Performance of TW
3.2. Identification of Metabolites in TW, OW and NW
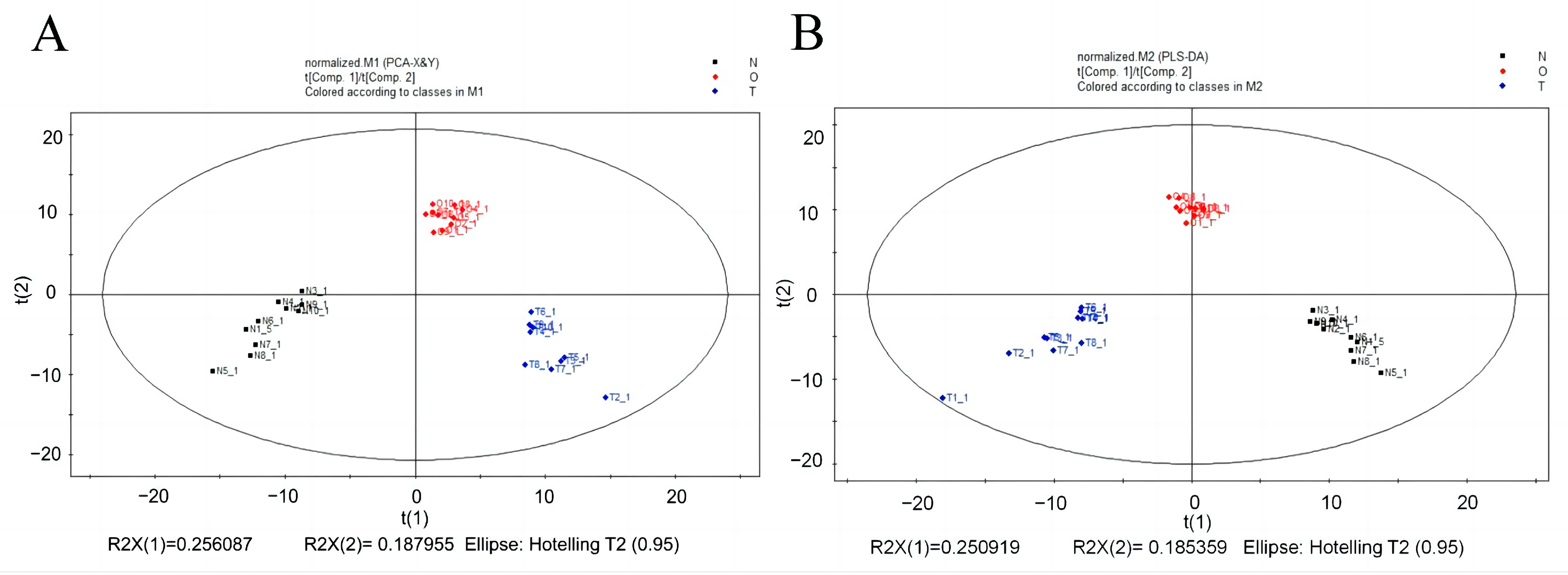
3.3. Metabolomics Analysis following Gravitational and Mechanical Bending Stimuli
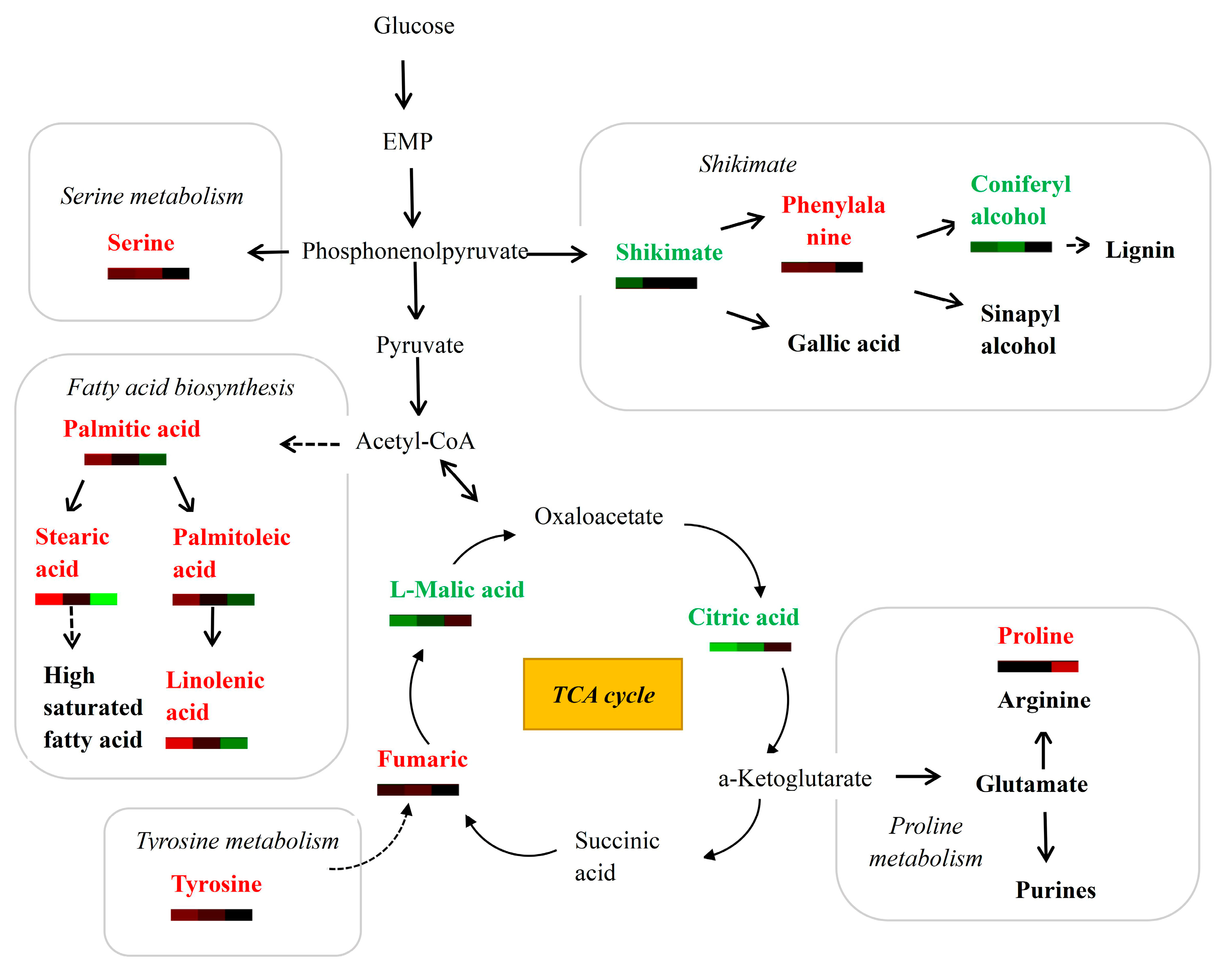
3.4. Analysis of Differential Metabolites Involved in Major Metabolic Pathways
4. Discussion
4.1. Unusual Growth and Metabolic Changes in TW, OW and NW
4.2. Metabolites Related to Cellulose and Lignin Biosynthesis Are Altered during Wood Formation
4.3. Metabolites of the TCA Cycle, Fatty-Acid Metabolism and Amino-Acid Metabolism Are Altered during Wood Formation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pauly, M.; Keegstra, K. Plant cell wall polymers as precursors for biofuels. Curr. Opin. Plant Biol. 2010, 13, 305–312. [Google Scholar] [CrossRef]
- Lachaud, S.; Catesson, A.M.; Bonnemain, J.L. Structure and functions of the vascular cambium. C. R. Acad. Sci. III 1999, 322, 633–650. [Google Scholar] [CrossRef] [PubMed]
- Roignant, J.; Badel, É.; Leblanc-Fournier, N.; Brunel-Michac, N.; Ruelle, J.; Moulia, B.; Decourteix, M. Feeling stretched or compressed? The multiple mechanosensitive responses of wood formation to bending. Ann. Bot. 2018, 121, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Scurfield, G. Reaction Wood: Its Structure and Function Lignification may generate the force active in restoring the trunks of leaning trees to the vertica. Science 1973, 179, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Jourez, A.; Riboux, A.; Leclercq, B. Anatomical characteristics of tension wood and opposite wood in young inclined stems of poplar (Populus euramericana cv ‘Ghoy’). Iawa J. 2001, 22, 133–157. [Google Scholar] [CrossRef]
- Nishikubo, N.; Awano, T.; Banasiak, A.; Bourquin, V.; Ibatullin, F.; Funada, R.; Brumer, H.; Teeri, T.T.; Hayashi, T.; Sundberg, B.; et al. Xyloglucan endo-transglycosylase (XET) functions in gelatinous layers of tension wood fibers in poplar--a glimpse into the mechanism of the balancing act of trees. Plant Cell Physiol. 2007, 48, 843–855. [Google Scholar] [CrossRef]
- Joseleau, J.P.; Imai, T.; Kuroda, K.; Ruel, K. Detection in situ and characterization of lignin in the G -layer of tension wood fibres of Populus deltoides. Planta 2004, 219, 338–345. [Google Scholar] [CrossRef]
- Gierlinger, N.; Schwanninger, M. Chemical imaging of poplar wood cell walls by confocal Raman microscopy. Plant Physiol. 2006, 140, 1246–1254. [Google Scholar] [CrossRef]
- Gritsch, C.; Wan, Y.; Mitchell, R.A.; Shewry, P.R.; Hanley, S.J.; Karp, A. G-fibre cell wall development in willow stems during tension wood induction. J. Exp. Bot. 2015, 66, 6447–6459. [Google Scholar] [CrossRef]
- McNeil, M.; Darvill, A.G.; Fry, S.C.; Albersheim, P. Structure and function of the primary cell walls of plants. Annu. Rev. Biochem. 1984, 53, 625–663. [Google Scholar] [CrossRef]
- Andersson-Gunnerås, S.; Mellerowicz, E.J.; Love, J.; Segerman, B.; Ohmiya, Y.; Coutinho, P.M.; Nilsson, P.; Henrissat, B.; Moritz, T.; Sundberg, B. Biosynthesis of cellulose-enriched tension wood in Populus: Global analysis of transcripts and metabolites identifies biochemical and developmental regulators in secondary wall biosynthesis. Plant J. 2006, 45, 144–165. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhao, X.; Bai, C.; Zhao, C.; Lu, G.; Xu, G. LC-MS-based metabonomics analysis. J. Chromatogr. B Analyt. Technol. Biomed Life Sci. 2008, 866, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Liu, Y.; Liu, J.; Ren, Y.; Liu, Y.; Sun, F.; Abozeid, A.; Tang, Z.; Mu, L. Comparison of Seasonally Adaptive Metabolic Response Strategies of TwoAcerSpecies. Forests 2022, 13, 2141. [Google Scholar] [CrossRef]
- Griffin, J.L.; Walker, L.A.; Garrod, S.; Holmes, E.; Shore, R.F.; Nicholson, J.K. NMR spectroscopy based metabonomic studies on the comparative biochemistry of the kidney and urine of the bank vole (Clethrionomys glareolus), wood mouse (Apodemus sylvaticus), white toothed shrew (Crocidura suaveolens) and the laboratory rat. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2000, 127, 357–367. [Google Scholar] [CrossRef]
- Fiehn, O. Extending the breadth of metabolite profiling by gas chromatography coupled to mass spectrometry. Trends Analyt. Chem. 2008, 27, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chang, Q.-Y.; Tang, Z.-H.; Wu, K.-X.; Abozeid, A.; Mu, L.-Q. The Interrelationship between Latitudinal Differences and Metabolic Differences in the Natural Distribution Area ofTilia amurensis Rupr. Forests 2022, 13, 1507. [Google Scholar] [CrossRef]
- Shulaev, V.; Cortes, D.; Miller, G.; Mittler, R. Metabolomics for plant stress response. Physiol. Plant. 2008, 132, 199–208. [Google Scholar] [CrossRef]
- Hong, J.; Yang, L.; Zhang, D.; Shi, J. Plant Metabolomics: An Indispensable System Biology Tool for Plant Science. Int. J. Mol. Sci. 2016, 17, 767. [Google Scholar] [CrossRef]
- Widodo; Patterson, J.H.; Newbigin, E.; Tester, M.; Bacic, A.; Roessner, U. Metabolic responses to salt stress of barley (Hordeum vulgare L.) cultivars, Sahara and Clipper, which differ in salinity tolerance. J. Exp. Bot. 2009, 60, 4089–4103. [Google Scholar] [CrossRef]
- Wu, T.; Zou, R.; Pu, D.; Lan, Z.; Zhao, B. Non-targeted and targeted metabolomics profiling of tea plants (Camellia sinensis) in response to its intercropping with Chinese chestnut. BMC Plant Biol. 2021, 21, 55. [Google Scholar] [CrossRef]
- Rastogi, S.; Pandey, M.M.; Kumar Singh Rawat, A. Medicinal plants of the genus Betula--traditional uses and a phytochemical-pharmacological review. J. Ethnopharmacol. 2015, 159, 62–83. [Google Scholar] [CrossRef]
- Ekman, R. The Suberin Monomers and Triterpenoids from the Outer Bark of Betula verrucosa Ehrh. Holzforsch.—Int. J. Biol. Chem. Phys. Technol. Wood 1983, 37, 205–211. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, N.; Gao, C.; Cui, Z.; Sun, D.; Yang, C.; Wang, Y. Comprehensive transcriptome analysis of developing xylem responding to artificial bending and gravitational stimuli in Betula platyphylla. PLoS ONE 2014, 9, e87566. [Google Scholar] [CrossRef]
- Lisec, J.; Schauer, N.; Kopka, J.; Willmitzer, L.; Fernie, A.R. Gas chromatography mass spectrometry-based metabolite profiling in plants. Nat. Protoc. 2006, 1, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.H.; Singhal, R.; Kachroo, A.; Kachroo, P. Fatty Acid- and Lipid-Mediated Signaling in Plant Defense. Annu. Rev. Phytopathol. 2017, 55, 505–536. [Google Scholar] [CrossRef] [PubMed]
- Mellerowicz, E.J.; Sundberg, B. Wood cell walls: Biosynthesis, developmental dynamics and their implications for wood properties. Curr. Opin. Plant Biol. 2008, 11, 293–300. [Google Scholar] [CrossRef]
- Rogers, L.A.; Dubos, C.; Cullis, I.F.; Surman, C.; Poole, M.; Willment, J.; Mansfield, S.D.; Campbell, M.M. Light, the circadian clock, and sugar perception in the control of lignin biosynthesis. J. Exp. Bot. 2005, 56, 1651–1663. [Google Scholar] [CrossRef] [PubMed]
- Schauer, N.; Semel, Y.; Roessner, U.; Gur, A.; Balbo, I.; Carrari, F.; Pleban, T.; Perez-Melis, A.; Bruedigam, C.; Kopka, J.; et al. Comprehensive metabolic profiling and phenotyping of interspecific introgression lines for tomato improvement. Nat. Biotechnol. 2006, 24, 447–454. [Google Scholar] [CrossRef]
- Endler, A.; Persson, S. Cellulose Synthases and Synthesis in Arabidopsis. Mol. Plant 2011, 4, 199–211. [Google Scholar] [CrossRef]
- Chang, S.S.; Clair, B.; Ruelle, J.; Beauchêne, J.; Di Renzo, F.; Quignard, F.; Zhao, G.J.; Yamamoto, H.; Gril, J. Mesoporosity as a new parameter for understanding tension stress generation in trees. J. Exp. Bot. 2009, 60, 3023–3030. [Google Scholar] [CrossRef]
- Delmer, D.P.; Haigler, C.H. The regulation of metabolic flux to cellulose, a major sink for carbon in plants. Metab. Eng. 2002, 4, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Joshi, C.P.; Mansfield, S.D. The cellulose paradox-simple molecule, complex biosynthesis. Curr. Opin. Plant Biol. 2007, 10, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Huang, H.; Ni, F.; Tong, Z.; Lin, E.; Zhu, M. RNA-Seq analysis of differential gene expression in Betula luminifera xylem during the early stages of tension wood formation. PeerJ 2018, 6, e5427. [Google Scholar] [CrossRef]
- Babb, V.; Haigler, C.H. Sucrose phosphate synthase activity rises in correlation with high-rate cellulose synthesis in three heterotrophic systems. Plant Physiol. 2001, 127, 1234–1242. [Google Scholar] [CrossRef]
- Holbrook, N.M.; PUTZ, F.E. Influence of neighbors on tree form—Effects of lateral shade and prevention of sway on the allometry of liquidambar-styraciflua (sweet gum). Amer. J. Bot. 1989, 76, 1740–1749. [Google Scholar] [CrossRef]
- Douglas, C.J. Phenylpropanoid metabolism and lignin biosynthesis: From weeds to trees. Trends Plant Sci. 1996, 1, 171–178. [Google Scholar] [CrossRef]
- Li, S.; Su, X.; Abdullah, M.; Sun, Y.; Li, G.; Cheng, X.; Lin, Y.; Cai, Y.; Jin, Q. Effects of Different Pollens on Primary Metabolism and Lignin Biosynthesis in Pear. Int. J. Mol. Sci. 2018, 19, 2273. [Google Scholar] [CrossRef]
- Deng, M.; Li, D.; Luo, J.; Xiao, Y.; Liu, H.; Pan, Q.; Zhang, X.; Jin, M.; Zhao, M.; Yan, J. The genetic architecture of amino acids dissection by association and linkage analysis in maize. Plant Biotechnol. J. 2017, 15, 1250–1263. [Google Scholar] [CrossRef]
- Alejandro, S.; Lee, Y.; Tohge, T.; Sudre, D.; Osorio, S.; Park, J.; Bovet, L.; Lee, Y.; Geldner, N.; Fernie, A.R.; et al. AtABCG29 is a monolignol transporter involved in lignin biosynthesis. Curr. Biol. 2012, 22, 1207–1212. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.C.; Liu, C.J. ATP-binding cassette-like transporters are involved in the transport of lignin precursors across plasma and vacuolar membranes. Proc. Natl. Acad. Sci. USA 2010, 107, 22728–22733. [Google Scholar] [CrossRef]
- Bonawitz, N.D.; Chapple, C. The genetics of lignin biosynthesis: Connecting genotype to phenotype. Annu. Rev. Genet. 2010, 44, 337–363. [Google Scholar] [CrossRef]
- Eloy, N.B.; Voorend, W.; Lan, W.; Saleme, M.L.; Cesarino, I.; Vanholme, R.; Smith, R.A.; Goeminne, G.; Pallidis, A.; Morreel, K.; et al. Silencing CHALCONE SYNTHASE in Maize Impedes the Incorporation of Tricin into Lignin and Increases Lignin Content. Plant Physiol. 2017, 173, 998–1016. [Google Scholar] [CrossRef]
- Vogt, T. Phenylpropanoid Biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef] [PubMed]
- Tzin, V.; Galili, G. New Insights into the Shikimate and Aromatic Amino Acids Biosynthesis Pathways in Plants. Molecular Plant 2010, 3, 956–972. [Google Scholar] [CrossRef]
- Owen, O.E.; Kalhan, S.C.; Hanson, R.W. The Key Role of Anaplerosis and Cataplerosis for Citric Acid Cycle Function. J. Biol. Chem. 2002, 277, 30409–30412. [Google Scholar] [CrossRef]
- Mcgarry, J.D. The regulation of ketogenesis from octanoic acid. The role of the tricarboxylic acid cycle and fatty acid synthesis. J. Biol. Chem. 1971, 246, 1149–1159. [Google Scholar] [CrossRef] [PubMed]
- Galili, G.; Amir, R.; Fernie, A.R. The Regulation of Essential Amino Acid Synthesis and Accumulation in Plants. Annu. Rev. Plant Biol. 2016, 67, 153–178. [Google Scholar] [CrossRef]
- Ghanati, F.; Morita, A.; Yokota, H. Deposition of suberin in roots of soybean induced by excess boron. Plant Sci. 2005, 168, 397–405. [Google Scholar] [CrossRef]
- Korpinen, R.I.; Kilpeläinen, P.; Sarjala, T.; Nurmi, M.; Saloranta, P.; Holmbom, T.; Koivula, H.; Mikkonen, K.S.; Willför, S.; Saranpää, P.T. The Hydrophobicity of Lignocellulosic Fiber Network Can Be Enhanced with Suberin Fatty Acids. Molecules 2019, 24, 4391. [Google Scholar] [CrossRef]
- Vélot, C.; Mixon, M.B.; Teige, M.; Srere, P.A. Model of a quinary structure between Krebs TCA cycle enzymes: A model for the metabolon. Biochemistry 1997, 36, 14271–14276. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Minteer, S. Krebs Cycle Metabolon: Structural Evidence of Substrate Channeling Revealed by Cross-Linking and Mass Spectrometry. Angew. Chem. Int. Ed. Engl. 2015, 54, 1851–1854. [Google Scholar] [CrossRef]
- Patil, N.K.; Bohannon, J.K.; Hernandez, A.; Patil, T.K.; Sherwood, E.R. Regulation of leukocyte function by citric acid cycle intermediates. J. Leukoc. Biol. 2019, 106, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Xu, X.; Xie, Y.; Huang, T.; Wang, W.; Zhao, L.; Ma, D. Comparative metabolomic analysis of the metabolismpathways under drought stress in alfalfa leaves—ScienceDirect. Environ. Exp. Bot. 2020, 183, 104329. [Google Scholar] [CrossRef]
- Hu, Z.; Yan, W.; Yang, C.; Huang, X.; Hu, X.; Li, Y.; Yang, J.; Xiang, S.; Yi, P.; Hu, R. Integrative analysis of transcriptome and metabolome provides insights into the underlying mechanism of cold stress response and recovery in two tobacco cultivars. Environ. Exp. Bot. 2022, 200, 104920. [Google Scholar] [CrossRef]
- Kallio, H. Aroma of birch syrup. Agric. Food Chem. 1989, 37, 1367–1371. [Google Scholar] [CrossRef]
- Reszczyńska, E.; Hanaka, A. Lipids Composition in Plant Membranes. Cell Biochem. Biophys. 2020, 78, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Tüfekci, F.; Karataş, Ş. Determination of geographical origin Turkish hazelnuts according to fatty acid composition. Food Sci. Nutr. 2018, 6, 557–562. [Google Scholar] [CrossRef]
- Ahokas, H. Cytokinins in the spring sap of curly birch (Betula pendula F. Carelica) and the noncurly form. Plant Physiol. 1985, 118, 33–39. [Google Scholar] [CrossRef]



| RT | Name | Relative Contents (N) | RT | Name | Relative Contents (O) | RT | Name | Relative Contents (T) |
|---|---|---|---|---|---|---|---|---|
| 20.1654 | Glucose 2 | 5020.921 | 20.1654 | Glucose 2 | 9314.5496 | 20.1654 | Glucose 2 | 6869.254 |
| 19.9109 | Glucose 2 | 4315.896 | 29.1436 | Isopropyl-beta-D-thiogalactopyranoside | 1330.0843 | 29.1436 | Isopropyl-beta-D-thiogalactopyranoside | 1570.597 |
| 23.0188 | Myo-inositol | 1051.433 | 14.2641 | Threonic acid | 1173.1317 | 23.1448 | Linoleic acid methyl ester | 1371.975 |
| 23.1448 | Linoleic acid methyl ester | 830.8744 | 23.0188 | Myo-inositol | 1166.3295 | 23.0188 | Myo-inositol | 1027.831 |
| 10.494 | Phosphate | 637.1568 | 23.1448 | Linoleic acid methyl ester | 910.87259 | 14.0999 | 4-aminobutyric acid 1 | 1012.181 |
| 29.1854 | Isopropyl-beta-D-thiogalactopyranoside | 603.3503 | 26.1339 | Lactobionic acid 1 | 518.13836 | 10.5093 | Ethanolamine | 601.9354 |
| 10.5093 | Ethanolamine | 580.8495 | 14.0999 | 4-aminobutyric acid 1 | 482.81804 | 26.1339 | Lactobionic Acid 1 | 518.7005 |
| 17.4188 | Glucose-1-phosphate | 70.06666 | 10.5093 | Ethanolamine | 455.46538 | 14.0543 | Oxoproline | 455.243 |
| 14.2641 | Threonic acid | 408.3579 | 29.1854 | Isopropyl-beta-D-thiogalactopyranoside | 403.35333 | 34.5937 | Galactinol 1 | 417.118 |
| 14.0543 | Oxoproline | 399.0452 | 14.0543 | Oxoproline | 329.90639 | 14.2641 | Threonic acid | 353.2065 |
| 14.0999 | 4-Aminobutyric acid 1 | 361.931 | 10.494 | Phosphate | 299.67554 | 8.58041 | Sarcosine | 310.7912 |
| 26.1339 | Lactobionic acid 1 | 360.4487 | 10.427 | Glycerol | 287.11954 | 18.237 | Shikimic acid | 110.15552 |
| 26.8392 | Thioctamide 1 | 332.7942 | 26.8392 | Thioctamide 1 | 174.71152 | 18.3135 | Mucic acid | 236.3835 |
| 34.5937 | Galactinol 1 | 234.7089 | 19.4666 | Fructose 1 | 160.4597 | 10.427 | Glycerol | 215.723 |
| 19.4666 | Fructose 1 | 174.0858 | 19.9109 | Glucose 2 | 152.188 | 10.494 | Phosphate | 203.4365 |
| 23.106 | Myo-inositol | 171.5473 | 18.3135 | Mucic acid | 146.27249 | 22.3688 | Palmitic acid | 193.1643 |
| 10.427 | Glycerol | 167.293 | 34.5937 | Galactinol 1 | 129.93732 | 20.2773 | Methyl palmitoleate | 187.0347 |
| 22.3688 | Palmitic acid | 147.4451 | 18.6615 | Citrulline 1 | 77.745466 | 18.6615 | Citrulline 1 | 163.7387 |
| 18.3135 | Mucic acid | 138.6985 | 20.2773 | Methyl palmitoleate | 121.8277 | 16.0655 | Fructose 1 | 101.4002 |
| 17.6124 | Glucose-1-phosphate | 131.2507 | 8.58041 | Sarcosine | 112.65931 | 29.1854 | Isopropyl-beta-D-thiogalactopyranoside | 90.85038 |
| 8.58041 | Sarcosine | 79.43472 | 18.237 | Shikimic acid | 244.1021 | 24.6155 | Linoleic acid | 147.2064 |
| 20.2773 | Methyl palmitoleate | 113.6915 | 13.81 | Aspartic acid 1 | 107.66232 | 16.2662 | 2,2-Dimethylsuccinic acid | 111.4056 |
| 24.6155 | Linoleic acid | 90.54071 | 16.0655 | Fructose 1 | 86.698988 | 13.81 | Aspartic acid 1 | 129.8358 |
| 14.6339 | 2-Deoxyerythritol | 72.07337 | 16.2662 | 2,2-Dimethylsuccinic acid | 72.220634 | 26.8392 | Thioctamide 1 | 129.7325 |
| 20.7827 | Gluconic lactone 2 | 85.46759 | 22.3688 | Palmitic acid | 74.378682 | 9.8543 | Valine | 118.6639 |
| 30.4995 | Xylitol | 82.69117 | 24.9673 | 3-Hydroxynorvaline 2 | 78.455361 | 19.4666 | Fructose 1 | 116.9403 |
| Number of Differentially Abundant Metabolites | |||
|---|---|---|---|
| TW vs. OW | TW vs. NW | OW vs. NW | |
| Total | 85 | 99 | 90 |
| Upregulated | 62 | 62 | 38 |
| Downregulated | 23 | 37 | 52 |
| RT | Name | PubChem | KEGG | KEGG Pathway | VIP | |
|---|---|---|---|---|---|---|
| T/O | 18.4685 | Citric acid | 311 | C00158 | TCA cycle | 1.79807 |
| 13.393 | cis-1,2-Dihydronaphthalene-1,2-diol | C04314 | Degradation of aromatic compounds | 1.78826 | ||
| 24.6155 | Linoleic acid | 3931 | C01595 | Linoleic-acid metabolism | 1.77608 | |
| 24.6943 | Linolenic acid | 860 | C06427 | Alpha-linolenic-acid metabolism | 1.75496 | |
| 25.8734 | N-Methyl-L-glutamic acid 3 | C01046 | Methane metabolism | 1.75403 | ||
| 27.2841 | Analyte 368 | 1.74 | ||||
| 23.74 | Pentadecanoic acid | 13,849 | 1.74 | |||
| 20.5483 | Methyl heptadecanoate | 1.72874 | ||||
| 22.3688 | Palmitic acid | 985 | C00249 | Fatty-acid biosynthesis | 1.72 | |
| 27.0315 | Stearic acid | 5281 | C01530 | Fatty-acid biosynthesis | 1.72 | |
| T/N | 28.3075 | Arbutin | C06186 | Glycolysis/gluconeogenesis | 1.65816 | |
| 13.393 | cis-1,2-Dihydronaphthalene-1,2-diol | C04314 | Degradation of aromatic compounds | 1.65002 | ||
| 19.9514 | Hexadecane | 10,459 | C08260 | 1.62956 | ||
| 11.4168 | Uracil | 1174 | C00106 | Pyrimidine metabolism | 1.61431 | |
| 28.5472 | 1-Monopalmitin | 1.5909 | ||||
| 8.58041 | Sarcosine | 1088 | C00213 | Arginine and proline metabolism | 1.58845 | |
| 9.8543 | Valine | 1182 | C00183 | Valine, leucine and isoleucine biosynthesis | 1.57943 | |
| 20.7827 | Gluconic lactone 2 | 7027 | C00198 | Pentose-phosphate pathway | 1.5779 | |
| 9.24869 | 4-aminobutyric acid 3 | 12,025 | C11118 | Arginine and proline metabolism | 1.56024 | |
| 19.629 | Gluconic lactone 1 | 7027 | C00198 | Pentose-phosphate pathway | 1.54458 | |
| O/N | 24.6155 | Linoleic acid | 3931 | C01595 | Linoleic-acid metabolism | 1.70565 |
| 20.0337 | 2-Keto-L-gulonic acid | 440,390 | 1.69295 | |||
| 24.6943 | Linolenic acid | 860 | C06427 | Alpha-linolenic-acid metabolism | 1.6928 | |
| 27.2841 | Analyte 368 | 1.66915 | ||||
| 15.2173 | (+-)-Dihydrocarveol | 1.66557 | ||||
| 22.3688 | Palmitic acid | 985 | C00249 | Fatty-acid metabolism | 1.66189 | |
| 24.4579 | Thymidine 5’-monophosphate 1 | 9700 | C00364 | Pyrimidine metabolism | 1.65926 | |
| 23.74 | Pentadecanoic acid | 13,849 | 1.63717 | |||
| 10.815 | 1,5-Anhydroglucitol | 64,960 | C07326 | 1.63427 | ||
| 10.6488 | Deoxyerythritol | 1.62735 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chi, Y.; Zhang, N.; Zou, A.; Yu, Y.; Wang, Y.; Wang, C. Tissue Metabolic Responses to Artificial Bending and Gravitation Stimuli in Betula platyphylla. Forests 2023, 14, 457. https://doi.org/10.3390/f14030457
Chi Y, Zhang N, Zou A, Yu Y, Wang Y, Wang C. Tissue Metabolic Responses to Artificial Bending and Gravitation Stimuli in Betula platyphylla. Forests. 2023; 14(3):457. https://doi.org/10.3390/f14030457
Chicago/Turabian StyleChi, Yao, Nan Zhang, Ao Zou, Ying Yu, Yucheng Wang, and Chao Wang. 2023. "Tissue Metabolic Responses to Artificial Bending and Gravitation Stimuli in Betula platyphylla" Forests 14, no. 3: 457. https://doi.org/10.3390/f14030457
APA StyleChi, Y., Zhang, N., Zou, A., Yu, Y., Wang, Y., & Wang, C. (2023). Tissue Metabolic Responses to Artificial Bending and Gravitation Stimuli in Betula platyphylla. Forests, 14(3), 457. https://doi.org/10.3390/f14030457






